Abstract
• Background and Aims The root apical meristems (RAM) of flowering plant roots are organized into recognizable pattern types. At present, there are no known ecological or physiological benefits to having one RAM organization type over another. Although there are phylogenetic distribution patterns in plant groups, the possible evolutionary advantages of different RAM organization patterns are not understood. Root caps of many flowering plant roots are known to release living border cells into the rhizosphere, where the cells are believed to have the capacity to alter conditions in the soil and to interact with soil micro-organisms. Consequently, high rates of border cell production may have the potential to benefit plant growth and development greatly, and to provide a selective advantage in certain soil environments. This study reports the use of several approaches to elucidate the anatomical and developmental relationships between RAM organization and border cell production.
• Methods RAM types from many species were compared with numbers of border cells released in those species. In addition, other species were grown, fixed and sectioned to verify their organization type and capacity to produce border cells. Root tips were examined microscopically to characterize their pattern and some were stained to determine the viability of root cap cells.
• Key Results The first report of a correlation between RAM organization type and the production and release of border cells is provided: species exhibiting open RAM organization produce significantly more border cells than species exhibiting closed apical organization. Roots with closed apical organization release peripheral root cap cells in sheets or large groups of dead cells, whereas root caps with open organization release individual living border cells.
• Conclusions This study, the first to document a relationship between RAM organization, root cap behaviour and a possible ecological benefit to the plant, may yield a framework to examine the evolutionary causes for the diversification of RAM organization types across taxa.
Keywords: Border cells, root caps, root apical organization, root meristem
INTRODUCTION
The root apical meristem (RAM) is the region within the root tip from which all primary root tissues and the root cap are derived. The RAM consists of many cells that are actively engaged in division, growth and differentiation. Tiers of more-or-less discrete initials that are self-perpetuating ‘stem’ cells remain at the pole of the meristem and function to produce cells for the RAM (Rost, 1994; Groot and Rost, 2001a, b; Groot et al., 2004). During the initial phases of root growth, the RAM develops a distinct cellular pattern that differs across taxa (Esau, 1965; Groot et al., 2004). In dicotyledonous plants these RAM patterns are diverse, but were originally categorized into two types, open and closed (von Guttenberg, 1968). Recently, Groot et al. (2004) fine-tuned these categories into three different organizational types (closed, basic-open and intermediate-open), which are defined by the arrangement of the initials and the spatial and apparent lineage relationships between the initials and the rest of the RAM.
Species exhibiting closed RAM organization are characterized by clonally distinct tiers of initials where cell files of a distinct tissue type can be traced back to the tier of origin. Generally in dicotyledonous angiosperms, there are three tiers of initials: one produces the vascular cylinder, another the cortex, and a third both the epidermis and the root cap. A classic example of a root with closed organization is that of Arabidopsis thaliana (Baum and Rost, 1996; Rost et al., 1996; Zhu et al., 1998a, b; Zhu and Rost, 2000; Wenzel and Rost, 2001; Baum et al., 2002). Comparatively, in roots exhibiting basic-open RAM organization, cell files originate from a relatively large zone of initials that lacks clonally distinct tiers (Rost and Baum, 1988; Rost et al., 1988; Groot et al., 2004). In this case, resulting cell types cannot be attributed to particular groups of initials. In intermediate-open organization, the cell files appear to converge on the meristem pole, but the initials are shared between the root cap and both cortex and vascular tissues (Wenzel et al., 2001; Groot et al., 2004). RAM organization in monocotyledonous angiosperms is quite different than found in the dicots and will not be considered in this report.
Border cells (formerly referred to as sloughed peripheral root cap cells) are so named in reference to their function as a physical and biological interface between the root and the soil (Hawes, 1991; Hawes et al., 2005). These living cells are programmed to separate from the root cap and from each other as they reach the cap periphery. This action is accomplished through the activity of cell-wall-degrading enzymes that solubilize the interconnections between cells while in most species, leaving the walls of individual cells intact (Hawes and Lin, 1990). In the majority of species, the detached root cap cells remain viable once they are shed into the external environment, and are henceforth referred to as border cells. The border cells undergo changes in morphology and gene expression. The cells elongate, produce lignified secondary walls and excrete proteins into the surrounding soil environment (Hawes et al., 2003). The number of border cells released from root caps is different for different species of plants. Examples of the extremes are Gossypium hirsutum (Malvaceae), which releases 8000–10 000 border cells per 24 h, and Brassica rapa (Brassicaceae), which releases no border cells (Hawes et al., 2003).
Like apical organization type, border cell production is conserved within taxa. Here, comparison is made of RAM types with the production and release of root cap border cells, and predictions of the hypothesis that border cell production might be related to RAM organization type are examined.
MATERIALS AND METHODS
The RAM types and border cell numbers for 19 dicotyledonous plant species were compared from the studies of Hawes et al. (2003) and Groot et al. (2004). In addition, three representative species exhibiting different apical organization types, Pisum sativum ‘Little Marvel’ (basic-open), Helianthus annuus ‘California Greystripe’ (intermediate-open) and Brassica napus (closed), were examined. Seeds were surface sterilized in a 25 % bleach solution with a small amount of Alconox (White Plains, NY, USA) detergent for 10 min, then rinsed three times in sterile glass-distilled water. The seeds were imbibed for a minimum of 4 h at room temperature and transferred to 1 % agar plates overlaid with no. 1 Whatman (Florham Park, NJ, USA) filter paper. The plates were wrapped in aluminium foil to exclude light and held in a growth chamber at 25 °C. Petri dishes were laid flat in the growth chamber for convenience; previous experiments with Arabidopsis thaliana showed that apical organization pattern was not affected by root orientation. Seeds were allowed to germinate and grow for a period of 4–7 d before root tips were harvested.
Root tips were excised and then fixed in a solution of 2·4 % glutaraldehyde, 0·3 % paraformaldehyde and 0·025 m PIPES [piperazine-N, N′-bis (2-ethanesulfonic acid)] buffer (pH 7·2) for a minimum of 24 h for scanning electron microscopy (SEM) examination. Specimens were rinsed three times in 0·025 m PIPES buffer and passed through an ethanol dehydration series from 10 % to 100 % at 10 % increments at 15-min intervals with three changes of absolute ethanol. Root tips were then processed for SEM in a Tousimis (Rockville, MD, USA) critical-point dryer. Specimens were mounted on aluminium stubs using adhesive carbon tabs and gold coated in a Denton Desk II cold sputter coater (Moorestown, NJ, USA). The specimens were viewed and photographed using a Hitachi (Schaumburg, IL, USA) S-3500N scanning electron microscope.
Root tips were excised, fixed and dehydrated using the procedure described above, with an additional staining step using 0·05 % fast green in 95 % ethanol prior to dehydration in absolute ethanol, for light microscopy examination. Specimens were infiltrated and embedded in JB-4 resin (Polysciences, Inc., Warrington, PA, USA) according to the manufacturer's directions. Specimens were trimmed, affixed to 1·5-cm pieces of wooden dowel and sectioned using a Reichert-Jung 2050 Supercut microtome (Hamburg, Germany) at 5-μm thickness. Sections were placed onto water droplets on gelatin-coated slides, dried on a 60 °C slide warmer and stained using the periodic acid/Schiff's reaction.
Whole root tips were excised and immediately stained in saturated aqueous fluorescein diacetate (FDA) for 4 h and mounted on slides with distilled water. Fluorescein diacetate is a vital stain designed to determine if the stained cells are living (O'Brien and McCully, 1981).
For border cell counts, seeds were surface sterilized, imbibed in water for 4 h, then plated onto water agar (1·0 %, w/v) overlaid with filter paper. When radicles were >25 mm in length (48–72 h, depending on species), border cells from 5–10 roots were harvested as follows. The apices (1–2 mm) were immersed in a droplet of water and placed on a hydrophobic surface (i.e. plastic Petri dish) for 1–2 min. The water was agitated briefly by taking up and releasing with a pasteur pipette, and an aliquot of cells was placed onto a microscope slide. Cell number was measured by direct counts, and was based on a mean of at least three samples of five roots per sample, as described (Hawes and Lin, 1990). Cell numbers given in Table 1 were rounded to the nearest 100.
Table 1.
Root apical meristem organization types and released border cell numbers
| Species | Family | Apical organization type* | Number of border cells† |
|---|---|---|---|
| Arabidopsis thaliana | Brassicaceae | c | 0 |
| Solanum melongena | Solanaceae | c | 50 |
| Petunia hybrida | Solanaceae | c | 100 |
| Nicotiana tabacum | Solanaceae | c | 100 |
| Capsicum annuum | Solanaceae | c | 100 |
| Lycopersicon esculentum | Solanaceae | c | 200 |
| Luffa cylindrica | Cucurbitaceae | b-o | 1500 |
| Helianthus annuus | Asteraceae | i-o | 2000 |
| Citrullus lanatus | Cucurbitaceae | b-o | 2400 |
| Daucus carota | Apiaceae | i-o | 2500 |
| Cucumis melo | Cucurbitaceae | b-o | 2500 |
| Cucumis sativa | Cucurbitaceae | b-o | 3100 |
| Phaseolus vulgaris | Fabaceae | b-o | 3500 |
| Glycine max | Fabaceae | b-o | 3700 |
| Sesbania exaltata | Fabaceae | b-o | 3900 |
| Pisum sativum | Fabaceae | b-o | 4500 |
| Sesbania javonica | Fabaceae | b-o | 4600 |
| Vigna unguiculata | Fabaceae | b-o | 6000 |
| Gossypium hirsutum | Malvaceae | i-o | 10 000 |
Abbreviations: c, closed; b-o, basic-open; i-o, intermediate-open. Data from Groot et al. (2004).
Data from Hawes et al. (2003).
RESULTS
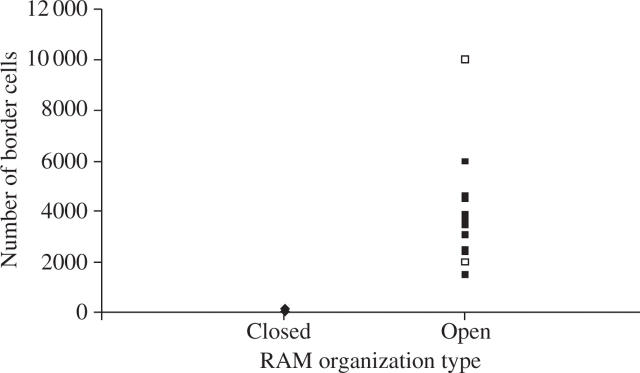
Initial comparison was made of Hawes et al.'s (2003) data on border cell counts from 19 plant species (of Brassicaceae, Solanaceae, Apiaceae, Fabaceae, Malvaceae, Asteraceae and Cucurbitaceae) with their respective RAM organization types (Groot et al., 2004). The results revealed that species with open (either basic-open or intermediate-open) organization produced significantly more border cells than species with closed organization (Table 1). All species exhibiting open RAM organization produced at least an order of magnitude more border cells in the same time period (24 h) than those with closed RAM organization (Fig. 1, Table 1). This information prompted an anatomical investigation of these examples and other species to verify this relationship and to examine the potential developmental causes for this correlation.
Fig. 1.
Graph illustrating that roots with closed organization (triangles) have few to no border cells, and those with open organization [intermediate-open (open squares) and basic-open (closed squares)] tend to release border cells. Border cell number data from Hawes et al. (2003) and root apical meristem organization data from Groot et al. (2004).
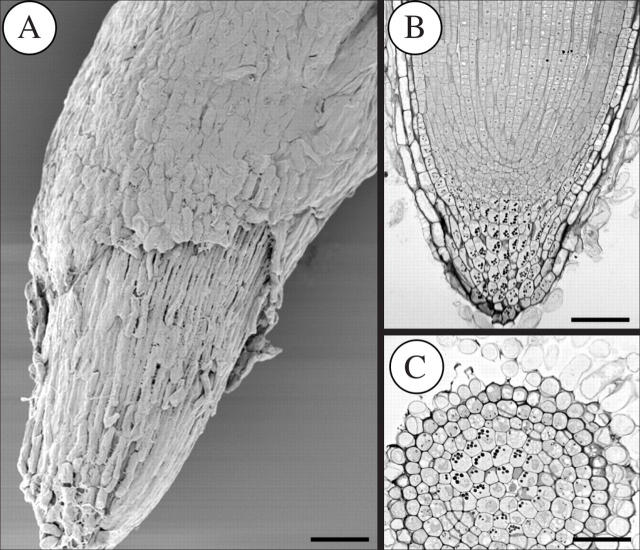
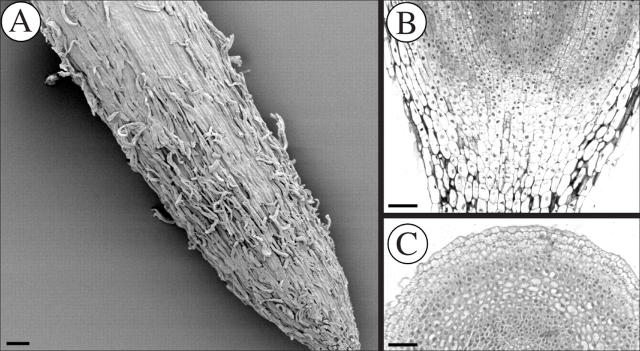
The structure and organization of the root tips of Brassica napus (closed), Helianthus annuus (intermediate-open) and Pisum sativum (basic-open) are shown in Figs 2–4. Roots with closed organization, such as those of B. napus, have discrete tiers of initials and show clear cell file connections to those initials (Fig. 2A). In such closed roots, the root cap tends to peel off in layers or groups of cells (Fig. 2A–C). H. annuus provides an example of a root with intermediate-open organization in which clearly defined initial tiers are not seen and root cap cells separate mostly as single or small groups of border cells (Fig. 3A–C). Roots like those of P. sativum with basic-open organization seemingly lack discrete initial tiers altogether and release many border cells (Fig. 4A–C).
Fig. 2.
(A) Scanning electron micrograph of the tip of a Brassica napus root showing external view of the root cap. (B) Longitudinal section of a closed root apical meristem showing initial tiers and root cap layers peeling off. (C) Transverse view. Individual border cells do not occur. Scale bars = 90 μm.
Fig. 3.
(A) Scanning electron micrograph of Helianthus annuus root tip. (B) An example of intermediate-open root apical meristem organization in longitudinal view. (C) Transverse view showing border cells. Scale bars = 60 μm (A), 90 μm (B and C).
Fig. 4.
(A) Scanning electron micrograph of Pisum sativum root tip. (B) Longitudinal view showing basic-open root apical meristem organization. (C) Transverse view showing released border cells. Scale bars = 105 μm.
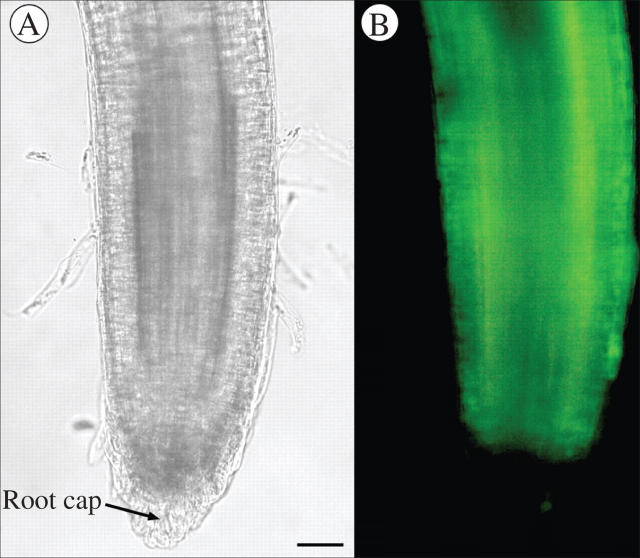
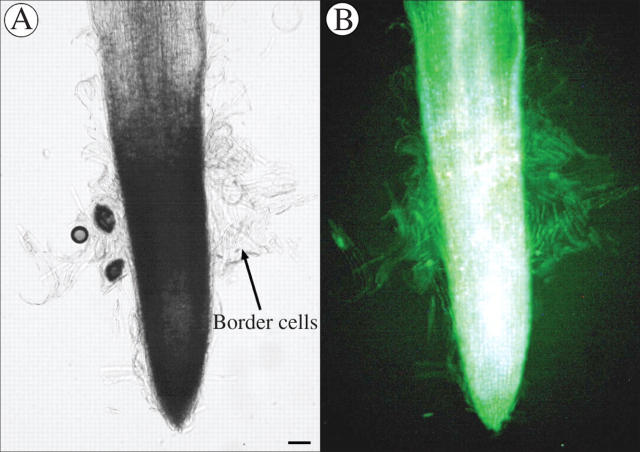
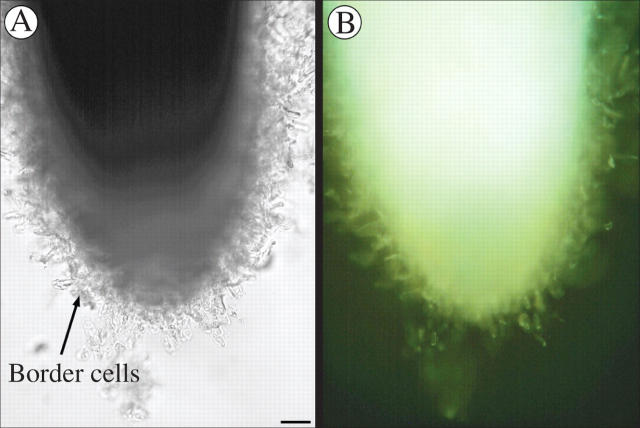
The same three species, B. napus, H. annuus and P. sativum, were examined with a vital stain to determine if the most peripheral cells of the root cap and the border cells, if released, tended to be dead or alive. In B. napus (Fig. 5) no border cells were observed and the cells on the root cap periphery showed no stain and were apparently not living. Both H. annuus and P. sativum (Figs 6 and 7) showed a positive reaction in the root cap periphery and the released border cells, suggesting that these cells were living.
Fig. 5.
(A) Whole mount of Brassica napus root tip. (B) Same root tip stained with fluoroscein diacetate shows no staining on the root cap periphery, indicating no living cells. Scale bar = 70 μm.
Fig. 6.
(A) Whole mount of Helianthus annuus root tip. (B) Same root tip showing positive fluoroscein diacetate staining of released border cells. Scale bar = 46 μm.
Fig. 7.
(A) Whole mount of Pisum sativum root tip. (B) Same root tip showing positive fluoroscein diacetate staining of border cells. Scale bar = 57 μm.
DISCUSSION
Border cell production and release and RAM organization type are conserved within taxa. This observation led to the idea that the capacity for border cell production and release could be correlated with RAM organization type. An initial comparison was made of Hawes et al.'s (2003) data on border cell counts from 19 plant species in seven different families (Brassicaceae, Solanaceae, Apiaceae, Fabaceae, Malvaceae, Asteraceae and Cucurbitaceae) with their respective RAM types from Groot et al. (2004). From this comparison, species with open RAM (either basic-open or intermediate-open) organization were found to produce significantly more border cells than species with closed organization. This information prompted an anatomical investigation to verify this relationship and to examine the potential developmental causes for this correlation.
RAM organization type tends to be a fairly specific character in plant families. Groot et al. (2004) studied RAM organization as a phylogenetic character in 35 dicotyledonous angiosperm families representing 23 different orders. RAM organization type was determined to be open (basic-open or intermediate-open) or closed based on the pattern of initials found in the root tip in median longitudinal view. Closed RAM organization in dicotyledonous angiosperms refers to roots where three specific tiers of initials are found for the epidermis/root cap, cortex and vascular cylinder, respectively. Basic-open organization is found in only two families—Fabaceae and Cucurbitaceae—and in that instance specific tiers of initials are absent. Intermediate-open RAM organization refers to cases where the initials are not clearly defined, i.e. two or more tissue systems may share undefined initials (Groot et al., 2004). The root tips studied by Groot et al. (2004) were sampled either from 1–2-week-old seedlings, or from verified drawings or photographs found in the literature. The three RAM organization types were then plotted by Groot et al. (2004) onto the Angiosperm Phylogeny Group cladogram (Soltis et al., 2000) and interpreted based on their distribution patterns in the different plant orders. The intermediate-open RAM type was found in basal eudicots such as the Ranunculales and was the ancestral condition. The closed RAM condition was derived once at the base of the core eudicots, and is found in its two large branches, the rosid and asterid clades. Intermediate-open and basic-open RAM types reappeared at different times as reversions.
Anatomical clues to border cell production and release from the RAM
Table 2 lists families for which at least some genera are known to have the closed RAM organization type. As shown in the current study, B. napus has closed organization and the cells on the periphery of the RAM showed no fluorescein diacetate (FDA) staining, indicating that the peripheral root cap cells are not living. In addition, peripheral root cap cells tend to be released in sheets and no living border cells were observed. This corresponds with the observation of Zhu and Rost (2000), who showed in Arabidopsis thaliana that apoptosis is triggered in the outer two layers of the root cap 2 weeks after germination. The symptoms of apoptosis observed by Zhu and Rost (2000) were nuclear degeneration, symplasmic isolation by reduction in numbers of plasmodesmata, and the appearance of dysfunctional plasmodesmata. Wang et al. (1996) observed apoptosis in peripheral root cap cells of tomato (Lycopersicon esculentum), another species with closed RAM organization. These observations in three different species in two families suggest the apoptosis occurs in the peripheral layers of the root cap of roots with closed RAM organization, thereby precluding the production and release of border cells. Root cap cells are released from B. napus (current study) and A. thaliana, but cells tend to peel off in layers or sheets of dead cells (Vicre et al., 2005). It is therefore incorrect to refer to these as border cells.
Table 2.
Families of dicotyledonous angiosperms with at least some genera with closed root apical meristem organization (data from Groot et al., 2004)
| Eurosid I clade | Eurosid II clade | Asterid clade |
|---|---|---|
| Oxalidaceae | Brassicaceae | Amaranthaceae |
| Linaceae | Sapindaceae | Polygonaceae |
| Euphorbiaceae | Rutaceae | Lamiaceae |
| Onagraceae | Haloragaceae | Veroniceae |
| Oleraceae | ||
| Solanaceae | ||
| Convolvulaceae |
Roots of H. annuus (intermediate-open) and P. sativum (basic-open) with open organization showed positive FDA staining and released living border cells from their root caps. The obvious difference between roots with open and closed RAM organization, therefore, is that roots with closed organization are programmed to trigger apoptosis in the outer layers of their root caps and roots with open organization are programmed instead to produce and release living border cells from their root caps.
Throughout years of study and literally hundreds of papers published on RAM organization (e.g. Rost, 1994; Rost and Bryant, 1996; Groot and Rost, 2001a, b; Groot et al., 2004), we are unaware of any reports that suggest any particular significance, ecological or otherwise, to RAM organization type. As far as we are aware, this is the first time a function for RAM organization has even been suggested. Are their any developmental clues to explain this difference in function?
Few studies have been published on the formation of the root caps in dicot plants. Baum and Rost (1996) and Wenzel and Rost (2001) reported on the precise development of the epidermis and root cap in A. thaliana. The peripheral root cap and epidermis are organized into modules of cells derived from a T-division of a root cap/protoderm (RCP) initial cell. This unique cell divides sequentially four times to produce 16 peripheral root cap cells and 16 protoderm cells per module. The RCPs divide around the RAM circumference in waves to form successive modules. The regularity of the pattern suggests a timing mechanism to co-ordinate the divisions of the RCP initials and the cells making up the modules of protoderm and peripheral root cap cells. Zhu et al. (1998a) showed that the cells of the peripheral root cap layers in A. thaliana had very few plasmodesmata and that these cells were basically symplasmically isolated. The outer layers of the peripheral root cap cells tend to be released in sheets, but as described earlier, these cells are not living border cells.
Wenzel et al. (2001) conducted a similar developmental study on the formation of the protoderm and peripheral root cap in white clover (Trifolium repens), a species with open RAM organization. Although this species has open RAM organization it still had RCP initials that regulated the formation of the protoderm and peripheral root cap. The RCP divided sequentially to form modules of protoderm and peripheral root cap cells in multiples of four, typically ending with 32 cells of each tissue in a module. The RCP cells divided around the circumference of the RAM producing rounds of peripheral root cap layers. The two studies above suggest that the developmental protocol followed to produce the protoderm and peripheral root cap is fundamentally the same in these two examples—A. thaliana, a closed root species, and T. repens, an open root species. These are only two examples, but they suggest no particular difference in the developmental process that structurally creates the peripheral root cap. The only essential difference remains that the outer layers in closed roots undergo apoptosis and open roots do not.
The dynamics of root growth offer another twist to understanding the nature of RAM organization patterns. Roots tend not to elongate indefinitely and RAM organization, size, and shape do not remain static throughout primary root growth in P. sativum, Gossypium hirsutum or A. thaliana (Gladish and Rost, 1993; Reinhardt and Rost, 1995; Baum et al., 2002). In roots such as those of A. thaliana with closed organization, the primary root stops elongating about 4 weeks after germination. At that time RAM organization changes from closed to intermediate-open (Baum et al., 2002), and the cells in the primary root meristem stop cycling new cells.
Chapman et al. (2003) made a comparative study of five different species of plants with closed RAM organization, and found that the following held true in each instance: primary roots tend to be determinate in that they eventually cease elongation and this is followed by a change in roots having closed RAM organization from closed to intermediate-open as a result of termination of the cell cycle in the RAM.
This means that in roots with closed RAM organization, in particular, the RAM pattern does not remain exactly the same over the entire life span of a root (Chapman et al., 2003). Two things are clear: (a) all primary roots regardless of RAM type grow for a finite period and then stop growth; and (b) roots with closed organization tend to lose their clear organization when they cease elongation. Do these older roots now with intermediate-open organization then produce border cells? The answer is no because the RAM in these roots has stopped producing new cells for the root cap. Even though roots with closed organization become open when the root stops elongating, border cells do not form, because coupled with ceasing elongation, cells in the root tip also tend to stop cycling and no new cells are made.
This is the first published account of the correlation between RAM organization type and border cell production. Although the investigative methods used elucidated no anatomical or morphological reason for this correlation outside of the presence of apoptosis in roots with closed organization, the correlation itself is a noteworthy addition to the limited literature on the topic of root caps and border cells.
What difference does the absence of border cells make?
Border cells comprise an unusual ‘tissue’, cells of which separate into the external environment where they release specific signals that modulate gene expression and growth in soil-borne bacteria and fungi (Hawes et al., 1998). Viability of border cells and their capacity to detach from the root into the rhizosphere appear to be critical to their role in modulating root infection by pathogenic fungi (Gunawardena et al., 2005). Living border cells also appear to play a role in the establishment of mycorrhizal symbiosis (Nagahashi and Douds, 2004). The number of border cells produced per root, in fact, is tightly correlated with the capacity to be infected with arbuscular mycorrhizal fungi, and species such as A. thaliana that do not produce border cells cannot form mycorrhizal associations (Niemira et al., 1996). In legumes, border cells produce signals that induce sporulation in pathogenic fungi and nodulation gene expression in Rhizobium species, and failure of nodulation is correlated with delayed production of border cells (Zhu et al., 1997; Woo et al., 2004; Gunawardena et al., 2005). If there is a causal relationship between border cell production and susceptibility of roots to infection by specific soil-borne organisms, then understanding the differences between plants with and without border cells may yield avenues for crop improvement.
Understanding why some families of plants have evolved roots with closed apical organization which do not produce border cells is an important problem. If such roots lack the capacity to interact with soil-borne micro-organisms, then either that ability was not needed to survive in their native environment or they have alternative means to deal with them. A future project will be to study the origins of the families listed in Table 2 to understand better their native rhizosphere conditions. In the meantime, it is now clear that dicotyledonous plants whose root tips have closed RAM organization do not produce border cells, and those with open organization do.
Acknowledgments
Sue Nichol (University of California, Davis) is acknowledged for her excellent microscopy work during the early stages of this project. T.L.R. acknowledges all of the graduate and undergraduate students, postdocs, technicians and visitors to the laboratory over the past several years for their fruitful and entertaining discussions and assistance.
LITERATURE CITED
- Baum SF, Rost TL. 1996. Root apical organization in Arabidopsis thaliana: 1. Root cap and protoderm. Protoplasma 192: 178–188. [Google Scholar]
- Baum SF, Dubrovsky JG, Rost TL. 2002. Apical organization and maturation events in Arabidopsis thaliana roots: developmental changes over time. American Journal of Botany 89: 908–920. [DOI] [PubMed] [Google Scholar]
- Chapman K, Groot EP, Nichol SA, Rost TL. 2003. Primary root growth and the pattern of root apical meristem organization are coupled. Journal of Plant Growth Regulation 21: 287–295. [Google Scholar]
- Esau K. 1965. Plant anatomy, 2nd edn. New York: John Wiley & Sons, 116–124.
- Gladish DK, Rost TL. 1993. The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisum sativum L., cv. “Alaska”). Environmental and Experimental Botany 33: 1–16. [Google Scholar]
- Groot EP, Rost TL. 2001a.. Cell division patterns and root apical organization. In: Francis D, ed. The plant cell cycle and its interfaces. Sheffield: Sheffield Academic Press Ltd, 137–158.
- Groot EP, Rost TL. 2001b.. Patterns of apical organization in roots of flowering plants. In: Proceedings, the 6th ISRR Symposium. Roots: the dynamic interface between plants and the earth. Nagoya, Japan: Japanese Society for Root Research, 8–9.
- Groot EP, Doyle JA, Nichol SA, Rost TL. 2004. Phylogenetic distribution and evolution of root apical meristem organization in dicotyledonous angiosperms. International Journal of Plant Science 165: 97–105. [Google Scholar]
- Gunawardena U, Rodriguez M, Straney D, Romeo JT, VanEtten D, Hawes MC. 2005. Tissue-specific localization of pea root infection by Nectria haematococcia. Mechanisms and consequences. Plant Physiology 137: 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Guttenberg H. 1968. Der primäre Bau der Angiospermenwurzel. In: Handbuch der Pflanzenanatomie, Band VIII, Teil 5. Berlin: Gebruder Borntraeger, 472 pp.
- Hawes MC. 1991. Living plant cells released from the root cap: a regulator of microbial populations in the rhizosphere? In: Keiser DL, Cregan PB, eds. The rhizosphere and plant growth. Dordrecht: Kluwer Academic Publishers, 51–59.
- Hawes MC, Lin H-J. 1990. Correlation of pectolytic enzyme activity with the programmed release of cells from root caps of pea (Pisum sativum). Plant Physiology 94: 1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes MC, Brigham LA, Wen F-S, Woo H-H, Zhu Y-M. 1998. Function of root border cells in plant health: pioneers in the rhizosphere. Annual Review of Phytopathology 36: 311–327. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Bengough G, Cassab G, Ponce G. 2003. Root caps and rhizosphere. Journal of Plant Growth Regulation 21: 352–367. [Google Scholar]
- Hawes MC, Woo H, Wen F. 2005. Root border cells: a delivery system for chemicals controlling plant health. Roots and Soil Management: Interactions between Roots and Soil, Agronomy Monograph no. 48, 107–117.
- Nagahashi G, Douds DD. 2004. Isolated root caps, border cells, and mucilage from host roots stimulate hyphal branching of the arbuscular mycorrhizal fungus, Gigaspora gigantea. Mycology Research 108: 1079–1088. [DOI] [PubMed] [Google Scholar]
- Niemira BA, Safir GR, Hawes MC. 1996. Arbuscular mycorrhizal colonization and border cell production: a possible correlation. Phytopathology 86: 563–568. [Google Scholar]
- O'Brien TP, McCully ME. 1981. The study of plant structure: principles and selected methods. Melbourne, Australia: Termarcarphi.
- Reinhardt DH, Rost TL. 1995. On the correlation of primary root growth and tracheary element size and distance from the tip in cotton seedlings grown under salinity. Environmental and Experimental Botany 35: 575–588. [Google Scholar]
- Rost TL. 1994. Root tip organization and the spatial relationships of differentiation events. In: Iqbal M, ed. Growth patterns in vascular plants. Portland: Dioscordes Press, Inc., 59–76.
- Rost TL, Baum S. 1988. On the correlation of primary root length, meristem size and protoxylem tracheary element position in pea seedlings. American Journal of Botany 75: 414–424. [Google Scholar]
- Rost TL, Bryant JA. 1996. Root organization and gene expression patterns. Journal of Experimental Botany 47: 1613–1628. [Google Scholar]
- Rost TL, Baum SF, Nichol S. 1996. Root apical organization in Arabidopsis thaliana and a comment on root cap structure. Plant and Soil 187: 91–95. [Google Scholar]
- Rost TL, Jones TJ, Falk RH. 1988. The distribution and relationship of cell division and maturation events in Pisum sativum (Fabaceae) seedling roots. American Journal of Botany 75: 1571–1583. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, et al. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381–461. [Google Scholar]
- Vicre, M, Santaella C, Blanchet S, Gateau A, Driouich A. 2005. Root border-like cells of Arabidopsis. Microscopical characterization and role in the interaction with Rhizobacteria. Plant Physiology 138: 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG. 1996. Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8: 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel CL, Rost TL. 2001. Cell division patterns in the root cap and epidermis of Arabidopsis thaliana roots. Protoplasma 218: 203–213. [DOI] [PubMed] [Google Scholar]
- Wenzel CL, Tong KL, Rost TL. 2001. Modular construction of the epidermis and peripheral root cap in the ‘open’ root apical meristem of Trifolium repens cv Ladino. Protoplasma 218: 214–224. [DOI] [PubMed] [Google Scholar]
- Woo HH, Hirsch AM, Hawes MC. 2004. Altered susceptibility to infection by Sinorhizobium meliloti and Nectria haematococca in alfalfa roots with altered cell cycle. Plant Cell Reports 22: 967–973. [DOI] [PubMed] [Google Scholar]
- Zhu T, Rost TL. 2000. Directional cell-to-cell communication in the Arabidopsis root apical meristem: III. Plasmodesmata turnover and apoptosis in meristem and root cap cells during four weeks post-germination. Protoplasma 213: 108–117. [Google Scholar]
- Zhu T, Lucas WJ, Rost TL. 1998a.. Directional cell-to-cell communication in the Arabidopsis root apical meristem: I. An ultrastructural and functional analysis. Protoplasma 203: 35–47. [Google Scholar]
- Zhu T, O'Quinn RL, Lucas WJ, Rost TL. 1998b.. Directional cell-to-cell communication in Arabidopsis root apical meristem. II. Dynamics of plasmodesmatal formation. Protoplasma 204: 84–93. [Google Scholar]
- Zhu Y, Pierson LS III, Hawes MC. 1997. Release of nodulation gene inducing chemicals from root border cells. Plant Physiology 115: 1691–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]