Abstract
• Background and Aims The negative logarithmic relationship between orthodox seed longevity and moisture content in hermetic storage is subject to a low-moisture-content limit (mc), but is mc affected by temperature?
• Methods Red clover (Trifolium pratense) and alfalfa (Medicago sativa) seeds were stored hermetically at 12 moisture contents (2–15 %) and five temperatures (–20, 30, 40, 50 and 65 °C) for up to 14·5 years, and loss in viability was estimated.
• Key Results Viability did not change during 14·5 years hermetic storage at −20 °C with moisture contents from 2·2 to 14·9 % for red clover, or 2·0 to 12·0 % for alfalfa. Negative logarithmic relationships between longevity and moisture contents >mc were detected at 30–65 °C, with discontinuities at low moisture contents; mc varied between 4·0 and 5·4 % (red clover) or 4·2 and 5·5 % (alfalfa), depending upon storage temperature. Within the ranges investigated, a reduction in moisture content below mc at any one temperature had no effect on longevity. Estimates of mc were greater the cooler the temperature, the relationship (P < 0·01) being curvilinear. Above mc, the estimates of CH and CQ (i.e. the temperature term of the seed viability equation) did not differ (P > 0·10) between species, whereas those of KE and CW did (P < 0·001).
• Conclusions The low-moisture-content limit to negative logarithmic relationships between seed longevity and moisture content in hermetic storage increased the cooler the storage temperature, by approx. 1·5 % over 35 °C (4·0–4·2 % at 65 °C to 5·4–5·5 % at 30–40 °C) in these species. Further reduction in moisture content was not damaging. The variation in mc implies greater sensitivity of longevity to temperature above, compared with below, mc. This was confirmed (P < 0·005).
Keywords: Red clover Trifolium pratense, alfalfa Medicago sativa, hermetic seed storage, seed longevity, seed viability equation, temperature, seed desiccation, critical moisture content, equilibrium relative humidity, Q10
INTRODUCTION
Some four decades ago, Nutile (1964) showed that whereas orthodox (Roberts, 1973) seeds survived desiccation to very low moisture contents (<1 %), evidence of desiccation damage to very dry seeds (<1 or 2 % moisture content, depending upon the species) emerged during 5 years subsequent storage at room temperature. The improved seed viability equation (Ellis and Roberts, 1980) demonstrated a negative logarithmic relationship between seed longevity and moisture content. That finding revealed that the benefit of considerable desiccation to seed survival was much greater than had been expected hitherto. However, how low can seeds be dried and not merely survive the immediate desiccation but also benefit their longevity?
In sesame (Sesamum indicum) seeds stored hermetically at 50 °C for 4 years, the negative logarithmic relationship was continuous down to 2 %, the lowest moisture content investigated (Ellis et al., 1986). Subsequent research with three contrasting species at the still warmer temperature of 65 °C discovered a low-moisture-content-limit (mc) to this relationship, below which further desiccation (within the range investigated) had no further effect on longevity (Ellis et al., 1988). Research with considerably more species confirmed these observations and showed that although mc varied greatly among species (from 2 to 6 %), true seed moisture status was more stable at approx. 10–12 % equilibrium relative humidity when sealed at 20 °C for subsequent hermetic storage (Ellis et al., 1989, 1990a, b, 1992). Roberts and Ellis (1989) posited that the difference in the response of longevity to moisture contents above and below mc was a consequence of the different types of water present in seeds. Thus, removal of weakly bound water improves longevity but, once this is all removed, the withdrawal of strongly bound water (below approx. −350 MPa) by further desiccation has no additional effect because strongly bound water has negligible chemical potential.
The implication from the above research that seeds should be dried to moisture contents in equilibrium with approx. 10–12 % relative humidity at 20 °C to maximize longevity in subsequent storage was countered by Vertucci and Roos (1990, 1991, 1993a, b). From their research on seed vigour and viability following short-term storage over saturated salt solutions at temperatures <50 °C, they concluded that such values are substantially below the optimum seed moisture content for storage and, moreover, that desiccation below the optimum greatly increases seed storage deterioration.
Here we report a 15 year investigation begun in 1989 of the effect of the temperature of hermetic storage on estimates of mc, and on relationships between seed longevity and moisture above and below mc, in two forage legumes. A 15 year study was required because of the considerable longevity of seeds stored at low moisture contents. Forage legumes were selected for study because they had not been included in our earlier research.
MATERIALS AND METHODS
Single seed lots of red clover (Trifolium pratense L.) ‘Granta’ and alfalfa (Medicago sativa) ‘Europe’ (Elsoms Seeds, UK) were selected for investigation. Both were stored hermetically at −20±2 °C (with initial moisture contents of 10·2 %) between receipt (March 1987) and the beginning of this research (August 1989).
Seed moisture content was adjusted to 12 (T. pratense) or 13 different values (M. sativa) between 2 and 15 % either by drying at 12–15 % relative humidity and 15 °C to about 7–8 % moisture content (approx. 5 days) and then further drying over regularly regenerated silica gel at 20 °C to about 2 % moisture content (approx. 10 days), or by humidification above water in a closed container at 20 °C. After equilibrating for 3 d at 3–5 °C in sealed containers, seed moisture contents were determined using two 3–4 g samples by the high constant temperature oven method (130 ± 3 °C) for 2 h (International Seed Testing Association, 1985, 2005). Moisture contents were calculated on the wet basis (wb). The equilibrium relative humidity of each moisture content treatment was determined at 20 °C using a Novasina Humidat IC1 (Zürich) previously calibrated at 11, 55 and 90 % relative humidity.
Eleven to 15 sub-samples, each of about 420 seeds for each species at each moisture content, were sealed in laminated aluminium foil packets (4 × 4 cm), and stored at 65, 50, 40, 30 ± 1 °C and −20 ± 2 °C. The temperature within each incubator was monitored and logged every 20 s using a data logging system (TempScan/1000A, IOtech, Ohio, USA) and the mean temperature during experimental storage was calculated. Samples were removed from storage at intervals varying from 20 min to 730 d, depending on temperature and moisture content, for periods up to 5277 d. All samples, together with controls (not stored) for each moisture content and species, were then tested for germination. Before testing these samples for germination, the seeds at <10 % moisture content were humidified above water at 20 °C for 1 d to avoid the possibility of imbibition injury during rehydration (Ellis et al., 1985). Seeds were tested on top of two filter papers (Whatman 181) moistened with 4·5 ml of deionized water, with six replicates of 70 seeds per test, at 20 °C. The test durations were extended from the prescribed 10 d (International Seed Testing Association, 2005) to 28 d in order to enable hard seeds to be identified, scarified after 14–21 d and tested for a further period to determine whether they were capable of germination. To scarify, seeds were gently rubbed on fine sand paper. All seedlings were evaluated according to the criterion of normal germination (International Seed Testing Association, 2005). The equilibrium relative humidity of the last seed samples tested in 2004 which had been stored at 40, 30 and −20 °C was determined using an AquaLab CX2 (Decagon Devices, Pullman, WA, USA) at 20 °C, previously calibrated at 11, 25, 50 and 75 % relative humidity.
The seed survival results were analysed by probit analysis (seed survival curves) and linear and multiple regression analysis (response to environment) using GENSTAT (Genstat 5, 1997) in accordance with the seed viability equation (Ellis and Roberts, 1980). This has two components. The first
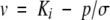 |
1 |
describes the seed survival curve where v is probit percentage viability, σ is the standard deviation of the frequency distribution of seed deaths in time (d), p is the period of storage (d) and Ki is the intercept. The second
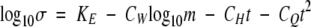 |
2 |
is the relationship between seed longevity (σ), storage temperature (t,°C) and moisture content (m, % wb), where KE, CW, CH and CQ are constants. At a single temperature, eqn (2) simplifies to
 |
3 |
where
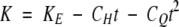 |
4 |
RESULTS
Equilibrium relative humidity
The isotherms for both species were quite similar (Fig. 1). In general, those treatments providing estimates of equilibrium relative humidity <15 % in 1989 were slightly greater in 2004, whereas those initially >30 % relative humidity were slightly reduced in 2004 (Table 1).
Fig. 1.
Relationships between seed moisture content (%, wb) and the equilibrium relative humidity (%) determined at 20 °C for Trifolium pratense and Medicago sativa.
Table 1.
Seed equilibrium relative humidity (%) estimated at 20 °C in 1989 (Novasina Humidat IC1) and 2004 (AquaLab CX2) for hermetic storage in laminated aluminium foil packets at different moisture contents and temperatures
| Species | Year | Temperature (°C) | Equilibrium relative humidity (%) determined at 20 °C (moisture content, %, wb) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. pratense | 1989 | 9·8 (2·2) | 10·1 (2·3) | 10·3 (2·5) | 10·5 (3·7) | 10·9 (4·1) | 11·7 (4·7) | 13·4 (5·5) | 16·3 (6·1) | 30·8 (7·6) | 53·8 (9·9) | 69·0 (12·3) | 79·8 (14·9) | ||
| 2004 | 40 | 13·4 | 14·1 | 14·3 | 13·4 | 13·2 | 13·3 | 14·0 | 15·7 | ||||||
| 30 | 14·1 | 14·1 | 13·8 | 14·1 | 13·4 | 13·7 | 15·7 | 16·6 | 21·3 | ||||||
| –20 | 16·9 | 16·8 | 15·9 | 25·9 | 18·2 | 17·0 | 17·3 | 27·7 | 32·5 | 49·0 | 59·5 | 66·7 | |||
| M. sativa | 1989 | 9·6 (2·0) | 9·9 (2·5) | 10·0 (2·9) | 10·2 (3·5) | 10·4 (4·2) | 11·2 (4·8) | 13·5 (5·5) | 16·7 (6·1) | 28·8 (7·0) | 44·1 (8·0) | 66·6 (10·2) | 76·1 (12·6) | 85·2 (15·2) | |
| 2004 | 40 | 13·3 | 13·8 | 12·8 | 12·9 | 12·5 | 14·1 | 13·9 | |||||||
| 30 | 13·9 | 14·0 | 13·7 | 13·9 | 13·7 | 13·8 | 14·0 | 15·7 | 18·6 | 18·7 | |||||
| –20 | 9·5 | 12·8 | 14·0 | 14·0 | 19·2 | 17·1 | 20·2 | 28·5 | 30·1 | 38·8 | 58·2 | 63·8 | 74·9 | ||
Seed survival in hermetic storage
The initial adjustment of moisture content (from 10·2 %) had no effect on viability (Fig. 2). During almost 15 years hermetic storage at −20 °C, no loss in seed viability (P > 0·25) was detected at moisture contents between 2·2 and 14·9 % in red clover, or between 2 and 12·6 % in alfalfa. However, 13 % loss of viability occurred in alfalfa at 15·2 % moisture content. Over this period at 30 °C, some 50–65 % of red clover seeds at 2·2–4·1 %, and some 80 or 85 % of alfalfa seeds at 2·9 down to 2·0 % moisture content, respectively, survived. In contrast, loss in viability was more rapid at greater moisture contents and/or warmer temperatures. The seed survival curves fitted (eqn 1) for each environment where considerable loss in viability occurred could be constrained to a common origin within each species (P > 0·05 and 0·10 for red clover and alfalfa, respectively). The resultant estimates of the seed lot viability constant (Ki) were 1·761 (s.e. 0·007) for red clover and 1·303 (s.e. 0·006) for alfalfa. Estimates for σ in each environment are shown in Fig. 3.
Fig. 2.
Ability to germinate (normal germination) of seeds of Trifolium pratense (A) and Medicago sativa (B) after adjustment of moisture content (from 10·2 %) and either before experimental storage or after 14·5 years of hermetic storage at –20 °C. Vertical bars indicate means ± s.e.
Fig. 3.
Relationships between seed moisture content (%, wb, logarithmic scale) and longevity [σ (days) logarithmic scale] for Trifolium pratense (A) and Medicago sativa (B) in hermetic storage at 65, 50, 40 or 30 °C. The critical moisture content mc at each temperature is the point of intersection between horizontal and diagonal dotted lines where observations <mc and >mc are denoted by open and filled symbols, respectively. Above mc, the broken lines represent models (eqn 2) fitted separately to each species, while the solid lines are where CH and CQ were constrained to common values. The models fitted are quantified in Table 2.
At a given storage temperature, seed longevity increased progressively (i.e. as described by eqn 2) with reduction in moisture content down to around 4–6 % (Fig. 3). Reduction in seed moisture content below this value had no further effect on longevity.
In order to determine the low-moisture-content limit (mc) to the viability equation, the observations at all moisture contents were analysed to determine the lower limit to eqn (3) for each temperature in turn. Longevity at moisture contents where eqn (3) applied (i.e. m > mc) are shown as filled symbols in Fig. 3, with open symbols where m < mc. The fitted lines show a negative logarithmic relationship (P < 0·005) between seed longevity and moisture content above mc, but no relationship (P > 0·25, i.e. constant longevity) below mc (where mc is the point of interception between fitted lines at one temperature). The negative effect of temperature on longevity is also evident. Estimates of mc varied from 4·0 (10·8 % e.r.h.) to 5·4 % (13·1 % e.r.h.) in red clover, and from 4·2 (10·4 % e.r.h.) to 5·5 % (13·5 % e.r.h.) in alfalfa depending upon storage temperature (Table 2).
Table 2.
Parameters of the relationships between longevity (σ, days), temperature (t, °C) and moisture content (%, wb) above and below the critical moisture content (mc)
| Relationships between longevity, temperature and moisture |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Above mc |
|||||||||
| Species | t (°C) | Below mc Intercept (s.e.) | mc (%, wb) (s.e.) | ERH at mc (%) | K (s.e.) | KE (s.e.) | CW (s.e.) | CH (s.e.) | CQ (s.e.) |
| T. pratense | 65·2 | 1·621 (0·010) | 4·0 (3·6–4·3) | 10·8 | 4·882 (0·227) | 5·452 (0·255) | |||
| 49·9 | 2·527 (0·010) | 4·7 (4·1–5·4) | 11·6 | 5·892 (0·329) | 5·008 (0·356) | ||||
| 39·9 | 3·106 (0·023) | 5·4 (4·9–6·0) | 13·1 | 7·051 (0·296) | 5·364 (0·256) | ||||
| 30·2 | 3·518 (0·014) | 5·4 (4·8–6·1) | 13·1 | 6·816 (0·263) | 4·516 (0·275) | ||||
| 30·2–65·2* | 8·593 (0·495) | 5·096 (0·162) | 0·0225 (0·0203) | 0·000602 (0·000208) | |||||
| 30·2–65·2+ | 8·866 (0·455) | 5·130 (0·195) | 0·0296 (0·0180) | 0·000561 (0·000184) | |||||
| M· sativa | 65·2 | 2·105 (0·020) | 4·2 (3·5–4·9) | 10·4 | 6·069 (0·491) | 6·402 (0·517) | |||
| 49·9 | 3·120 (0·020) | 5·3 (4·4–6·4) | 12·9 | 8·230 (0·624) | 7·070 (0·637) | ||||
| 39·9 | 3·654 (0·025) | 5·5 (4·8–6·4) | 13·5 | 8·840 (0·473) | 6·963 (0·498) | ||||
| 30·2 | – | – | – | 8·753 (0·647) | 6·042 (0·638) | ||||
| 30·2–65·2* | 11·065 (0·771) | 6·739 (0·300) | 0·0385 (0·0303) | 0·000508 (0·000309) | |||||
| 30·2–65·2+ | 10·713 (0·310) | 6·682 (0·323) | 0·0296 (0·0180) | 0·000561 (0·000184) | |||||
ERH = equilibrium relative humidity.
Estimates of KE, CW, CH and CQ fitted independently to each species.
Estimates of CH and CQ constrained to common values for both species, KE and CW not constrained.
All observations above mc were then analysed in accordance with eqn (2) to quantify the effect of temperature (30–65 °C) as well as moisture content. The temperature term did not differ (P > 0·10) between species, with common values for CH (0·0296) and for CQ (0·000561), but KE (P < 0·001) and Cw varied considerably (P < 0·001) (Table 2).
DISCUSSION
The ability of orthodox seeds to survive considerable desiccation and the substantial longevity of the dry and very dry seeds in hermetic storage are confirmed by this research.
Above mc, the basic tenets of the seed viability equation (Ellis and Roberts, 1980) were confirmed: a common Ki for the survival curves (negative cumulative normal distributions) of one seed lot stored in different environments; a negative logarithmic relationship between longevity and moisture content; a curvilinear, negative semi-logarithmic relationship between longevity and temperature, etc.
In quantitative terms, the estimates of Ki are equivalent to initial viabilities of 96 % (red clover) and 90 % (alfalfa), and so are at the top end of the range of estimates of viability provided by the germination tests (Fig. 2). Also, the estimates of Cw are not dissimilar but are slightly higher than those for starchy grain legumes such as pea (Pisum sativum L.), Cw = 5·39 (Ellis et al., 1989), and cowpea (Vigna unguiculata [L.] Walp.), Cw = 4·715 (Ellis et al., 1982) or 5·487 (Ellis et al., 1990b). Finally, the large error associated with fitting the temperature term (–CHt – CQt2) is not surprising given the somewhat limited temperature range (35 °C). Nevertheless, the conclusion that the quantitative response to temperature does not differ between the two species and the parameter values are compatible with earlier studies (e.g. Ellis and Roberts, 1981; Dickie et al., 1990).
The observations during storage at −20 °C with 15·2 % moisture content for alfalfa (Fig. 2) are beyond the application of the seed viability equation. This damage is typical of high moisture content seeds at sub-zero temperature: the free water present freezes and so damages viability (Roberts and Ellis, 1989). The equilibrium relative humidity of these seeds was 85·2 %, and so within the range where such damage is highly probable (Zewdie and Ellis, 1991).
We detected no evidence whatsoever of the damage that emerged over Nutile's 5 year study following extreme desiccation. The two studies' results are compatible, however, since the lowest moisture content investigated here (2 %) was greater than the moisture contents to which Nutile (1964) had to dry seeds to detect damage during storage.
The discontinuity in the relationships between seed longevity and moisture content for red clover and alfalfa detected at 65 °C (Fig. 3)—specifically, no response where m < mc, a negative logarithmic relationship where m > mc, with values of mc in equilibrium with about 11 % relative humidity at 20 °C when prepared for hermetic storage – was anticipated. It is very similar to that detected in previous reports in a wide variety of other species at 65 °C (e.g. Ellis et al., 1988, 1989, 1990a, b, 1992). The almost 15 year duration of this study has enabled us to overcome, at least in part, the problem in seed survival research of the very considerable longevity of low moisture content seeds at cooler temperatures. It is now apparent that the discontinuous response seen at 65 °C is also evident at 30, 40 and 50 °C, albeit that longevity is very much greater. In the extreme comparison, seed storage longevity in red clover is around 80 times greater at 30 °C compared with 65 °C.
To what extent then is to the low-moisture-content limit to the negative logarithmic relationhip between seed longevity and moisture content in hermetic storage affected by the temperature of storage? The individual estimates of mc varied from 4·0 to 5·5 %, with lower estimates at the highest storage temperature and the higher estimates at cooler temperatures (Fig. 4). Analyses of this trend showed no significant difference between these two forage legumes (P > 0·05) and a significant curvilinear (quadratic), negative relationhip (r2 = 0·756, P < 0·01, 5 d.f.). The curvilinear relationhip was superior to a linear relationship (r2 = 0·724, P < 0·01, 5 d.f.) particularly in terms of residuals (Fig. 4), indicating that while mc increases with reduction in storage temperature, the increase diminishes at cooler temperatures. Clearly, therefore, Fig. 4 provides good evidence of a negative relationship between mc and storage temperature.
Fig. 4.
Variation in mc with storage temperature (°C) for Trifolium pratense and Medicago sativa. The solid line describes the quadratic relationship fitted to estimates at 40–65 °C (Medicago sativa) or 30–65 °C (Trifolium pratense), whereas the broken line represents extrapolation to cooler temperatures. A poorer fitting linear relationship (dotted line), fitted and extrapolated, is also shown. The widening gap between extrapolations to cooler temperatures of the two relationships is an indicator of uncertainty in the values of mc at such temperatures.
These results are of considerable practical relevance to seed storage in general and ex situ plant genetic resources conservation by long-term seed storage in particular. In such seed banks, orthodox seeds are stored dry (approx. 3–7 % moisture content) and cool (approx. −18 °C) with a requirement to maintain high viability for considerable periods. Some time ago, we suggested ‘that any variation [of mc] with storage temperature is likely to be minor …[but that] … further work is required to verify this assumption’ (Ellis et al., 1989). Our earlier investigations (Ellis et al., 1988, 1989, 1990a, b, 1992), or rather the possible practical implications of the conclusions, attracted considerable controversy (Vertucci and Roos, 1990, 1991, 1993a, b; Vertucci et al., 1994). Summaries of the respective differing standpoints and supporting evidence have been provided elsewhere (Ellis, 1998; Walters, 1998), while Smith (1992) discussed the relevance of this debate to seedbank management.
Extrapolation is always dangerous. However, it is beyond human working lives to determine seed longevity of these two species in hermetic storage at −20 °C with low moisture contents. Accordingly, extrapolation has some utility in this context. Figure 4 shows extrapolation to cooler temperatures of each of the relationships fitted. The widening gap between the two relationships with reduction in seed storage temperature provides a visual indication of the zone of uncertainty regarding mc at cool or cold temperatures. Certainly, this variation in mc is not negligible. Moreover, only if the curvilinear extrapolation were to prove valid could our earlier suggestion that the variation is likely to be minor be accepted (albeit with a generous interpretation of the word minor).
Vertucci and Roos (1990, 1991) were therefore correct to challenge our suggestion (Ellis et al., 1989) that this variation would be no more than minor. On the other hand, their suggestions that: (a) there is a single (or narrow range) optimum moisture content at which seed storage determination is minimized; and (b) even limited desiccation below this value results in greater damage are not supported by the current results. Rather, and as suggested from research at only 65 °C originally (Ellis et al., 1989), whether the storage temperature is 30, 40, 50 or 65 °C, reduction in m below mc is not damaging to either viability (Fig. 2) or subsequent survival (Figs 2 and 3). Indeed, close examination where m < mc at the coolest temperatures (30 °C, red clover; 40 °C, alfalfa) in Fig. 3 shows that individual estimates of longevity were greatest at the lowest moisture content investigated (2·0–2·2 %). Moreover, at the low moisture contents for alfalfa seeds stored at 30 °C, where loss in viability was too small to be quantified, the estimates of viability after 14·5 years were greatest at the lowest (2·0 %) moisture content studied. Hence, the information above and the results for 14·5 years hermetic storage at −20 °C (Fig. 2) together with those from more practically oriented studies such as those of Ellis et al. (1996), Hong et al. (2005) and Steiner and Ruckenbauer (1995) should provide considerable reassurance to those running seedbanks which meet the international standards (FAO/IPGRI, 1994): those standards provide an environment for considerable seed longevity which cannot be considered damaging to orthodox seeds.
Finally, a question arises as a consequence of variation in mc with temperature, i.e. as a result of the geometry of responses to temperature and moisture content fitted above and below mc: is the sensitivity of seed longevity to temperature variant or invariant above and below mc? If mc does not vary with temperature, then seed longevity is equally sensitive to temperature above and below mc; but, if mc varies, then the quantitative response of longevity to temperature must differ above and below mc. Accordingly, we reanalysed the complete data set (i.e. the observations shown in Fig. 3) simultaneously using the FITNONLINEAR directive in GENSTAT to enable temperature sensitivity to vary, or not, above and below mc. The difference was highly significant (P < 0·005), the estimates of Q10 (temperature coefficient for change in rate of loss in viability per 10 °C) being much greater above mc than below: 7·16 compared with only 3·64 below mc. These estimates are subject to considerable error, because convergence in the optimization routine within the FITNONLINEAR directive was difficult, and of course Q10 varies with temperature range (Ellis and Roberts, 1981). Nevertheless, the considerably reduced sensitivity to temperature below mc confirms, via a different method of analysis, the negative curvilinear trend of mc with temperature shown in Fig. 4.
We suggest two possible explanations for this reduced sensitivity to temperature below mc which are not mutually exclusive. First, moisture content is only one measure of seed water status, equilibrium relative humidity being another. Relationships between moisture content and equilibrium relative humidity are also influenced by temperature (Pixton and Warburton, 1971). Now, Roberts and Ellis (1989) showed that the sensitivity of longevity to temperature (above mc) in lettuce (Lactuca sativa L.) was less if equilibrium relative humidity replaced the (logarithm of) moisture content in eqn (2). Perhaps, therefore, the coefficient of the temperature term in eqn (2) may be quantifying part of the response of longevity to change in seed moisture status as well as to temperature. Specifically, it may be quantifying an indirect effect of temperature on longevity via its effect on relationships among the different measures of moisture in seeds. Secondly, the water remaining within seeds below mc has very little chemical potential (Roberts and Ellis, 1989), and so there may be less scope for an increase in temperature to be quite so damaging compared with above mc where the weakly bound water is present.
We conclude that the low-moisture-content limit to negative logarithmic relationships between seed longevity and moisture content shows a negative (probably curvilinear) relationship with the temperature of hermetic storage. Further desiccation, by 2 % or so, is not damaging to survival.
Acknowledgments
We thank The University of Reading for providing an environment in which long-term research was possible, and Dr Mike Gooding for his help with the FITNONLINEAR directive in GENSTAT.
LITERATURE CITED
- Dickie JB, Ellis RH, Kraak HL, Ryder K, Tompsett PB. 1990. Temperature and seed storage longevity. Annals of Botany 65: 197–204. [Google Scholar]
- Ellis RH. 1998. Longevity of seeds stored hermetically at low moisture contents. Seed Science Research 8, Supplement No. 1: 9–10. [Google Scholar]
- Ellis RH, Roberts EH. 1980. Improved equations for the prediction of seed longevity. Annals of Botany 45: 13–30. [Google Scholar]
- Ellis RH, Roberts EH. 1981. The quantification of ageing and survival in orthodox seeds. Seed Science and Technology 9: 373–409. [Google Scholar]
- Ellis RH, Osei-Bonsu K, Roberts EH. 1982. The influence of genotype, temperature and moisture on seed longevity in chickpea, cowpea and soyabean. Annals of Botany 50: 69–82. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1985. Handbook of seed technology for genebanks. Volume I. Principles and methodology. Rome: International Board for Plant Genetic Resources.
- Ellis RH, Hong TD, Roberts EH. 1986. Logarithmic relationship between moisture content and longevity in sesame seeds. Annals of Botany 57: 499–503. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1988. A low-moisture-content limit to logarithmic relations between seed moisture content and longevity. Annals of Botany 61: 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1989. A comparison of the low-moisture-content limit to the logarithmic relation between seed moisture content and longevity in twelve species. Annals of Botany 63: 601–611. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1990a Moisture content and the longevity of seeds of Phaseolus vulgaris. Annals of Botany 66: 341–348. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH, Tao K-L. 1990b Low moisture content limits to relations between seed longevity and moisture. Annals of Botany 65: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1992. The low moisture content limit to the negative logarithmic relation between seed longevity and moisture content in three subspecies of rice. Annals of Botany 69: 53–58. [Google Scholar]
- Ellis RH, Hong TD, Astley D, Pinnegar AE, Kraak HL. 1996. Survival of dry and ultra-dry seeds of carrot, groundnut, lettuce, oilseed rape, and onion during five years' hermetic storage at two temperatures. Seed Science and Technology 24: 347–358. [Google Scholar]
- FAO/IPGRI 1994. Genebank standards. Rome: Food and Agriculture Organization of the United Nations, International Plant Genetic Resources Institute.
- Genstat 5 Committee. 1997. Genstat 5 release 3 reference manual. Oxford: Clarendon Press.
- Hong TD, Ellis RH, Astley D, Pinnegar AE, Groot SPC, Kraak HL. 2005. Survival and vigour of ultra-dry seeds after ten years of hermetic storage. Seed Science and Technology 33: 449–460. [Google Scholar]
- International Seed Testing Association. 1985. International rules for seed testing. Seed Science and Technology 13: 1–513. [Google Scholar]
- International Seed Testing Association. 2005. International rules for seed testing. Edition 2005. CH-Switzerland: The International Seed Testing Association.
- Nutile GE. 1964. Effect of desiccation on viability of seeds. Crop Science 4: 325–328. [Google Scholar]
- Pixton SW, Warburton S. 1971. Moisture content/relative humidity equilibrium of some cereal grains at different temperatures. Journal of Stored Products Research 6: 283–293. [Google Scholar]
- Roberts EH. 1973. Predicting the storage life of seeds. Seed Science and Technology 1: 499–514. [Google Scholar]
- Roberts EH, Ellis RH. 1989. Water and seed survival. Annals of Botany 63: 39–52. [Google Scholar]
- Smith RD. 1992. Seed storage, temperature and relative humidity. Seed Science Research 2: 113–116. [Google Scholar]
- Steiner AM, Ruckenbauer P. 1995. Germination of 110-year-old cereal and weed seeds, the Vienna sample of 1877. Verification of effective ultra-dry storage at ambient temperature. Seed Science Research 5: 195–199. [Google Scholar]
- Vertucci CW, Roos EE. 1990. Theoretical basis of protocols for seed storage. Plant Physiology 94: 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW, Roos EE. 1991. Seed moisture content, storage, viability and vigour: response. Seed Science Research 1: 277–279. [Google Scholar]
- Vertucci CW, Roos EE. 1993a Theoretical basis of protocols for seed storage. II. The influence of temperature on optimal moisture levels. Seed Science Research 3: 201–213. [Google Scholar]
- Vertucci CW, Roos EE. 1993b Seed storage temperature and relative humidity: response. Seed Science Research 3: 215–216. [Google Scholar]
- Vertucci CW, Roos EE, Crane J. 1994. Theoretical basis of protocols for seed storage. III. Optimum moisture contents for pea seeds stored at different temperatures. Annals of Botany 74: 531–540. [Google Scholar]
- Walters C. 1998. Ultra-dry technology: perspective from the National Seed Storage Laboratory, USA. Seed Science Research 8, Supplement No. 1: 11–14. [Google Scholar]
- Zewdie M, Ellis RH. 1991. Response of tef and niger seed longevity to storage temperature and moisture. Seed Science and Technology 19: 319–329. [Google Scholar]






