Abstract
• Background and Aims Neotyphodium lolii is a fungal endophyte of perennial ryegrass (Lolium perenne), improving grass fitness through production of bioactive alkaloids. Neotyphodium species can also affect growth and physiology of their host grasses (family Poaceae, sub-family Pooideae), but little is known about the mechanisms. This study examined the effect of N. lolii on net photosynthesis (Pn) and growth rates in ryegrass genotypes differing in endophyte concentration in all leaf tissues.
• Methods Plants from two ryegrass genotypes, Nui D and Nui UIV, infected with N. lolii (E+) differing approx. 2-fold in endophyte concentration or uninfected clones thereof (E−) were grown in a controlled environment. For each genotype × endophyte treatment, plant growth rates were assessed as tillering and leaf extension rates, and the light response of Pn, dark respiration and transpiration measured in leaves of young (30–45 d old) and old (>90 d old) plants with a single-chamber open infrared gas-exchange system.
• Key Results Neotyphodium lolii affected CO2-limited rates of Pn, which were approx. 17 % lower in E+ than E− plants (P < 0·05) in the young plants. Apparent photon yield and dark respiration were unaffected by the endophyte (P > 0·05). Neotyphodium lolii also decreased transpiration (P < 0·05), but only in complete darkness. There were no endophyte effects on Pn in the old plants (P > 0·05). E+ plants grew faster immediately after replanting (P < 0·05), but had approx. 10 % lower growth rates during mid-log growth (P < 0·05) than E− plants, but there was no effect on final plant biomass (P > 0·05). The endophyte effects on Pn and growth tended to be more pronounced in Nui UIV, despite having a lower endophyte concentration than Nui D.
• Conclusions Neotyphodium lolii affects CO2 fixation, but not light interception and photochemistry of Pn. The impact of N. lolii on plant growth and photosynthesis is independent of endophyte concentration in the plant, suggesting that the endophyte mycelium is not simply an energy drain to the plant. However, the endophyte effects on Pn and plant growth are strongly dependent on the plant growth phase.
Keywords: Lolium perenne, Neotyphodium lolii, perennial ryegrass, grass endophyte, net photosynthesis, tillering rate, leaf extension rate, plant fitness
INTRODUCTION
Foliar endophytes of grasses of the genus Epichloë (and their anamorphs of the genus Neotyphodium) are fungal symbionts of cool-season grasses (Poaceae; sub-family Pooideae). Endophyte infection has been shown to benefit the host plant through the production of mycotoxins, protecting the grass host from mammalian and insect herbivores (Bush et al., 1997; Clay and Schardl, 2002; Schardl et al., 2004). Endophyte infection has also been shown to modulate growth, morphology, nitrogen assimilation, resource allocation and mineral uptake of the host plant (Latch et al., 1985; De Battista et al., 1990b; Lyons et al., 1990; Belesky and Fedders, 1996; Malinowski and Belesky, 2000; Ahlholm et al., 2002; Pan and Clay, 2002; Cheplick, 2004). However, whether endophyte infection generally benefits plant growth has been the subject of debate. Results of earlier studies had suggested that endophyte infection improves host reproductive fitness by enhancing plant growth (Latch et al., 1985; Belesky et al., 1987; De Battista et al., 1990b). However, recent studies on a wider range of symbiotic associations and environmental conditions revealed much more variable effects of the endophytes on grass performance (Cheplick et al., 1989; Marks et al., 1991; Groppe et al., 1999; Ahlholm et al., 2002; Morse et al., 2002; Cheplick, 2004; Hesse et al., 2004).
How environmental and genetic factors interact with endophyte infection to alter plant growth is still poorly understood. Growth of plant and endophyte is highly synchronized (Tan et al., 2001), and production of reactive oxygen species by a fungal NADPH oxidase plays a key role in maintaining this synchrony as well as apical dominance in the plant, and host control of in planta endophyte concentration (Tanaka et al., 2006). Host genotypes often differ in tissue concentration of the endophyte, commonly assessed by light microscopy as hyphal density in tissues, endophyte-linked β-glucuronidase (GUS) reporter gene activity, quantitative polymerase chain reaction (PCR) of endophyte DNA, and immunological methods (Hiatt and Hill, 1997; Groppe et al., 1999; Tan et al., 2001; Spiering et al., 2005). The endophyte mycelium represents a metabolic sink for carbohydrates, nitrogen and other nutrients to the host plant, and effects due to the energy requirements of the fungal myclium have been implicated in changes of growth of endophyte-infected plants (Ahlholm et al., 2002). However, only very few studies have investigated the effects of host differences in endophyte concentration on plant growth (Groppe et al., 1999), and there are no previous studies of the effect of these differences on photosynthesis. Moreover, end-point measurements of plant biomass have often been used to assess endophyte effects on growth, thus neglecting the effects of the endophytes on the temporal dynamics of plant growth. Changes in growth dynamics due to the endophyte are important to consider, as they provide a more complete picture of the range of endophyte effects on plant growth in a given host–endophyte association.
The physiological basis of the various endophyte effects on plant growth is so far unknown. Endophyte-produced plant hormones, glycosidases and proteases (De Battista et al., 1990a; Lam et al., 1995; Reddy et al., 1996; Yue et al., 2000) may affect metabolic pathways in the plant, and may be responsible for changes in net photosynthesis, stomatal conductance or osmotic adjustment (Belesky et al., 1987; Elmi and West, 1995; Marks and Clay, 1996; Morse et al., 2002; Newman et al., 2003; Monnet et al., 2005). However, similar to plant growth, endophyte effects on these parameters are very variable and have not always been directly correlated with rates of plant growth.
Neotyphodium lolii is symbiotic with the forage grass, perennial ryegrass (Lolium perenne L.) (Christensen et al., 1993). We have recently determined in planta concentration and distribution of the N. lolii strain KS1, a derivative of the strain Lp 19, containing the GUS reporter gene (Tan et al., 2001) as a molecular marker, and N. lolii-produced alkaloids in perennial ryegrass genotypes differing in in planta endophyte concentration (Spiering et al., 2005). Using the same experimental system and two of the host genotypes differing in tissue concentration of the endophyte, we have now tested the effect of N. lolii on rates of net photosynthesis (Pn), transpiration and plant growth.
MATERIALS AND METHODS
Endophyte strains, host genotypes and growth conditions
The host genotypes used, Nui D and Nui UIV, were of the perennial ryegrass (L. perenne L.) ‘Grasslands Nui’; both genotypes were infected with N. lolii [(Latch, Christensen, & Samuels) Glenn, Bacon & Hanlin] strain KS1, a transformant of the strain Lp19 containing a constitutively expressed reporter gene (the Escherichia coli GUS gene) (Tan et al., 2001). Extensive analysis of 16 tissues, including all mature and emerging leaves as well as the true stem, has previously shown that tissue concentrations of KS1, assessed as GUS activity, are approx. 2-fold higher in Nui D than in Nui UIV (Spiering et al., 2005). Plants were generated by transplanting a single grass tiller into 1·4 L pots containing potting mix (AgResearch Grasslands) with Osmocote slow-release fertilizer (Grace Sierra, Australia) and watered with tap water as needed (approx. 2–3 times per week). After 1 month of growth, each plant was supplied weekly with 80 mL of nutrient solution (Thrive, Yates, New Zealand), containing 2 g of nitrogen supplied from nitrate, ammonium and urea, 413 mg phosphate, 663 mg potassium L−1 and trace elements (Co, Mn, Mb, Zn, Cu). The symbioses (and endophyte-free controls, see below) were grown in a controlled environment (Temperzone Ltd, New Zealand). An area of 110 × 120 cm was illuminated with two 1000 W halogen lamps and six 400 W HPI-T lamps (Philips, NY, USA). Light intensity at canopy height was 650 ± 50 μmol photons m−2 s−1 [measured with a quantum sensor (LI-190S, LiCor, Lincoln, NE, USA) and recorded on a solar monitor (LI-1776, Li-Corp)]. A 12/24 h light cycle and 15 ± 2 °C temperature regime was used. Each genotype–treatment combination was grown in three replicates. Each replicate consisted of one plant grown from a single tiller. Plants were regenerated by transplanting single tillers into new pots every 3–4 months, and equally spaced and rearranged weekly at random, to minimize possible effects of environmental shifts across locations within the cabinet.
Measurements of endophyte concentration in grass tissues
Endophyte concentration in host tissues was quantitatively determined by counting aniline blue-stained endophyte hyphae in leaf cross-sections. This method is a reliable and direct measure of the amount of viable endophyte mycelium in grass leaves (Tan et al., 2001; Spiering et al., 2005), because N. lolii hyphae are sparsely branched and oriented mainly in parallel to the leaf axis, and show essentially identical morphology in the two host genotypes examined (M. J. Spiering and J. Schmid, unpubl. res.). Leaf sheath tissues (between 2 and 5 cm in length) were first cleared of leaf pigments and then stained with the fungus-specific stain aniline blue. Hyphae were then counted in cross-sections, cut from either end of a sheath tissue, by light microscopy at × 400 magnification (Tan et al., 2001). All hyphae in a cross-section were counted, and counts were carried out in duplicate; the endophyte concentration in the sheath tissue was determined as the average calculated from the hyphal counts in the two cross-sections.
Generating endophyte-free plants
To obtain endophyte-free, clonal material of Nui D and Nui UIV, the commercially available fungicide Benlate {containing benomyl (methyl[1-butylamino carbonyl]-1H-benzimidazol-2-yl]carbamate, DuPont, Wilmington, DE, USA} was used. Benomyl was chosen as no phytotoxic effects have been detected on perennial ryegrass (Dernoeden and McIntosh, 1991). Tillers were removed from the soil and thoroughly rinsed with water to remove all organic matter attached to the roots. Leaves and roots were trimmed to 2–4 cm length, and tillers were completely submersed in tap water containing 2 g L−1 Benlate and incubated for 6 h at room temperature. Each tiller was then planted into 150 mL pots with 300 g of sand (with Osmocote slow-release fertilizer), containing 40 mL of Benlate solution (200 μg g−1 benomyl), and grown in the greenhouse. After 4–5 weeks of growth, newly emerged tillers were examined for the presence of fungal mycelium by light microscopy (at ×400 magnification) of aniline blue-stained leaf sheath strips. Each endophyte-free tiller was transferred to regular potting mix without fungicide and grown until 3–4 new tillers had emerged. The newly emerged tillers were checked for endophyte infection as before, and no endophyte-infected tillers were observed. The tillers were individually transplanted into new pots as before and transferred into the controlled environment (see above). All plants were grown and regenerated (as described above) in the controlled environment for at least 6 months before the start of experiments. The infection status of each treatment was verified before and after experiments.
Plant growth measurements
The number of tillers in a plant was recorded by manual counting; new tillers were included as soon as they appeared between leaves of a parent tiller. To assess plant dry mass (Md), all aerial tissues were harvested as follows: whole plants were removed from pots and all root material carefully separated from the aerial tissues, which consisted of all leaf and crown tissues. The roots were discarded and all remaining tissues were freeze-dried for 48 h, and immediately weighed. Leaf extension rates (LERs) were determined by marking with a small ink drop the area of the emerging leaf just above the encircling mature leaves; after growth for 24 h, the distance of the ink dot from this point was measured with a ruler. LERs were measured on 4–6 tillers per plant replicate and the values obtained were used to calculate the average LER of a replicate.
Measurements of leaf net photosynthesis and transpiration
Measurements of leaf Pn were performed with a single-chamber open infrared gas-exchange system. A brass metal leaf chamber and experimental set-up for measurements of Pn and transpiration, control of temperature and data recording were used as described by Laing et al. (2002). Water vapour pressure in the leaf chamber was controlled by passing incoming air through a fine bubbler in a refrigerated water bath (LTD6; Grant Instruments, Cambridge, UK), and determined from dew point measurements (Dew Point Hygrometer, 1100DP; General Eastern Instruments Corporation, Watertown, MA, USA). All measurements were made at ambient CO2 (360 μmol mol−1), on the youngest mature leaves in two tillers from a plant replicate that had been light induced for >2 h before the measurements. The vapour pressure deficit (VPD) in experiments ranged from 0·7 to 0·8 kPa. A 50 W tungsten–halogen light source (Philips) situated above the leaf chamber was used to illuminate the leaves, and a fan installed between the chamber and light source was used to dissipate excess heat. A thermistor inserted directly below the leaf was used to measure incident leaf temperature during measurements. Leaf photosynthesis was equilibrated at 20–25 °C leaf temperature for 15–20 min, and a photon flux (PF) of 1900–2000 μmol m−2 s−1. To determine the response of Pn and transpiration to changes in PF, neutral-density filters (Coherent Scientific Pty, Ltd., Unley, Australia) of decreasing light transmittance, calibrated before and after experiments, were successively interposed between the light source and leaf chamber. Leaf temperature was maintained at 20 °C throughout the measurements by adjusting the temperature of air entering the leaf chamber to eliminate temperature changes due to the variation in light intensity. CO2 exchange and transpiration per unit leaf area were calculated from the raw data with the leaf area determined after each measurement.
Data analyses
A P-value <0·05 was considered statistically significant for detecting differences between means or treatment effects. To fit non-linear regression curves to the response of Pn to variation in light intensity, the equation Pn(PF) = Pmax × Tanh (I × α × Pmax−1) − Rd was used (Greer et al., 2004). In this formula, Pmax corresponds to light-saturated maximum Pn, Tanh is the hyperbolic tangent, I the photon flux, α the apparent photon yield and Rd the dark respiration. This function permits very good data fits (average r2 = 0·9936; range: 0·9867–0·9973), and the parameters contain meaningful information relating to the physiological responses of leaf photosynthesis to light intensity (Greer et al., 2004): Pmax is the photosynthetic capacity of the leaf at saturating light intensities where Pn is CO2 limited, α the linear slope of the curve where Pn is light limited, and Rd the rate of respiration at PF = 0.
To determine rates of plant growth (as doubling time), the exponential growth equation T(t) = S × exp(K × t) was fitted to tillering data from plants in exponential growth phase (after 25 d of culture). In this equation, S corresponds to the initial number of tillers, K is the rate constant (used to calculate doubling time = 0·69 × K−1) and t is the time in days. We chose this equation as it gave very reliable fits (average r2 = 0·9934; range: 0·9778–0·9997) and takes into account that growth of forage grasses is exponential (Bélanger and Gastal, 2000). Significant differences in best-fit values of parameters between treatments were tested with an extra-sum-of-squares F-test, testing the null hypothesis that the variance of a model including data from all treatments has a variance that is not significantly different from the variance of models fitted to each of the treatments. The statistical significance of differences between means was tested with two-tailed t-tests, and the significance of treatment effects by two-way analysis of variance (ANOVA). The significance of treatment effects on LERs at different time points was tested by repeated-measures ANOVA. All analyses were performed in GraphPad Prism 4.0c (GraphPad Software, Inc.; http://www.graphpad.com/prism/Prism.htm).
RESULTS
Endophyte infection decreases net photosynthesis at high light intensities, but has no effects on apparent photon yield and dark respiration
Two grass genotypes, Nui D and Nui UIV, both infected with the same N. lolii strain, KS1, were chosen for this study, because results of a previous study (Spiering et al., 2005) had indicated an approx. 2-fold higher concentration of KS1 in Nui D than in Nui UIV. This was confirmed by counting hyphae in the leaf sheaths of plants used in our experiments (389 ± 33 and 208 ± 17 hyphae per leaf cross-section; mean of five replicates ± s.e. in Nui D and Nui UIV, respectively; P < 0·01). As controls, we generated endophyte-free plants (E−) of Nui D and Nui UIV, by eradicating the endophyte through fungicide treatment (see Materials and methods).
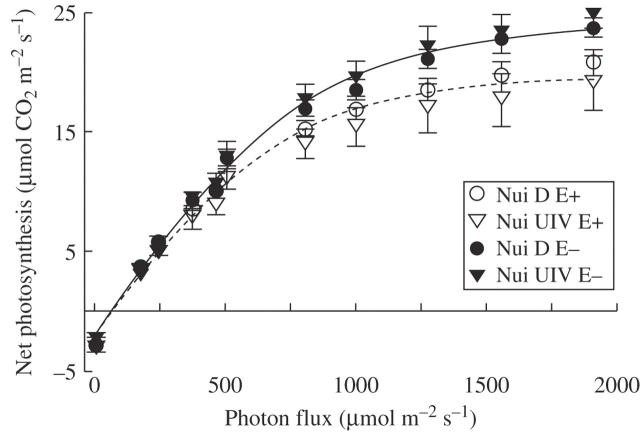
Following growth for 6 months under controlled conditions, we measured Pn and transpiration in leaves of vegetative, 30- to 45-day-old (from last planting) plants, growing in mid-log phase (see below). Pn light–response curves were fitted to the data from each replicate with non-linear regression (r2 in all regressions ≥0·99), to determine maximum Pn (Pmax) and apparent photon yield in each treatment, and rates of dark respiration were also measured (Table 1). No statistically significant effects of plant genotype or genotype × endophyte interactions on the three parameters were detected. E+ plants had significantly lower Pmax than E− plants (Fig. 1 and Table 1), indicating an impact of the endophyte on leaf photosynthesis under saturating light intensities where photosynthesis is CO2 limited (Vogelmann, 2002). In the E+ plants, Pmax was decreased on average by 11 % in Nui D and 23 % in Nui UIV. Similar differences in light responses between E+ and E− plants were also observed by Belesky et al. (1987). However, the apparent photon yield, indicating the photosynthetic efficiency of light interception and conversion processes, and rates of dark respiration were similar in E+ and E− plants, and no statistically significant effect of endophyte infection on these parameters was detected (Table 1).
Table 1.
Maximum net photosynthesis (Pn), apparent photon yield (α) and dark respiration in endophyte-infected (E+) and endophyte-free (E–) plants of Nui D and Nui UIV
| Nui D |
Nui UIV |
|||
|---|---|---|---|---|
| Parameter | E+ | E– | E+ | E– |
| Maximum Pn (μmol CO2 m−2 s−1) | 22·6 ± 1·3 | 25·5 ± 0·7 | 20·8 ± 2·4 | 27·2 ± 0·9 |
| Apparent photon yield [μmol CO2 (μmol photons)−1] | 3·06 ± 0·17 × 10−2 | 3·11 ± 0·17 × 10−2 | 2·97 ± 0·26 × 10−2 | 3·23 ± 0·14 × 10−2 |
| Dark respiration (μmol CO2 m−2 s−1) | 2·87 ± 0·08 | 2·35 ± 0·58 | 2·88 ± 0·22 | 2·63 ± 0·79 |
Data shown are the means of 3–5 replicates ± s.e.; parameters were determined by non-linear regression of the light response of Pn in each replicate, except dark respiration, which was measured directly at PF = 0. ANOVA detected a significant effect of endophyte infection on maximum Pn (P < 0·05); no other significant effects of the treatments and interactions on any of the parameters were detected at α = 0·05.
Fig. 1.
Light response of Pn in endophyte-infected (E+) and endophyte-free (E–) L. perenne plants of Nui D and Nui UIV. All measurements were at a leaf temperature of 20 °C. Least-square regression lines for E+ (broken line) and E– plants (solid line) were fitted to the data from both genotypes (r2 > 0·99 for both regressions on data from E+ and E– plants averaged across the genotypes). Each data point represents the mean of 3–5 replicates ± s.e.
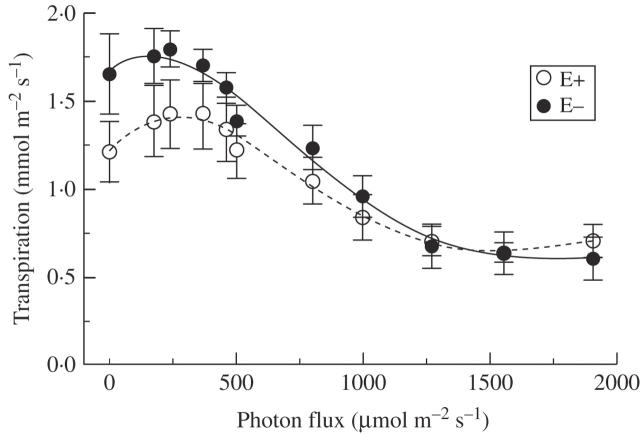
Rates of transpiration gradually increased with decreasing PF, and the curve shapes of the increase and overall rates were similar in both genotypes, with the exception of complete darkness in which Nui UIV had significantly (P < 0·05) higher transpiration than Nui D. No significant correlation between transpiration and Pn was detected by linear regression (P > 0·05). The combined data from the two genotypes, given in Fig. 2, indicated very similar transpiration rates in E+ and E− plants at higher PF (>500 μmol m−2 s−1), while transpiration tended to be lower in E+ plants at lower PF (<500 μmol m−2 s−1). Non-linear regression showed a significant (P < 0·05) difference in the y-intercept, which was 1·22 and 1·67 mmol m−2 min−1 for E+ and E− plants, respectively; therefore, E+ plants had significantly lower rates of transpiration in darkness.
Fig. 2.
Light response of transpiration in E+ and E– L. perenne plants. All measurements were at a leaf temperature of 20 °C. Least-square lines were fitted to the data of E+ (broken line) and E– (solid line) plants from both genotypes (r2 ≥ 0·99 for both regressions on data averaged from both genotypes), using fourth-order polynomial equations; a significant (P < 0·001) difference was detected between the y-intercepts of the E+ and E– curves, but no significant differences between E+ and E– curves were detected for the other terms (P > 0·05). Each data point represents the mean of 5–8 replicates ± s.e.
To see whether age and physiological status of the plant interacted with endophyte infection, rates of Pn were also measured in 90- to 120-day-old E+ and E− plants from both genotypes. Vegetative reproductive growth was strongly impaired in these plants due to restrictions caused by plant size (≥150 tillers per plant) and pot dimensions. Pn rates were 13·6 ± 1·5 and 14·8 ± 1·5 μmol CO2 m−2 s−1 for E+ and E− plants from Nui D, respectively, and 10·3 ± 0·7 and 8·8 ± 0·8 μmol CO2 m−2 s−1 for E+ and E− Nui UIV plants, respectively (mean of five replicates ± s.e.). The rates of Pn in the older, non-growing plants from both genotypes were significantly lower than in the 30- to 45-day-old plants (P < 0·05). Moreover, unlike the younger plants, there was no significant effect of the endophyte on Pn in the non-growing plants (P > 0·05; ANOVA) and the genotypes differed significantly (P < 0·05) in Pn.
Endophyte infection affects rates of tillering and leaf extension, but not final tiller number or plant and tiller dry mass
Given that endophyte infection inhibited photosynthesis in growing plants, we wanted to test if endophyte infection also affected plant growth. E+ and E− plants from both genotypes used in the photosynthesis experiments were grown for 50 d, and final tiller number, plant Md and average tiller Md were determined (Table 2). Plants from both genotypes had similar biomass (Md per plant), but in Nui D this biomass was made up from a larger number of tillers with an average tiller Md lower than that of the Nui UIV tillers. There were no statistically significant differences in any of the parameters between E+ and E− plants. However, this did not provide information about possible endophyte effects on the temporal dynamics of plant growth.
Table 2.
Tiller number per plant, dry mass (Md) of each plant and tiller Md in endophyte-infected (E+) and endophyte-free (E–) plants of Nui D and Nui UIV used in the light response experiment (Fig. 1) after growth for 50 d
| Nui D |
Nui UIV |
|||
|---|---|---|---|---|
| Parameter | E+ | E– | E+ | E– |
| Tiller number | 58·7 ± 14·4 | 75·0 ± 15·3 | 44·5 ± 9·5 | 44·7 ± 9·8 |
| Plant Md (g) | 6·0 ± 1·4 | 7·6 ± 1·5 | 6·6 ± 0·45 | 6·4 ± 1·8 |
| Tiller Md (g) | 0·103 ± 0·001 | 0·101 ± 0·002 | 0·152 ± 0·023 | 0·138 ± 0·009 |
Data shown are the means of three replicates ± s.e. A significant difference in tiller Md was detected between Nui D and Nui UIV (P < 0·01); no other significant differences were detected at α = 0·05.
To determine plant growth rates, we measured the rate with which new tillers appeared and LERs at different time points. Very similar results were obtained for two separate growth experiments; the results of one experiment are shown in Fig. 3. Growth kinetics were very similar in the plants from both genotypes. As seen in the earlier experiment (see above), Nui D plants tended to produce more tillers at each time point than Nui UIV plants—at the last measurement (day 58), Nui D plants had an on average twice as many tillers per plant as Nui UIV (P < 0·001). In both genotypes, the presence of the endophyte induced newly planted tillers to initiate re-growth more quickly: between days 8 and 25, E+ plants had an on average 30 % more tillers than E− plants (P < 0·01; ANOVA on the least-square mean tiller number from days 8, 13, 16 and 25). Once exponential growth had set in (after day 25), however, E− plants produced tillers faster than E+ plants: doubling time, determined by non-linear regression (r2 in all regressions ≥0·98) during log phase, i.e. between days 25 and 58, was 10·49 ± 0·26 and 9·69 ± 0·17 d in E+ and E− plants, respectively, of Nui D, and 13·01 ± 0·74 and 11·57 ± 0·42 d in E+ and E− plants, respectively, of Nui UIV (mean of three replicates ± s.e.). Endophyte infection and plant genotype both had significant effects on doubling time (P < 0·05 and P < 0·01, respectively, ANOVA). On average, the endophyte increased doubling time by 8 % in Nui D and 11 % in Nui UIV.
Fig. 3.
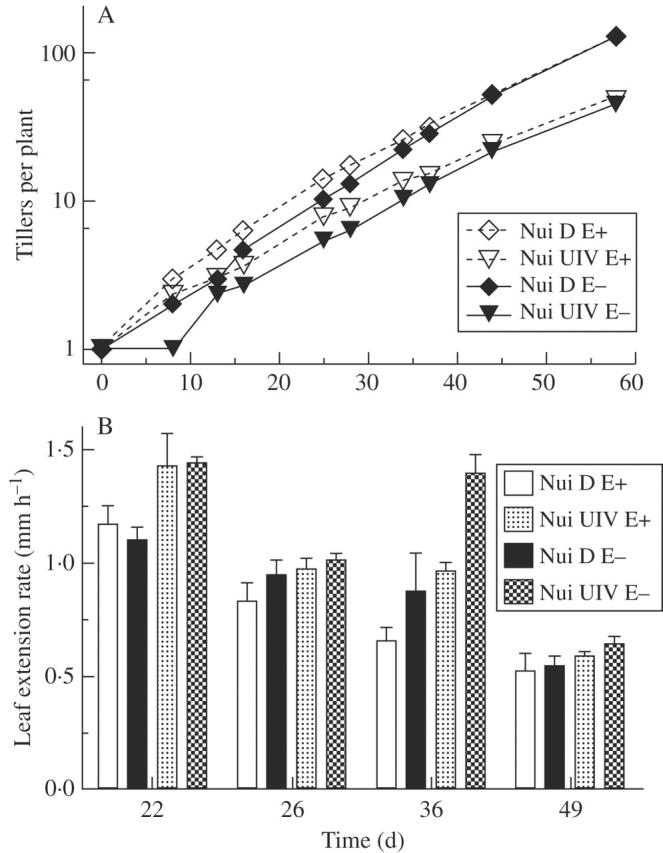
Plant growth and leaf extension rates in endophyte-infected (E+) and endophyte-free (E–) L. perenne plants of Nui D and Nui UIV. The graphs show (A) tiller number in each plant and (B) leaf extension rate (LER) at different times during plant growth. Each data point represents the mean of three replicates ± s.e.; LER was determined from 4–6 tillers in each replicate.
LERs, measured during logarithmic plant growth (Fig. 3), were highest during early log growth, and declined significantly (P < 0·0001; ANOVA) with increasing plant age, and differed significantly (P < 0·05) between the genotypes (least-square means across all time points for Nui D and Nui UIV were 0·84 and 1·05 mm h−1, respectively). Endophyte infection decreased LER at all times in Nui UIV and at all but one time point in Nui D. This effect was most pronounced at day 36, i.e. during early mid-log growth. The average LER decrease in E+ plants was very similar (9–11 %) in both genotypes, but a significant (P = 0·01; ANOVA) difference in LER between E+ and E− plants was detected only for Nui UIV.
DISCUSSION
Neotyphodium lolii significantly decreased CO2-limited leaf Pn and tillering rates in actively growing L. perenne plants. There was no evidence that an increase in endophyte concentration led to stronger effects on Pn and plant growth: the effects of N. lolii on Pn, tillering and LER tended to be more pronounced in Nui UIV plants, which had an approx. 2-fold lower tissue concentration of N. lolii than Nui D. We do not know the reason for this difference, but our results suggest that physiological changes of the plant in response to endophyte infection are independent of in planta endophyte concentration, and that endophyte effects on Pn and plant growth rate are much more strongly influenced by plant growth phase, indicated by a lack of an effect of endophyte infection on Pn in old plants, and also by plant genotype. A very similar decrease in Pn in E+ relative to E− plants has been observed in five genotypes of the related grass, tall fescue (Lolium arundinaceum Darbyshire = Festuca arundinacea Schreb.), in symbiosis with Neotyphodium coenophialum grown under similar environmental conditions (Belesky et al., 1987). This suggests that the decrease in Pn in response to endophyte infection is common under these conditions.
The lack of an effect of in planta endophyte concentration on Pn, similar rates of dark respiration and final biomass in E+ and E− plants, and the observation that endophyte infection both increased (in lag-phase of plant growth) and decreased (in log-phase of plant growth) tillering rates, suggest that these changes are not simply due to the fungal mycelium being an energy drain for the plant as previously suggested (Ahlholm et al., 2002). This is also supported by a recent study investigating the effect of different isolates of N. coenophialum on germination and growth of tall fescue (Belesky and Burner, 2004), and effects of the related endophyte, Epichloë bromicola, on vegetative growth of its host, Bromus erectus (Groppe et al., 1999). As also found in previous studies (Cheplick, 1997, 2004), the endophyte-induced changes in growth dynamics and Pn were subtle compared with the effects of plant genotype and age. This does not preclude the possibility, however, that endophyte effects may become more pronounced in other endophyte–plant associations or environments.
For example, the more rapid lag-phase growth of the E+ plants compared with the E− plants might enable E+ plants to outcompete E− plants in environments with frequent disturbances of grass communities, such as human activity or feeding by animals, causing localized plant death or injury with subsequent vegetative regrowth of grasses into the areas affected by these disturbances. There is indeed increasing evidence that E+ plants can outcompete E− plants in such environments (reviewed by Malinowski and Belesky, 2000; Clay and Schardl, 2002), and the more rapid regrowth of E+ plants might be one possible reason. However, we caution that our findings are based on only two endophyte–host associations grown under one set of environmental conditions, allowing only limited conclusions about how these findings may apply to populations in the field. Nonetheless, while constrained by sample size, our approach examining selected physiological processes coupled with quantitative measurement of the endophyte in the plant has provided a first insight into the role of endophyte concentration for several plant growth parameters that are significant for grass yield and persistence. Similar approaches may be feasible to identify the biochemical basis for the endophyte-induced changes in vegetative growth observed here and in previous studies (Groppe et al., 1999; Ahlholm et al., 2002; Pan and Clay, 2002; Cheplick, 2004; Hesse et al., 2004).
The Pn responses to light suggested that the endophyte had no effect on the light interception and conversion processes of photosynthesis, supported by the lack of a difference between E+ and E− plants in apparent photon yield. In contrast, light-saturated Pn was markedly impaired by the endophyte, with rates 11–23 % lower than in E− plants. Generally, the light-saturated portion of a photosynthetic light–response curve is CO2 limited (Vogelmann, 2002). This suggests that CO2 fixation rather than light interception and photochemistry were affected by the endophyte. For example, endophyte-expressed invertases and glucanases (Lam et al., 1995; Moy et al., 2002) might increase the concentration of monomeric sugars in plant tissues, which has been shown to decrease the activities of photosynthesis enzymes in the Calvin cycle (Scholes et al., 1994). However, amounts of ribulose 1,5 bisphosphate carboxylase/oxygenase (Rubisco), catalysing the first step in the Calvin cycle (Woodrow and Berry, 1988), were unaffected by the endophyte (M. J. Spiering and J. Schmid, unpubl. res.). Neotyphodium lolii could have also affected leaf conductance, regulating CO2 entry into the leaves (Woodrow and Berry, 1988). However, rates of transpiration, which are also affected by stomatal conductance, were very similar in the E+ and E− plants (Fig. 2) under the light intensities giving the most pronounced differences in Pn between E+ and E− plants (Fig. 1), and there was no correlation between rates of Pn and transpiration. Effects of fungal hyphae on CO2 diffusion within the leaf are also unlikely, given the low concentration of N. lolii in leaf tissues [<0·2 % in Nui D (Tan et al., 2001)] and the lack of an effect of genotype differences in endophyte concentration on Pn. Therefore, changes in leaf biochemistry seem to be the most probable explanation for the endophyte-induced changes in Pn, but further experiments would be required to determine the exact mechanisms.
Neotyphodium lolii also decreased tillering rate in log growth and LER. Tillering rate depends on the phyllochron (the time period between the appearance of new leaves), which is correlated with LER (Skinner and Nelson, 1995; Gautier et al., 1999; Fournier et al., 2005). It is unclear whether the endophyte decreased the tillering rate by decreasing the LER, since multiple factors can affect the tillering rate (Gautier et al., 1999), and Nui UIV had higher leaf extension but lower tillering rates than Nui D. However, the endophyte effect on LER was most pronounced in early mid-log growth when the tillering rate began to decrease in E+ plants. Furthermore, the decreases in LER and tillering caused by the endophyte were similar, i.e. both rates were decreased by approx. 10 % lower in E+ compared with E− plants. This suggests that the decreases in LER and tillering rate may be related. Endophytes possess enzymes or cause alterations to plant cells that might affect LERs. For example, endophyte infection affects host nitrogen metabolism by increasing the free amino acid concentration in plant tissues (Lyons et al., 1990) and the elasticity of plant cell walls (White et al., 1992). In addition, endophyte-expressed glycolytic and proteolytic enzymes (Lam et al., 1995; Reddy et al., 1996) may increase in planta carbohydrate and free amino acid concentrations and thereby affect host protein and nitrogen composition. In particular, the concentration of available nitrogen strongly influences the LER of grass leaves, while concentrations of carbohydrates seem to have less of an effect (Volenec and Nelson, 1984). However, in L. perenne, the leaf growth zone is supplied mainly with carbohydrates immediately after photosynthesis (Lattanzi et al., 2005). Given that the light intensities in the controlled environment were high enough to cause differences in Pn between E+ and E− plants, this raises the possibility that the endophyte-induced decrease in Pn might lead to a reduction in carbohydrates supplied to the leaf growth zone, causing the decrease in LER.
The endophyte decreased Pn in young, actively growing plants (30–45 d old), but not in older, non-growing plants (3–4 months old). In earlier studies, plant age has not been considered explicitly as a factor interacting with endophyte infection. This, along with variation among host genotypes, might explain some of the large variability in endophyte effects on Pn found in previous studies (Belesky et al., 1987; Richardson et al., 1993; Marks and Clay, 1996; Amalric et al., 1999; Morse et al., 2002; Newman et al., 2003; Monnet et al., 2005). Plant age also affected the endophyte effects on LER, as differences in LER between E+ and E− plants were most pronounced in young, very actively growing plants. The plant age effect on Pn and LER may also be present among tillers of different age; this was not examined further in this study, but may be important to consider in future studies. Synchronous growth of host and endophyte (Tan et al., 2001) probably requires extensive signalling between the symbionts, especially in very actively growing plants, but perhaps less so in old plants in which growth is stationary. The recent discovery of a signalling pathway for endophyte–host growth synchrony and functional development of the symbiosis (Tanaka et al., 2006), and future discoveries of other pathways for signalling between the symbionts will be invaluable for further studies of symbiont growth strategies under different physiological and environmental conditions.
CONCLUSIONS
Our results have shown that infection by N. lolii decreases net photosynthesis and, depending on growth phase, both increases and decreases growth rates of its host, perennial ryegrass. The endophyte decreased CO2-limited leaf Pn, and rates of tillering and leaf extension in actively growing L. perenne plants, but there were no effects on photosynthesis in old, non-growing plants. Our findings suggest that endophyte effects on photosynthesis and plant growth are not positively correlated with the amount of endophyte mycelium in the plant. To our knowledge, this is the first study that has assessed endophyte effects on photosynthesis along with quantitative measurement of endophyte concentration in its host. Endophyte enzymes and compounds, as well as signalling between the symbionts, might cause some of the observed alterations in photosynthesis and plant growth, but further studies will be required to test these possibilities. The observed strong interaction of endophyte effects with the temporal dynamics of plant growth is significant for future studies. It highlights the need for extensive control, including close monitoring of the plant growth dynamics, use of selected host–endophyte associations and defined environmental conditions, in experiments aimed at identifying the biochemical mechanisms of the physiological changes in the plant caused by the endophyte.
Acknowledgments
We thank Yong Y. Tan and Taha Al-Samarrai for assistance with the plant maintenance, and Michael J. Christensen for help and advice on generating endophyte-free plants. We also thank two anonymous reviewers for their helpful comments on this manuscript. M.J.S. was supported by a Massey University PhD Scholarship. This work was supported in part by the New Zealand Foundation for Research, Science and Technology.
LITERATURE CITED
- Ahlholm JU, Helander M, Lehtimaki S, Wali P, Saikkonen K. 2002. Vertically transmitted fungal endophytes: different responses of host–parasite systems to environmental conditions. Oikos 99: 173–183. [Google Scholar]
- Amalric C, Sallanon H, Monnet F, Hitmi A, Coudret A. 1999. Gas exchange and chlorophyll fluorescence in symbiotic and non-symbiotic ryegrass under water stress. Photosynthetica 37: 107–112. [Google Scholar]
- Bélanger G, Gastal F. 2000. Nitrogen utilization by forage grasses. Canadian Journal of Plant Science 80: 11–20. [Google Scholar]
- Belesky DP, Burner DM. 2004. Germination and seedling development of a tall fescue cultivar in response to native and novel endophyte. In: Kallenbach R, Rosenkrans, C, Lock, TR, eds. Proceedings of the 5th International Symposium on Neotyphodium/grass interactions. Fayetteville, AR, USA, Poster Abstract 305: 1–3.
- Belesky DP, Fedders JM. 1996. Does endophyte influence regrowth of tall fescue? Annals of Botany 78: 499–505. [Google Scholar]
- Belesky DP, Devine OJ, Pallas JE, Stringer WC. 1987. Photosynthetic activity of tall fescue as influenced by a fungal endophyte. Photosynthetica 21: 82–87. [Google Scholar]
- Bush LP, Wilkinson HH, Schardl CL. 1997. Bioprotective alkaloids of grass–fungal endophyte symbioses. Plant Physiology 114: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheplick GP. 1997. Effects of endophytic fungi on the phenotypic plasticity of Lolium perenne (Poaceae). American Journal of Botany 84: 34–40. [Google Scholar]
- Cheplick GP. 2004. Recovery from drought stress in Lolium perenne (Poaceae): are fungal endophytes detrimental? American Journal of Botany 91: 1960–1968. [DOI] [PubMed] [Google Scholar]
- Cheplick GP, Clay K, Marks S. 1989. Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytologist 111: 89–97. [Google Scholar]
- Christensen MJ, Leuchtmann A, Rowan DD, Tapper BA. 1993. Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis) and perennial ryegrass (Lolium perenne). Mycological Research 97: 1083–1092. [Google Scholar]
- Clay K, Schardl C. 2002. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. American Naturalist 160: S99–S127. [DOI] [PubMed] [Google Scholar]
- De Battista JP, Bacon CW, Severson R, Plattner RD, Bouton JH. 1990a. Indole acetic acid production by the fungal endophyte of tall fescue. Agronomy Journal 82: 878–880.
- De Battista JP, Bouton JH, Bacon CW, Siegel MR. 1990b. Rhizome and herbage production of endophyte removed tall fescue clones and populations. Agronomy Journal 82: 651–654. [Google Scholar]
- Dernoeden PH, McIntosh MS. 1991. Seasonal responses of perennial ryegrass as influenced by fungicides. Hortscience 26: 1181–1183. [Google Scholar]
- Elmi AA, West CP. 1995. Endophyte infection effects on stomatal conductance, osmotic adjustment and drought recovery of tall fescue. New Phytologist 131: 61–67. [DOI] [PubMed] [Google Scholar]
- Fournier C, Durand JL, Ljutovac S, Schäufele R, Gastal F, Andrieu B. 2005. A functional–structural model of elongation of the grass leaf and its relationships with the phyllochron. New Phytologist 166: 881–894. [DOI] [PubMed] [Google Scholar]
- Gautier H, Varlet-Grancher C, Hazard L. 1999. Tillering responses to the light environment and to defoliation in populations of perennial ryegrass (Lolium perenne L.) selected for contrasting leaf length. Annals of Botany 83: 423–429. [Google Scholar]
- Greer DH, Seleznyova AN, Green SR. 2004. From controlled environments to field simulations: leaf area dynamics and photosynthesis of kiwifruit vines (Actinidia deliciosa). Functional Plant Biology 31: 169–179. [DOI] [PubMed] [Google Scholar]
- Groppe K, Steinger T, Sanders I, Schmid B, Wiemken A, Boller T. 1999. Interaction between the endophytic fungus Epichloë bromicola and the grass Bromus erectus: effects of endophyte infection, fungal concentration and environment on grass growth and flowering. Molecular Ecology 8: 1827–1835. [DOI] [PubMed] [Google Scholar]
- Hesse U, Hahn H, Andreeva K, Förster K, Warnstorff K, Schöberlein W, Diepenbrock W. 2004. Investigations on the influence of Neotyphodium endophytes on plant growth and seed yield of Lolium perenne genotypes. Crop Science 44: 1689–1695. [Google Scholar]
- Hiatt EE, Hill NS. 1997. Neotyphodium coenophialum myclial protein and herbage mass effects on ergot alkaloid concentration in tall fescue. Journal of Chemical Ecology 23: 2721–2736. [Google Scholar]
- Laing WA, Greer DH, Campbell BD. 2002. Strong responses of growth and photosynthesis of five C3 pasture species to elevated CO2 at low temperature. Functional Plant Biology 29: 1089–1096. [DOI] [PubMed] [Google Scholar]
- Lam CK, Belanger FC, White JF, Daie J. 1995. Invertase activity in Epichloë/Acremonium fungal endophytes and its possible role in choke disease. Mycological Research 99: 867–873. [Google Scholar]
- Latch GCM, Hunt WF, Musgrave DR. 1985. Endophytic fungi affect growth of perennial ryegrass. New Zealand Journal of Agricultural Research 28: 165–168. [Google Scholar]
- Lattanzi FA, Schnyder H, Thornton B. 2005. The sources of carbon and nitrogen supplying leaf growth. Assessment of the role of stores with compartmental models. Plant Physiology 137: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons PC, Evans JJ, Bacon CW. 1990. Effects of the fungal endophyte Acremonium coenophialum on nitrogen accumulation and metabolism in tall fescue. Plant Physiology 92: 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski DP, Belesky DP. 2000. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science 40: 923–940. [Google Scholar]
- Marks S, Clay K. 1996. Physiological responses of Festuca arundinacea to fungal endophyte infection. New Phytologist 133: 727–733. [Google Scholar]
- Marks S, Clay K, Cheplick GP. 1991. Effects of fungal endophytes on interspecific and intraspecific competition in the grasses Festuca arundinacea and Lolium perenne. Journal of Applied Ecology 28: 194–204. [Google Scholar]
- Monnet F, Vaillant N, Hitmi A, Sallanon H. 2005. Photosynthetic activity of Lolium perenne as a function of endophyte status and zinc nutrition. Functional Plant Biology 32: 131–139. [DOI] [PubMed] [Google Scholar]
- Morse LJ, Day TA, Faeth SH. 2002. Effect of Neotyphodium endophyte infection on growth and leaf gas exchange of Arizona fescue under contrasting water availability regimes. Environmental and Experimental Botany 48: 257–268. [Google Scholar]
- Moy M, Li HJM, Sullivan R, White JF, Belanger FC. 2002. Endophytic fungal β-1,6-glucanase expression in the infected host grass. Plant Physiology 130: 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JA, Abner ML, Dado RG, Gibson DJ, Brookings A, Parsons AJ. 2003. Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: growth, photosynthesis, chemical composition and digestibility. Global Change Biology 9: 425–437. [Google Scholar]
- Pan JJ, Clay K. 2002. Infection by the systemic fungus Epichloë glyceriae and clonal growth of its host grass Glyceria striata. Oikos 98: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PV, Lam CK, Belanger FC. 1996. Mutualistic fungal endophytes express a proteinase that is homologous to proteases suspected to be important in fungal pathogenicity. Plant Physiology 111: 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MD, Hoveland CS, Bacon CW. 1993. Photosynthesis and stomatal conductance of symbiotic and nonsymbiotic tall fescue. Crop Science 33: 145–149. [Google Scholar]
- Schardl CL, Leuchtmann A, Spiering MJ. 2004. Symbioses of grasses with seedborne fungal endophytes. Annual Review of Plant Biology 55: 315–340. [DOI] [PubMed] [Google Scholar]
- Scholes JD, Lee PJ, Horton P, Lewis DH. 1994. Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytologist 126: 213–222. [Google Scholar]
- Skinner RH, Nelson CJ. 1995. Elongation of the grass leaf and its relationship to the phyllochron. Crop Science 35: 4–10. [Google Scholar]
- Spiering MJ, Lane GA, Christensen MJ, Schmid J. 2005. Distribution of the fungal endophyte Neotyphodium lolii is not a major determinant of the distribution of fungal alkaloids in Lolium perenne plants. Phytochemistry 66: 195–202. [DOI] [PubMed] [Google Scholar]
- Tan YY, Spiering MJ, Scott V, Lane GA, Christensen MJ, Schmid J. 2001. In planta regulation of extension of an endophytic fungus and maintenance of high metabolic rates in its mycelium in the absence of apical extension. Applied and Environmental Microbiology 67: 5377–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Christensen MJ, Takemoto D, Park P, Scott, B. 2006. Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. Plant Cell 18: 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann TC. 2002. Photosynthesis: physiological and ecological considerations. In: Taiz L, Zeiger E, eds. Plant physiology. Sunderland, MA: Sinauer Publishers, 171–192.
- Volenec JJ, Nelson CJ. 1984. Carbohydrate metabolism in leaf meristems of tall fescue II. Relationship to leaf elongation rates modified by nitrogen fertilization. Plant Physiology 74: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RH, Engelke MC, Morton SJ, Johnson-Cicalese JM, Ruemmele BA. 1992. Acremonium endophyte effects on tall fescue drought tolerance. Crop Science 32: 1392–1396. [Google Scholar]
- Woodrow IE, Berry JA. 1988. Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annual Reviews of Plant Physiology and Plant Molecular Biology 39: 533–594. [Google Scholar]
- Yue Q, Miller CJ, White J.F, Richardson MD. 2000. Isolation and characterization of fungal inhibitors from Epichloë festucae. Journal of Agricultural and Food Chemistry 48: 4687–4692. [DOI] [PubMed] [Google Scholar]