Abstract
• Background and Aims Global warming is gaining significance as a threat to natural and managed ecosystems since temperature is one of the major environmental factors affecting plant productivity. Hence, the effects of moderate temperature increase on the growth and development of the tomato plant (Lycopersicon esculentum) were investigated.
• Methods Plants were grown at 32/26 °C as a moderately elevated temperature stress (METS) treatment or at 28/22 °C (day/night temperatures) as a control with natural light conditions. Vegetative growth and reproductive development as well as sugar content and metabolism, proline content and translocation in the androecium were investigated.
• Key Results METS did not cause a significant change in biomass, the number of flowers, or the number of pollen grains produced, but there was a significant decrease in the number of fruit set, pollen viability and the number of pollen grains released. Glucose and fructose contents in the androecium (i.e. all stamens from one flower) were generally higher in the control than METS, but sucrose was higher in METS. Coincidently, the mRNA transcript abundance of acid invertase in the androecium was decreased by METS. Proline contents in the androecium were almost the same in the control and METS, while the mRNA transcript level of proline transporter 1, which expresses specifically at the surface of microspores, was significantly decreased by METS.
• Conclusions The research indicated that failure of tomato fruit set under a moderately increased temperature above optimal is due to the disruption of sugar metabolism and proline translocation during the narrow window of male reproductive development.
Keywords: Lycopersicon esculentum, moderately elevated temperature stress, microsporogenesis, mean daily temperature, fruit set, pollen release, male reproductive development, tapetum, hexose, sucrose, acid invertase, proline transporter
INTRODUCTION
The Intergovernmental Panel on Climate Change (IPCC, 2001) announced that the average global atmospheric temperature may rise 2·6 °C by 2050 relative to 1990, and 5·8 °C by 2100. Many reports have envisioned that the increase of average global temperature would cause significant changes on natural and managed ecosystems. Atmospheric temperature is a major environmental factor determining plant growth and productivity, hence there are a number of researchers investigating high temperature effects on plant productivity. Many reports, however, have focused on the effects of acute high temperature, known as heat shock, which is far above the normal growth temperature range of most domesticated agricultural plant species (Dash and Mohanty, 2001; Gong et al., 2001; Harndahl and Sundby, 2001; Bowen et al., 2002; Park and Hong, 2002). Research of heat shock effects on plant physiology is important since the IPCC has reported that environmental changes on a global scale would bring more extreme weather phenomena, such as droughts, floods and heat waves. At the same time, information on the effects of moderate increases in average temperature on plant physiology is also essential to develop strategies for coping with global warming, since the amount of predicted atmospheric temperature increase due to global warming is gradual. From an agricultural viewpoint, the organ of interest depends on each plant. For instance, rice (Oryza sativa) plants have higher biomass accumulation and rapid leaf expansion at 34 °C day temperature compared with 28 °C (Baker et al., 1992). The grain yield, however, decreases 10 % as temperature increases 1 °C from 28 °C. Higher biomass accumulation at elevated temperatures could be an advantageous trait for some agricultural crops, such as forage and leaf vegetables. However, for others, such as grains and fruit crops, biomass increase not accompanied by an increase of the organ to be harvested is of no benefit.
It has been reported that reproductive development is more sensitive to high temperature stress than vegetative development, but the process sensitive to the stress appears to be different for each crop. In tomato (Lycopersicon esculentum), temperature increase from 28/22 (day/night)°C to 32/26 °C significantly decreased fruit set, but did not affect photosynthetic rate or night respiration in both high temperature-sensitive and -tolerant cultivars (Sato et al., 2000). The reduction of fruit set under moderately elevated temperature stress was mostly due to a reduction in pollen release and viability but not in pollen production. In groundnut (Arachis hypogaea), however, a reduction in pod number by high temperature was caused by a reduction in pollen number and viability (Vara Prasad, 1999). Peet et al. (1997) reported that the yield of tomato decreases as mean daily temperature increases, and the magnitude of the effect was the same in day and night temperatures. In the experiment under artificial light conditions, a moderate increase of average temperature did not affect vegetative growth of tomato (i.e. photosynthesis, night respiration and biomass) but impaired reproductive development. Specifically, pollen viability and release were affected adversely and became major factors limiting fruit set (Sato et al., 2000, 2005). The narrow window of male reproductive development, 8–13 d prior to anthesis, is the period most sensitive to moderately elevated temperature stress (Sato et al., 2002).
Sucrose, a major form of carbohydrate transport in tomato, imported by anthers is cleaved into hexoses before being routed to several biochemical pathways including starch synthesis (Ho, 1988). Prior to anthesis, developing pollen grains and anthers accumulate starch temporarily, but a moderate temperature increase reduces starch concentration in developing pollen grains, and pollen viability decreases (Pressman et al., 2002). Proline is reported to be correlated with pollen viability of sorghum (Sorghum bicolor) (Lansac et al., 1996) and represents over 70 % of total free amino acids in tomato (Schwacke et al., 1999). However, the relation of carbohydrate and proline with pollen viability under moderately elevated temperature stress is still unclear. The aim of the current studies was to investigate the effects of moderately elevated temperature stress on biochemical and molecular events during male reproductive development, which have crucial roles for fruit set in tomato.
MATERIALS AND METHODS
Plant growth and temperature treatments
Tomato (Lycopersicon esculentum ‘Momotaro’, Takii Seed, Kyoto, Japan) was used in the experiments. In a preliminary experiment, Momotaro was compared with ‘NC8288’, a cultivar used in previous experiments (Sato et al., 2000, 2002, 2005). The results indicated that the sensitivity to moderately elevated temperature stress of both cultivars was similar in biomass, fruit set, pollen production, release and viability, hence Momotaro was used in the current experiment because of availability.
In expt 1, on 25 March 2004, seeds were placed in a Petri dish, supplied with de-ionized water and placed in an incubator maintained at 25 °C. Germinated seeds were transplanted into plastic pots (50 mm diameter) filled with commercial soil media (Metromix 250, The Scotts Company, Marysville, OH, USA) on 29 March and placed in a glasshouse. On 30 April, 24 plants were moved into two phytotrons with natural lighting (i.e. 12 plants in each, Koito Industries, Tokyo, Japan) set at 28/22 °C day/night temperatures.
The temperature treatment started on 15 May 2004. The temperature in one of the two phytotrons was set at 32 °C during the day and 26 °C at night, providing the moderately elevated temperature stress treatment (METS), while the other remained at 28 °C during the day and 22 °C at night as the control (CONT). Irradiance and light and dark periods were left as in natural conditions. A half-strength commercial nutrient solution (Otsuka Chemical Co. Ltd, Tokyo, Japan; EC 1·4 dS m−1; N, P, K, Ca and Mg = 9·3, 2·6, 4·3, 4·1 and 1·5 me L−1, respectively) was applied to the plants throughout the culture two to five times daily by drip irrigation until drainage was observed during each irrigation. The number of flowers and the number of fruit set were recorded throughout the culture. A home-made electric pollinating rod was applied to stimulate pollination, which is a common practice in protected tomato culture. Shoot length and dry weight were also investigated after the culture was terminated on 30 July 2004.
In expt 2, another set of plants was prepared from 21 September and subjected to temperature stress from 8 October. In this experiment, buds and flowers were used to investigate pollen viability, number of pollen grains produced and released, sugar and proline content, transcripts of acid invertase and proline transporter 1, as indicated below.
Pollen counting and in-vitro pollen germination
To count the number of pollen grains released, a 1·5-mL microtube was attached to an anther of a flower that had opened on the day of sampling, then an electric pollinating rod was applied for 10 s. Pollen grains released into the microtube by vibrating the pollinating rod were considered as released pollen grains. Samples were stored at −4 °C until the in-vitro germination test and the count of pollen grains as described in Sato et al. (2000) were performed. In short, the tube containing the released pollen grains was incubated at 25 °C for 12 h after 300 μL of cultural media was added. After incubation, the pollen grain suspension was loaded into a haemocytometer plate (Hausser Scientific Co., Horsham, PA, USA) for the total number of pollen grains. The number of viable pollen grains (i.e. germinated) was also recorded at the same time.
The androecium (i.e. all stamens in one flower) of the same flower that was used to count the number of pollen grains released was collected into another microtube for investigation of the number of pollen grains retained in the anther. The total number of pollen grains produced was calculated by adding the number of pollen grains released from the anther and the number of pollen grains retained in the anther. Samples were collected between 1000 h and 1200 h. The analysis described herein was repeated three times.
Sugar and proline contents in androecium
Flower buds at various developmental stages were harvested and immediately frozen with liquid nitrogen and stored at −20 °C. Prior to analysis, the androecium was separated from the rest of the organ under a dissecting microscope, its size was compared with the chart by Sawhney and Bhadula (1988), and the approximate developmental stage of the anther was identified. Two androecia of the same length were placed in a 1·5-mL microtube, to which 500 μL each of ultra-pure water and 100 % ethanol were added. Samples were homogenized and centrifuged at 15000 g for 5 min. Three hundred and sixty microlitres of supernatant were collected and mixed with 40 μL of 1 n NaOH. Supernatant mix was subjected to ultrafiltration with Ultrafree-MC 5000 NMWL Filter (Millipore, MA, USA) to remove all substances ≥5000 mol. Wt and then subjected to a multi-channel capillary electrophoresis measuring system (CAPI-330; Otsuka Electronics Co., Ltd, Osaka, Japan). Buffer solution was made by following the instructions from the manufacturer. In short, 1·47 g of 2-pyridinecarboxylic acid, 12·11 g of 3-pyridinecarboxylic acid, 0·58 g of lithium hydroxide monohydrate and 60 μL of tetradecyltrimethylammonium bromide solution were added to 180 mL of ultra-filtrated water. The solution was thoroughly stirred and then the final volume was brought up to 200 mL with ultra-filtrated water. Capillary tubes 800 mm long with an internal diameter of 75 µm were chosen and capillary temperature was set at 25 °C. The absorbance of sugars and proline was measured at 230 nm. Standard solutions containing identical concentrations of glucose, fructose, sucrose and proline in the range 0·005–5 mm were used for quantification of sample concentrations. The analysis described herein was repeated three times each for the meiosis, immature microspore and mature pollen grain stages.
Real time RT–PCR analysis of acid invertase and proline transporter 1 expression in anther
Flower buds at meiosis, immature microspore and mature pollen grain stages were harvested, submerged in 500 mL of RNAlater (Ambion, TX, USA), incubated overnight at 4 °C and stored at −20 °C until the analysis. Developmental stages were decided by comparing with the chart by Sawhney and Bhadula (1988). For analysis, an androecium in a microtube was homogenized for total RNA extraction with RNeasy plant mini-kit (QIAGEN K. K., Tokyo, Japan). Fifty to one thousand nanograms of extracted total RNA were reverse-transcribed to cDNA by using Superscript III First-Strand Synthesis System for RT–PCR according to the manufacturer's instructions (Invitrogen Japan K. K., Tokyo, Japan). Two sets of primers shown in Table 1 were designed for the analysis of acid invertase (GenBank accession number Z12027) and proline transporter 1 (GenBank accession number AF014808) using 7300/7500 real-time PCR system (Applied Biosystems, CA, USA). The analysis described herein was repeated five times each for the meiosis, immature microspore and mature pollen grain stages.
Table 1.
Name of the genes studied and their corresponding primer sequence for real-time PCR analysis
| Target gene (accession number) | Name | Sequence | Size of PCR product (bp) |
|---|---|---|---|
| Acid invertase (Z12027) | SLE 30–1 | Forward-CGACAAGAA-GACAGGGACACATCT | 102 |
| SLE 30–2 | Reverse-CCTGGTTGAA-GATCGACTTGCTTA | ||
| Proline transporter 1 (AF014808) | LE100–1 | Forward-GTGCGATTGG-AAACCTTTTCTTTG | 104 |
| LE100–2 | Reverse-GTTAAGCGCT-TTCACCATGTTTCC |
Statistical analysis
Figures show means with standard error bars. All data were compared between the two temperature treatments and significant differences between the treatments were tested by t-test. Percentage data were confirmed to be normally distributed, therefore transformation was not necessary.
RESULTS
Temperature effects on vegetative and reproductive growth
A significant difference between CONT and METS was not found in shoot dry weight (Fig. 1A), shoot length (Fig. 1B), the number of flowers (Fig. 1C) or the number of pollen grains produced (Fig. 1E). However, significant differences were observed in the number of fruit set (Fig. 1D, P = 0·05), the number of pollen grains released (Fig. 1F) and the percentage of viable pollen grains (Fig. 1G). At anthesis, it was observed that the stigma was hidden in CONT, but protruded from the anther cone in METS (Fig. 2A); this phenomenon is known as ‘stigma elongation’ in horticulture. In METS, stigma protrusion out of the anther was obvious when petals were removed (Fig. 2B). In fact, it was the anther that was shortened instead of the stigma being elongated by METS. When stamen and carpel were separated, it was clear that METS affected male reproductive development rather than female development (Fig. 2C, D). Also, anther, rather than filament, was shortened by the stress. A statistical difference was found in the length of the stamen (P = 0·001) but not in the stigma (Fig. 2E)
Fig. 1.
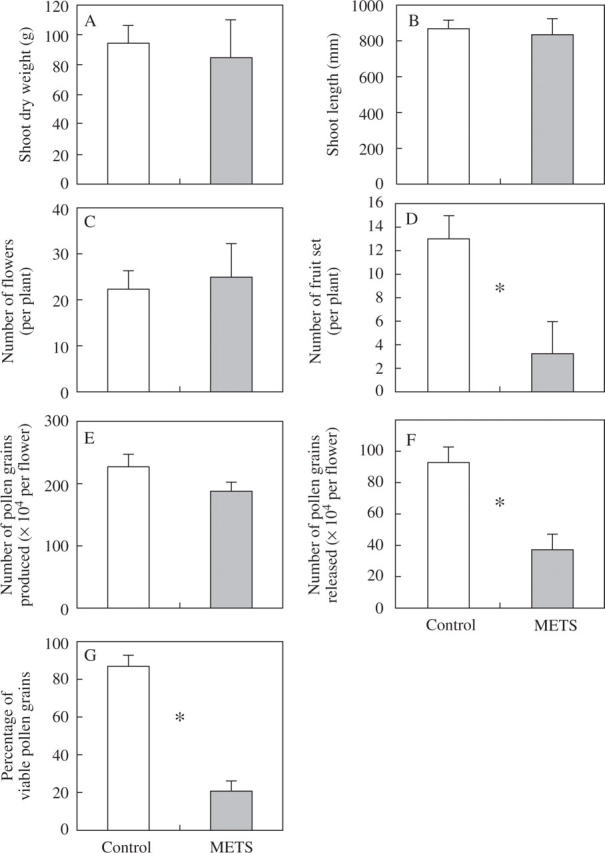
Temperature effects on vegetative and reproductive development of tomato. Shoot dry weight (A), shoot length (B) and number of fruit set (D) were investigated on 30 July 2004, 128 d after seeding. Twelve plants were used for the investigation. The number of flowers (C) was examined three times a week throughout culture. The number of pollen grains produced (E), number of pollen grains released (F) and the percentage of viable pollen grains (G) were investigated three times. A single asterisk indicates where a significant difference was found between the treatment and the control by t-test (P = 0.05). Control: 28/22 °C day/night temperatures; METS (moderately elevated temperature stress): 32/26 °C day/night temperatures.
Fig. 2.
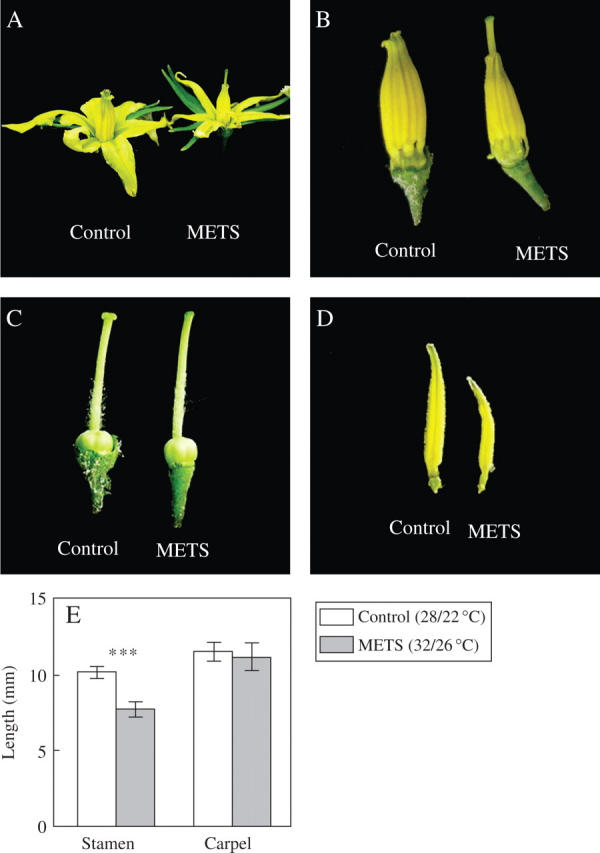
Temperature effects on tomato flower development: (A) whole flower at anthesis stage; (B) anthers and pistil (calyx and corolla were removed); (C) pistil; (D) anther; (E) average length of stamen and pistil. Data are expressed as averages with standard error bars. Three asterisks indicate where significance was found by t-test (P = 0.001, n = 24).
Temperature effects on sugar and proline contents
In CONT, glucose contents in stamens were 49·4, 201·9 and 784·2 nmol per androecium at meiosis, immature microspore and mature pollen grain stages, respectively (Fig. 3A). In METS, they were 48·7, 84·9 and 469·8 nmol per androecium. Significant differences were found at immature microspore and mature pollen grain stages (P = 0·05). A similar pattern was observed in fructose contents as well (Fig. 3B); 46·7, 211·9 and 806·3 nmol per androecium in CONT and 63·8, 116·4 and 539·7 nmol per androecium in METS. In general, the reducing sugar contents were higher in CONT than in METS, while the observed pattern was opposite for sucrose. The sucrose contents in CONT were 175·8, 158·6, and 82·0 nmol per androecium at meiosis, immature microspore and mature pollen grain stages, respectively (Fig. 3C). In METS, the contents were 65·0, 194·6 and 195·1 nmol per androecium, being significantly higher in METS at immature microspore and mature pollen grain stages (P = 0·05). Proline contents of both treatments gradually increased as the anthers developed (Fig. 3D). Significant difference was found only at the meiosis stage; 147·8 and 239·2 nmol per androecium in CONT and METS, respectively (P = 0·05).
Fig. 3.
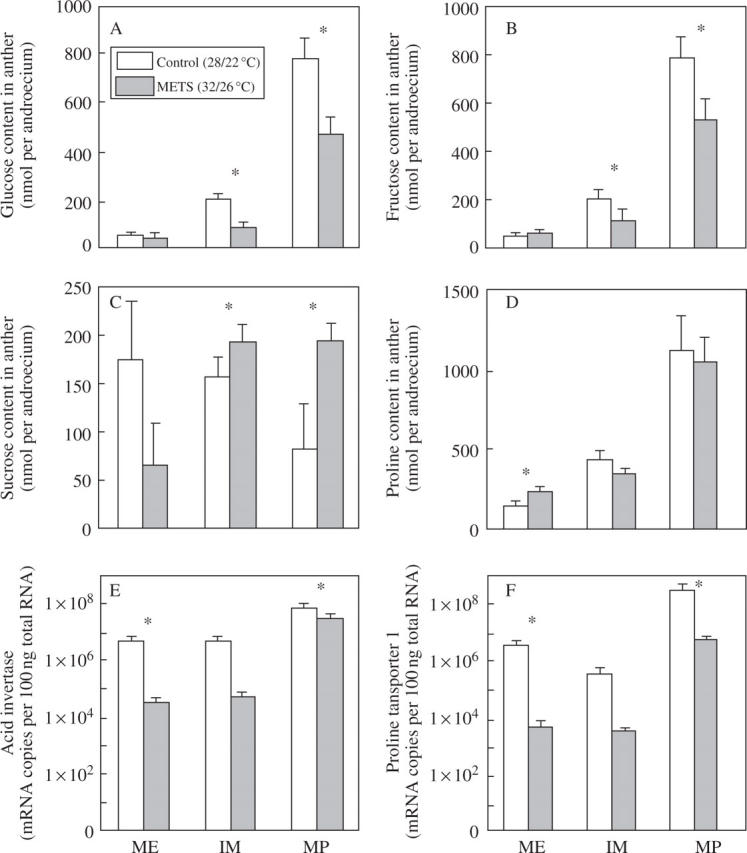
Temperature effects on sugar and proline contents, and mRNA abundance of acid invertase and proline transporter 1 in tomato androecium. All stamens from one flower were subjected to mRNA extraction. Measurement was replicated three times for sugars and five times for proline and mRNA analysis. A single asterisk indicates that a significant difference was found between the treatment and control by t-test (P = 0.05). ME, IM and MP stand for meiosis, immature microspore and mature pollen grain stages, respectively.
Real time RT–PCR analysis of acid invertase and proline transporter 1 in male reproductive development
The copy numbers of acid invertase mRNA (Fig. 3E) tended to be lower in METS throughout the stages investigated. Significant differences were found at the meiosis stage and mature pollen grain stage but not at the immature microspore stage. The copy numbers of proline transporter 1 mRNA (Fig. 3F) indicated a very similar pattern to acid invertase; they tended always to be lower in METS than in CONT, with significant differences found at meiosis and mature pollen grain stages but not at the immature microspore stage.
DISCUSSION
Moderately elevated temperature stress (METS) did not affect vegetative growth (Fig. 1A, B) or reproductive development such as the number of flowers (Fig. 1C) and the number of pollen grains produced (Fig. 1E), but significantly reduced the number of fruit set (Fig. 1D). The reduction of fruit set under the stress was likely to be caused by reduction in pollen viability (Fig. 1F) and release (Fig. 1G); this agrees with previous reports (Sato et al., 2000, 2005) in which plants were grown under artificial light conditions. Photosynthetic ability under high temperature stress has been a major research topic in various agricultural crops, including tomatoes (Bar-Tsur et al., 1985; Camejo et al., 2005; Nautiyal et al., 2005). However, this experiment, conducted under natural light condition with the same temperature regimes as in the previous report of Sato et al. (2005), indicates that the source strength such as photosynthetic ability is not a major limiting factor of tomato fruit set under moderately elevated temperature stress regardless of the light conditions. Carbon dioxide (CO2) is one of the major sources of global warming. It is known that higher CO2 concentration enhances growth rate in various plants (Pooter et al., 1996). The present results, however, imply that the productivity of grain crops and fruit vegetables might not be enhanced by high CO2 since reproductive development is much more sensitive even to a moderate increase in temperature.
In the present experiment, tomato fruit set correlated with pollen grain viability and pollen release (Fig. 1). Pollen grains need to accumulate enough reserve to be viable. A major form of reserve in mature pollen grains of tomato is starch (Polowich and Sawhney, 1993). Prior to starch accumulation, sucrose, a major form of carbohydrate transport in tomato, must be transferred from source organs (i.e. photosynthetically active leaves) to sink organs (i.e. flowers, or more specifically anthers) via phloem. After sucrose is transported, and prior to starch synthesis, sucrose is hydrolysed by invertase to produce hexoses, because a basic component of starch is the α-1,4 linkage of glucose. Several isoforms of invertase have been isolated in various plants, including one uniquely expressed in tomato fruit vascular tissue (GenBank accession number Z12027). The present analysis indicated that tomato plants grown at METS had lower hexose (glucose and fructose) content in stamens at immature microspore and mature pollen grain stages (Fig. 3A, B), but higher sucrose content (Fig. 3C). Furthermore, the copy number of acid invertase mRNA tended to be lower in METS than CONT throughout the investigation (Fig. 3F). Therefore, it seems that METS did not affect carbohydrate transport from the source to the anther, but disrupted sucrose hydrolysis and prevented normal pollen development, leading to the loss of pollen viability and the reduction in fruit set.
Disturbed sugar metabolism might have affected anther development as observed (Fig. 2E); the length of stamen was approx. 26 % reduced in METS when compared with CONT. As a result of reduced anther growth, the stigma protruded out of the anther cone (Fig. 2A, B). Such a phenomenon is often observed in summer tomato production and has been known as ‘stigma elongation’. The present results, however, indicate that the phenomenon is ‘anther shortening’ rather than stigma elongation.
Proline has been reported as one of the factors affecting pollen viability (Zhang and Croes, 1983; Lansac et al., 1996). In tomato, proline concentration in reproductive organs was found to be six to ten times higher than in the rest of the plant (Schwacke et al., 1999). In the present experiment, proline content in anthers did not change between the treatments, except in the meiosis stage. Since a developing anther is a sink organ, most amino acids, including proline, are transported from source organs—as discussed on sucrose above. Sink–source translocation requires trafficking between symplastic and apoplastic environments and vice versa, because nutrients produced in the symplast of source tissues (i.e. most likely the photosynthetic leaves) have to cross a plasma membrane for translocation via phloem. After translocation to sink organs (i.e. developing flower buds, anther and microspores), nutrients move to a symplastic environment, because most nutrients supplied to developing microspores will be carried out through tapetum cells. Tapetum is a cell layer which takes a crucial role in supplying nutritional storage during pollen development and degrades around the time of pollen mitosis in a programmed cell death fashion (Scott et al., 2004). Prior to and during degradation, tapetum cells supply various nutrients such as amino acids, lipids and carbohydrate. Therefore, proline has to pass a plasma membrane at least a few times before reaching pollen grains. In the current experiment, it was observed that the proline content of the anthers was not significantly different between CONT and METS, except at the meiosis stage (Fig. 3D). The copy number of proline transporter 1, which is expressed specifically at the pollen surface (Schwacke et al., 1999), was significantly lower in METS at meiosis and mature pollen grain stages (Fig. 3F). Therefore, it is reasonable to conclude that the disruption of proline transport into the developing pollen grain is part of the reason for the loss of pollen viability under METS.
Tapetum cell development and degradation should be precisely orchestrated with pollen development (Twell, 2002) and any alteration in initiation and/or duration of tapetum degradation should influence pollen development. Evidence that a large number of male sterile mutants are related with defects of tapetum cell differentiation (Kaul, 1988; Van der Meer et al., 1992; Chaudhury, 1993; Aarts et al., 1997; Taylor et al., 1998; Sanders et al., 1999) may suggest the impairment of tapetum degradation could participate in the loss of pollen viability which caused fruit set reduction under METS. In heat shock research, Iwahori (1965) reported that tomato plants subjected to 40 °C for 3 h for two consecutive days did not have tapetum degradation but indicated the loss of pollen viability. Under METS, however, tapetum degradation still occurred, although it was delayed. Therefore, the impact of moderately elevated temperature stress on plant reproduction differs from the impact of heat shock, or acute high temperature stress.
In the present experiment, a possible consequence of global warming to agricultural production has been presented and explained at a physiological and molecular level; moderate increases of atmospheric average temperature are likely to cause a significant reduction in tomato fruit set, which can be attributed to the disruption of specific physiological and molecular events during the narrow window of male reproductive development.
Acknowledgments
Authors thank Andrea M. J. Sato, APHIS-USDA, for carefully proofreading and advising on the technical writing of the manuscript.
LITERATURE CITED
- Aarts MGM, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, et al. 1997. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complex. The Plant Journal 12: 615–623. [DOI] [PubMed] [Google Scholar]
- Baker JT, Allen Jr LH, Boote KJ. 1992. Response of rice to carbon dioxide and temperature. Agricultural and Forest Meteorology 60: 153–166. [Google Scholar]
- Bar-Tsur A, Rudich J, Bravdo B. 1985. High temperature effects on CO2 gas exchange in heat-tolerant and -sensitive tomatoes. Journal of the American Society for Horticultural Science 110: 582–586. [Google Scholar]
- Bowen J, Lay-Yee M, Plummer K, Ferguson I. 2002. The heat shock response is involved in thermotolerance in suspension-cultured apple fruit cells. Journal of Plant Physiology 159: 599–606. [Google Scholar]
- Camejo D, Rodríguez P, Morales MA, Dell'Amico JM, Torrecillas A, Alarcón JJ. 2005. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. Journal of Plant Physiology 162: 281–289. [DOI] [PubMed] [Google Scholar]
- Chaudhury AM. 1993. Nuclear genes controlling male fertility. The Plant Cell 5: 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S, Mohanty N. 2001. Evaluation of assays for the analysis of thermo-tolerance and recovery potentials of seedlings of wheat (Triticum aestivum L.) cultivars. Journal of Plant Physiology 158: 1153–1165. [Google Scholar]
- Gong M, Chen B, Li ZG, Guo LH. 2001. Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. Journal of Plant Physiology 158: 1125–1130. [Google Scholar]
- Harndahl U, Sundby C. 2001. Does the chloroplast small heat shock protein protect photosystem II during heat stress in vitro? Physiologia Plantarum 111: 273–275. [DOI] [PubMed] [Google Scholar]
- Ho LC. 1988. Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Annual Review of Plant Physiology and Plant Molecular Biology 39: 355–378. [Google Scholar]
- IPCC 2001. Climate change 2001: impacts, adaptation and vulnerability—technical summary (http://www.ipcc.ch/)
- Iwahori S. 1965. High temperature injuries in tomato. IV. Development of normal flower buds and morphological abnormalities of flower buds treated with high temperature. Journal of the Japanese Society for Horticultural Science 34: 33–41. [Google Scholar]
- Kaul LH. 1988. Male sterility in higher plants. Berlin: Springer-Verlag.
- Lansac AR, Sullivan CY, Johnson BE. 1996. Accumulation of free proline in sorghum (Sorghum bicolor) pollen. Canadian Journal of Botany 74: 40–45. [Google Scholar]
- Nautiyal PC, Shono M, Egawa E. 2005. Enhanced thermotolerance of the vegetative part of MT-sHSP transgenic tomato line. Scientia Horticulturae 105: 393–409. [Google Scholar]
- Park SM, Hong CB. 2002. Class I small heat-shock protein gives thermotolerance in tobacco. Journal of Plant Physiology 159: 25–30. [Google Scholar]
- Peet MM, Willits DH, Gardner R. 1997. Response of development and post-pollen production processes in sterile tomatoes to chronic sub-acute high temperature stress. Journal of Experimental Botany 48: 101–111. [Google Scholar]
- Polowick PL, Sawhney VK. 1993. An ultrastructural study of pollen development in tomato (Lycopersicon esculentum). Canadian Journal of Botany 71: 1048–1055. [Google Scholar]
- Poorter H, Roumet C, Campbell BD. 1996. Interspecific variation in the growth response of plants to elevated CO2: a search for functional types. In: Korner C, Bazzaz FA, eds. Carbon dioxide, populations, and communities. New York: Academic Press, 375–412.
- Pressman E, Peet MM, Pharr DM. 2002. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany 90: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, et al. 1999. Anther developmental defects in Arabidopsis thaliana male sterile mutants. Sexual Plant Reproduction 11: 297–322. [Google Scholar]
- Sato S, Peet MM. 2005. The effects of moderately elevated temperature stress on the timing of pollen release and germination in tomato (Lycopersicon esculentum Mill.). Journal of Horticultural Science and Biotechnology 80: 23–28. [Google Scholar]
- Sato S, Peet MM, Thomas JF. 2000. Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant, Cell and Environment 23: 719–726. [Google Scholar]
- Sato S, Peet MM, Thomas JF. 2002. Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. Journal of Experimental Botany 53: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Sawhney VK, Bhadula SK. 1988. Microsporogenesis in the normal and male-sterile stamenless-2 mutant of tomato (Lycopersicon esculentum). Canadian Journal of Botany 66: 2013–2021. [Google Scholar]
- Schwacke R, Grallath S, Breitkreuz KE, Stransky E, Stransky H, Frommer WB, et al. 1999. LeProT1, a transporter for proline, glycine betaine, and γ-amino butyric acid in tomato pollen. The Plant Cell 11: 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Dickinson HG. 2004. Stamen structure and function. The Plant Cell 16: S46–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PE, Glover JA, Lavithis M, Craig S, Singh MB, Knox RB, et al. 1998. Genetic control of male fertility in Arabidopsis thaliana: structural analyses of postmeiotic developmental mutants. Planta 205: 492–505. [DOI] [PubMed] [Google Scholar]
- Twell D. 2002. The developmental biology of pollen. In: O'Neill DS, Roberts JA, eds. Plant reproduction. Annual Plant Reviews, Vol. 6. Sheffield: Sheffield Academic Press, 87–153.
- Van der Meer IM, Stam ME, van Tunen AJ, Mol JN, Stuije AR. 1992. Antisense inhibition of flavonoid biosynthesis in petunia anthers results in male sterility. The Plant Cell 4: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara Prasad PV, Craufurd PQ, Summerfield RJ. 1999. Sensitivity of peanut to timing of heat stress during reproductive development. Crop Science 39: 1352–1357. [Google Scholar]
- Zhang H, Croes AF. 1983. Protection of pollen germination from adverse temperatures: a possible role for proline. Plant, Cell and Environment 6: 471–476. [Google Scholar]


