Abstract
• Background Roots growing in soil encounter physical, chemical and biological environments that influence their rhizospheres and affect plant growth. Exudates from roots can stimulate or inhibit soil organisms that may release nutrients, infect the root, or modify plant growth via signals. These rhizosphere processes are poorly understood in field conditions.
• Scope and Aims We characterize roots and their rhizospheres and rates of growth in units of distance and time so that interactions with soil organisms can be better understood in field conditions. We review: (1) distances between components of the soil, including dead roots remnant from previous plants, and the distances between new roots, their rhizospheres and soil components; (2) characteristic times (distance2/diffusivity) for solutes to travel distances between roots and responsive soil organisms; (3) rates of movement and growth of soil organisms; (4) rates of extension of roots, and how these relate to the rates of anatomical and biochemical ageing of root tissues and the development of the rhizosphere within the soil profile; and (5) numbers of micro-organisms in the rhizosphere and the dependence on the site of attachment to the growing tip. We consider temporal and spatial variation within the rhizosphere to understand the distribution of bacteria and fungi on roots in hard, unploughed soil, and the activities of organisms in the overlapping rhizospheres of living and dead roots clustered in gaps in most field soils.
• Conclusions Rhizosphere distances, characteristic times for solute diffusion, and rates of root and organism growth must be considered to understand rhizosphere development. Many values used in our analysis were estimates. The paucity of reliable data underlines the rudimentary state of our knowledge of root–organism interactions in the field.
Keywords: Rhizosphere, roots, soil, organisms, signals, exudates, diffusion, growth, development, Pseudomonas, Rhizoctonia
INTRODUCTION
The overarching goal of root and rhizosphere research is to understand how roots function in the field, either to develop better crops and practices in agricultural or horticulture, or to understand how natural systems work (Welbaum et al., 2004; Malamy, 2005). This goal is challenging because of the chemical, physical and biological variability inherent to soils, and the abilities of plants and plant communities to respond to these in space and time (e.g. Ho et al., 2005; James and Richards, 2005). Field soils have marked physicochemical heterogeneity in pH, water content, hardness, oxygen levels and nutrient concentrations. Roots growing in the field may encounter open spaces within the soil, including biopores that contain roots from current plants and dead roots remnant from previous plants. Roots also encounter a wide range of soil organisms. It is in the area of rhizosphere biology where our understanding of field-grown roots is particularly limited. Laboratory research has dealt predominantly with adding single types of micro-organisms to roots, and has characterized the number or ‘quorum’ of microbial cells needed for particular plant responses. However, we cannot predict accurately when and where these and other organisms occur in the rhizospheres of field-grown plants.
The types of organisms reported in the rhizosphere are diverse. Their numbers depend on the soil conditions, plant species, root growth and development, and uptake and release of solutes from the root (Young, 1998; Garbeva et al., 2004). Relatively well-studied micro-organisms in agricultural systems include the fungal pathogens Gaemannomyces gramminis (the take-all fungus) and Rhizoctonia solani, and bacteria such as Pseudomonas spp. (Bowen and Rovira, 1999). Some of the well-studied bacteria can be deleterious to shoot and root growth, whereas others are beneficial through processes including nutrient mineralization and pathogen inhibition. Other important organisms in agricultural systems are the symbionts rhizobia and vesicular arbuscular myccorhiza (VAM) (Lekberg and Koides, 2005). In natural systems notable micro-organisms include ectomycorrhizal fungi, rhizobia, actinorrhizal filamentous bacteria, free-living nitrogen-fixing bacteria and phosphorus-solubilizing bacteria, and fungi. Microfauna including protozoa and nematodes (Gupta, 1994; Bonkowski, 2004), and macroarthropods such as worms and ants (Doube and Brown, 1998), also occur in the rhizosphere. Despite this diversity of rhizosphere organisms already reported, approximately 80 % of soil bacteria and 90 % of soil microarthropods have yet to be characterized (André et al., 2002; Hugenholtz, 2002).
Carbon-rich exudates from living roots and metabolites released from dead roots feed the organisms in the rhizosphere (Kuzyakov, 2002; Bertin et al., 2003). Carbon exudation is closely linked to photosynthesis (within hours of fixation), but is also tightly linked to import into the roots (Dilkes et al., 2004). Most recently fixed carbon is released from the root tips (McCully and Canny, 1985), but older regions of roots also provide substantial substrates for organisms (e.g. Jaeger et al., 1999). Signals, including antibacterial and antifungal compounds, play critical roles in regulating organism numbers in the rhizosphere and establishing infection of the root (Bais et al., 2004). Some signals such as auxin are common to microbial organisms, roots and nodules (Brown, 1972; Mathesius, 2003). Others, such as the quorum sensing signals produced by Gram-negative bacteria, are perceived by the roots of some species, which in turn produce exudates that ‘mimic’ the bacterial signals (Bauer and Mathesius, 2004). The activity of exudates and external signals is poorly understood under field conditions. Sampling and experimentation in the field require an understanding of the spatial and temporal dynamics of root growth and differentiation, of the colonization by organisms of the rhizosphere, and of the traffic of chemical signals and nutrients that pass between roots and rhizosphere organisms.
This paper attempts to provide a general framework for exploring how roots interact with soil organisms, and considers how rates of root elongation, ageing and differentiation influence root exudates and the growth rate of various organisms, especially bacteria and fungi, in different soil conditions. We draw on Huisman's (1982) analysis of the importance of root growth rate and root diseases, the models of bacterial population dynamics around growing roots by Darrah (1991a) and Zelenev et al. (2000), the model of Kim et al. (1999) of pH profiles around growing roots, and the review of Jones et al. (2004), in particular their figure 3, in which they show a series of ageing events in a volume of soil after arrival of a root tip.
We first describe the soil as an environment for a growing root, and the organisms, both living and dead, in a developing rhizosphere. Second, we consider the time it takes for solutes to diffuse between roots and responsive soil organisms, and show how distance is critical. Third, we compile published values for rates of movement and growth of soil organisms. Finally, we consider root extension rates and some related spatial and temporal aspects of root and rhizosphere development. We use this spatial and temporal framework to highlight two important factors influencing rates of root and organism growth: soil hardness and contact with other roots.
THE SOIL
Biological components
Roots are the largest fraction of the biological material in most arable soils (Table 1). A large proportion of visible roots may be dead, either from a living plant or remnant from previous plants. For example, the density of dead wheat roots from a preceding crop in the soil can be 2 cm cm−3 prior to sowing, similar to the density that the living crop will reach in that soil. For other crops the density of live roots ranges from 0·5 cm cm−3 for pea to 11 cm cm−3 for rice, depending on season and depth in the soil (from minirhizotron studies; see Pierret et al., 2005). The density of dead roots would reach high levels in grasslands, in surfaces of irrigated rice fields where roots tend to grow to acquire oxygen, and in areas prone to waterlogging where soil has been ploughed into mounds above the soil surface (‘raised beds’) to protect roots from flooding events. In Banksia woodlands dense mats of living and dead proteoid roots, soil particles and leaf litter form in the upper layers of nutrient-poor sandy soils. New proteoid roots colonize these mats annually, probably acquiring substantial amounts of phosphorus from the dead proteoid roots and leaf litter, either directly by exuding phosphatases or indirectly by promoting mineralization by bacteria (Pate and Watt, 2002).
Table 1.
Examples of biological components of arable soils
| Roots | 170–900 kg dry weight ha−1* |
| Living (topsoil) 0·3–10 cm cm−3† | |
| Dead (topsoil) 2 cm cm−3‡ | |
| Living (subsoil) 0·2 cm cm−3‡ | |
| Dead (subsoil) 0·2 cm cm−3‡ | |
| Bacteria | 30–90 kg C ha−1§ |
| 104–107 cells per cm root¶ | |
| 106–109 cells per g rhizosphere soil¶ | |
| 106 cells per mm3 rhizosphere close to the root¶ | |
| 170–3780 µg C g−1 soil and 6–56 % active** | |
| Fungi | 4–70 kg C ha−1§ |
| Protozoa | 50 kg ha−1 |
| 101–106 per g bulk soil†† | |
| Nematodes | 0·01–0·24 kg C ha−1§ |
| Microarthropods | 0·01–0·19 kg C ha−1§ |
| Macroarthropods | 0–0·1 kg C ha−1§ |
| Enchytraeids | 0·03 to 0·21 kg C ha−1§ |
| Earthworms | 0–13·5 kg C ha−1§ |
Wheat at 168 days past sowing (Vincent and Gregory, 1989).
Wheat (Pierret et al., 2005).
Soil extracted in a core from wheat field in south-east Australia, and roots divided into living and dead based on visual features under a dissecting microscope, and length measured with a scanner and image analysis software.
Zwart and Brussard (1991); ranges from different cereal crops at six sites around the world.
Ranges from wheat roots from the field and direct counts using fluorescent oligonucleotide probes or dye and calculated from Watt et al. (2003, 2006a).
Darrah (1991).
Bacteria and fungi are the next largest fraction of the biological material in soil (Table 1). Population densities of bacteria can vary by three orders of magnitude depending on environment. Even for the same environment, estimates vary because culturing can underestimate numbers of bacteria by two orders of magnitude when compared with direct counts by microscopy (e.g. Hugenholtz, 2002; Watt et al., 2003).
Microfauna such as protozoa and nematodes are a small proportion of the biological material compared with roots and micro-organisms (Gupta, 1994). Microarthropods such as mites and collembola, and the macroarthropods, are also a minor portion, when averaged over a large area. These larger organisms, however, can function in small pockets of soil to affect plant growth. For example, protozoa proliferating on decomposing root remnants eat bacteria and then, because they have a lower carbon-to-nitrogen ratio than the bacteria, release nitrogen (Bonkowski, 2004). This nitrogen may be taken up by living roots, but can be quickly used up by other soil organisms (Hodge et al., 1998). Insects can feed on roots, altering root longevity, growth and anatomy (Wells et al., 2002). Earthworms and ants disperse micro-organisms, make important physical structures such as burrows, and deposit castings or faeces that support high numbers of micro-organisms that may stimulate or inhibit plant growth (Doube and Brown, 1998).
Spaces, water movement, aeration and size exclusion limits
Typical diameters of soil pores, and distances between roots and other soil components, are given in Table 2. We focus on pores and distances smaller than 2 mm, around roots and their rhizospheres. Diameters of pores strongly affect how quickly water can move (see Nobel, 1983, chapter 2). A critical diameter is about 30 μm, because water can drain quickly out of larger pores. A day or two after watering, only pores smaller than 30 μm will still be filled with water; the soil water suction will be about 10 kPa; and the soil is said to be at ‘field capacity’ (Hanks and Ashcroft, 1980). At the other end of the scale of available soil water, only pores smaller than 0·2 μm in diameter will retain water at a suction of about 1·5 MPa, approaching the practical limit for the extraction of water by crop roots. Thus, only pores ranging in diameter between 0·2 and 30 μm contain water that is available to plants.
Table 2.
Indicative spaces and distances between components of the soil relevant to root and rhizosphere function
| μm | Feature | Significance |
|---|---|---|
| ≤ 0·2 | Pores between clay particles (or in remnant cell walls) | Roots unable to access water in these spaces* |
| Some bacteria this diameter, including filamentous bacteria | ||
| 1–2 | Pores within soil microaggregates | Bacterial diameters |
| Fungal hyphae and root hairs can flatten to this diameter | ||
| 2–30 | Thickness of mucilaginous film retaining bacteria on field-grown roots† | Matrix (film) of gel-like compounds from roots and organisms that is water stable and may hold bacteria close to the root and soil |
| 5–10 | Distances within bulk soil microaggregates | Some large bacteria |
| Hydrated fungal hypha | ||
| Turgid root hairs | ||
| 1–170 (mean 90) | Distances between bacteria on field-grown roots within clusters† | Quorum signals between Gram-negative bacteria can diffuse quickly over this distance§ |
| 10–30 | Pores between soil microaggregates (mesopores) | Pores that can retain water against gravity for a day or two, i.e. largest water-filled pores at ‘field capacity’* |
| Nematodes | ||
| 6–500 | Pores between soil aggregates in the rhizosheath; see Fig. 1A‡ | |
| 7–250 (mean 50) | Distances between neighbouring hairs close to root in rhizosheaths‡ | Protozoa range between 2 and 1000 µm |
| 40–300 | Distances between neighbouring hairs in outer rhizosheaths‡ | |
| 30–2000 | Pores in soil created by roots or macrofauna or cracks | Water drains and flows rapidly in these pores |
| from wetting or drying or freezing (macropores) | Successive generations of roots colonize these pores (biopores) (see Fig. 2A) | |
| Organisms can move readily in these spaces | ||
| Diameters of roots, depending on type and species | ||
| Diameters of insects | ||
| 400–1000 | Distance between root surface and outer edge of rhizosheath in cereals‡ | Soil more tightly bound to the root in dry soil compared with wet;¶ depends on species where barley is 1·6-fold wider than wheat |
| Root hair lengths |
Direct measurements using image analysis software (AnalySIS, Soft Imaging Systems, GmbH, Münster, Germany) of distances within the rhizosheaths of field-grown wheat and barley roots such as those shown in Fig. 1A that were frozen in the field in liquid nitrogen and observed with a cryo-scanning electron microscope (see Watt et al., 2005, for methods).
Water flow may carry micro-organisms and mobile ions such as nitrate rapidly for long distances before they become adsorbed to a surface. Pores larger than about 30 μm frequently occur between the bound soil aggregates and root hairs of wheat and barley roots (Table 2, Fig. 1A) in the volume of adhered soil termed the rhizosheath (see McCully, 1999). When such pores are filled with water, during rainfall or irrigation, organisms may be rapidly transported to the surface of roots that are taking up water.
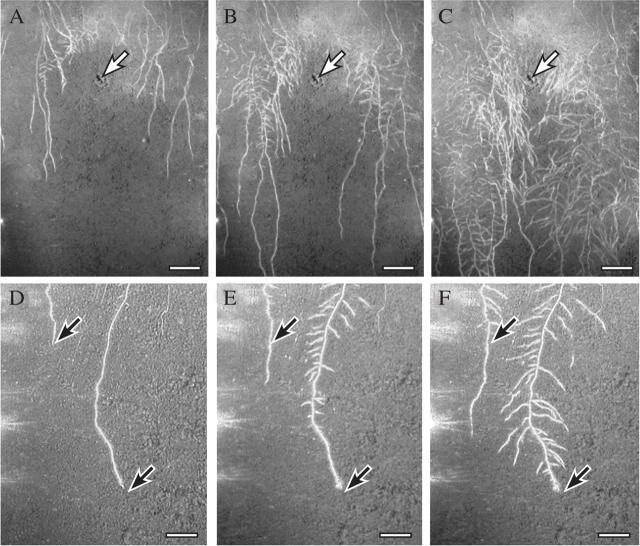
Fig. 1.
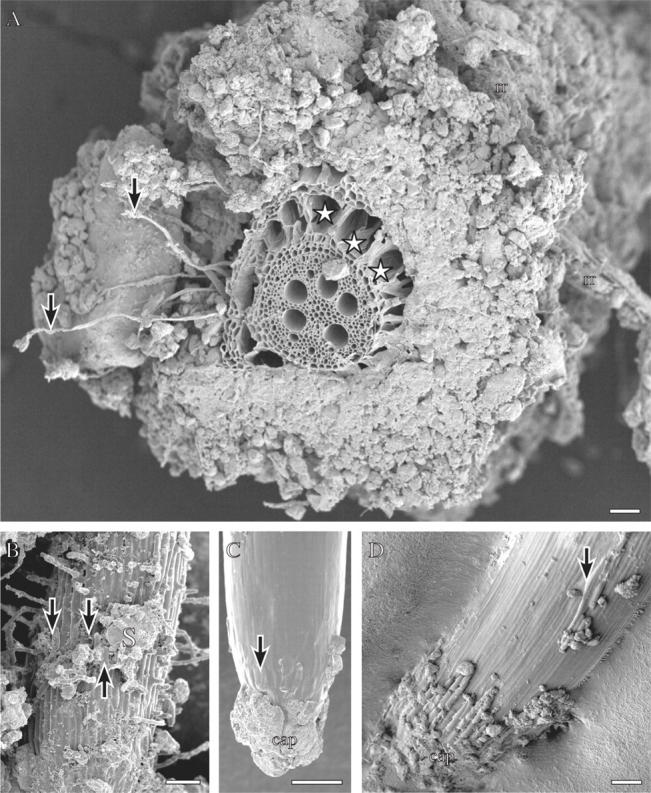
Wheat roots grown in the field (A, D), or in the same field soil in a rhizobox (B, C), frozen in liquid nitrogen and viewed frozen with a cryo-scanning electron microscope (see Watt et al., 2005, for details of method). (A) A nodal root sectioned by hand on a piece of wax with a single edged blade before freezing. Arrows indicate root hairs extending the length of the bound rhizosheath soil. A dead remnant root is caught in this soil (rr). Spaces (stars) have developed in the cortex. Scale bar = 300 µm. (B) Branch root with root hairs extending from the surface, bound soil (S) and root cap cells (arrows) along the surface. Scale bar = 100 µm. (C) Tip of the branch root in B. Root cap cells have collapsed (arrow) and soil adheres to the cap. Scale bar = 100 µm. (D) Tip of a nodal root. Sausage-shaped root cap cells are turgid on the cap and where sloughed along the elongation zone (arrow). Reproduced with permission from Watt et al. (2005). Scale bar = 200 µm.
The diameters of pores in soil determine where roots and organisms can fit, i.e. their size exclusion limit. Bacteria can reside in pores as small as 1 μm, thus between clay particles, although presumably not in the interlamellar spaces of clays, which may be much thinner than 1 μm. Fungal hyphae diameters range from about 2 to 5 μm, depending on species and soil type (e.g. Drew et al., 2003). Larger organisms such as nematodes may be physically excluded from dense soil. Alternatively, some macrofauna such as insects, termites and earthworms may burrow through dense soil and make pores where roots and other organisms can grow and move (Fig. 2A). Roots of many species including the branch roots of maize (McCully, 1999) and Arabidopsis (Malamy and Benfey, 1997) are thinner than 100 μm. Field-grown seminal and branch roots of wheat can have both thick (1000 μm) and thin (100 μm) regions along their lengths, possibly to fit into spaces in the soil (Watt et al., 2005). Root hairs are around 10 μm in diameter but can flatten to fit into pores of less than 2 μm (M. Watt, unpublished observation).
Fig. 2.
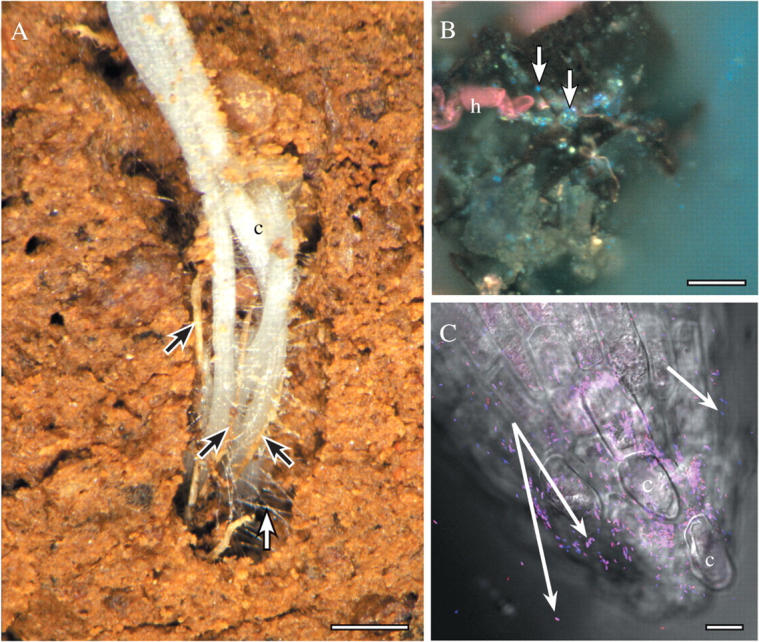
(A) Roots of canola (c) in the field, growing into a pore, in close contact with each other and dead roots of wheat (black arrows). Many root hairs (white arrow) extend from the new white, canola roots to bind to soil, and other living and dead remnant roots. Scale bar = 3 mm. (B) Root hair of wheat (h) associated with a piece of dark soil organic matter, bacteria (bright blue spots; some indicated by arrows) and mineral soil particles. Sample harvested from the field and prepared with the periodic-acid Schiff's reactions followed by DAPI (4,6 diamidine-phenyl indole), and viewed with UV fluorescence optics (see Watt et al., 2003). Scale bar = 20 μm. (C) Tip of wheat seminal root that was growing on agar and had Pseudomonas bacteria applied to the tip. Bacteria are hybridized to Bacteria- and Pseudomonas-specific oligonucleotide probes that are conjugated to fluorescent dyes, and viewed mounted in water, with a confocal microscope (reproduced from Watt et al., 2006a; see also for details of method). Some bacteria are bound to the root cap, and others are retained in hydrated mucilage behind the tip (white arrows). C, cap cell. Scale bar = 10 µm.
Distances and characteristic time for diffusion of solutes
Distances between roots and other soil organisms determine how far solutes must move if the roots and the organisms are to influence each other. Here we present examples from wheat and barley roots (Table 2). The bacteria on the root surface can reside within a mucilage film approx. 30 µm thick, and are clustered on average 80 µm apart, but often are in direct contact with each other (Fig. 2B, C; Watt et al., 2006a). The mucilage is produced by root cap cells and some rhizosphere organisms (McCully, 1999). Sloughed root cap cells are seen within 100 µm of each other along a branch root in Fig. 1B. Root hairs and associated bound soil aggregates form the rhizosheath that extends 1000 µm from the wheat root surface (Fig. 1A). Within the rhizosheath, the average distance between root hairs is 50 µm (Fig. 1A, B). Field roots often grow closely associated with other roots in soil pores (Fig. 2A; discussed in more detail below). Root hairs, in particular, can be in direct contact with those of another living root, or with the surface of a dead root from a dead plant, in addition to anchoring to the soil particles of the pore edges. Hairs of the living roots are often heavily colonized by organisms from neighbouring dead roots (Watt et al., 2006a), and possibly stimulate bacteria on associated dead organic material within 20 µm of the hair (Fig. 2C). Thus, distances between biological components in soil can be of tens of micrometres, approximately the thickness of the mucilage layer produced by the root and the organisms, if roots are in direct contact with each other.
Distance has a large effect on the time it takes for solutes, such as exudates and signals, to diffuse between roots and soil organisms. The characteristic time, t*, for diffusion over a distance, a, is given by
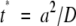 |
(1) |
where D is the diffusivity of the solute in a given medium (Crank, 1975). This relationship implies that a given amount of diffusion of a solute over a distance a will take four times longer over a distance of 2a, and nine times longer over a distance of 3a, etc. Thus, distance is critical to estimating when sufficient solute is likely to reach a point in the soil to stimulate a soil organism to germinate and grow towards the root. This equation also highlights that organisms close to the root, within, for example, the mucilage layer 30 µm thick, will receive signals from the root much sooner, indeed about 1000 times sooner, than those at 1000 µm from the root surface, at the edge of the rhizosheath. Similarly, the root receives signals much sooner from neighbouring than from distant organisms.
The characteristic time depends inversely on the diffusivity of the solute in water (Table 3). The diffusivity depends on the charge and size of the molecule. Ions such as Na+ and Cl− diffuse about twice as fast as glucose in water. Root cap mucilage of maize diffuses approximately 170 times slower than glucose in water (Sealey et al., 1995). The diffusivity in soil depends strongly on the soil water content, roughly as a function of the square of the water content (Olesen et al., 2000). For example, Olesen et al. (2000) measured a diffusivity of glucose in a loamy sand that was about 30 times smaller than that in water when the soil had a volumetric water content of 20 %, and was 200 times smaller than that in water when the soil water content was 10 %. Nichol and Silk (2001) found an even greater dependence of proton diffusivity on water content: protons had a diffusivity in agar 1000 times greater than the measured diffusivity in sandy soil at 7 % water content. Because solutes diffuse so much faster in agar than in soil, signal exchange in agar must be different from that in soil, and agar is not a realistic medium to study relationships among root growth, differentiation and exudation, and organism responses.
Table 3.
Diffusivities (D, mm2 s−1) of different solutes in water, agar, and soil
| Medium |
|||
|---|---|---|---|
| Soil |
|||
| Solute | Water or agar | 10 cm cm−3 SWC | 20 cm cm−3 SWC |
| H+* | 4·6 × 10−3 | 4·7 × 10−6 | n.d. |
| O2† | 2·1 × 10−3 | ||
| KNO3‡ | 1·8 × 10−3 | 9·2 × 10−6 | 5·5 × 10−5 |
| Citrate‡ | 6·6 × 10−4 | 3·3 × 10−6 | 2·0 × 10−5 |
| Glucose‡ | 5·2 × 10−4 | 2·6 × 10−6 | 1·5 × 10−5 |
| Maize root cap mucilage§ | 4·0 × 10−6 | 4·0 × 10−8 | 1·2 × 10−7 |
Direct measurements in agar (Shantz and Lauffer, 1962) and sand with a pH microprobe (Nichol and Silk, 2001).
In water (Cook and Knight, 2003), 104 times faster in air.
Direct measurements in water (Hodgman et al., 1961), and soil values estimated for a loamy soil from Oleson et al. (2000) by multiplying by the ratio Ds/D0 = 0·005 for 10 cm cm−3 SWC (soil water content), and Ds/D0 = 0·03 for 20 cm cm−3 SWC.
Direct measurements on 1 % agar from Sealey et al. (1995), and estimated for loamy soil as in ‡.
n.d., not determined.
An additional complication is variation in rhizosphere moisture content. Depending on rainfall, irrigation, transpiration, root growth and water efflux from roots, rhizosphere moisture can vary hourly and in different parts of a root system (Topp et al., 1996; Vrugt et al., 2001; Boyer and Silk, 2004). Rhizosphere moisture has a dramatic effect on hydraulic conductivity as well as on the diffusivities mentioned above, and the growth and motility of micro-organisms, as discussed in the following section.
Any tendency for solutes to bind to soil surfaces or be taken up by soil organisms, including roots, will strongly influence how far solutes travel in the rhizosphere. Phosphorous, in particular (see Bar-Yosef, 1996), binds to soil surfaces strongly (‘effective soil diffusitivities’ range from 10−6 to 10−9 mm2 s−1; Hinsinger et al., 2005). Furthermore, exudates that mobilize P move slowly in soil. For example, citrate diffuses up to 1 mm from the surfaces of the cluster roots in soil before it is consumed or bound to a calcareous soil (Dinkelaker et al., 1989). Jones and Brassington (1998) found that greater than 80 % of organic anions applied in solution bind within 10 min to soil surfaces, regardless of pH. The enzyme phytase is also rapidly sorbed to acidic soils within 10 min, but is desorbed by increasing pH from 4·5 to 7·5 (George et al., 2005). The sorption of phytase was found to be unaffected by micro-organisms. Jones et al. (1996) demonstrated that half of 14C-labelled malate is consumed by micro-organisms within 6 h in field soils at 25 °C, and within 48 h at 4 °C. These studies suggest that adsorption, more than organism consumption, influences the distance that organic anions and phytase travel from the root, and that this limits the activity of these solutes to the immediate vicinity of the root. Indeed, Kinraide et al. (2005) suggest that detoxification of Al by malate at wheat root tips occurs in the epidermal layer, rather than outside the root.
The distance from which soil organisms are stimulated and grow towards a root indicates the distance that stimulatory root exudates have diffused (Huisman, 1982). These distances are determined from densities of a fungal inoculum in soil and infection densities on the root. Huisman (1982) concluded that most response distances are within 1 mm of the root, but reported that Rhizoctonia solani can respond at 5 mm, Gaemannomyces gramminis at 12 mm, and VAM from 1·6 to 6 mm of the root. The distances that the solutes travel depend on the type of fungal inoculum within a genus or species, water content, the organic matter in the soil, which may support microbes that suppress fungal growth, and root type (reviewed in Bowen and Rovira, 1999).
Given the large variation in distances that root exudates may travel, the radial limits of the rhizosphere need to be defined in the context of the chemical or biological processes they regulate (Hinsinger et al., 2005). The characteristic time (eqn 1) draws attention to the strong effect of distance on the timescale of processes mediated by biological exudates. Both space and time must be considered with organism growth and motility rates to predict the succession of rhizosphere organisms on roots. Growth rates are reviewed in the following section.
ORGANISM EXTENSION, MOBILITY AND DIVISION RATES
Hyphal extension rates reported for root fungal pathogens range from 0·4 to 17 mm d−1, depending on species, temperature, moisture, colonized surface and direction along a root (Table 4). Temperature has a large effect on extension. For example, Pythium ultimum grows four times faster on agar at 16 °C than at 8 °C. Hyphae of G. gramminis extend three times faster along a wheat root at 19 °C than at 10 °C. Interestingly, at the higher temperature, hyphae growing towards the base of the root extend ten times faster than those extending towards the tip of the root, but at the lower temperature, this directional growth is absent (Gilligan, 1980). Wet soil reduces G. gramminis extension compared with moist soil (Grose et al., 1996). R. solani hyphal growth is also greater in drier, aerated soil, but is fastest along surfaces of wet sand and across gaps in soil (Otten et al., 1999, 2004). Thus, structure and aeration strongly influence extent of fungal soil colonization and potential for contact with roots.
Table 4.
Soil organism motility and extension rates
| Organism | Species | Environment | Motility or extension rate* (mm d−1) |
|---|---|---|---|
| Bacterial cells | Undefined general populations† | Aqueous media | 1700–3400 |
| Fungal hyphae | Pythium ultimum‡ | Solid agar medium, 16 °C | 17 |
| Solid agar medium, 8 °C | 4 | ||
| Gaemannomyces graminis | Surface of wheat seminal roots,§ | Towards root tip, 0·4 | |
| 19 °C | Away from root tip, 4·4 | ||
| Surface of wheat seminal roots,§ | Towards and away from root tip, 1·6 | ||
| 10 °C | |||
| Surface of slides buried in soil¶ | Moist soil (−8 kPa matrix potential), 2·4–9 | ||
| Wet soil (−2 kPa), 1 | |||
| Rhizoctonia solani | Sandy soil** | Within wet (−2 kPa), 0·5 | |
| Within moist (−6 kPa), 2 | |||
| On the surface, wet, 3·5 | |||
| Across a gap, 5 | |||
| Surface of wheat seminal roots†† | 1·6 (0·45–2·3) | ||
| Insect larvae | Sitona lepidus | Intact soil towards clover nodule‡‡ | 43 |
Motilities for bacteria and insect larvae; extension rates for fungal hyphae.
From Sherwood et al. (2003).
Gilligan (1980). Direct quantification of hyphal front on wheat roots using microscopy.
Grose et al. (1996, from Blair 1943).
Refshauge (unpubl. res.). Means of growth at different temperatures and tissue types. Direct quantification of hyphal front on wheat roots using microscopy.
Johnson et al. (2004), from measurements in intact soil using X-ray microtomography.
Many bacteria swim quickly in aqueous media (Table 4). Pseudomonas fluorescence swims at between 1·7 and 6·0 m d−1 in water (Arora and Gupta, 1993), three orders of magnitude faster than hyphal extension. These rates suggest that some bacteria move rapidly through water-filled spaces in the soil and on roots. Maize roots in dry soil release water (6–13 µL cm−1 d−1), hydrating their rhizosheath to between 25 and 48 % (v/v) soil water content during the night (Topp et al., 1996), such that the surfaces of these roots, in particular in the grooves between epidermal cells, may have water for substantial movement of bacteria. It is unclear how the polymer molecules of mucilage and the bound soil on the root surface would affect such movement. Camper et al. (1993) found that flagella function and cell size had no effect on the rate of movement of a P. fluorescence strain through a water-filled column of glass beads. However, starving the bacteria strongly increased their adhesive properties. Bacteria may adhere to some roots more strongly than others depending on the type and amount of substrate, and certain populations may be very transient at a given position on a root, and may wash easily from that position, compared with others.
Estimates of bacterial division rates in rhizosphere models in Scott et al. (1995), Zelenev et al. (2000) and Darrah (1991a) range from 1 to 4 cells per cell per day. Death rates range between 1·2 and 1·4 cells per cell per day. Different bacteria may divide at different rates in the rhizosphere: for example, Pseudomonas at 4·6 cells per cell per day, and Bacillus at 0·6 cells per cell per day (Bowen and Rovira, 1999). We did not find reports of division and death rates, and thus extension rates, of filamentous bacteria.
Compared with fungal hyphae extension, insect larvae may move quickly through soil, for example 40 mm d−1 for Sitona lepidus (Johnson et al., 2004), but slowly compared with the rates at which bacteria can swim in aqueous solution (Table 4). In the following two sections, we compare soil organism growth and motility rates against rates of root growth and differentiation.
ROOT EXTENSION RATES AND TIME FOR RHIZOSPHERE DEVELOPMENT
The extension rates for the types of roots in a root system—primary axis, seminal axes, adventitious roots and branch roots—and their gravitropism, regulate when and where roots develop their rhizospheres in the soil profile. A good understanding of when types of roots of the same species grow in the soil profile may help to predict which organisms are likely to dominate in the rhizospheres of different roots at different depths in the profile. Different root types can be colonized by different bacteria and fungi. For example, maize adventitious and seminal roots had similar numbers of bacteria, but the adventitious roots had fewer fungi (Sivasithamparam et al., 1979). VAM infect the first-order branch roots of subclover twice as quickly as the primary roots, per unit length (Walker and Smith, 1984). The rootlets of cluster roots have bacterial populations specific to different stages of development and anion efflux, and these differ from populations found on non-proteoid roots (Marschner et al., 2002). Thus, as genes regulating developmental pathways are identified to generate root systems with different root types (e.g. in maize, Hochholdinger et al., 2004), it may be possible to design plants that favour specific rhizosphere organisms by selecting root types in the root system.
Extension rates
Most reports of root extension rates are of the primary, seminal roots of seedlings (examples are given in Tables 5 and 6). These depend on species, and are affected by abiotic factors such as temperature, dryness and salinity. There is some knowledge of fine root longevity (reviewed in Eissenstat et al., 2000) but comparatively little knowledge of extension rates of branch roots of a root system, and how these vary with plant and root age. This is a major gap in root research, as branch roots comprise by far the greatest length in a root system (Fig. 3A–C; Pierret et al., 2005). Here, we used time-lapse photography to examine the elongation of the seminal and primary branch roots of more mature wheat root systems (Fig. 3). The seminal axes grew 1·5 times more quickly when the plants were young, and generally the branch roots grew more slowly than their parent axes. When their seminal tips were impeded, however, either by a fresh patch of high ammonia or other soil factor, the branch roots doubled their elongation rate (Table 6). This was also observed along barley seminal axes impeded by closely packed glass beads, which developed branch roots twice as long as those in looser beads (Goss, 1977).
Table 5.
Root extension and estimation of distance and time to the end of the growth zone or root hair emergence
| Onset of root hair emergence or end of growth zone |
||||
|---|---|---|---|---|
| Plant species and root type | Growth conditions | Rate of tip extension (mm d−1) | Distance from tip (mm) | Time since tip arrival (d) |
| Wheat seminal root* | Loose, 15 °C | 24·5 | 8·6 | 0·35 |
| Hard, 15 °C | 8·16 | 1·4 | 0·17 | |
| Wheat seminal root† | Loose, 15 °C | 35 | 5·15 | 0·15 |
| Loose, 7 °C | 10·1 | 5·5 | 0·54 | |
| Maize‡ | 29 °C | 69·6 | 12·5 | 0·18 |
| 16 °C | 25 | 12·3 | 0·5 | |
| Maize§ | Vermiculite, moist (3·7 %; −0·03 MPa), 29 °C | 74·4 | 10·5 | 0·14 |
| Vermiculite, Dry (0·1 %; −1·6 MPa), 29 °C | 26·4 | 6 | 0·22 | |
| Arabidopsis primary root¶ | Agar | 8·6 | 1·3 | 0·15 |
Watt et al. (2003); onset of root hair emergence measured.
Refshauge (unpubl. res.); onset of root hair emergence measured.
Pahlavian and Silk (1988); end of growth zone measured, length growth zone not related to extension rate.
Sharp et al. (1988); end of growth zone measured.
van der Weele et al. (2003); end of growth zone from Fig. 3; see also their fig. 7 for other species.
Table 6.
Time to emergence of branch roots and branch root extension rates
| Onset of branch emergence |
|||||
|---|---|---|---|---|---|
| Species and root type | Growth conditions | Rate of tip extension (mm d−1) | Distance from tip (mm) | Time since tip arrival (d) | Rate of growth of emerged branches (mm d−1) |
| Wheat* | Rhizoboxes, soil, | Young plants: 20 | Young plants: 100 | 5 | 3–6 |
| seminal axes | Older plants: 13 | Older plants: 65 | |||
| Impeded tip | 0 | n.d. | n.d. | 11 | |
| Wheat† | Packed core, loose, 15 °C | 24·5 | 85 | 3·5 | |
| Hard, 15 °C | 8·16 | 19 | 2·3 | n.d. | |
| Cotton‡ | Hydroponics, low salt (0–100 mmol NaCl) | 25·2 | 16·5 | 0·66 | n.d. |
| Hydroponics, high salt (200 mmol NaCl) | 2·4 | 5 | 2·4 | n.d. | |
| Maize§ | Aeroponics | 4·32 | 42 | 0·32 | n.d. |
| 43·9 | 212 | 16 | n.d. | ||
Values from root movies represented in Fig. 3. Images measured over time with AnalySIS image analysis software (Soft Imaging Systems) to get rates of growth. Young seedlings (leaf 1) have faster seminal roots; older plants (leaves 3 to 4) have slower seminal roots. Impeded tips due to a patch of ammonia or an unidentified soil obstacle; see Fig. 3.
n.d., not determined.
Fig. 3.
Images from time-lapse movies of wheat roots growing in a rhizobox (10 × 25 × 50 mm) with field soil, taken with green light from diodes and a Nikon Coolpix 5400 digital camera. (A–C) Roots responding to a patch of anhydrous ammonia. One day prior to sowing seeds (five seeds along the top), ammonia (1 mL of 14 m solution) was injected into the soil at the position indicated by the arrow. The ammonia diffused approximately 50 mm from the centre within minutes of injection, and the soil was very alkaline. (A) Image at 13 d after sowing seeds and 9 d after seminal axes in the middle of the box had reached the upper border of the N patch. Seminal axes on the sides grew past the patch. (B) Image taken 5 d after (A), and branch roots from the seminal axes that were inhibited by the ammonia have started to grow into the patch. Note that their tips are directed downwards, while branch roots from the uninhibited axes on the sides tended to grow more horizontally with weaker gravitropic responses. (C) Image taken 8 d after (A). Branch roots have almost completely filled the area of the nitrogen patch. Rate of growth of seminal roots along the edges was 20 mm d−1; branch roots from these axes approx. 6 mm d−1 and branch roots growing through the patch approx. 11 mm d−1. See Wetselaar et al. (1972) for nitrogen reactions. (D–F) Different movie to (A–C), without an N patch. Two seminal axes of wheat, and 2 d between images. Arrows indicate the tips of the axes; axis on the left is elongating 12 mm d−1; axis on the right is impeded by something in the soil, and branch roots emerge closer and closer to the tip. Scale bar = 50 mm for A–B; 18 mm for D–F.
The rates of fungal extension in Table 4 can be compared with those of root extension in Tables 5 and 6 to speculate if root tips can grow away from hyphae, or become ‘caught’ by hyphae, depending on environment and root type. Apparently, extending root tips keep ahead of hyphae in many conditions, except when severely impeded (e.g. cool, very hard soil), or when plant (or organism) signals stop growth or induce root determinacy, favouring extensive colonization, which occurs in ectomycorrhizal roots. Interestingly, Arabidopsis root hairs extend within the rates reported for hyphae, at 0·7 mm d−1 as they first bulge from the epidermal cells, and at 3·6 mm d−1 when they are extending fastest (Dolan et al., 1994; roots on agar with 2 % sucrose). Both root hairs and hyphae extend by tip growth, perhaps explaining the similarity in rates.
The movement of bacteria is potentially much faster than root extension, but is difficult to predict, given the complications of possible adhesion to the root surface molecules. For bacteria attached to an element of root tissue within a root growth zone, estimating the bacterial density involves integrating the bacterial division rate over time and dividing by the growth strain rate (expansion) of the tissue element, as explained below in the section ‘Events at the root tip’.
Time from tip arrival to differentiation events in a volume of soil
In a volume of soil, the extension rate of the root tip, V(t) can be used to calculate the time (t) between the arrival of the tip and the arrival of a location (L) along a root axis (see also Fig. 4):
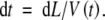 |
(2) |
This gives the time that the rhizosphere has been developing at that location (van Bruggen et al., 2000; Watt et al., 2003). Increasing L corresponds to such events as the passing of the root cap and the end of the growth zone, emergence of branch roots, onset of senescence and, for example, shedding of cortex. After elongation ceases, eqn (2) is no longer useful. Times must be recorded directly, and other developmental indices must be chosen to stage the rhizosphere through events including root decomposition. Values for time to end of growth zone (Table 5) and onset of branch emergence (Table 6), calculated either from our data or from values in the literature, show that time to these events varies. This is because both the rate of extension of the axis and the location of the different zones vary with the environmental condition. Consider the time for a soil particle to experience the end of the growth zone, relative to the arrival of the root tip of wheat. In hard soil, the growth zone is shorter than in soft soil; the end of the growth zone is therefore reached three times sooner than in cool soil, although extension rates are similar (Table 5).
Fig. 4.
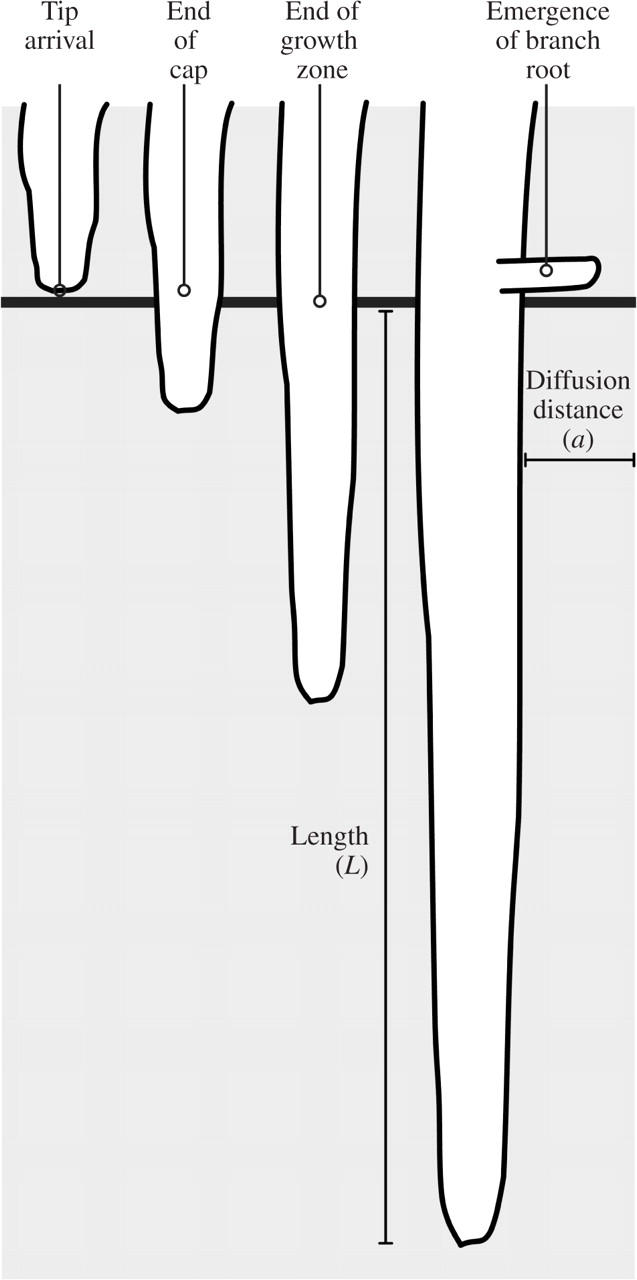
Diagram illustrating the movement of a root tip past a position in the soil (solid horizontal line), and how the extension rate of the root tip (V) can be used to calculate the time (t) between the arrival of the tip and the arrival of a location or length (L) along a root axis in the equation: t = L/V. This gives the time that the rhizosphere has been developing at that location. Increasing L corresponds to such events as the root cap passing, reaching the end of the growth zone, and emergence of branch roots. If the root tip stops elongating, t = L/V is not longer useful in estimating the time the rhizosphere has been developing.
The time between different events on a root allows an estimate of the distance that exudates from the length of root can diffuse. The characteristic diffusion time (eqn 1) may be appropriate for such estimations. Alternatively, if growth and root transport processes are steady (constant in time, when viewed from the root tip), then the Peclet number may be used to describe the ratio of the time scales for diffusion and growth-associated convection. The Peclet number, V·C/D, where V is root elongation rate, C is the diameter of the root and D is the diffusivity, characterizes the development of the plume of exudates from a growing root (Kim et al., 1999). This plume surrounds the root tip, is carried forward with the root tip through the soil and may prime the rhizosphere ahead of the advancing tip (Darrah, 1991b). A lower root elongation rate or a higher diffusivity may produce a plume of larger diameter, depending on rates of efflux and influx of the exudates. In these cases of steady-state growth, the time required for a particle on the soil ahead of the root tip to experience the advancing plume is simply calculated using eqn (2) with dL in this case representing the distance between the soil particle and the present location of the root tip.
For both main axes and branch roots, application of eqn (2) requires knowledge of the elongation rate. On a time scale of hours, and distance scale of millimetres, these rates are often constant under controlled conditions in the laboratory, so that root length increases linearly with time for many hours. With higher spatial and temporal resolution, growth rate oscillations are apparent. For instance, Walter et al. (2002) tracked maize primary root elongation in solution at high resolution and found similar elongation rates during the day and night. The roots constantly wave back and forth. They increase in growth rate, first approximately 2 mm behind the tip, within 1 h of experiencing a 5 °C temperature increase. In field conditions roots are very likely to encounter variations in physical, chemical or biological conditions that cause changes in rate of elongation (see Fig. 3). Roots dug from the field have many distortions, suggesting that their tips expand radially at different rates in response to soil conditions (Watt et al., 2005). The availability of good imaging technology with high computational power should facilitate future studies of non-steady growth, and how these are related to developmental events along roots, in the field.
Exudation and uptake rates can vary with developmental events along a root. For example, carbon efflux is generally highest at the root tips, from mainly sloughed root cap cells and their mucilage (McCully and Canny, 1985; Iijima et al., 2000). Nitrate uptake varies within and beyond the growth zones of maize roots (Taylor and Bloom, 1998). In legumes, young root hairs and the cortical cells around emerging branch roots release flavonoids to trigger nodules in only those regions (Mathesius et al., 2000). Efflux of the amino acid tryptophan is associated with branch roots but not with the more apical region of the primary root where sucrose is released (Jaeger et al., 1999). The cell types on the root surface to which fungi and bacteria can adhere also change with development. Gochnauer et al. (1989) found an association between microbial populations and developmental events on maize axes sampled from the field. The young regions, covered in rhizosheaths bound to living root hairs and epidermal cells, had higher numbers of Pseudomonas and Cytophaga spp., whereas the older regions with abscised sheaths and cortices had much higher numbers of actinomytetes. However, developmental events along a root do not always relate to changes in bacterial populations. For example, Semenov et al. (1999) found no relationship between branch roots and oligotrophic and copiotrophic bacteria, and the authors proposed that their numbers increase and decrease along the root axis relative to waves in birth and death rates, initiated by carbon from the root tip.
EVENTS AT THE ROOT TIP
Rate of growth and kinematics of differentiation at the root apex affect the density of bacteria along the growth zone and the inocula of the rhizosphere at onset of differentiation in the stationary part of the root. The density of bacteria on the growth zone may influence the nutrients available to the root, either through direct competition for nutrients between the bacteria and the root, or via the consumption of nutrient-mobilizing exudates by the bacteria. The bacterial density may determine the exchange of quorum-sensing molecules between bacteria to induce a process that affects the plant (Steidle et al., 2001), and provide protection from invasion from a fungus (‘biocontrol’).
The tips of elongating roots can be divided into functional zones. These include a cap, with its own meristem plus expanding cells that are being released (sloughed) as the root moves forward (‘border’ cells) (Figs 1C, D and 2C). The root proper has a growth zone containing an apical meristem with dividing and expanding cells, and a rapidly elongating zone with cells that have stopped dividing and are expanding primarily in the axial direction while developing vacuoles. Functional phloem develops in or near the meristem. Cells within the rapidly elongating zone are also beginning to differentiate into cells for the vascular, cortical or epidermal tissues. Root hairs may develop from some epidermal cells in the basal part of the elongating zone. Behind the elongating zone, cells continue to differentiate biochemically and anatomically, forming functional xylem, Casparian strips and branch roots.
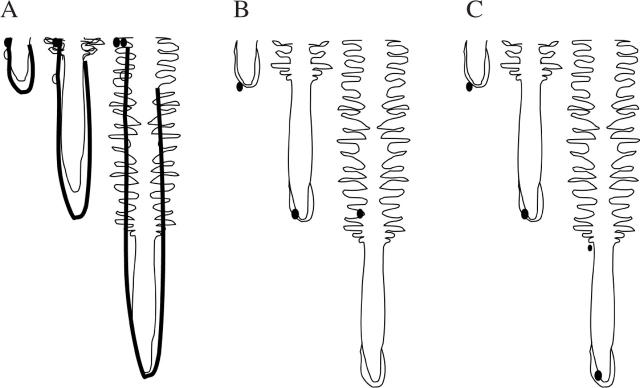
The time that the root apex and a soil organism experience each other can be presented in several scenarios (Fig. 5). In Scenario 1 (Fig. 5A), the apex meets an organism, but the organism fails to anchor with the tip, is perhaps sloughed from the tip immediately with the cap, and the tip grows past. In Scenario 2 (Fig. 5B), the organism joins the root tip, and is carried forward with the root ‘particle’ to which it is anchored, during its decelerating downward trajectory. Once the root particle and its attached micro-organism have been displaced past the boundary of the elongating zone, they remain stationary in the soil profile but interact with both the surrounding soil and the developing root tissue in the continuing development of the rhizosphere. In Scenario 3 (Fig. 5C), organisms swim at the same rate as the root elongation rate. These organisms maintain their positions near a particular root developmental zone as that zone moves downward through the soil profile.
Fig. 5.
Diagram illustrating scenarios possible between a growing root and bacteria relatively immobile in the soil. Bacteria are depicted as dark dots; the root tip is shown at three stages relative to the bacteria. (A) Scenario 1: the root tip grows past the bacterium. (B) Scenario 2: the bacterium anchors to the root cap and is carried forward with the tip as it extends (see also Fig. 6). The root cap mucilage is shown as a dark line in (A), suggesting how it covers the root in hard soil, and is shown as a light line in (B), suggesting how it covers the root tip in loose soil (see text for details and additional explanation). (C) Scenario 3: the bacterium swims to maintain its position on a particular root developmental zone.
The three scenarios can result in large differences in the time that an organism and zones of the root apex experience each other before the organism joins the stationary part of the root. In Scenario 1, the time the apex, or regions of the apex, and organisms experience each other can be described as in eqn (2) used with biochemical and anatomical data on the position of the zones of interest. The quotient of the length of a zone of interest and the elongation rate of the root is the time that nearby organisms experience exudates from that zone (e.g. cap or growth zone) (see Table 5, Fig. 4).
Scenario 2 can be visualized from the point of view of an organism ‘sitting’ on the root (Silk and Erickson, 1979). If the organism is shed at the tip with the cap cells to join the soil, then the organism experiences the fate of organisms in Scenario 1, and the apex grows past. If it is not quickly shed with the cap, but becomes embedded within the mucilage of the cap (see Fig. 2B), it can remain anchored to the root particle for many hours. During this time the micro-organism will be displaced from the root apex by the expansion of the more apical tissue. The ‘growth trajectory’ in the moving reference frame attached to the tip equates to the plot of distance from the tip to the attached particle versus time (Fig. 6). The growth trajectory shows that the micro-organism with its host root particle will accelerate to a final constant velocity of displacement from the tip. The final displacement velocity is equal to the root elongation rate. Organisms that become anchored to the root meristems have more time to divide and consume exudates from the growth zone than organisms anchored to more basal regions within the growth zone, as the residence time for a particle in a zone is inversely proportional to the velocity of displacement from the apex.
Fig. 6.
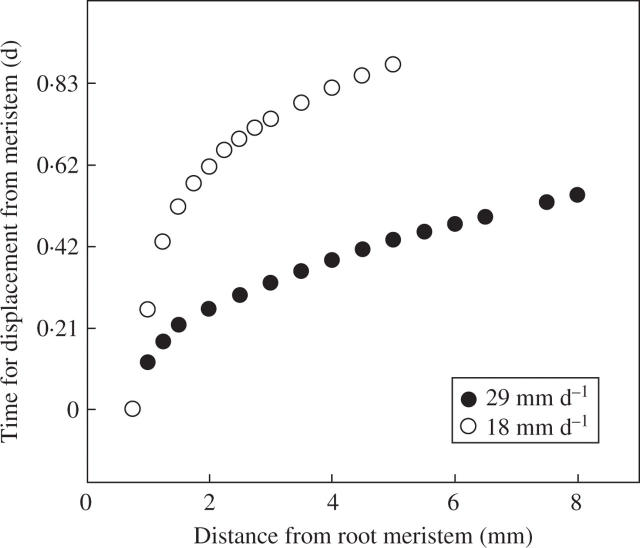
The time it takes for a ‘particle’ or bacterium to be displaced from the beginning of the elongation zone to different distances from the tip of wheat seminal roots to eventually arrive at the stationary region of the root. These values were calculated from cell lengths of root tips grown in soil in rhizoboxes, frozen, planed and viewed with a cryo-scanning electron microscope (Silk et al., 1989; Huang et al., 1994). Root elongation rates were measured daily before freezing. The time the organism is bound to the elongation zone can be estimated from kinematic analyses where the organism is treated as a ‘particle’ or ‘element’ attached to the surface of the elongation zone. The particle is initially anchored 0·5 mm from the tip of the root growing 29 mm d−1, and 0·75 mm from the tip of the root growing 18 mm d−1. When the root is growing at a moderate rate of 18 mm d−1, the time to reach the stationary region is 20 h, whereas on the roots growing at 29 mm d−1 it is 12 h.
The growth trajectory can be determined by following natural or applied marks on the root surface over time, or by using indirect methods such as anatomical measurements where direct measurements are difficult, such as in soil (Silk et al., 1989). Anatomical measurements reveal the developmental characteristics of the different zones associated with the trajectory. Once the growth trajectory is known, there is a predictable but non-linear temporal sequence during which the micro-organism will be associated with root cap activity, cell division, rapid expansion, vascular differentiation, etc. The time bound to the root cap will depend on the ability of the organism to ‘swim’ into the cap, and remain there without becoming sloughed. The sloughing rate of the cap increases with soil hardness (Iijima et al., 2000) and perhaps moisture (Sealey et al., 1995).
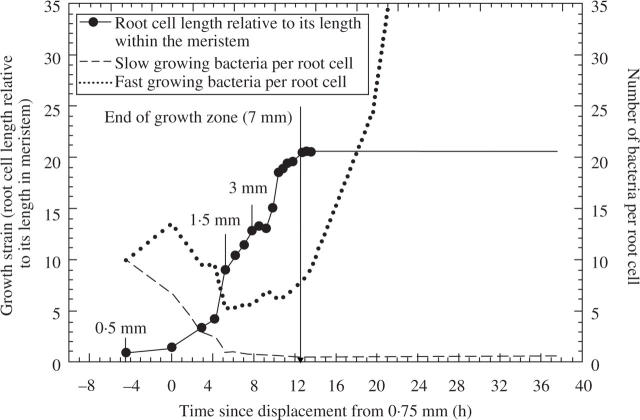
Assuming that bacterial relative growth rates can range from 0·029 to 0·05 h−1 (2 to 3 per day), we speculate that most bacteria will keep pace if they are anchored and dividing in the quiescent centre, but not if they are associated with the meristem and rapidly elongating regions of the growth zones of the roots (Fig. 6). The growth of a tissue element during its displacement through the elongating zone, together with calculated bacterial numbers, is shown as a function of time after displacement from the meristem in Fig. 7. Each point on the graph represents growth strain plotted against time to reach the position associated with the elongating zone. The time to reach 1·5, 2·0, 2·5 mm, etc., is found from the growth trajectory of the faster growing roots in Fig. 6. The growth strain, or relative growth of the tissue element at each position, is found by dividing cell length there by the cell length in the meristem. Root cells grow about 20-fold after displacement from the meristem, and they take about 13 h to be displaced from the meristem to the base of the growth zone. If exudates from the elongating zone are required for bacterial growth, then even the fastest growing bacteria (0·163 h−1 relative growth rate) will be considerably diluted during displacement through the elongating zone and might not reach critical colony sizes before they reach the stationary part of the root. If they can thrive on exudates from the stationary zone, then the fast-growing bacteria will rapidly increase in density as root cell elongation slows near the base of the elongating zone, whereas the slow-growing bacteria (0·01 h−1 relative growth rate) will not multiply much during several days of displacement.
Fig. 7.
Dilution of bacteria by root growth. A group of bacteria, originally ten in number, as they might be tracked during their attachment to a root cell that is experiencing expansion and displacement from the base of the root meristem through the growth zone. See ‘Events at the root tip’ section for additional details.
In Scenario 3, bacteria on the elongating zone may keep pace by swimming toward the root apex. Chemotactic mechanisms might facilitate this co-ordination and permit growth of bacterial colonies so that the growing colonies move downward in the soil profile, remaining near a particular zone but not physically attached to a root cell.
TWO EXAMPLES OF APPLICATION OF THE SPATIAL TEMPORAL FRAMEWORK
Root tips in hard soil and organisms in the rhizosphere
For nearly 30 years, researchers have known that conservation farming, which includes ‘direct-drilling’ the seed into unploughed soil, can inhibit the growth of wheat seedlings, and only occasionally improves growth despite improved soil porosity and less water evaporation from the soil surface (Kirkegaard, 1995; Lekberg and Koide, 2005; Watt et al., 2006b). Soil loosening and sterilizing intact, unploughed soil relieve constraints to wheat seedling growth, indicating roles for both soil hardness and soil organisms in this constraint. Organisms that inhibit direct-drilled wheat include some deleterious Pseudomonas species. These are specific to the rhizospheres of direct-drilled roots, as they were not isolated from the rhizospheres of roots from ploughed soil (Simpfendorfer et al., 2002). Infection by the pathogenic fungus, R. solani also increases in direct-drilled conditions (Jarvis and Brennan, 1986).
Field and laboratory studies with a semi-dwarf cultivar of wheat showed that soil hardness and rate of root growth interact to alter bacterial numbers in the rhizosphere (Watt et al., 2003). Slow-growing roots in hard, unploughed soil have 20 times more Pseudomonas around their tips than faster growing roots in loose, ploughed soil. These studies explain how rhizosphere Pseudomonas increase in direct-drilled conditions. The mechanism by which these Pseudomonas inhibit plant growth, however, is unclear. Studies in intact field soil (Watt et al., 2005), and direct application of Pseudomonas to roots in soil (M. Watt, unpublished data), show that they limit leaf growth but not root growth. Although Pseudomonas inhibits root extension in agar bioassays, this is unlikely to be occurring in soil where native numbers are much less than those inoculated in agar (reviewed in Watt et al., 2006a). Direct quantification with fluorescence in situ hybridization (FISH) indicated that Pseudomonas comprise about 10 % of the total bacteria on root caps, and 15 % of those on the elongation zone (Watt et al., 2006a). Pseudomonas may produce a toxin that stimulates roots to send signals that inhibit shoot growth. The toxin concentration would probably increase with Pseudomonas density.
Here we apply the temporal and spatial relationships above for root tip and organism growth, to help understand what is regulating Pseudomonas density on roots growing in loose and hard soil (see Watt et al., 2003, fig. 3). The possibilities are: Scenario 1, the Pseudomonas do not attach to the tip, or are quickly sloughed with the root cap, and the tip grows past; Scenario 2, the Pseudomonas bind to the tip; or Scenario 3, the Pseudomonas swim in the soil adjacent to the tip to keep pace with it (Fig. 5). If the bacteria bind to the tip or swim to keep pace with it, more bacteria would be on the tip than behind. This is the case for the fast-growing roots, but not for the slow-growing roots in the hard soil where the region 5–10 mm from the root tip has more bacteria, preferentially Pseudomonas. This is Scenario 1, where the tip has moved past the bacterium. We also know that when the bacteria per root length are expressed per unit time (Watt et al., 2003, fig. 4), the Pseudomonas population doubles between 0·5 and 1 d from arrival of the root tip, whereas those along the roots in loose soil halve in numbers. The slow-growing roots probably release more exudates for the bacteria to proliferate in that time. Given the extreme shortening of the elongation zone in roots in hard soil, and the short time that it takes for it to pass the bacteria compared with how long the root cap or meristem would take, it appears that the exudates come from the cap or meristem. The bacteria take a half a day to respond to these exudates, and thus the proliferation is observed behind the cap in the zone 5–10 mm from the tip. Hard soil increases the release of maize seedling root cap cells and mucilage carbon (Iijima et al., 2000). This suggests the wheat roots in hard direct-drilled soil may have had higher root cap exudation, to which the Pseudomonas responded by dividing.
The experimental line Vigour 18, developed for rapid leaf growth by crossing a wheat with a large embryo with one with high specific leaf area (Richards and Lukacs, 2002), is not inhibited in early growth by soil organisms in unploughed soil (Watt et al., 2005). It also does not accumulate Pseudomonas around its root tips when growing slowly in the field, or when grown in hard soil in a controlled environment experiment (M. Watt, unpubl. res.). Based on the analysis above, the likely explanation for these observations are that the root tip exudates for Vigour 18 do not stimulate Pseudomonas growth because they are less abundant or they counteract other exudates.
R. solani is a necrotrophic pathogen that invades wheat roots, and rots the tissue such that plant growth is severely impaired. Hard soil with conservation farming, and low temperatures, exacerbate infection possibly due to slower root growth in the surface soil where Rhizoctonia inocula are concentrated on plant debris (Neate, 1987). The extension rates of Rhizoctonia hyphae approach that of wheat seminal roots in hard and cool soil (Tables 4 and 5), suggesting that the Rhizoctonia hyphae could ‘catch’ root tips when impeded in, for example, the ends of soil pores. It is not known, however, if the fungus preferentially infects root tips or if hard, cool soil enhances root exudates that stimulate Rhizoctonia hyphae, leading to more rapid and severe infection. Evidence from strawberry (Husain and McKeen, 1963) suggests that amino acids in exudates released preferentially from roots at low soil temperatures stimulate Rhizoctonia.
Root–root interactions
As highlighted in Table 1 and ‘The Soil’ section, roots grow through soil dominated by other roots. New roots frequently contact these roots; for example, at least half of wheat root length was found to be in contact with dead roots from previous crops, and contact tended to increase with direct-drilling or when the previous species was perennial (Watt et al., 2005). The processes at the contact points and consequences for the living plant are not clear. Recent work in wheat is showing that the dead roots are a source of nitrogen (McNeill et al., 1999; Kahn et al., 2001, 2003) and phosphorus (Nuruzzaman et al., 2005). Wheat grown after chickpea accessed 14 % of labelled plant nitrogen from chickpea root remnants, but much less from its shoot remnants incorporated near the soil surface (McNeill et al., 1999). Kahn et al. (2001) found with similar field studies that wheat accessed about 5 % of labelled nitrogen in root remnants of fababean, chickpea and barley, and that absolute amounts from each species were related to the total nitrogen in the tissues, chickpea being the highest by a factor of three owing to higher absolute amounts of root. The remnants would continue to contribute nitrogen to following crops provided it is not leached from the soil (Crews and Peoples, 2005). The amount of nitrogen in the root remnants varies with the type of root tissue. Nodules can be 6 % nitrogen (M. B. Peoples, personal communication). Fine roots of both Trifolium subterraneum and Medicago sativa have 3–4 % nitrogen, whereas coarse roots are about 1 % nitrogen (Bolger et al., 2003). Bolger et al. (2003) found 70 % of the root mass of subclover was fine root, whereas only 15 % of the alfalfa root system was fine, suggesting that choosing a species with a high proportion of fine roots will stimulate nitrogen transfer to subsequent crops via root–root interactions.
In certain circumstances, especially in the unploughed surface soil of conservation farming systems, and in undisturbed subsoil, living and dead roots are constrained in cracks and pores (Fig. 2A). The effects of the root–root contact on plant growth, especially in cracks and pores where roots do not have good contact with mineral particles of soil (van Noordwijk et al., 1993), and where water can flow, are unclear. Stirzaker et al. (1996) found that barley leaf growth was highest in uniformly mixed soil packed to a medium bulk density, lowest in uniformly packed hard soil, and intermediate in soil where pores were made artificially or by plant roots. Effects depended on plant species, with alfalfa and ryegrass promoting better barley growth than the clover and artificial pores. Thus, contact with soil, and the nature of the roots, affected growth. In addition, Pierret et al. (1999) showed that removing pores by mixing, and uniformly packing the soil to a similar bulk density, doubled wheat leaf growth and water use. The soil 1–3 mm from the edges of these pores had a bacterial and fungal population that used a wider range of substrates than those of the bulk soil, and tended to have higher levels of the fungus Pythium (Pierret et al., 1999). These results suggest that the microbiology of the pores, in addition to contact with the soil and dead root remnants, affects plant growth.
Dead remnants can be colonized by saprophytic, pathogenic organisms such as Rhizoctonia (Neate, 1987). They are also heavily colonized by bacteria, including approx. 50 % of which are filamentous (Watt et al., 2006a). When new roots make direct contact with remnants, they become colonized by filamentous bacteria. If the junction between the remnants and new roots is heavily colonized by bacteria that are only decomposing the remnant material, and are not themselves inhibitory to the crop growth, the new roots will have access to nutrients from those organisms, provided it is not used by other micro-organisms first. Nutrient acquisition by roots from mineralization by micro- and macro-organisms will be greatest when the nutrient-to-carbon ratio is high in the remnants (Hodge et al., 2000). It also will be greatest when the distance between the roots and available, mineralized nutrients is short (eqn 1).
How nutrients are transferred from remnants to new roots via organisms is not clear. Mineral nutrients from the remnants can become available from the death and lysing of bacterial cells, or released from protozoa that have consumed the bacteria and release excess nitrogen. Moisture and high temperatures favour microbial growth rates, and dry soil favours death rates, but not diffusion. At moisture levels greater than field capacity, pores wider than about 30 µm will drain rapidly, and water will flow past the root–remnant junctions, possibly leaching nutrients such as nitrogen before roots can take them up. We can speculate from the analyses above on distances and their relationship to diffusion, and spaces and their relationship to flow and water movement, that continuous production of root length and surface area (root hairs) within cracks and pores would increase contact between roots (new and dead) and the soil of the pore edges that adsorbs mineralized nutrients. These would favour uptake of nutrients from mineralization by the new roots, and may minimize flow and stimulate decomposition and uptake of mineralized nutrients before they are captured by nearby micro-organisms. It is unclear how disease and deleterious organisms will respond.
CONCLUSIONS
This review highlights the kinematics of developing rhizospheres. We have shown how knowledge of growth rates, and the anatomical and biochemical patterns associated with root development, improves understanding of the interactions between roots and soil in field conditions. Genetic analyses of soil communities and in situ visualization of organisms in the rhizosphere use very small samples compared with an entire root system. Such studies would benefit from a knowledge of spatial and temporal aspects of the rhizosphere and root system development.
Expression of root and rhizosphere processes in similar units of distance and time facilitates understanding of physiological mechanisms, and the development of predictive models. When roots are growing, the tips carry a chemical micro-plume with chemistry and geometry determined by the diffusivity of the soil and the root exudation, uptake, and elongation rates. In the simplest cases, the elongation rate of the root gives the transport rate of the micro-plume in the soil profile. Distances are critical to predicting the time it takes for newly produced solutes to move between roots and other soil components. The characteristic time (eqn 1) shows that diffusion occurs much more rapidly over short than longer distances. This suggests that: (1) exchanges between roots and organisms on (or within) the root, in the ‘rhizoplane’, occur much more rapidly than those in the outer rhizosphere, and may be more important to plant function; and (2) roots that grow into biopores and contact dead and living roots that are heavily colonized by micro-organisms will form microbial interactions much more quickly than roots in the bulk soil.
High-resolution spatial data on roots, soil and micro-organisms have recently been obtained by using microscopy with in situ imaging of responding organisms with inducible reporter systems (Bringhurst et al., 2001). Non-invasive imaging techniques from medical research, in intact soil environments, are promising as resolution continues to improve for larger volumes of soil (Johnson et al., 2004). Temporal resolution has been improved with time-lapse imaging methods that resolve events in seconds or minutes (Walter et al., 2002), rather than in days (most minirhizotron studies). The improved spatial and temporal resolution within the rhizosphere can be combined with new molecular and classical culturing techniques to identify the more than 80 % of soil micro-organisms still uncharacterized (Amann et al., 1995; Janssen et al., 2002). The convergence of these new technologies suggests the time is ripe for research to understand the biology of the rhizosphere and its link to plant growth in the field.
Acknowledgments
We thank Linda Magee, Cheng Huang, the CSIRO Microscopy Centre, and Shaun Cunningham, for help with root and rhizosphere measurements. Margaret McCully kindly provided Fig. 3A. We are grateful to John Kirkegaard for stimulating discussions and field experiments. M.W. is funded by the Grains Research and Development Corporation (GRDC) of Australia. W.K.S. had support from the Kearney Foundation for Soil Science, and her visit to CSIRO was partially funded by the GRDC and grant 00-35100-9531 from the NRI competitive grants program/USDA.
LITERATURE CITED
- Amann RI, Ludwig W, Schleifer KH. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiological Reviews 59: 143–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André HM, Ducarme X, Lebrun P. 2002. Soil biodiversity: myth, reality or conning? Oikos 96: 3–24. [Google Scholar]
- Arora DK, Gupta S. 1993. Effect of different environmental conditions on bacteria chemotaxis toward fungal spores. Canadian Journal of Microbiology 39: 922–931. [Google Scholar]
- Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM. 2004. How plants communicate using the underground superhighway. Trends in Plant Science 9: 26–32. [DOI] [PubMed] [Google Scholar]
- Bar-Yosef B. 1996. Root excretions and their environmental effects: influence on availability of phosphorus. In: Waisel Y, Eshel A, Kafkaki U, eds. Plant roots: the hidden half, 2nd edn. New York: Marcel Dekker Inc., 581–605.
- Bauer WD, Mathesius U. 2004. Plant responses to bacterial quorum sensing signals. Current Opinion in Plant Biology 7: 429–433. [DOI] [PubMed] [Google Scholar]
- Bertin C, Yang X, Weston LA. 2003. The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil 256: 67–83. [Google Scholar]
- Bolger TP, Angus JF, Peoples MB. 2003. Comparison of nitrogen mineralisation patterns from root residues of Trifolium subterraneum and Medicago sativa. Biology and Fertility of Soils 38: 296–300. [Google Scholar]
- Bonkowski M. 2004. Protozoa and plant growth: the microbial loop in soil revisited. New Phytologist 162: 617–631. [DOI] [PubMed] [Google Scholar]
- Bowen GD, Rovira AD. 1999. The rhizosphere and its management to improve plant growth. Advances in Agronomy 66: 1–102. [Google Scholar]
- Boyer JS, Silk WK. 2004. Hydraulics of plant growth. Functional Plant Biology 31: 761–773. [DOI] [PubMed] [Google Scholar]
- Bringhurst RM, Cardon ZG, Gage DJ. 2001. Galactosides in the rhizosphere: utilization by Sinorhizobium meliloti and development of a biosensor. Proceedings of the National Academy of Sciences of the USA 98: 4540–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME. 1972. Plant growth substances produced by micro-organisms of soil and rhizosphere. Journal of Applied Bacteriology 35: 443–451. [Google Scholar]
- van Bruggen AHC, Semenov AM, Zelenev VV. 2000. Wavelike distributions of microbial populations along an artificial root moving through soil. Microbial Ecology 40, 250–259. [DOI] [PubMed] [Google Scholar]
- Camper AK, Hayes JT, Sturman PJ, Jones WL, Cunningham AB. 1993. Effects of motility and adsorption rate coefficient on transport of bacteria through saturated porous media. Applied and Environmental Microbiology 59: 3455–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook FJ, Knight JH. 2003. Oxygen transport to plant roots: modeling for physical understanding of soil aeration. Soil Science Society of America Journal 67: 20–31. [Google Scholar]
- Crank J. 1975. The Mathematics of Diffusion, 2nd edn. Oxford: Clarendon Press.
- Crews TE, Peoples MB. 2005. Can the synchrony of nitrogen supply and crop demand be improved in legume and fertilizer-based agroecosystems? A review. Nutrient Cycling in Agroecosystems 72: 101–120. [Google Scholar]
- Darrah PR. 1991a. Models of the rhizosphere. I. Microbial population dynamics around a root releasing soluble and insoluble carbon. Plant and Soil 133: 187–199. [Google Scholar]
- Darrah PR. 1991b. Models of the rhizosphere. II. A quasi three-dimensional simulation of the microbial population dynamics around a growing root releasing soluble exudates. Plant and Soil 138: 147–158. [Google Scholar]
- Dilkes NB, Jones DL, Farrar J. 2004. Temporal dynamics of carbon partitioning and rhizodeposition in wheat. Plant Physiology 134: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. 1989. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell and Environment 12: 285–292. [Google Scholar]
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, et al. 1994. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 120: 2465–2474. [Google Scholar]
- Doube BM, Brown GG. 1998. Life in a complex community: functional interactions between earthworms, organic matter, microorgansims and plants. In: Edwards CA, ed. Earthworm ecology. Boca Raton, FL: St Lucie Press,179–211.
- Drew EA, Murray RS, Smith SE, Jakobsen I. 2003. Beyond the rhizosphere: growth and function of arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant and Soil 251: 105–114. [Google Scholar]
- Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL. 2000. Building roots in a changing environment: implications for root longevity. New Phytologist 147: 33–42. [Google Scholar]
- Garbeva P, van Veen JA, van Elsas JD. 2004. Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology 42: 243–270. [DOI] [PubMed] [Google Scholar]
- George TS, Richardson AE, Simpson RJ. 2005. Behaviour of plant-derived extracellular phytase upon addition to soil. Soil Biology and Biochemistry 37: 977–988. [Google Scholar]
- Gilligan CA. 1980. Dynamics of root colonization by the take-all fungus, Gaeumannomyces graminis. Soil Biology and Biochemistry 12: 507–512. [Google Scholar]
- Gochnauer MB, McCully ME, Labbe H. 1989. Different populations of bacteria associated with sheathed and bare regions of roots of field-grown maize. Plant and Soil 114, 107–120. [Google Scholar]
- Goss MJ. 1977. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). I. Effects on the elongation and branching of seminal root axes. Journal of Experimental Botany 28: 96–111. [Google Scholar]
- Grose MJ, Gilligan CA, Spencer D, Goddard BVD. 1996. Spatial heterogeneity of soil water around single roots: use of CT-scanning to predict fungal growth in the rhizosphere. New Phytologist 133: 261–272. [DOI] [PubMed] [Google Scholar]
- Gupta VVSR. 1994. The impact of soil and crop management practice on the dynamics of soil microfauna and mesofauna. In: Pankhurst CE, Doube BM, Gupta VVSR, Grace PR, eds. Soil biota. Management in sustainable farming systems. Melbourne: CSIRO Publishing, 107–124.
- Hanks RJ, Ashcroft GL. 1980. Applied Soil Physics. Berlin: Springer.
- Hinsinger P, Gobran GR, Greogry PJ, Wenzel WW. 2005. Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytologist 168: 293–303. [DOI] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. 2005. Root architectural tradeoffs for water and phosphorus acquisition. Functional Plant Biology 32: 737–748. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Sauer M, Woll K. 2004. From weeds to crops: genetic analysis of root development in cereals. Trends in Plant Science 9: 42–48. [DOI] [PubMed] [Google Scholar]
- Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH. 1998. Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytologist 139: 479–494. [DOI] [PubMed] [Google Scholar]
- Hodge A, Robinson D, Fitter A. 2000. Are micro-organisms more effective than plants at competing for nitrogen? Trends in Plant Science 5: 304–308. [DOI] [PubMed] [Google Scholar]
- Hodgeman CD, Weast RC, Shankland RS, Selby SM. 1961. Handbook of Chemistry and Physics, 44th edn. Cleveland, OH, USA: Chemical Rubber Publishing Co.
- Huang CX, Canny MJ, Oates K, McCully ME. 1994. Planing frozen hydrated plant specimens for SEM observation and EDX analysis. Microscopy Research Technique 28: 67–74. [DOI] [PubMed] [Google Scholar]
- Hugenholtz P. 2002. Exploring prokaryotic diversity in the genomic era. Genome Biology 3: reviews 0003.1–0003.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman OC. 1982. Interrelations of root growth dynamics to epidemiology of root-invading fungi. Annual Review of Phytopathology 20: 303–327. [Google Scholar]
- Husain SS, McKeen WE. 1963. Interactions between strawberry roots and Rhizoctonia fragariae. Phytopathology 53, 541–545. [Google Scholar]
- Iijima M, Griffiths B, Bengough AG. 2000. Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytologist 145: 477–482. [DOI] [PubMed] [Google Scholar]
- Jaeger III C, Lindow S, Miller S, Clark E, Firestone M. 1999. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Applied and Environmental Microbiology 65: 2685–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JJ, Richards JH. 2005. Plant N capture from pulses: effects of pulse size, growth rate, and other soil resources. Oecologia 145: 113–122. [DOI] [PubMed] [Google Scholar]
- Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M. 2002. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria and Verrucomicrobia. Applied and Environmental Microbiology 68: 2391–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis RJ, Brennan RF. 1986. Timing and intensity of surface cultivation and depth of cultivation affect rhizoctonia patch and wheat yield. Australian Journal of Experimental Agriculture 26: 703–708. [Google Scholar]
- Johnson SN, Read DB, Gregory PJ. 2004. Tracking larval insect movement within soil using high resolution X-ray microtomography. Ecological Entomology 29: 117–122. [Google Scholar]
- Jones DL, Brassington DS. 1998. Sorption of organic acids in acid soils and its implications in the rhizosphere. European Journal of Soil Science 49: 447–455. [Google Scholar]
- Jones DL, Prabowo AM, Kochian LV. 1996. Kinetics of malate transport and decomposition in acid soils and isolated bacterial populations: the effect of micro-organisms on root exudation of malate under Al stress. Plant and Soil 182: 239–247. [Google Scholar]
- Jones DL, Hodge A, Kuzyakov Y. 2004. Plant and mycorrhizal regulation of rhizodeposition. New Phytologist 163: 459–480. [DOI] [PubMed] [Google Scholar]
- Khan DF, Herridge DF, Schwenke GD, Chen D, Peoples MB. 2001. The application of 15N-shoot labeling techniques to field-grown crops to study the decomposition and fate of shoot or root residue N in a legume-cereal rotation. In: 11th Nitrogen Workshop Book of Abstracts. France: INRA, 121–122.
- Khan DF, Peoples MB, Schwenke GD, Felton WL, Chen D, Herridge DF. 2003. Effects of below-ground nitrogen on N balances of field-grown fababean, chickpea and barley. Australian Journal of Agricultural Research 54: 333–340. [Google Scholar]
- Kim TK, Silk WK, Cheer AY. 1999. A mathematical model for pH patterns in the rhizospheres of growth zones. Plant, Cell and Environment 22: 1527–1538. [Google Scholar]
- Kinraide TB, Parker DR, Zobel RW. 2005. Organic acid secretion as a mechanism of aluminium resistance: a model incorporating the root cortex, epidermis, and the external unstirred layer. Journal of Experimental Botany 56: 1853–1865. [DOI] [PubMed] [Google Scholar]
- Kirkegaard JA. 1995. A review of trends in wheat yield responses to conservation cropping in Australia. Australian Journal of Experimental Agriculture 35: 835–848. [Google Scholar]
- Kuzyakov Y. 2002. Factors affecting rhizosphere priming effects. Journal of Plant Nutrition and Soil Science 165: 382–396. [Google Scholar]
- Leach LD. 1947. Growth rates of host and pathogen as factors determining the severity of preemergence damping-off. Journal of Agricultural Research 75, 161–179. [Google Scholar]
- Lekberg Y, Koide RT. 2005. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytologist 168: 189–204. [DOI] [PubMed] [Google Scholar]
- Malamy JE. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment 28: 67–77. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. 1997. Down and out in Arabidopsis: the formation of lateral roots. Trends in Plant Science 2: 390–396. [Google Scholar]
- Marschner P, Neumann G, Kania A, Weiskopf L, Lieberei R. 2002. Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant and Soil 246: 167–174. [Google Scholar]
- Mathesius U. 2003. Conservation and divergence of signaling pathways between roots and soil microbes. The Rhizobium–legume symbiosis compared to the development of lateral roots, mycorrhizal interactions and nematode-induced galls. Plant and Soil 255: 105–119. [Google Scholar]
- Mathesius U, Weinman JJ, Rolfe BG, Djordjevic MA. 2000. Rhizobia can induce nodules in white clover by ‘hijacking’ mature cortical cells activated during lateral root development. Molecular Plant–Microbe Interactions 13: 170–182. [DOI] [PubMed] [Google Scholar]
- McCully ME. 1999. Roots in soil: unearthing the complexities of roots and their rhizospheres. Annual Review of Plant Physiology and Plant Molecular Biology 50: 695–718. [DOI] [PubMed] [Google Scholar]
- McCully ME, Canny MJ. 1985. Localisation of translocated 14C in roots and root exudates of field-grown maize. Physiologia Plantarum 65: 380–392. [Google Scholar]
- McNeill A, Unkovich M, Zhu C, Pinchand T. 1999. The N benefit from above-ground and below-ground legume crop residues. In: The Twelfth Australian Nitrogen Fixation Conference Book of Abstracts. Australia: The Australian Society for Nitrogen Fixation, 99–100.
- Neate SM. 1987. Plant debris in soil as a source of inoculum of Rhizoctonia in wheat. Transactions of the British Mycological Society 88: 157–162. [Google Scholar]
- Nichol SA, Silk WK. 2001. Empirical evidence of a convection-diffusion model for pH patterns in the rhizospheres of root tips. Plant, Cell and Environment 24: 967–974. [Google Scholar]
- Nobel PS. 1983. Biophysical Plant Physiology and Ecology. New York: W.H. Freeman and Company.
- van Noordwijk M, Schoonderbeek D, Kooistra MJ. 1993. Root–soil contact of field-grown winter wheat. Geoderma 56: 277–286. [Google Scholar]
- Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ. 2005. Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant and Soil 271: 175–187. [Google Scholar]
- Olesen T, Moldrup P, Yamaguchi T, Nissen HH, Rolston DE. 2000. Modified half-cell method for measuring the solute diffusion coefficient in undisturbed, unsaturated soil. Soil Science 165: 835–840. [Google Scholar]
- Otten W, Gilligan CA, Watts CW, Dexter AR, Hall D. 1999. Continuity of air-filled pores and invasion thresholds for a soil-borne fungal plant pathogen, Rhizoctonia solani. Soil Biology and Biochemistry 31: 1803–1810. [Google Scholar]
- Otten W, Harris K, Young IM, Ritz K, Gilligan CA. 2004. Preferential spread of the pathogenic fungus Rhizoctonia solani through structured soil. Soil Biology and Biochemistry 36: 203–210. [Google Scholar]
- Pahlavaian AM, Silk WK. 1988. Effect of temperature on spatial and temporal aspects of growth in the primary maize root. Plant Physiology 87: 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate JS, Watt M. 2002. Roots of Banksia spp. (Proteaceae) with special reference to functioning of their specialized proteoid root clusters. In: Waisel Y, Eshel A, Kafkaki U, eds. Plant roots: the hidden half, 3rd edn. New York: Marcel Dekker Inc., 989–1006.
- Pellerin S, Tabourel F. 1995. Length of the apical unbranched zone of maize axile roots: its relationship to root elongation rate. Environmental and Experimental Botany 35: 193–200. [Google Scholar]
- Pierret A, Moran CJ, Pankhurst CE. 1999. Differentiation of soil properties related to the spatial association of wheat roots and soil macropores. Plant and Soil 211: 51–58. [Google Scholar]
- Pierret A, Moran CJ, Doussan C. 2005. Conventional detection methodology is limiting our ability to understand the roles and functions of fine roots. New Phytologist 166: 967–980. [DOI] [PubMed] [Google Scholar]
- Reinhardt DH, Rost TL. 1995. Developmental changes of cotton root primary tissues induced by salinity. International Journal of Plant Sciences 156: 505–513. [Google Scholar]
- Richards RA, Lukacs Z. 2002. Seedling vigour in wheat—sources of variation for genetic and agronomic improvement. Australian Journal of Agricultural Research. 53: 41–50. [Google Scholar]
- Schantz EJ, Lauffer MA. 1962. Diffusion measurements in agar gel. Biochemistry 1: 658–663. [DOI] [PubMed] [Google Scholar]
- Scott EM, Rattray EAS, Prosser JI, Killham K, Glover LA, Lynch JM, Bazin MJ. 1995. A mathematical model for dispersal of bacterial inoculants colonizing the wheat rhizosphere. Soil Biology and Biochemistry 27: 1307–1318. [Google Scholar]
- Sealey LJ, McCully ME, Canny MJ. 1995. The expansion of maize root-cap mucilage during hydration.1. Kinetics. Physiologia Plantarum 93: 38–46. [Google Scholar]
- Semenov AM, van Bruggen AHC, Zelenev VV. 1999. Moving waves of bacterial populations and total organic carbon along roots of wheat. Microbial Ecology 37: 116–128. [DOI] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsaio TC. 1988. Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiology 87: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood JL, Sung JC, Ford RM, Fernandez EJ, Maneval JE, Smith JA. 2003. Analysis of bacterial random motility in a porous medium using magnetic resonance imaging and immunomagnetic labeling. Environmental Science and Technology 37: 781–785. [DOI] [PubMed] [Google Scholar]
- Silk WK, Erickson RO. 1979. Kinematics of plant growth. Journal of Theoretical Biology 76: 481–501. [DOI] [PubMed] [Google Scholar]
- Silk WK, Lord EM, Eckard KJ. 1989. Growth patterns inferred from anatomical records. Plant Physiology 90: 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasithamparam K, Parker CA. 1979. Rhizosphere micro-organisms of seminal and nodal roots of wheat grown in pots. Soil Biology and Biochemistry 11: 155–160. [Google Scholar]
- Simpfendorfer S, Kirkegaard JA, Heenan DP, Wong PTW. 2002. Reduced early growth of direct drilled wheat in southern New South Wales – role of root inhibitory pseudomonads. Australian Journal of Agricultural Research 53: 323–331. [Google Scholar]
- Steidle A, Sigl K, Schuhegger R, Ihring A, Schmid M, Gantner S, et al. 2001. Visualization of N-acylhomoserine lactone-mediated cell–cell communication between bacteria colonizing the tomato rhizosphere. Applied and Environmental Microbiology 67: 5761–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirzaker RJ, Passioura JB, Wilms Y. 1996. Soil structure and plant growth: impact of bulk density and biopores. Plant and Soil 185: 151–162. [Google Scholar]
- Taylor AR, Bloom AJ. 1998. Ammonium, nitrate, and proton fluxes along the maize root. Plant, Cell and Environment 21: 1255–1263. [Google Scholar]
- Topp GC, Watt M, Hayhoe HN. 1996. Point specific measurement and monitoring of soil water content with an emphasis on TDR. Canadian Journal of Soil Science 76: 301–316. [Google Scholar]
- Vincent CD, Gregory PJ. 1989. Effects of temperature on the development and growth of winter wheat roots. II. Field studies of temperature, nitrogen and irradiance. Plant and Soil 119: 99–110. [Google Scholar]
- Vrugt JA, van Wijk MT, Hopmans JW, Simunek J. 2001. One-, two-, and three-dimensional root water uptake functions for transient modeling. Water Resources Research 37: 2457–2470. [Google Scholar]
- Walker NA, Smith SE. 1984. The quantitative study of mycorrhizal infection. II. The relation of rate of infection and speed of fungal growth to propagule density, the mean length of the infection unit and the limiting value of the fraction of the root infected. New Phytologist 96: 55–69. [Google Scholar]
- Walter A, Spies H, Terjung S, Küsters R, Kirchgeßner N, Schurr U. 2002. Spatio-temporal dynamics of expansion growth in roots: automatic quantification of diurnal course and temperature response by digital image sequence processing. Journal of Experimental Botany 53: 689–698. [DOI] [PubMed] [Google Scholar]
- Watt M, McCully ME, Canny MJ. 1994. Formation and stabilisation of maize rhizosheaths: effect of soil water content. Plant Physiology 106: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M, McCully ME, Kirkegaard JA. 2003. Soil strength and rate of root elongation alter the accumulation of Pseudomonas spp. and other bacteria in the rhizosphere of wheat. Functional Plant Biology 30: 482–491. [DOI] [PubMed] [Google Scholar]
- Watt M, Kirkegaard JA, Rebetzke GJ. 2005. A wheat genotype developed for rapid leaf growth copes well with the physical and biological constraints of unploughed soil. Functional Plant Biology 32: 695–706. [DOI] [PubMed] [Google Scholar]
- Watt M, Hugenholtz P, White R, Vinall K. 2006a. Numbers and locations of native bacteria on field grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environmental Microbiology Vol 8(5) in press. (doi 0.1111/j.1462-2920.2005.00973.x) [DOI] [PubMed]
- Watt M, Kirkegaard JA, Passioura JP. 2006b. Rhizosphere biology and crop productivity. Australian Journal of Soil Research Vol 44(4): in press.
- van der Weele CM, Jiang HS, Palaniappan KK, Ivanov VB, Palaniappan K, Baskin TI. 2003. A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiology 132: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum GE, Sturz AV, Dong Z, Nowak J. 2004. Managing soil micro-organisms to improve productivity of agroecosystems. Critical Reviews in Plant Sciences 23: 175–193. [Google Scholar]
- Wells CE, Glenn DM, Eissenstat DM. 2002. Soil insects alter fine root demography in peach (Prunus persica). Plant, Cell and Environment 25: 431–439. [Google Scholar]
- Wetselaar R, Passioura JB, Singh BR. 1972. Consequences of banding nitrogen fertilizers in soil. I. Effects on nitrification. Plant and Soil 36: 159–175. [Google Scholar]
- Young IM. 1998. Biophysical interactions at the root–soil interface: a review. Journal of Agricultural Science, Cambridge 130: 1–7. [Google Scholar]
- Zelenev VV, van Bruggen AHC, Semenov AM. 2000. BACWAVE, a spatial–temporal model for traveling waves of bacterial populations in response to a moving carbon source in soil. Microbial Ecology 40: 260–272. [DOI] [PubMed] [Google Scholar]
- Zwart SB, Brussaard. 1991. Soil fauna and field crops. In: Firbank LG, ed. The ecology of temperate cereal fields. Oxford: Blackwell Scientific Publications, 139–168.