Abstract
• Background and Aims The date palm is a dioecious perennial species of the Arecaceae for which in vitro micropropagation is essential to ensure the renewal of palm plantations. This study presents a histocytological analysis of the traditional Mauritanian Amsekhsi cultivar beginning from the initial callogenesis and continuing up to the establishment of the cellular embryogenic cell suspensions. The formation of somatic embryos and their development into rooted plants are also described.
• Methods Foliar segments of seedlings cultured in the presence of 2,4-D produced primary calli that were chopped to produce fine friable granular calli that subsequently produced cellular suspensions when transferred to liquid medium. The somatic proembryos that developed after removal of the 2,4-D were plated on agar medium where they developed into rooted plants. Thin sections of tissue fragments taken at each stage of the process were stained using Periodic Acid Schiff and Naphthol Blue-Black.
• Key Results The first cellular divisions were localized close to the vascular vessels of the leaf. The primary calli were obtained within 2 months. Fine friable granular calli grew quickly after the primary calli were chopped. Individual embryogenic cells were identified that rapidly started to divide and developed into globular proembryos. In addition, in the microcalli, breaking zones appeared in the thick pectocellulosic walls which delimited the pluricellular proembryos. The anatomy of somatic embryos is similar to that of zygotic embryos despite a deficit in the accumulation of intracellular proteins. When rooted with NAA, the vitroplants developed a strong orthotropic taproot.
• Conclusions This study contributes to understanding the whole process of somatic embryogenesis, but two specific questions remain to be answered: what factors are involved in the reactivation of the somatic cells at the beginning of the initial callogenesis, and why do the somatic embryos not accumulate proteins in their tissues during maturation?
Keywords: Histocytology, callogenesis, somatic embryos, cell culture, micropropagation, date palm, Phoenix dactylifera, vitroplants, zygotic embryo, acclimatation
INTRODUCTION
The date palm (Phoenix dactylifera) is a dioecious ‘tree’ of the Arecaceae that is the mainstay of agriculture in oasis zones where many food crops can be grown in combination with it. This oasis agricultural system is very common in the arid regions of the Middle East, North Africa and in the Sahara as far as central Mauritania (Munier, 1973). Traditionally, palm groves were planted from off-shoots produced by the date palms in the early part of their life. In-vitro micropropagation thus soon became an essential and effective means to ensure the renewal and the extension of palm plantations (Smith and Aynsley, 1995). Vegetative multiplication of the date palm by somatic embryogenesis was developed at the end of 1970s, and studies were published by Reuveni (1979), Reynolds and Murashige (1979), Tisserat (1979) and Tisserat and DeMason (1980), starting from zygotic embryos, from axillary buds or from immature leaves. Drira and Benbadis (1985) reported that the reversion of female floral buds into vegetative buds also enabled vegetative multiplication. Cultures of the apical zone taken from the apical bud of the off-shoots (Rhiss et al., 1979) or from seedlings (Gabr and Tisserat, 1985) led to the formation of axillary buds which have been used up until now for in-vitro cloning.
Histological analysis of embryogenic calli obtained from zygotic embryos cultivated on semi-solid medium allowed Tisserat and DeMason (1980) to attribute a unicellular origin to the somatic embryos of date palm.
Embryogenic cultures of date palm tissues in liquid medium initiated from shoot tips, young off-shoot leaves or from immature inflorescences have already been used with success for true-to-type propagation of some commercial varieties cultivated in North Africa like Medjoul, and Barhé (Daguin and Letouzé, 1988), Deglet Nour (Fki et al., 2003) or Bousthami and Jihel (Zouine et al., 2005). However, in these cases the process needs as long as 6 months of culture on semi-solid medium before primary calli are obtained.
To date, no procedure has been developed for cloning progenies of traditional cultivars potentially suitable for subtropical environments, such as Amsekhsi, an early-flowering local cultivar from central Mauritania. The aim of cloning progenies is to select the best early-flowering male and female individuals in a multi-clonal and multi-site field trial.
Up to now, no precise histological analysis of the different stages of development has been performed during the regeneration process from cellular suspensions of date palm. This study presents a histo-cytological description of the tissues of date palm from the initial callogenesis until the establishment of the embryogenic cellular suspensions, followed by the formation and the development of somatic embryos to the production of rooted clonal plants of the Amsekhsi cultivar.
MATERIALS AND METHODS
Plant material and preparation of explants
The study was conducted using seeds of the early flowering Phoenix dactylifera L. ‘Amsekshi’ selected and harvested directly in palm groves in the Atar region of Mauritania (20–21°N; 012–013°W).
The seeds were sterilized with 96 % H2SO4 for 10 min then rinsed with sterile distilled water. They were then soaked in sterile water for 24 h before being placed in glass tubes (25 × 150 mm) containing 20 ml of agar (Difco Agar) (8 g L−1). After 1 month of culture in a controlled-culture room with a 12 h/12 h photoperiod and an irradiance of 80 μE s−1 m−2, at 27 ± 0·2 °C constant temperature, the seedlings where dissected. Young white to yellowish leaves were cut into segments 1 cm in length. The apices where dissected separately. All the explants were placed in a range of different conditions for callus induction on various 2,4-D concentrations.
Primary and secondary calli
The explants were placed on a basic medium composed of Murashige and Skoog solution (Murashige and Skoog, 1962), FeEDTA, Morel and Wetmore vitamins (Morel and Wetmore, 1951), biotine (0·01 mg L−1), sodium ascorbate (100 mg L−1) and myo-inositol (100 mg L−1). Sucrose (30 g L−1), agar (Difco Agar) (8 g L−1) and 2,4-dichlorophenoxy-acetic acid (2,4-D) (2 mg L−1) were added to the basic medium. The primary calli obtained after 2 months of culture were chopped with a scalpel blade according to the method described by Teixeira et al. (1995) then transferred on the same medium. After 1 month of culture, the secondary calli grown from the chopped primary calli were used to prepare cellular suspensions. They were placed in 250-ml Erlenmeyer flasks containing 50 mL liquid medium of the same composition but without agar, and placed on an orbital shaker at 90 rpm in the same conditions.
Initiation of embryogenesis and development of the somatic embryos
The process of regeneration was adapted from the procedure described by de Touchet et al. (1991) and Aberlenc-Bertossi et al. (1999) for oil palm somatic embryogenesis. Each month, 300 mg fresh weight of cell suspensions were transferred in a liquid medium containing the same basic medium described above, supplemented with 20 g L−1 of glucose and 2 mg L−1 of 2,4-D. To produce somatic embryos, the suspensions were then cultivated for 1 month in a liquid medium of the same composition as the basic medium but without 2,4-D, on the same orbital shaker. The cell suspensions were then filtered through a double nylon mesh (1 and 2 mm). Fifty milligrams fresh weight of sieved microcalli were transferred onto a filter paper in a 9-cm-diameter Petri dish containing 20 mL basic medium enriched with 0·5 mg L−1 of benzyladenine (BA) and gelled with agar 8 g L−1. The filter paper with the culture was transferred weekly on a new medium without hormone for 5 weeks. The somatic embryos that developed (length 10–11 mm) were transferred for germination in individual glass tubes (25 × 150 mm) on Murashige and Skoog medium (Murashige and Skoog, 1962), with or without naphthalene acetic acid (NAA) (1 mg L−1) for rooting.
Histological analysis
Samples of ten tissue fragments were taken at each developmental stage. They were fixed using a solution containing for 100 mL, 4 mL of a 25 % glutaraldehyde solution, 50 mL of phosphate buffer at pH 7·2, 20 mL of 10 % paraformaldehyde solution, 1 g of caffeine and 26 mL of distilled water (Schwendiman, 1988). Progressive dehydration with ethanol and impregnation in methyl methacrylate was performed for each sample and these were then embedded in epoxy resin (Historesin from Reichert-Jung). Serial sections, 3·5 µm thick, were stained with Periodic Acid Schiff (PAS) combined with Naphthol Blue Black (NBB) according to the method described by Fisher (1968).
RESULTS
Formation of primary and secondary calli
After 2 weeks of culture in the presence of 2,4-D, the histological sections from apices and foliar explants revealed the presence of degenerating parenchymatous tissues. Embryogenic cells (30–40 µm length) were observed close to the vascular tissues (Fig. 1A). These peri-vascular cells were characterized by small vacuoles and dense cytoplasm, where soluble proteins were stained blue by NBB. After 4 weeks of culture, histological sections showed the formation of many individual spherical globules ranging from 250 to 500 µm in diameter located near the vascular tissues (Fig. 1B). These globular calli were composed of small meristematic cells ranging from 8 to 20 µm in diameter. NBB staining showed the intensely stained cytoplasm and a large and dark nucleus. The compact globular calli growing from internal tissues of the explant became clearly visible at the eighth week of culture (Fig. 1C). Calli were observed on 89 % of the apices and 63 % of foliar-cultured explants.
Fig. 1.
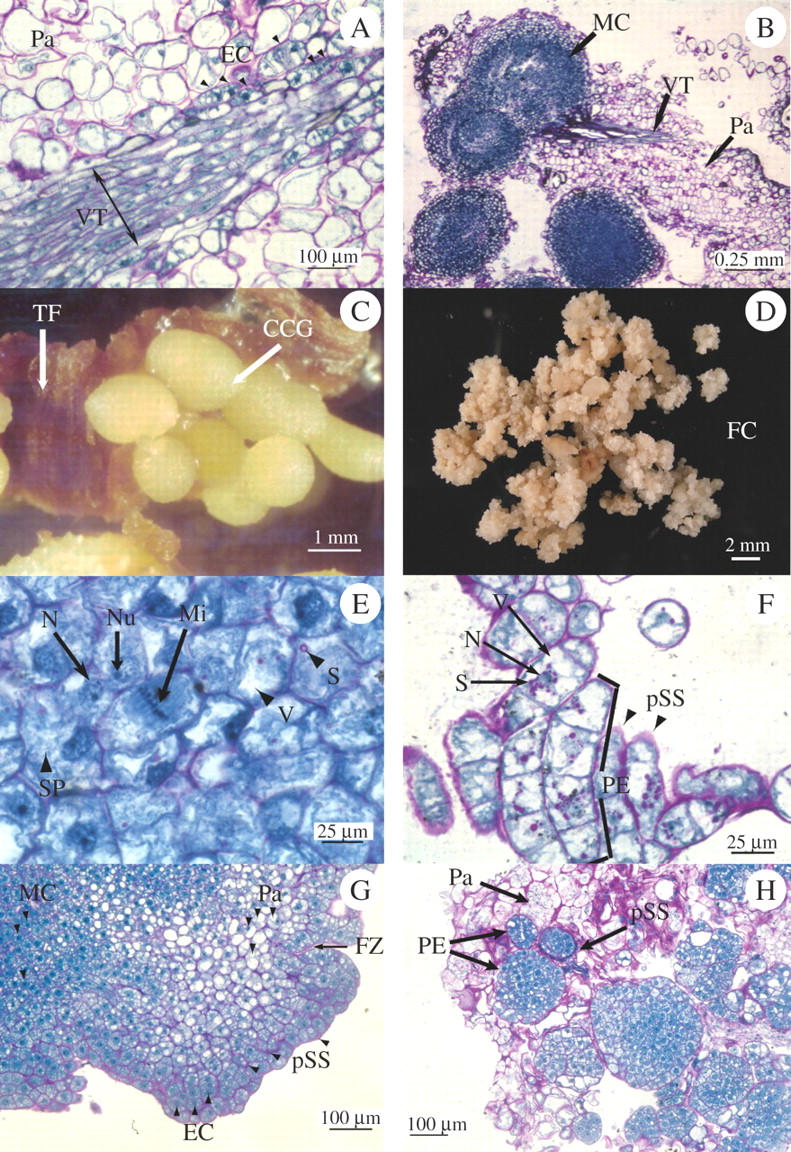
(A) After 2 weeks of culture in 2 mg L−1 2,4-D, a primary explant shows a vascular tissue (VT) in a longitudinal section of the parenchyma tissue (Pa) of a young leaf. Newly formed embryogenic cells (EC) indicated by black triangles, are located in the perivascular area. (B) In same explant type after 1 month of culture, vestiges of the vascular tissues (VT) are still visible in the parenchyma (Pa). Many spherical globules, 250–500 μm in diameter, made up of meristematic cells (MC) have developed. (C) After 2 months of culture, these globules developed into a compact nodular callus (CCG) which can be seen emerging through the surface of the leaf tissue (TF) (see Supplementary Information). (D) Friable granular callus (FC) developed on medium enriched with 2 mg L−1 2,4-D, 2 months after chopping the compact nodular callus (CCG). (E) Histological structure of the friable granular callus showing cells that are very rich in soluble proteins (SP), with small vacuoles (V), with a large nucleus (N) and easily visible nucleole (Nu). This tissue is in active division (Mi). Some cells present starch grains (S) stained pink by APS (see Supplementary Information). (F) When cultivated in liquid medium with 2 mg L−1 of 2,4-D, the friable granular calli resulted in cellular suspensions made up of individualized embryogenic cells or, after division, proembryogenic clusters (PE). These proembryos are embedded in a thick polysaccharidic sheath (pSS) that is stained pink by APS. The embryogenic cells are also characterized by many small vacuoles (V) and are rich in starch (S) grains grouped around the nucleus (N) (see Supplementary Information). (G) Three months later, the suspensions developed into embryogenic microcalli with several distinct areas. Meristematic cells (MC) in the central area and embryogenic cells (EC) are shown surrounded by a polysaccharidic sheath (pSS) and presenting fracture zones (FZ) in the peripheral area. In between, the ells show a large vacuole, and the tissue appears to be parenchymatic (see Supplementary Information). (H) Four months later, individual globular proembryos (PE) are clearly visible in their polysaccharidic sheath (pSS), and the parenchyma (Pa) which surrounds them is in the process of degenerating (see Supplementary Information).
When the globular and compact primary calli were chopped with a scalpel blade and cultivated on the same medium, they divided actively and gave rise to friable granular calli composed of embryogenic cells after 6–8 weeks of culture (Fig. 1D, E). These cells showed very small vacuoles and a large quantity of soluble proteins in the cytoplasm stained blue with NBB.
Proliferation of the cellular suspensions and embryogenesis
Under the experimental conditions described above, the proliferation of the cellular suspensions initiated from friable granular calli became effective only at the end of the second subculture on liquid medium enriched with 2,4-D. From then on, histological observation of the embryogenic structures at different stages of development allowed the following events to be identified:
After 2 months of culture in liquid medium, the suspension consisted of embryogenic cells whose cytoplasm contained starch and lipoproteic storage grains (Fig. 1F). Their thick polysaccharide walls were stained pink by PAS. Some isolated thick-walled embryogenic cells were observed in addition to aggregates made of two to several cells, 15 µm in diameter, that may result from the division of an initial single cell.
After culture for 5 months, the cell clusters that had proliferated into microcalli comprised two zones of cells with a large nucleus (Fig. 1G and Supplementary Information). In the inner part of the microcalli, the cells were meristematic and small (20 µm) with a dense cytoplasm which was very rich in soluble proteins stained blue by NBB and without any visible vacuole. In the outer part, the cells were embryogenic with a thickened outer wall stained pink by PAS. Their less-dense cytoplasm appeared to be rich in starch and lipoprotein storage grains. Fragmentation zones were visible between cells with a thickened wall.
After 9 months, small individualized embryogenic masses and globular proembryos were observed embedded in degenerating parenchyma (Fig. 1H). The clusters of embryogenic cells were surrounded by a thick polysaccharide outer wall. The cells appeared to be very rich in storage proteins stained in dark blue by NBB. After the 10th month, they developed into individual globular structures surrounded by an epidermal cell layer (Fig. 2A).
Fig. 2.
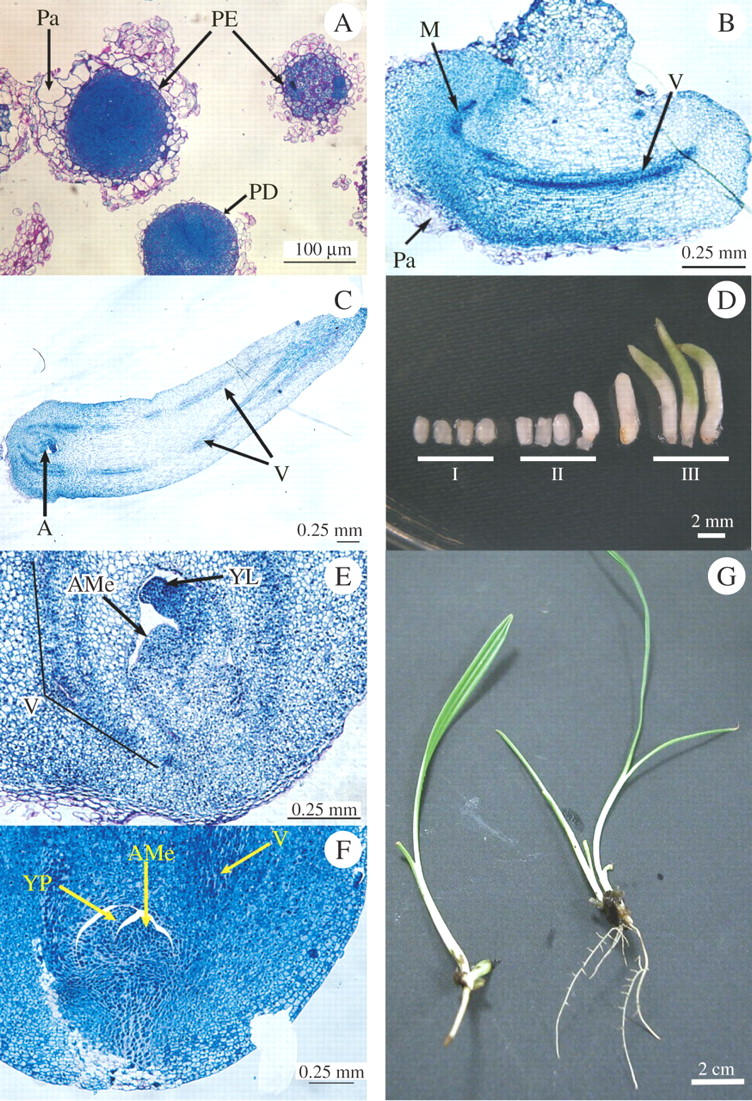
(A) Within 1 month, the parenchymatous matrices (Pa) have completely degenerated, the proembryos (PE) have become independent and are surrounded by an epidermic cell layer (PD). (B) Longitudinal section of a stage-II somatic embryo composed of a polarized structure which consists in a meristematic end (M) and a parenchyma comprising a vascular trend (V) visible the entire length of the embryo. (C) Longitudinal section of a stage-III somatic embryo presenting a well-differentiated vascular system (V) and the shoot apical meristem (SAM). (D) Developmental stages of somatic embryos: at stage I, polar embryos are <2 mm in diameter; at stage II, they begin to lengthen to between 2 and 4 mm; at stage III, the cotyledon develops and becomes green. When the first leaf appears through the cotyledon, the embryo is considered as germinating (GE) (see Supplementary Information). (E and F) Details of the apices of a stage-III somatic embryo (E) and a zygotic embryo after 1 d of imbibition (F) showing the similarity in the structure of the shoot apical meristem (SAM), the leaf primordium (LP) and the vascular system (V) in the two embryos. In the zygotic embryo only, the abundance of proteinic reserves (stained dark blue by NBB) is remarkable (see Supplementary Information). (G) Rooted vitroplants 14 months after in-vitro culture of the primary explant was started. Rooting was on medium enriched with 1 mg L−1 NAA (left) and on medium without hormone (right) (see Supplementary Information).
Development of the somatic embryos
The cellular suspensions were cultured for 1 month in a liquid medium without hormone, and then plated on the MS agar medium described in the Materials and methods. One week after plating on BA-containing medium, somatic embryos reached stage I. Stage-II embryos were clearly visible at the end of the 2nd week of culture on hormone-free MS medium. During this period, 40 mg of cell suspension were able to produce an average of 30 stage-II embryos. They were ovoid in shape, 1·5 mm in length, 1 mm in diameter, with a pearly epidermis (Fig. 2D). Stage-II embryos were polarized structures consisting of parenchyma tissue comprised of a vascular bundle and a meristematic area located at one end of the embryo (Fig. 2B). The development of somatic embryos from stage II to stage III (length 4–5 mm, diameter 1·5 mm) occurred between the 3rd and the 4th week of culture (Fig. 2D). At this developmental stage, longitudinal sections of stage III embryos displayed a well-differentiated vascular system along the cotyledon and a fully organized shoot apex (Fig. 2C). The shoot apex consisted of a meristematic dome surrounded by a leaf primordium (Fig. 2E) similar to that of a zygotic embryo after 1 d of imbibition (Fig. 2F). In both embryos, the root meristem is a diffuse area that is less organized than the shoot apex meristem. In zygotic embryos, cells showed many more storage proteins stained dark blue by NBB than in somatic embryos. After the 6th week, stage-III somatic embryos lengthened and started to accumulate chlorophyll (length 10–11 mm, diameter 1·7–2 mm) (Fig. 2D).
Germination of the somatic embryos and rooting of the vitroplants
The germination rate of stage III somatic embryos was 82 % after transfer onto MS medium enriched with 1 mg L−1 NAA. Under these conditions, they developed leaves and a taproot system whose morphology was identical to that of seedlings. In contrast, rooting without hormone resulted in the development of fine ramified roots (Fig. 2G) that were unable to survive when planted in a nursery.
DISCUSSION
Adaptation of the procedure of in vitro regeneration developed for oil palm (de Touchet et al., 1991; Aberlenc-Bertossi et al., 1999) to P. dactylifera enabled production of vitroplants through indirect somatic embryogenesis in liquid medium. This process required two steps for the production of calli before cell suspensions could be obtained. The results of the present study showed that the immature leaf and apical tissues used as starting explants provided better material for the regeneration of somatic embryos than the segments of roots of young seedlings (D. Sané, unpubl. res.).
During the transition from somatic to embryogenic status, cells have to dedifferentiate and activate their cell division cycle (Fehér et al., 2003). In date palm, dividing small cells had an embryogenic appearance, with a protein-rich cytoplasm, small vacuoles and a large nucleus as described in coconut (Verdeil et al., 2001). Cellular divisions were observed in the area of the peri-vascular parenchyma in leaf explants and apices after 15 d. This suggests that these cells could be the origin of the compact globular primary calli that were visible in the peri-vascular area of leaf explants after 2 months of culture. Proliferation of the calli near the vascular tissues has also been observed in leaf explants from other Arecaceae such as Elaeis guineensis (Schwendiman et al., 1988) and Cocos nucifera (Buffard-Morel et al., 1992), but also in other species like Gossypium hirsutum (Gawel et al., 1986) or Acacia raddiana (Sané et al., 2000).
It is well established that 2,4-D plays a role in the induction of somatic embryogenesis which is presumably mediated by a signal cascade triggered by this exogenous auxin (Zuo et al., 2002). However, the whole sequence of events is poorly understood even if a number of genes have been identified that promote vegetative to embryogenic transition, e.g. SERK (Schmidt et al., 1997) or WUSCHEL/PGA6 (Zuo et al., 2002), and are involved in the regulation of somatic embryo development, e.g. AGL15 (Harding et al., 2003), BBM (Boutilier et al., 2002) or LEC2 (Stone et al., 2001).
Moreover, Barbier-Brygoo et al. (1989) showed that the multiplication of cells which occurs during callus formation begins by a hyperpolarization of membrane polypeptides under the action of the auxin. According to Goldsworthy and Rathore (1985), this membrane hyperpolarization is the consequence of a destabilization of the polarity of the cellular electric fields which could be the cause of the disorganized growth observed in the presence of the 2,4-D during callus development. However, Schwendiman et al. (1988) suggested that the ability of the cells to divide could also be related to the genotype and even to the origin of the explant used due to the effect of environmental factors during in-vitro culture.
Histological analysis of the compact primary calli showed that they were made up of small meristematic cells with dense cytoplasm and were very rich in soluble protein. In date palm, subsequent development is different from callogenesis in oil palm described by Schwendiman et al. (1988), in which growth of the primary calli continues in a regular way throughout the subcultures. Indeed, the compact globular calli obtained in date palm were characterized by very slow growth. In addition, they started to degenerate and necrotize very quickly at the end of the third subculture on 2,4-D-enriched media.
In agreement with Teixeira et al. (1995), in the present study chopping the calli was necessary to enable the appearance and the growth of granular secondary calli after 1 month of culture on the same medium. After transfer to liquid medium, the chopped calli showed a very embryogenic behaviour. Similar observations were made by Buffard-Morel et al. (1992) then by Kamo et al. (2004), respectively, in Cocos nucifera and Rosa hybrida. In these two species, the authors also observed that after the primary calli had divided, the development of secondary calli was accompanied by an increase in the embryogenic potential of the tissues being cultured.
Under the present experimental conditions, two subcultures were necessary to initiate and establish the cellular suspensions on liquid media enriched with 2,4-D. Histological monitoring of the development of the embryogenic cell clusters through ten subcultures enabled the different pathways of pro-embryogenesis, which have been already observed in many other plant species such as oil palm (Schwendiman et al., 1990), coconut (Verdeil et al., 2001) and Acacia tortilis (Sané et al., 2000), to be described for the date palm. The first developmental pathway is a typical embryogenesis of unicellular origin where the embryogenic cell is actively dividing inside a thick polysaccharide outer wall providing physical insulation from adjacent cells. This isolation may be essential to the cells for the expression of the embryogenic potential (Lowe et al., 1985). In the second pathway, embryogenesis shows a pluricellular origin. In the date palm, the embryogenic microcalli present fragmentation lines which, from place to place, delimit territories of meristematic cells by breaking the thick polysaccharide cell wall. These clusters of cells evolve towards proembryos. Later on, the peripheral cell layer of these proembryos differentiates into protoderm, thus delimiting pluricellular embryos. This pathway resembles the primitive zygotic embryogenesis of the coconut described by Haccius and Philip (1979).
In addition, the orientation towards one or the other of the two embryogenesis pathways is accompanied by a significant accumulation of starch and/or proteins which could be a good indicator of the development of the tissues towards embryogenesis as already observed (Lu and Vasil, 1985; Schwendiman et al., 1988; Verdeil et al., 2001). In the present study, the formation of the embryos was only possible after transferring the cell suspensions to a 2,4-D-free medium. This is in agreement with observations on oil palm (Aberlenc-Bertossi et al., 1999) and many other species.
Increasing the proportion of cytokinin in the hormonal balance is thought to promote the expression of somatic embryogenesis and the later development of the embryos (Dhedh' A et al., 1994). In oil palm, Aberlenc-Bertossi et al. (1999) observed that cultivating the cell suspensions for 1 month in a large quantity of liquid hormone-free medium, followed by plating the cells on BA-enriched medium, promoted the growth of proembryos which then developed from the globular stage to form bipolar stage embryos. The importance of BA during this phase of development was confirmed by experiments with date palm (D. Sané, unpubl. res.). Indeed, the histological sections of date palm tissues showed that the transient application of this cytokinin promoted the appearance of meristematic territories in the tissues whose successive divisions led to the polarization of the proembryo within 3–4 weeks of culture.
A fundamental difference between the development of somatic embryos and zygotic embryos is the very weak accumulation of reserves during the development of the former. In Brassica napus embryogenesis, Crouch (1982) observed that storage proteins were of the same nature in both types of embryos. In oil palm, Morcillo et al. (1998) demonstrated the greatly different amounts of the same 7S globulins during the maturation phase of somatic and zygotic embryos. In oil palm, storage proteins are accumulated more precociously during the development of the somatic embryo, but in lower quantities than in the zygotic embryo. The results with date palm appear to be similar, given the differential NBB staining of the somatic and zygotic embryos tissues (D. Sané, unpubl. res.).
In short, in date palm, during the early stages of embryogenesis in liquid medium, it was possible to observe a significant accumulation of starch and proteins. This observation is in agreement with observations made in oil palm by Schwendiman et al. (1988), who noted that the early accumulation of the lipid reserves could be a good indicator of the acquisition of the embryogenic potential of the tissues. In agreement with observations made in other species, the late stages of somatic embryogenesis leading to the development of the embryos are characterized by extremely low reserves.
In the present study, the complete process of regeneration of isolated somatic embryos is described. As far as is known, this is the first precise histocytological description of the different stages of somatic embryogenesis in the date palm: the division of perivascular cells on primary explants, the secondary callogenesis after mechanical stress of the primary calli, the unicellular or pluricellular origin of the embryos, the absence of accumulation of reserves during the maturation of the somatic embryos, and finally the development of these embryos into rooted vitroplants. Two significant questions remain to be addressed: (1) What factors, including a possible genotypic effect, are involved in the reactivation of somatic cells at the beginning of the initial callogenesis? (2) What are the optimal maturation conditions which allow a good accumulation of reserves in somatic embryos of date palm? These two questions need to be studied with the long-term objective of creating artificial clonal seeds of date palm cultivars.
SUPPLEMENTARY INFORMATION
A supplementary set of 27 pictures is available at http://perso.wanadoo.fr/alain.borgel/biblio.htm. These figures complement Figs 1 and 2 in the text.
Acknowledgments
We thank the Support and Training Department (DSF) of the French Institut de Recherche pour le Développement (Paris) for supplying the grant for this work. The research was partially supported by the International Foundation for Science, Stockholm, Sweden and United Nations University (UNU), Tokyo, Japan, through a grant to Dr Djibril Sané. The University of Nouakchott (Mauritania) and M. Saleck facilitated access to germplasm in the Atar region. We also thank Dr Tim Tranbarger for critically reading the manuscript.
LITERATURE CITED
- Aberlenc-Bertossi F, Noirot M, Duval Y. 1999. BA enhances the germination of oil palm somatic embryos derived from embryogenic suspension cultures. Plant Cell, Tissue and Organ Culture 56: 53–57. [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klambt D, Ghislain M, Guern J. 1989. Functional Evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proceedings of the National Academy of Sciences of the USA 86: 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, et al. 2002. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. The Plant Cell, 14: 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffard-Morel J, Verdeil J-L, Pannetier C. 1992. Embryogenèse somatique du cocotier (Cocos nucifera L.) à partir d'explants foliaires: étude histologique. Canadian Journal of Botany 70: 735–741. [Google Scholar]
- Crouch ML. 1982. Non-zygotic embryos of Brassica napus L. contain embryo-specific storage proteins. Planta 156: 520–524. [DOI] [PubMed] [Google Scholar]
- Daguin F, Letouze R. 1988. Regeneration of date palm (Phoenix dactylifera) by somatic embryogenesis: improved efficiency by shaking in liquid medium. Fruits 43: 191–194. [Google Scholar]
- Dhed'A D, Dumortier F, Panis B, Vuylsteke D, Langhe E. 1991. Plant regeneration in cell suspension cultures of the cooking banana cv. ‘Bluggoe’ (Musa spp. ABB group). Fruits 46: 125–135. [Google Scholar]
- Drira N, Benbadis A. 1985. Vegetative multiplication of date palm (Phoenix dactylifera L.) by reversion of in vitro cultured female flower buds. Journal of Plant Physiology 119: 227–235. [Google Scholar]
- Fehér A, Pasternak TP, Dudits D. 2003. Transition of somatic plant cell to an embryogenic state. Plant Cell, Tissue and Organ Culture 74: 201–228 [Google Scholar]
- Fisher DB. 1968. Protein staining of ribboned epon sections for light microscopy. Histochemie 16: 92–96. [DOI] [PubMed] [Google Scholar]
- Fki L, Masmoudi R, Drira N, Rival A. 2003. An optimised protocol for plant regeneration from embryogenic suspension cultures of date palm, Phoenix dactylifera L., cv. Deglet Nour. Plant Cell Reports 21: 517–524. [DOI] [PubMed] [Google Scholar]
- Gabr MF, Tisserat B. 1985. Propagating palms in vitro with special emphasis on the date palm (Phoenix dactylifera L.). Scientia Horticulturae 25: 255–262. [Google Scholar]
- Gawel NJ, Rao AP, Robacker CD. 1986. Somatic embryogenesis from leaf and petiole callus cultures of Gossypium hirsutum L. Plant Cell Reports 5: 457–459. [DOI] [PubMed] [Google Scholar]
- Goldsworthy A, Rathore KS. 1985. Electrical control of growth in plant tissue cultures: the polar transport of auxin. Journal of Experimental Botany 36: 1134–1141. [Google Scholar]
- Haccius B, Philip VJ. 1979. Embryo development in Cocos nucifera L.: a critical contribution to a general understanding of palm embryogenesis. Plant Systematics and Evolution 132: 91–106. [Google Scholar]
- Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE. 2003. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiology 133: 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo K, Jones B, Castillon J, Bolar J, Smith F. 2004. Dispersal and size fractionation of embryogenic callus increases the frequency of embryo maturation and conversion in hybrid tea roses. Plant Cell Reports 22: 787–792. [DOI] [PubMed] [Google Scholar]
- Lowe K, Taylor DB, Ryan P, Paterson KE. 1985. Plant regeneration via organogenesis and embryogenesis in the maize inbred line B73. Plant Science 41: 125–132. [Google Scholar]
- Lu CY, Vasil IK. 1985. Histology of somatic embryogenesis in Panicum maximum (Guinea grass). American Journal of Botany 72: 1908–1913. [Google Scholar]
- Morcillo F, Aberlenc-Bertossi F, Hamon S, Duval Y. 1998. Accumulation of storage protein and 7S globulins during zygotic and somatic embryo development in Elaeis guineensis. Plant Physiology and Biochemistry 36: 509–514. [Google Scholar]
- Morel G, Wetmore RM. 1951. Fern callus tissue culture. American Journal of Botany 38: 141–143. [Google Scholar]
- Munier P. 1973. Le palmier dattier. Techniques Agricoles et Productions Tropicales. Paris: Maisonneuve et Larose.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Reuveni O. 1979. Embryogenesis and plantlets growth of date palm (Phoenix dactylifera L.) derived from callus tissue. Plant Physiology 63 (Suppl.): 138.
- Reynolds JF, Murashige T. 1979. Asexual embryogenesis in callus cultures of palms. In vitro 15: 383–387. [Google Scholar]
- Rhiss A, Poulain C, Beauchesne G. 1979. In vitro culture for the vegetative propagation of date palms Phoenix dactylifera L. Fruits 34: 551–554. [Google Scholar]
- Sané D, Borgel A, Verdeil J-L, Gassama YK. 2000. Plantlet regeneration via somatic embryogenesis in immature zygotic embryo callus from a tree species adapted to arid lands: Acacia tortilis subsp. raddiana (Savi.) Brenan. Acta Botanica Gallica 147: 257–266. [Google Scholar]
- Schmidt DL, Guzzo F, Toonen MAL, de Vries SC. 1997. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124: 2049–2062 [DOI] [PubMed] [Google Scholar]
- Schwendiman J, Pannetier C, Michaux-Ferriere N. 1988. Histology of somatic embryogenesis from leaf explants of the oil palm Elaeis guineensis. Annals of Botany 62: 43–52. [Google Scholar]
- Schwendiman J, Pannetier C, Michaux-Ferriere N. 1990. Histology of embryogenic formations during in vitro culture of oil palm Elaeis guineensis Jacq. Oléagineux 45: 409–418. [Google Scholar]
- Smith RJ, Aynsley JS. 1995. Field performance of tissue cultured date palm (Phoenix dactylifera) clonally produced by somatic embryogenesis. Principes 39: 47–52. [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, et al. 2001. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proceedings of the National Academy of Sciences of the USA 98: 11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira JB, Sondahl MR, Nakamura T, Kirby EG. 1995. Establishment of oil palm cell suspensions and plant regeneration. Plant Cell, Tissue and Organ Culture 40: 105–111. [Google Scholar]
- Tisserat B. 1979. Propagation of date palm (Phoenix dactylifera L.) in vitro. Journal of Experimental Botany 30: 1275–1283. [Google Scholar]
- Tisserat B, DeMason DA. 1980. A histological study of development of adventive embryos in organ culture of Phoenix dactylifera L. Annals of Botany 46: 465–472. [Google Scholar]
- de Touchet B, Duval Y, Pannetier C. 1991. Plant regeneration from embryogenic suspension cultures of oil palm (Elaeis guineensis Jacq.). Plant Cell Reports 10: 529–532. [DOI] [PubMed] [Google Scholar]
- Verdeil J-L, Hocher V, Huet C, Grosdemange F, Escoute J, Ferrière N, Nicole M. 2001. Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Annals of Botany 88: 9–18. [Google Scholar]
- Zouine J, El Bellaj M, Meddich A, Verdeil J-L, El Hadrami I. 2005. Proliferation and germination of somatic embryos from embryogenic suspension cultures in Phoenix dactylifera. Plant Cell, Tissue and Organ Culture 82: 83–92. [Google Scholar]
- Zuo JR, Niu QW, Frugis G, Chua NH. 2002. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. The Plant Journal 30: 349–359. [DOI] [PubMed] [Google Scholar]


