Abstract
• Background and Aims Heterodichogamy differs from normal dichogamy, in that it involves two mating types (protogyny and protandry) that occur at a 1 : 1 ratio in a population. Flowering phases of the two mating types are synchronized and reciprocal, which was considered to ensure between-type outcrossing. This study aims to quantify the flowering pattern and pollination efficacy in Juglans mandshurica, a wind-pollinated heterodichogamous tree.
• Methods The pattern of flowering phenology was monitored within individual trees and pollen traps were used to measure air-borne pollen loads during the spring in 2003 and 2004. Pollen longevity was determined by staining technique. Also a pollen supplementation experiment was performed in 2004 to assess pollen limitation of fruit production.
• Key Results There was no overlap between sexual functions within individual trees. Flowering periods of the two mating types were reciprocal and synchronous in both 2003 and 2004. Air-borne pollen loads were large, and protogynous and protandrous individuals each produced a high pollination peak, consistent with the two blooming periods. Maximum pollen longevity was about 4 h for protandrous individuals, and 3 h for protogynous individuals. Pollen supplementation did not increase fruit production in either protogynous or protandrous individuals.
• Conclusions Heterodichogamous flowering in Juglans mandshurica effectively avoids selfing, promotes between-type outcrossing, and leads to efficient pollination in a natural population.
Keywords: Heterodichogamy, Juglans mandshurica, protandry, protogyny, pollination efficacy
INTRODUCTION
Dichogamy refers to differences in the timing of female and male functions at the flower, inflorescence or plant level. In general, it includes two types of flowering system: protogyny (female function before male) and protandry (male function before female). Both systems are widespread among flowering plants, with several entire families characterized by one system or the other (Knuth, 1906). Notably, some species exhibit a phenotypic dimorphism, in which protogynous and protandrous individuals co-occur in one population, typically at a ratio of 1 : 1 (Renner, 2001). This system was denominated by Errera and Gevaert (1878) as ‘heterodichogamy’, and has been documented in a few genera of flowering plants such as Corylus (Muller, 1875), Juglans (Delpino, 1874; Knuth, 1906; Stout, 1928; Wood, 1934; Gleeson, 1982; Kimura et al., 2003), Carya (Thompson and Romberg, 1985; McCarthy and Quinn, 1990), Acer (Gabriel, 1968; Asai, 2000; Sato, 2002), Grayia (Pendleton et al., 1988), Thymelaea (Dommee et al., 1990), Alpinia (Li et al., 2001) and Hernandia (Endress and Lorence, 2004). In heterodichogamous species, flowering phases of the two mating types are synchronous and reciprocal, so their mating patterns have been characterized as disassortative mating (Gleeson, 1982; Pendleton et al., 1988; Dommee et al., 1990).
Darwin's explanation of heterodichogamy as an outcrossing mechanism has become widely accepted since he first formulated it (Darwin, 1889). Dichogamy, or the temporal separation in male and female flowering within an individual, is sufficient to reduce selfing, but insufficient to avoid inbreeding through sib-mating. Furthermore, a population of only a single mating type might face some problems in successful fertilization (Gleeson, 1982). In the extreme, if the entire population completely separates its male and female flowering phases in the same sequence simultaneously, no fertilization occurs (Proctor and Yeo, 1972). It can be inferred that very early and/or late individuals in dichogamous populations would suffer reduced reproductive success, and selection acting both to reduce selfing by increasing within individual separation and to reduce between individual variation in flowering time, could lead to reduced fertilization in the population as a whole. On the other hand, heterodichogamy circumvents this fertilization problem (and inbreeding as well) by enabling each mating type to utilize both the pollen and receptive ovules of the reciprocal type. Therefore, heterodichogamous species are expected to show both little within-individual overlap in male and female flowering and high pollination efficacy (i.e. seed production is less likely to be pollen-limited). However, there have been few empirical tests of pollen limitation in heterodichogamous plants; the only exception is McCarthy and Quinn (1990).
In this paper, the flowering and pollination patterns of Juglans mandshurica, a widespread wind-pollinated heterodichogamous species in northern China, are examined. The objectives of the study are: (a) to describe the flowering pattern in terms of both flowering phenology and pollen fluxes; (b) to evaluate the pollination efficacy for the two mating types by measuring the air-borne pollen loads and performing a pollen supplementation experiment.
MATERIALS AND METHODS
Study species
Juglans mandshurica Maxim. is a monoecious, wind-pollinated and deciduous tree, mainly distributed in northern and north-eastern China, and often located along a brook. Trees are about 10–20 m tall and commence flowering in early spring (April/May). Staminate inflorescences (catkins) are pendulous, 9–20 cm long, and have 200–300 male flowers; pistillate inflorescences (spikelets) are erect, 3–5 cm long, and typically consist of 10–18 flowers arranged spirally on the floral axis. Flowers mature acropetally. The gynoecium has a single ovule and two feathery stigmas. Protogynous and protandrous trees are randomly distributed in a population. It has been suggested that Juglandaceae are entirely self-compatible (Thompson and Romberg, 1985; Renner, 2001).
Study sites
The present study was conducted in the Dongling Mountain, 100 km west of Beijing, China (39° 58′N, 115° 26′E; 1120 m a.s.l.). This region is dominated by mountain brown earth, with a temperate continental monsoon climate. The mean annual temperature is 4·8 °C (January –10·1 ° C and July 18·3 ° C). Annual precipitation is 612 mm year−1, 78 % of which occurs in June–August (Li and Chen, 1999). At this site, a riverside population was selected, in which J. mandshurica was dominant in the canopy layer. The shrub layer included Spiraea pubescens, Deutzia parviflora and Rhamnus parvifolia.
Flowering phenology
A thorough investigation was performed on the sex expression of each of the flowering individuals studied in 2002. In this study, mating types were classified into protogyny and protandry. For each tree, inter-annual changes in the mating types were evaluated by a 3-year consecutive observation (from 2002 to 2004). The dates of onset and termination of male- and female-flowering were recorded for 24 trees (14 protogynous and 10 protandrous) and 25 trees (13 protogynous and 12 protandrous) in 2003 and 2004, respectively. Two to four female inflorescences and two or three branches with male catkins were tagged in each tree. The flowering phenology was observed every morning from 3 to 31 May 2003 and 23 April to 23 May 2004. The period of female flowering was determined by the colour of the stigmas, namely red to dark red. The period of male flowering was determined as that from the date of pollen shedding to the date when pollen was completely shed for the individuals monitored.
Air-borne pollen loads
To explore the efficacy of wind pollination in this species, pollen traps were used to measure air-borne pollen loads during the spring in 2003 and 2004. The trapping sites were located on the branches 2 m above ground. A glass microscope slide covered with a sticky tape (1 cm × 8 cm) was placed on each of 20 selected sites. Traps were placed in the field at the beginning of flowering and collected every 24 h. Pollen grains adhering to the sticky surface were easily identified and counted under a microscope at × 50 magnification. The mean, with standard error, of pollen grain numbers was computed over 20 traps each day. The measurement lasted from 10 May to 2 June 2003 and from 24 April to 29 May 2004. The measurement in 2003 was taken 1 week later than the start of flowering.
Pollen longevity
Pollen longevity was determined at the two pollination peaks for protandrous and protogynous trees by the MTT test method (Rodriguez-Riano and Dafni, 2000). Ten male catkins in each of five, either protandrous or protogynous, individuals were selected, and pollen was collected when about half of male flowers in the sampled catkins had their anthers dehisced. All the catkins from each mating type were shaken by hand to shed the pollen grains to a mixed general sample. Using a teasing needle, a small amount of pollen was put into a 5- to 10-μL droplet of stain [100 mg 2,5-diphenyl monotetrazolium (MTT) dissolved in 5 mL of 5 % sucrose] on the microscope slide. Then ten fields were randomly chosen by microscope, the number of stained (viable) and unstained (unviable) pollen grains counted every 15 or 20 min until all the pollen grains were unviable. The pollen grain was considered viable if it turned deep pink. The mean and standard error of the percentage of the dyed pollen grains were computed over ten fields.
Pollen supplementation
Pollen limitation of fruit production was assessed through a pollen supplementation experiment in spring 2004. Because each tree produces many hundreds of female flowers, it was not possible to subject entire plants to experimental treatments. Instead, two branches of similar size and canopy position on each of seven protogynous trees and ten protandrous trees were selected. Then one branch was assigned randomly to pollen supplementation treatment, with the other as control. All selected branches had 50–100 female flowers and were open to natural pollination. The branches subject to pollen supplementation also had all their flowers hand-pollinated with mixed outcross-pollen from several nearby trees. The number of flowers and the number of matured fruits were counted. A paired t-test is used to assess the treatment effect on the proportion of flowers producing mature seeds, executed by SPSS 10·0.
RESULTS
Flowering phenology
Protogynous and protandrous mating types occurred in roughly equal proportions at the study site, as observed from 2002 to 2004. A total of 133 protogynous and 138 protandrous flowering trees were observed in 2002, which were randomly dispersed both at the study site and with respect to each other. Unisexual mating types (male or female) were not detected and the mating type of individual plants was consistent from year to year.
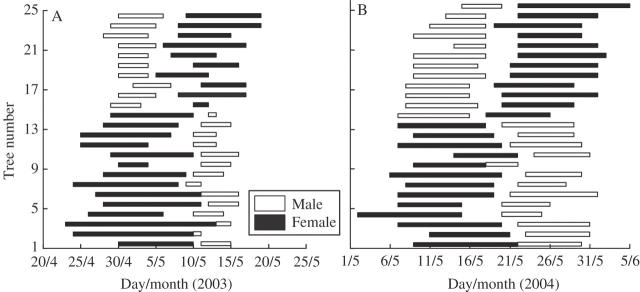
The stigma of female flowers turned dark red and wilted after 6–7 d (protandry) or 7–9 d (protogyny) from opening, while the pollen shedding of male catkins lasted only 1–2 d. For all the plants sampled, there was no overlap of sexual functions. More than 1 d separation of male and female flowering times was observed in 21 out of 24 trees in 2003, and 23 out of 25 in 2004 (Fig. 1). Exceptions were all protogynous trees (nos 2, 3 and 6 in 2003 and 1 and 9 in 2004).
Fig. 1.
Flowering period of female and male inflorescence for 24 and 25 individuals of Juglans mandshurica at a site in Dongling Mountain, Beijing, in 2003 (A) and 2004 (B), respectively.
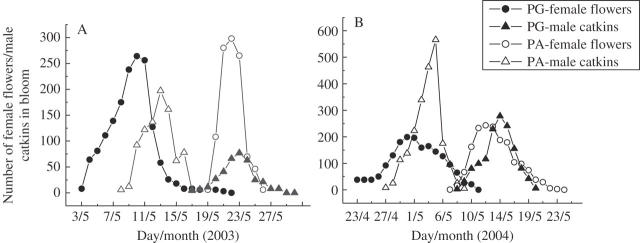
Flowering peak dates were 13 May (male) and 22 May (female) for the protandrous type and 10 May (female) and 23 May (male) for the protogynous type in 2003; 4 May and 14 May for the protandrous type and 30 April and 12 May for the protogynous type in 2004. Figure 2 illustrates the reciprocity and synchronization of the flowering periods of the two mating types. The dates of female flowering peaks were consistently earlier than those of corresponding male flowering peaks for both flowering periods in both years.
Fig. 2.
Number of female flowers and male catkins in bloom for each mating type for each day in 2003 (A) and 2004 (B). PG, protogynous; PA, protandrous.
Pollen loads and pollen longevity
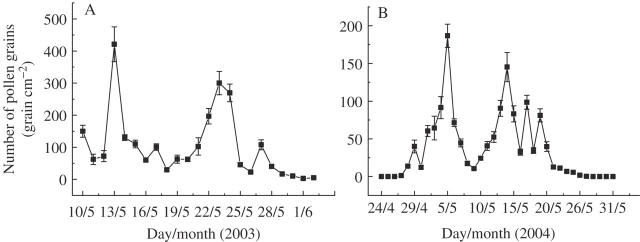
Jugulans mandshurica pollen was present in the atmosphere between April and June and traps collected large numbers of air-borne pollen grains (Fig. 3). Peak count dates in 2003 were 13 May (420 grains cm−2) and 23 May (300 grains cm−2). In 2004, the dates were 5 May (186 grains cm−2) and 14 May (145 grains cm−2). Protandrous and protogynous individuals each produced a peak of pollen quantity in the air (Fig. 3), consistent with their respective male flowering peaks.
Fig. 3.
Average number of pollen grains collected from 20 traps for each day of the flowering period in 2003 (A) and 2004 (B).
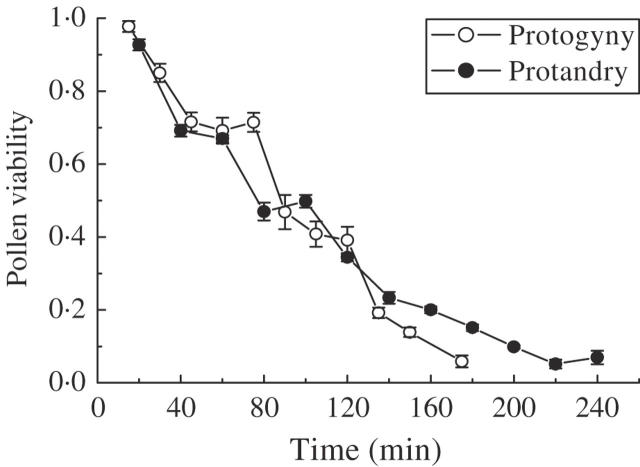
Pollen viability decreased gradually with time for the two mating types and the trend was very similar (Fig. 4). Approximately half of the pollen lost its viability after 90 min in protogynous trees and 100 min in protandrous trees; only <5 % appeared to be viable after 175 min in protogynous trees and 220 min in protandrous trees (Fig. 4). Pollen longevity of protandrous individuals was about 4 h, and that of protogynous individuals was 3 h.
Fig. 4.
Pollen longevity of protandrous and protogynous individuals as measured by percentage viability in 2003.
Pollen limitation
No evidence was found for pollen-limited fruit production in J. mandshurica of either protandrous or protogynous mating type. In protandrous plants, fruit set of pollen-supplemented flowers (0.41 ± 0.08, mean ± s.e.) was higher than control (0.27 ± 0.06), but this was not significant (pair-wise t test: T = –1.776; d.f. = 9; P = 0·110). In protogynous plants, pollen supplementation had a negligible effect on the fruit set (0·20 ± 0·08 versus 0·20 ± 0·07; T = 0·075; d.f. = 6; P = 0·942).
DISCUSSION
Selfing avoidance within individual trees
Temporal separation of the individual's male and female functions was complete in the population of J. mandshurica studied, with a separation of at least 1 d being observed for all sampled protandrous plants and most protogynous plants (Fig. 1). While geitonogamy (fertilization by other flowers on the same plant) appears highly unlikely for the protandrous mating type, self-pollination may be possible in some protogynous plants if their stigmas remained receptive when their own pollen began to be released. A short period of overlapping in protogynous individuals was also observed for other heterodichogamous species (Pendleton et al., 1988; Sato, 2002; Kimura et al., 2003). But selfing can occur only when outcross pollen is limited, which is apparently not the case in this study. Therefore, the mating system of J. mandshurica can be characterized as predominantly outcrossing (Gleeson, 1982). Rink et al. (1994) studied allozyme variation in a natural population and two seed orchards of black walnut and obtained multi-locus outcrossing rate estimates of 0·880 to 0·928. Therefore, heterodichogamy in walnuts may represent an effective way of reducing self-pollination while still retaining high pollination efficacy.
Between-type outcrossing within a population
The sexual functions of the two mating types (protogyny and protandry) are synchronous and reciprocal within a population in view of flowering phenology (Fig. 2) or air-borne pollen loads (Fig. 3), consistent with the flowering behaviour observed for J. regia (Knuth, 1906), J. hindsii (Gleeson, 1982) and J. ailanthifolia (Kimura et al., 2003). Potential mating probabilities were substantially higher between the two mating types than within each mating type. In Grayia brandegei, Pendleton et al. (1988) estimated the frequency of between-type matings at 95·1 % for the protandrous type and 85·8 % for the protogynous type. Here, just a rough description of the mating pattern from flowering phenology is given, and the exact frequency of between-type or within-type matings could be determined by paternity analysis in the future.
In a 3-year study focused on the sex expression of J. ailanthifolia, Kimura et al. (2003) found not only monoecious mating types (protogyny and protandry) but also unisexual mating types (female and male) in two natural populations. Since the changes in mating category occurred mainly from unisexual to monoecious types and inverse changes were very few, Kimura et al. (2003) believed that unisexuality is a temporary trait expressed only when small or under conditions of limited environmental resources. Although unisexual mating types (male or female) were not observed in the present study population and the sex expression of the monitored plants was invariant from year to year, a few very young trees bearing only male catkins were noticed in the surrounding areas of the study site.
The dates of female flowering peaks were earlier than those of male flowering peaks for the two flowering periods in both 2003 and 2004. The reason may be that if the male flowering peak was earlier than the female, most pollen released from the earlier flowers would not reach a stigma and the plant may suffer a loss in reproductive success through male function. On the contrary, if female flowers remain unfertilized, unfertilized stigmas continue to grow and may remain receptive for a few days. In addition, weather conditions may affect the degree of reciprocity and synchronization of flowering periods of the two mating types, especially for wind pollinated species. In the present case, a series of rainstorms in 2003 temporarily delayed flowering, particularly the male function.
Pollination efficacy
Pollen supplementation did not increase fruit production. This result, along with the large number of pollen grains collected by pollen traps (Fig. 3), indicates that J. mandshurica is not pollen-limited. Although a pollen supplementation experiment was conducted only in 2004, the air-borne pollen loads in 2003 were two times higher than that in 2004 (Fig. 3), implying even less likelihood of pollen limitation.
Recent reviews (e.g. Knight et al., 2005) suggest that pollen limitation is very common among flowering plants. There is also experimental evidence that reproduction in populations of wind-pollinated trees can at times be pollen-limited (Nilsson and Wastljung, 1987; Allison, 1990; Perry and Knowles, 1990; Holm, 1994; Knapp, 2001; Satake and Iwasa, 2002; Sork, 2002). Gleeson (1982) suggested that heterodichogamy has an advantage over dichogamy in effective pollination. Preliminary studies of J. mandshurica seem to be consistent with Gleeson's (1982) expectation that heterodichogamy results in relatively high pollination efficacy. Until now there have been very few studies on pollination efficacy of heterodichogamous species. As far as is known, only McCarthy and Quinn (1990) performed pollen supplementation experiments for heterodichogamous Carya ovata and C. tomentosa, and they also found that pollen was not limiting fruit set of either species. In a recent study of the genetic structure of black walnut, Victory et al. (2006) reported high levels of gene flow, which provided some indirect evidence for high pollination efficacy. Clearly, more studies of heterodichogamous species are needed to confirm the insight of Gleeson (1982).
Experimental studies of pollen limitation have been criticized for frequently being based on supplementally pollinating a relatively small number of flowers in some inflorescences and comparing fruit production from these flowers with production from flowers that did not receive supplemental pollen. The argument (Webb, 1984; Zimmerman and Pyke, 1988) is that inflorescences receiving supplemental pollen may have higher fruit production due to reallocation of resources from other parts of the plant to the experimental inflorescences, which, because they have initiated more fruits, are a stronger resource sink. Consequently, greater fruit production in experimental inflorescences may be compensated for by reduced fruit production throughout the remainder of the plant, with no change in fruit production at the whole-plant level. Despite the potential problems, well-designed experiments have conclusively demonstrated pollen limitation in many species (Burd, 1994). Branches with relatively large numbers of flowers per branch were used as experimental units and supplementally pollinated all flowers on the branches; tree branches are at least partially autonomous (Sprugel et al., 1991). Most importantly, although resource reallocation may result in false support for pollen limitation, it cannot yield false support for what we found in the natural population of J. mandshurica, the absence of pollen limitation. Furthermore, pollen traps in the present study collected large numbers of air-borne pollen grains, suggesting highly successful pollination of female flowers.
It was noted that the fruit set of pollen-supplemented flowers was higher than in the control in protandrous plants, although non-significantly, while it was nearly identical in protogynous plants. Whitehead (1983) pointed out that atmospheric conditions influence pollen release, which usually occurs under dry conditions and before leafing. Pollen release in the first flowering period of J. mandshurica was before leafing, while after leafing in the second flowering period. In addition, pollen longevity of protandrous individuals (4 h) was longer than that of protogynous individuals (3 h). Desiccation risks are considered a main factor affecting pollen longevity (Dafni and Firmage, 2000). With temperature increasing, pollen grains were more vulnerable to desiccation. So pollination conditions for J. mandshurica in the first flowering period was better than in the second flowering period.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30125008) and Beijing Normal University.
LITERATURE CITED
- Allison TD. 1990. The influence of deer browsing on the reproductive biology of Canada yew (Taxus canadensis Marsh.). II. Pollen limitation: an indirect effect. Oecologia 83: 530–534. [DOI] [PubMed] [Google Scholar]
- Asai T. 2000. Dichogamy in fullmoon maple (Acer japonicum Thunb.). Bulletin of the Hokaido Forestry Research Institute 37: 27–40. [Google Scholar]
- Burd M. 1994. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review 60: 83–139. [Google Scholar]
- Dafni A, Firmage D. 2000. Pollen viability and longevity: practical, ecological and evolutionary implications. Plant Systematics and Evolution 222: 113–132. [Google Scholar]
- Darwin C. 1889. Different forms of flowers on plants of the same species. New York, NY: D. Appleton and Co.
- Delpino F. 1874. Ulteriori osservazioni e considerazioni sulla dicogamia nel regno vegetale. Appendice. Dimorfismo nel noce (Juglans regia) e pleiontismo nelle piante. Atti della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale (Milan) 17: 402–407.
- Dommee B, Bompar J-L, Dnelle N. 1990. Sexual tetramorphism in Thymelaea hirsuta (Thymelaeaceae): evidence of the pathway from heterodichogamy to dioecy at the infraspecific level. American Journal of Botany 77: 1449–1462. [Google Scholar]
- Endress PK, Lorence DH. 2004. Heterodichogamy of a novel type in Hernandia (Hernandiaceae) and its structural basis. International Journal of Plant Sciences 165: 753–763. [Google Scholar]
- Errera L, Gevaert G. 1878. Sur la structure et les mode de fecondation des fleurs. Societe Royale de Botanique de Belgique, 17: 38–181. [Google Scholar]
- Gabriel WJ. 1968. Dichogamy in Acer saccharum. Botanical Gazette 129: 334–338. [Google Scholar]
- Gleeson SK. 1982. Heterodichogamy in walnuts: inheritance and stable ratios. Evolution 36: 892–902. [DOI] [PubMed] [Google Scholar]
- Holm S. 1994. Reproductive variability and pollen limitation in three Betula taxa in nothern Sweden. Ecography 17: 73–81. [Google Scholar]
- Kimura M, Seiwa K, Suyama Y, Ueno N. 2003. Flowering system of heterodichogamous Juglans ailanthifolia. Plant Species Biology 18: 75–84. [Google Scholar]
- Knapp EE. 2001. Pollen-limited reproduction in blue oak: implications for wind pollination in fragmented populations. Oecologia 128: 48–55. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, et al. 2005. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution and Systematics 36: 467–497. [Google Scholar]
- Knuth P. 1906. Handbook of flower pollination (English translation), 3 volumes. London: Clarendon Press.
- Li HT, Chen LZ. 1999. Study on the microclimate in the mountain forest in the warm temperate zone. Acta Phytoecologica Sinica 23: 139–147. [Google Scholar]
- Li Q-J, Xu Z-F, Kress W, Xia Y-M, Zhang L, Deng X-B, et al. 2001. Flexible style that encourages outcrossing. Nature 410: 432. [DOI] [PubMed] [Google Scholar]
- McCarthy BC, Quinn JA. 1990. Reproductive ecology of system of two sympatric tree species. American Journal of Botany 77: 261–273. [DOI] [PubMed] [Google Scholar]
- Muller H. 1875. Flowering of the hazel. Nature 12: 26 [Google Scholar]
- Nilsson SG, Wastljung U. 1987. Seed predation and cross-pollination in mast-seeding beech (Fagus sylvatica) patches. Ecology 68: 260–265. [Google Scholar]
- Pendleton RL, McArthur ED, Freeman DC, Blauer AC. 1988. Heterodichogamy in Grayia brandegei (Chenopodiaceae): report from a new family. American Journal of Botany 75: 267–274. [Google Scholar]
- Perry DJ, Knowles P. 1990. Evidence of high self-fertilization in natural populations of eastern white cedar (Thuja occidentalis). Canadian Journal of Botany 68: 663–668. [Google Scholar]
- Proctor M, Yeo P. 1972. The pollination of flowers. New York, NY: Taplinger.
- Renner SS. 2001. How common is heterodichogamy? Trends in Ecology and Evolution 11: 595–597. [Google Scholar]
- Rink G, Zhang G, Jinghua Z, Kung FH, Carroll ER. 1994. Mating parameters in Juglans nigra L. seed orchard similar to natural population estimates. Silvae Genetica 43: 261–263. [Google Scholar]
- Rodriguez-Riano T, Dafni A. 2000. A new procedure to assess pollen viability. Sexual Plant Reproduction 12: 241–244. [Google Scholar]
- Satake A, Iwasa Y. 2002. Spatially limited pollen exchange and a long-range synchronization of trees. Ecology 83: 993–1005. [Google Scholar]
- Sato T. 2002. Phenology of sex expression and gender variation in a heterodichogamous maple, Acer japonicum. Ecology 83: 1226–1238. [Google Scholar]
- Sork VL. 2002. Pollen movement in declining populations of California valley oak, Quercus lobata: where have all the fathers gone? Molecular Ecology 11: 1657–1668. [DOI] [PubMed] [Google Scholar]
- Sprugel DG, Hinckley TM, Schaap W. 1991. The theory and practice of branch autonomy. Annual Review of Ecology and Systematics 22: 309–334. [Google Scholar]
- Stout AB. 1928. Dichogamy in flowering plants. Bulletin of Torrey Botanical Club 55: 141–153. [Google Scholar]
- Thompson TE, Romberg LD. 1985. Inheritance of heterodichogamy in pecan. Journal of Heredity 76: 456–458. [Google Scholar]
- Victory ER, Glaubitz JC, Rhodes OE, Woeste K. 2006. Genetic homogeneity in Juglans nigra (Juglandaceae) at nuclear microsatellites. American Journal of Botany 93: 118–126. [Google Scholar]
- Webb CJ. 1984. Pollination specialization and protogyny in Myrrhidendron donellsmithii (Umbelliferae). Systematic Botany 9: 240–246. [Google Scholar]
- Whitehead D. 1983. Wind pollination: some ecological and evolutionary perspectives. In: Real L, ed. Pollination biology. New York, NY: Academic Press, 97–108.
- Wood MN. 1934. Pollination and blooming habits of the Persian walnut in California. US Department of Agriculture, Technical Bulletin No. 387.
- Zimmerman M, Pyke GH. 1988. Reproduction in Polemounium: assessing the factors limiting seed set. American Naturalist 131: 723–728. [Google Scholar]