Abstract
• Background and Aims Prunus, subgenus Padus, exhibits two completely different calcium oxalate crystal macropatterns in mature leaves. Foliar macropattern development has been described previously in P. virginiana, representing one version. Prunus serotina, in the group exhibiting the second macropattern, is described here. The goal was to describe developmental details for comparison with P. virginiana, and to extend the sparse current knowledge of crystal macropatterns.
• Methods Leaves at various developmental stages were removed from local trees and from herbarium specimens. Early leaf stages and freehand leaf and stem sections were mounted directly in aqueous glycerine; larger leaves were processed whole or in representative pieces in household bleach, dehydrated in alcohol/xylol, and mounted in Permount. Crystals were detected microscopically between crossed polarizers.
• Key Results Bud scales have a dense druse population. Druses appear first at the stipule tip and proliferate basipetally but soon stop forming; growing stipules therefore have a declining density of druses. Druses appear at the tip of leaves <1 mm long, then proliferate basipetally in the midrib. Lamina druses appear in the distal marginal teeth of leaves 3 cm long; from here they proliferate basipetally and towards midrib along major veins. In about two-thirds-grown leaves (6–9 cm length) druses are all adaxial to veins of most orders; a shift occurs then to formation of prisms, which appear first abaxial to, then all around, veins. Mature leaves have virtually all prisms encrusting all major veins, more sparsely along smaller minor veins. Late season leaves form epitactic crystals on existing prismatics.
• Conclusions The developing and mature macropattern of P. serotina is almost the reverse of the pattern described previously in P. virginiana, and shows that two closely related species can develop radically different modes of crystallization. The few detailed macropattern studies to date reveal striking variations that indicate a new level of organization that must be integrated with the anatomical, physiological and molecular approaches that have been dominant so far.
Keywords: Black cherry, calcium oxalate, choke cherry, crystals, crystal macropattern, druses, prismatics, Prunus serotina, Prunus virginiana, Rosaceae
INTRODUCTION
The large genus Prunus exhibits considerable diversity of types and locations of calcium oxalate crystals in its leaves, but they are not distributed haphazardly. Lersten and Horner (2000) surveyed 131 of the variously estimated 150–400 species of Prunus and found six major calcium oxalate crystal macropatterns (this term includes crystal types and their tissue distribution), which showed a systematically significant trend among the five commonly recognized subgenera. Subgenus Padus was especially interesting because it has two quite different macropatterns.
One group of Padus species has druses only along leaf veins and large prismatic crystal idioblasts restricted to palisade mesophyll cells. The crystal macropattern development was described in choke cherry (Prunus virginiana) which is in this group (Lersten and Horner, 2004).
Another group of Padus species exhibits a quite different crystal macropattern: small prismatic crystals (with a scattering of small druses) encrust all but the smallest veins, while mesophyll cells remain almost crystal-free. A member of this group is black cherry (Prunus serotina), a widespread species of central and eastern North America (Cronquist, 1990), which was investigated for crystal macropattern development in the present study for comparison with the previously described macropattern of P. virginiana. It was found that these related species are remarkably dissimilar in both crystal macropattern development and mature crystal macropattern, which serves as the basis for the present study.
MATERIALS AND METHODS
Trees of Prunus serotina Ehrh. growing locally in Ames, Iowa and on the Iowa State University campus provided abundant study material. Leaves at various stages of development were also removed from vegetative branch tips of selected specimens in the Iowa State University (ISC) Ada Hayden Herbarium [B.O. Wolden 746, Emmet Co., IA (28 V 1923); H.C. Phillips and E. Wofford 02661, Lyon Co., KY (13 IV 1967); Verhoek-Williams, Davis, Luikart 96, Mo. Bot. Garden, St Louis, MO (4 V 1970)].
Cross-sections of leaves and young stems, as well as some whole leaves from primordia to about 1 cm long, were mounted directly in 50 % aqueous glycerine on glass slides and coverslipped. Additional similar young leaves were processed in the following manner, but not bleached. All larger leaf stages were placed whole, or cut into smaller pieces, into 95 % ethanol to remove all or most chlorophyll, hydrated to deionized water, then placed into full-strength household bleach for 15 min to 1 h or so, depending on the time required to bleach the samples white.
Bleached specimens were rinsed in deionized water (about 15 min), then dehydrated in an ethanol series (50 %, 70 %, 95 %, 100 %, 100 %, 1 : 1/100 % : xylol; about 10 min in each step). After a final transfer to xylol (no time limit), samples were mounted in Permount and coverslipped. The bleaching technique used was a modified version of that of Frank (1972). Leaf and stem sections were cut freehand with a razor blade and mounted in 50 % aqueous glycerine.
All preparations were viewed in bright-field mode or between crossed polarizers, and selected images were captured digitally using a Zeiss MRc digital camera, processed in Adobe PhotoShop 7.0, and organized into plates with Adobe Illustrator 10 software.
RESULTS
Leaves of local trees of Prunus serotina are lanceolate, 10–12 cm long and 2.5–3.0 cm wide when full-grown. Major venation is semicraspedodromous (Hickey, 1973), with 20–25 rather inconspicuous main lateral veins. In each lamina half, these veins extend acropetally at an angle of about 70 degrees from the midvein towards the margin. Each vein near the margin turns parallel to it and generates a few smaller veins that terminate in nearby leaf teeth (Fig. 1J). The two slender stipules per leaf grow rapidly, attain their final length of about 1.5 cm when the leaf blade is only about 1.0 cm long, and abscise when the leaf is about full length. Internally, the mature leaf has two layers of rather short palisade mesophyll cells and a larger region of lacunate spongy mesophyll (Fig. 2E).
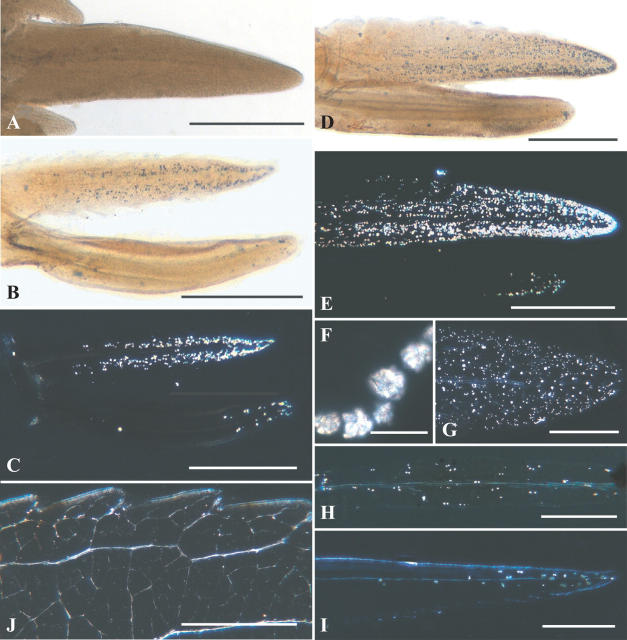
Fig. 1.
(A–I) Bright field (BF) and polarized light (PL) views of clearings of Prunus serotina young leaves and bud scales and crystals. (A) Young leaf 735 μm long with a small pair of stipules, no crystals are evident; BF. (B) Young leaf (bottom) about 1 mm long with one similarly long stipule (top); BF. (C) Same as (B) but crystals are visible; PL. (D) Young leaf about 2 mm long with longer stipule (top); BF. (E) Same as (D) but with further development of crystals; PL. (F) Close up of large druses in stipule; BF. (G) Older stipule 1.2 cm long displays some reduction in druses; PL. (H) Old stipule displays sparsely distributed druses; PL. (I) Young leaf 6-mm long displays distal crystals; PL. (J) Margin of young leaf 3.3 cm long; druses occur in leaf teeth and adjacent veins; PL. Scale bars: A = 250 μm; B–E and I = 0.5 mm; F = 40 μm; G = 250 mm; H = 0.5 mm; J = 1.0 mm.
Fig. 2.
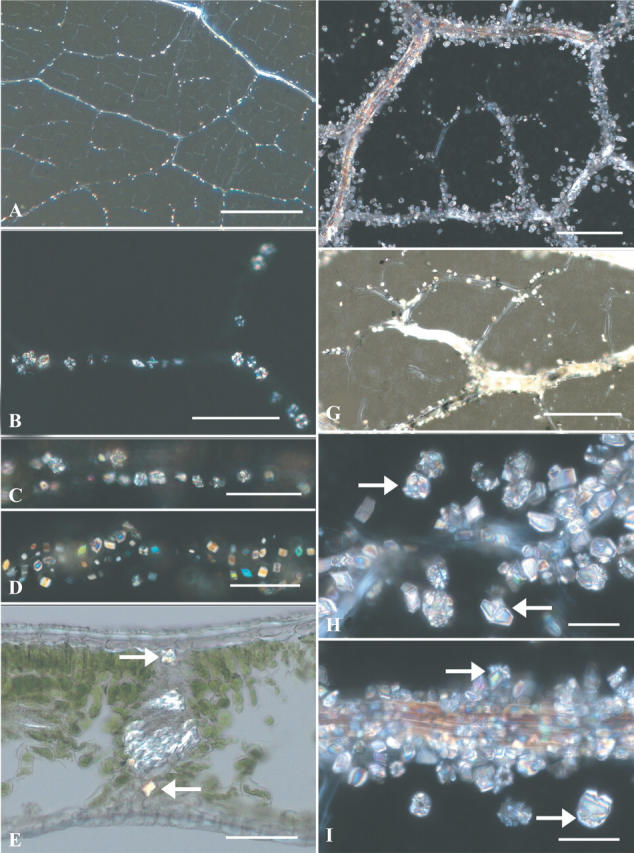
(A–I) Bright field (BF) and polarized light (PL) views of clearing of Prunus serotina young and old leaves. (A) Young leaf showing larger veins with crystals; PL. (B) A two-thirds-grown leaf with adaxial vein druses; PL. (C) Close-up of adaxial vein druses; PL. (D) Same image as C, but at a different plane of focus showing abaxial prisms; PL. (E) Vibratome cross-section of almost full-length leaf showing vein with an adaxial druse (right-pointing arrow) and an abaxial prism (left-pointing arrow); partial PL. (F) Face view of old leaf areole showing irregularly encrusted large veins and minor veins with sparse crystals; PL. (G) Close up of minor veins with few or no crystals; partial PL. (H) Old leaf vein displays prisms with a few associated druses, and some prisms with epitactic crystals (arrows); PL. (I) Portion of old leaf vein irregularly encrusted with prisms and a few druses, and some prisms with epitactic crystals (arrows); PL. Scale bars: A = 0.5 mm; B–E = 50 μm; F and G = 200 μm; H and I = 40 μm.
Crystallization events during macropattern development are not correlated precisely with leaf length, but they occur instead within a range of leaf lengths. This variability can be attributed to climatic differences within the geographical range of the species, different locations of buds and leaves on an individual tree, and probably even differences in final leaf size if each had been allowed to complete its development. Crystallization events as described here, however, followed each other predictably in all series of leaves studied.
Leaves up to at least 800 μm long lack crystals, but by this length they have a midvein, have at least initiated the lamina, and have a tiny pair of stipules (Fig. 1A). Stipules grow more rapidly than the leaf blade, and the first crystals to appear are at the stipule tip; from here they quickly proliferate basipetally in both mesophyll cells and along veins. By the time a few druses appear later at the tip of an 800- to 1000-μm leaf blade, the almost equally elongate stipules already have a dense population of druses that extends three-quarters of the way to its base (Fig. 1B, C). Crystals therefore appear in stipules and leaf when they are both slightly <1 mm long.
Stipules at 2 mm have only reached about 15 % of their full length, but the druse population already appears to be at maximum density (Fig. 1D–F). The associated leaf blade, which is slightly shorter, still has only a small cluster of apical druses, mostly in the midrib, but a few druses also occur along the most distal major lateral veins, which have just matured enough to accommodate them. A decrease in crystal density was noted in stipules as short as 3 mm because, while stipules continue to grow, the rate of crystal formation either declines or ceases. Cessation must occur at a fairly early stage of stipule expansion because druses are thinly distributed in stipules of intermediate length (Fig. 1G), and even sparser per unit area in mature stipules 1.2–1.5 cm long (Fig. 1H).
Crystals appear first in stipules instead of in the leaf blade during the earliest period of leaf growth; even in a leaf about 6 mm long only a few druses occur distally, and they have just begun to proliferate basipetally in the midrib (Fig. 1I). It is only in leaf blades 0.8–1.0 cm long that the file of midrib druses finally extends to the leaf base and reaches the druses that have probably proliferated acropetally in the petiole (observations were obscured here because of its greater thickness).
Although some lamina development is evident in leaves about 6 mm long (Fig. 1I), no lamina crystals appear until leaves are 3–4 cm long, when the first druses appear in distal leaf teeth, along the offshoot veins. From these loci druses proliferate progressively basipetally among leaf teeth and towards the midrib along maturing major veins (Fig. 1J). Crystallization in the lamina therefore seems to follow the general pattern of lamina maturation, but only after a crystal pathway extends through midrib and petiole.
Until leaves are between one-half and three-quarters (6–9 cm long) grown, only druses were seen. These occur mostly in rather regularly spaced, single files adaxial to major and even larger minor veins in the lamina (Fig. 2A, B). Minor veins, which seem to be fully formed in leaves of this range of lengths, remain free of crystals or have only an occasional adaxial druse (Fig. 2A). But somewhere within this range of leaf lengths an abrupt change occurs in the type and location of newly formed crystals. Prisms form instead of druses, and they occur abaxial to the now mature phloem sclerenchyma fibre strand of major veins (Fig. 2C–E). This new abaxial crystallization pattern spreads throughout the leaf, but we could not ascertain how closely it follows the earlier basipetal and margin-to-midrib progression of druse formation.
This shift in crystallization was easily detected in bleached leaf samples 6–9 cm long by first focusing on the earlier-formed druses above any of the larger veins (Fig. 2C), then refocusing directly below the vein onto the prisms (Fig. 2D). Cross-sections of leaves also revealed the segregation of crystals (Fig. 2E).
Leaves reach full size sometime in May and remain on the tree until mid-October. During the intervening months new prisms and a tiny percentage of druses continue to be deposited, at first only abaxially, and later all around major veins, until by late in the growing season all vein orders except the very smallest have become unevenly encrusted with crystals (Fig. 2F, I). The smallest minor veins remain either free of crystals or only sparsely populated by prisms (Fig. 2F, G).
Also seen late in the season were small, mostly druse-like, epitactic crystals (Fig. 2H, I) attached to many prisms. Epitactic crystals were seen in leaves collected from mid-August to mid-October, indicating that it is a late season phenomenon.
In mature midribs and petioles, a mixture of druses and prisms were found in parenchyma cells surrounding the central vascular bundle (Fig. 3A, B). In the stem and in the leaf traces, however, only druses occur, but here they form in the phloem as well as in cortical parenchyma cells (Fig. 3C). It was not possible to determine where, in the transition from stem to leaf, the crystal configuration changed.
Fig. 3.
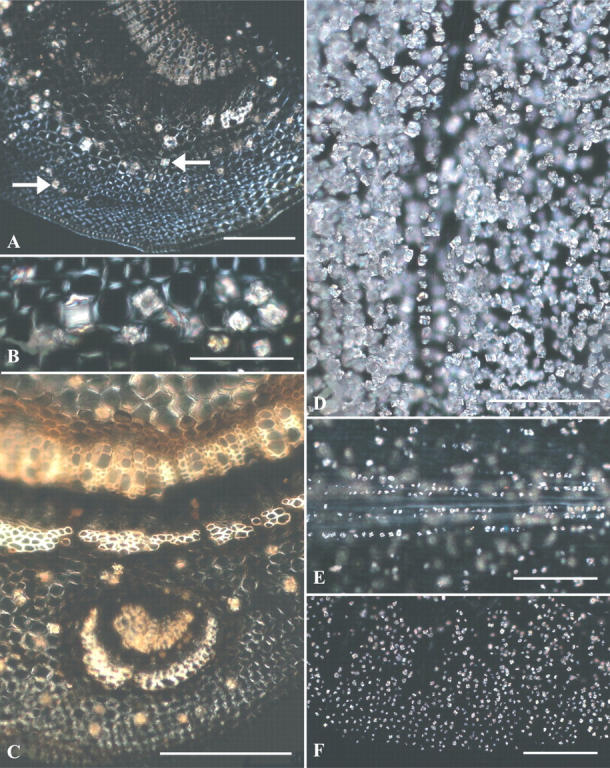
(A–F) Bright field (BF) and polarized light (PL) views of Prunus serotina stem sections and clearings of scales showing crystals. (A) Stem cross-section displays cortical druses (left-pointing arrow) and prisms (right-pointing arrow); PL. (B) Enlarged region of (A) displays both druses and prisms; PL. (C) Vascular leaf trace (below) in stem cross-section displays only druses in cortex; PL. (D) Thick outer bud scale filled with crystals; PL. (E) Inner bud scale showing midvein druses (in focus) and scattered druses elsewhere (out of focus); PL. (F) Thin inner bud scale margin with druses in all cell types; PL. Scale bars: A, C–F = 200 μm; B = 100 μm.
After all of the current season's leaves have been initiated, the shoot apex produces several bud scales before it begins forming next season's leaf primordia. The first few outer scales are thick and decidedly scale-like, with every interior cell layer densely populated by druses and a miniscule number of small prisms (Fig. 3D). Inner bud scales are thinner, with a somewhat more leaf-like shape and a more developed venation system. They have virtually only druses, which also occur in all cell layers as well as along veins (Fig. 3E, F).
DISCUSSION
This is the third descriptive study of crystal macropattern development, following those on Prunus virginiana (Lersten and Horner, 2004) and Punica granatum (Lersten and Horner, 2005b). This trio comprises the total literature on such detailed studies. Results from just these three species already show that there are distinctive and as yet largely enigmatic crystallization patterns superimposed on the cellular level of organization. Crystallization events at this level will probably require a different kind of explanation from that which is useful at the cellular level, just as architecture is more than knowledge of structural components and how they are put together, and paintings are more than the chemical analysis of their pigments. These studies in Prunus illustrate the difficulty of integrating the two levels.
Prunus serotina might be expected to show at most only subtle differences from the related P. virginiana, but instead it is dissimilar in almost all respects. Comparison with the results from P. virginiana (Lersten and Horner, 2004) is therefore necessary.
Bud scales in both species are densely populated by crystals, but P. serotina has virtually only druses, P. virginiana only prisms. Similarly, stipules of P. serotina have only druses, those of P. virginiana only prisms. Stipules of both species, however, initiate crystals apically before the leaf blade and, in both, the subsequent crystals proliferate basipetally and occupy all cell layers except epidermis. Prunus serotina stipules at maturity have an attenuated population of druses but mature P. virginiana stipules retain a dense population of prisms.
Crystals in both species appear in the apex of the leaf blade before it is 1 mm long: druses in P. serotina, prisms in P. virginiana. Prunus serotina druses proliferate basipetally in the midrib somewhat later, but in P. virginiana no further crystal proliferation occurs until a leaf reaches at least 1 cm.
Druses in P. serotina appear next above veins in and near distal marginal teeth of leaves about 3 cm long, and from these loci they proliferate both basipetally along the margin and towards the midrib adaxially along major veins. In sharp contrast, P. virginiana leaves 1.0–1.5 cm long produce tiny prisms in palisade mesophyll idioblasts that are distributed throughout the lamina. Prunus virginiana also differs because druses proliferate upward from the petiole into the midrib instead of basipetally from the apex, and crystallization only reaches the apex when the leaf is about half-grown.
The remarkable shift of crystallization in P. serotina, from adaxial druses to abaxial prismatics in leaves one-half to two-thirds mature is a previously unknown phenomenon, unless the brief mention by Schimper (1888) that early druses are later replaced by prisms in the mesophyll of developing leaves of a Crataegus (Rosaceae) species is a similar example. Prunus virginiana leaves show no such shift; instead, the early widely scattered palisade crystal idioblasts are supplemented by continued formation of small to intermediate-size prisms in interspersed palisade mesophyll cells.
Continued production of small prisms in mature P. serotina leaves eventually encrusts major veins, but smaller minor veins remain either crystal-free or else have only a few prisms associated with them. In P. virginiana, however, druses form mostly in single file only along veins, and mostly after the leaf is full size. The largest druses are generally associated with the smallest veins. Late in the growing season, crystals in both species develop epitactic crystals but in P. serotina they are mostly druse-like whereas in P. virginiana they are mostly tiny prisms.
These macropattern comparisons emphasize the drastic differences between P. serotina and P. virginiana, but are they really closely related? Most contemporary systematists recognize Padus as a valid subgenus (Lersten and Horner, 2000), but a study of internal transcribed spacers of nuclear ribosomal DNA of 40 Prunus s.l. species (Lee and Wen, 2001) found that this molecular evidence showed species of subgenus Padus to be intermingled with subgenus Cerasus and Laurocerasus, all three subgenera comprising a recognizable major subgroup within Prunus. But aside from the systematics of Padus, P. serotina and P. virginiana appeared adjacent to each other, both in the strict concensus tree and the maximum likelihood tree. Another study by Bortiri et al. (2001), using sequence analysis of ITS and chloroplast trnL-trnF spacer DNA data from 48 Prunus species, yielded a well-supported clade comprising subgenera Cerasus, Padus and Laurocerasus. Within this clade, P. serotina and P. virginiana occur in a more weakly supported subclade, but not as sister-species These latter two studies emphasize their close relationship within Prunus and deepen the mystery of how they came to deviate so greatly from each other in their crystal macropatterns.
It should be mentioned here that the only previously published report that includes a description of crystals in P. serotina (Holm, 1909) mentioned, but did not illustrate, that crystals of undescribed shape surround all leaf veins from base to tip, and that they ‘abound’ in the spongy mesophyll but are relatively sparse in palisade parenchyma. His report regarding crystals around veins agrees with the authors', but his observation concerning mesophyll crystals is contrary to their findings in the present investigation as well as previous findings from a study of leaves from 11 herbarium specimens representing the geographic range of the species (Lersten and Horner, 2000).
The adaptive significance of most aspects of the crystal macropatterns in P. serotina and P. virginiana is difficult to interpret. It can be speculated that the dense accumulation of druses in bud scales helps these appendages to better protect next year's leaf primordia. In both species, the late season appearance of epitactic crystals may indicate that older leaves are unable to initiate new crystal cells, but can add on to existing crystals. Other aspects of the developing and mature macropattern of P. serotina do not support speculation at present. The shift from early druse formation to later prismatic production in P. serotina is particularly puzzling. The present study, therefore, adds to this speculation that has been previously posed regarding the role(s) of calcium oxalate crystals and crystal idioblasts in plants and fungi in general (Franceschi and Horner, 1980; Horner and Wagner, 1995; Nakata, 2003; Franceschi and Nakata, 2005).
The study of crystal macropatterns in leaves is in its infancy. Besides the present contribution, only two previous studies have correlated crystal macropattern development with measured leaf stages: Lersten and Horner (2004) on Prunus virginiana and Lersten and Horner (2005b) on Punica granatum. In two other studies, crystallization has been described more generally, without reference to specific leaf stages: Borchert (1984) on the progressive appearance of vein, mesophyll and epidermal crystals in the legume, Gleditsia triacanthos, and Borchert (1990) on Carya ovata (Juglandaceae), which was concerned mostly with crystallization in the lamina during leaf growth and maturation. These five studies describe five different scenarios, which mean that the true range of crystal macropattern development among angiosperms must be vastly greater.
Also scarce are detailed studies of mature crystal macropatterns. The Lersten and Horner (2000) survey of Prunus subgenera and the Cervantes-Martinez et al. (2005) survey of 69 species of 14 genera of two groups within the legume subtribe Glycininae (Tribe Phaseoleae) each give a detailed description of macropatterns in mature leaves of related taxa and show variations of potential usefulness for systematics. Lersten and Horner (2005a) studied Quillaja (Quillajaceae) stem and leaf in detail and showed that styloids and druses are segregated in both organs and form a previously unknown macropattern.
These few recent contributions demonstrate that there is a great deal of descriptive information at the whole organ level yet to be discovered that must be integrated with knowledge from cell and molecular biology to eventually yield a more coherent, although certainly more complex, general theory of plant crystallization.
LITERATURE CITED
- Borchert R. 1984. Functional anatomy of the calcium-excreting system of Gleditsia triacanthos L. Botanical Gazette 145: 474–482. [Google Scholar]
- Borchert R. 1990. Ca2+ as developmental signal in the formation of Ca-oxalate crystal spacing patterns during leaf development in Carya ovata. Planta 182: 339–347. [DOI] [PubMed] [Google Scholar]
- Bortiri E, Oh S-H, Jiang J, Baggett S, Granger A, Weeks C, et al. 2001. Phylogeny and systematics of Prunus (Rosaceae) as determined by sequence analysis of ITS and the chloroplast trnL-trnF spacer DNA. Systematic Botany 26: 797–807. [Google Scholar]
- Cervantes-Martinez T, Horner HT, Palmer RG, Hymowitz T, Brown AHD. 2005. Calcium oxalate crystal macropatterns in leaves of species from groups Glycine and Shuteria (Glycininae; Phaseoleae; Papilionoideae; Fabaceae). Canadian Journal of Botany 83: 1410–1421. [Google Scholar]
- Cronquist A. 1990. Manual of vascular plants of northeastern United States and adjacent Canada, 2nd edn. Bronx, NY: New York Botanical Garden.
- Frank E. 1972. The formation of crystal idioblasts in Canavalia ensiformis DC. at different levels of calcium supply. Zeitschrift fur Pflanzenphysiologie 67: 350–358. [Google Scholar]
- Franceschi VR, Horner Jr HT. 1980. Calcium oxalate crystals in plants. Botanical Review 46: 361–427. [Google Scholar]
- Franceschi VR, Nakata PA. 2005. Calcium oxalate in plants: formation and function. Annual Review of Plant Biology 56: 41–71. [DOI] [PubMed] [Google Scholar]
- Hickey LJ. 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany 60: 17–33. [Google Scholar]
- Holm T. 1909. No. 33. Prunus serotina Ehrh. Merck's Report 18: 287–290. [Google Scholar]
- Horner HT, Wagner BL. 1995. Calcium oxalate crystal formation in higher plants. In: Khan S, ed. Calcium oxalate in biological systems. Boca Raton, FL, USA: CRC Press, 53–72.
- Lee S, Wen J. 2001. A phylogenetic analysis of Prunus and the Amygdaloideae (Rosaceae) using ITS sequences of nuclear ribosomal DNA. American Journal of Botany 88: 150–160. [PubMed] [Google Scholar]
- Lersten NR, Horner HT. 2000. Calcium oxalate crystal types and trends in their distribution patterns in leaves of Prunus (Rosaceae: Prunoideae). Plant Systematics and Evolution 224: 83–96. [Google Scholar]
- Lersten NR, Horner HT. 2004. Calcium oxalate crystal macropattern development during Prunus virginiana (Rosaceae) leaf growth. Canadian Journal of Botany 82: 1800–1808. [Google Scholar]
- Lersten NR, Horner HT. 2005a Macropattern of styloid and druse crystals in Quillaja (Quillajaceae) bark and leaves. International Journal of Plant Sciences 166: 705–711. [Google Scholar]
- Lersten NR, Horner HT. 2005b. Development of the calcium oxalate crystal macropattern in pomegranate (Punica granatum; Punicaceae). American Journal of Botany 92: 1935–1941. [DOI] [PubMed] [Google Scholar]
- Nakata PA. 2003. Advances in our understanding of calcium oxalate formation and function in plants. Plant Science 164: 901–909. [Google Scholar]
- Schimper AFW. 1888. Ueber Kalkoxalatebildung in den Laubblattern. Botanische Zeitung 46: 64–69, 80–89, 97–107, 113–123, 129–139, 145–153. [Google Scholar]