Abstract
• Background and Aims Rice (Oryza sativa) is an aquatic plant with a characteristic of forming iron plaque on its root surfaces. It is considered to be the most Al-tolerant species among the cereal crops. The objective of this study was to determine the effects of root surface iron plaque on Al translocation, accumulation and the change of physiological responses under Al stress in rice in the presence of iron plaque.
• Methods The japonica variety rice, Koshihikari, was used in this study and was grown hydroponically in a growth chamber. Iron plaque was induced by exposing the rice roots to 30 mg L−1 ferrous iron either as Fe(II)-EDTA in nutrient solution (6 d, Method I) or as FeSO4 in water solution (12 h, Method II). Organic acid in root exudates was retained in the anion-exchange resin and eluted with 2 m HCl, then analysed by high-performance liquid chromatography (HPLC) after proper pre-treatment. Fe and Al in iron plaque were extracted with DCB (dithionite–citrate–bicarbonate) solution.
• Key Results and Conclusions Both methods (I and II) could induce the formation of iron plaque on rice root surfaces. The amounts of DCB-extractable Fe and Al on root surfaces were much higher in the presence of iron plaque than in the absence of iron plaque. Al contents in root tips were significantly decreased with iron plaque; translocation of Al from roots to shoots was significantly reduced with iron plaque. Al-induced secretion of citrate was observed and iron plaque could greatly depress this citrate secretion. These results suggested that iron plaque on rice root surfaces can be a sink to sequester Al onto the root surfaces and Fe ions can pre-saturate Al-binding sites in root tips, which protects the rice root tips from suffering Al stress to a certain extent.
Keywords: Rice (Oryza sativa), formation of iron plaque, root surface, Fe(II)-EDTA, aluminium (Al) stress, citrate secretion, DCB (dithionite–citrate–bicarbonate), root tip, Al translocation
INTRODUCTION
Approximately 40–50 % of the world's potentially arable soils are acidic, and aluminium (Al) toxicity has been recognized as one of the most important limiting factors of plant productivity on acidic soils (Kochian et al., 2004). Al toxicity was also deemed to be one of the major concerns of low rice productivity in acid upland and lowland acid sulfate soils (IRRI, 1978; Fageria and Carvalho, 1982; Vasconcelos et al., 2002).
Rice (Oryza sativa) is an aquatic plant with the characteristic of forming iron plaque on its root surfaces due to its capability to release oxygen to the rhizosphere, and that results in the oxidation of ferrous to ferric iron and the precipitation of iron oxide/hydroxide on the root surface. Rice cultivars, growth stage and soil type were significantly related to the amount of iron plaques on field-grown rice roots (Chen et al., 1980a). In wetland fields, iron (Fe) forms precipitates on root surfaces, which is composed mainly of goethite for Juncus bulbosus (Chabbi, 1999) or predominantly of ferrihydrite (approx. 63 %) for Phalaris arundinacea (Hansel et al., 2001) depending on the species and growing conditions. In field and laboratory conditions, iron plaque was present as a continuous precipitate (Hansel et al., 2001) or an amorphous coating on roots with an uneven distribution (Batty et al., 2000, 2002).
It has been proposed that the presence of iron plaque may act as a barrier, buffer or something else to reduce the uptake of potentially phytotoxic metals and metalloids into plant tissues (Greipsson, 1994, 1995; Batty et al., 2000; Liu et al., 2004a). Rice is generally considered to be the most Al-tolerant species among the cereal crops (Foy, 1988; Wagatsuma et al., 1988). There have been several studies to explore the Al tolerance mechanisms on genetic bases (Wu et al., 2000; Nguyen et al., 2001, 2002; Ma et al., 2002). Iron plaque on aquatic plant roots is ubiquitous (Hansel et al., 2001); however, the response of rice with root surface iron plaque under Al stress remains unknown. Thus the objective of this study was to determine the effects of root surface iron plaque on Al translocation, accumulation and the change of physiological responses under Al stress in rice in the presence of iron plaque.
MATERIALS AND METHODS
Plant materials and seedling growth
The Oryza sativa japonica variety rice, ‘Koshihikari’, was used in this study. Seeds were soaked in deionized water for 24 h and then germinated on nets that were floated on 0·5 mm CaCl2 (pH 4·5) solution. After growth at 30 °C for 5 d, uniform seedlings were selected and transplanted to 1 L pots (15 seedlings per pot) containing half-strength Kimura B nutrient solution (modified from Kimura B macronutrients and Arnon micronutrients). The nutrient solution contained the macronutrients (mm): (NH4)2SO4 (0·18), MgSO4.7H2O (0·27), KNO3 (0·09), CaNO3.4H2O (0·18) and KH2PO4 (0·09), and the micronutrients (μm): Na2EDTA-Fe(II) (20), MnCl2.4H2O (9), H3BO3 (46), Na2MoO4.4H2O (9), ZnSO4.7H2O (0·7) and CuSO4.5H2O (0·3). The pH of this solution was adjusted to 4·5 using 0·1 m HCl; the solution's volume was restored daily with the same nutrient solution and renewed every 3 d. The plants were grown in a controlled environment growth chamber (Microclim-1000, Snijders Scientific, Tilburg, The Netherlands) with a 14 h/30 °C day and 10 h/20 °C night regime, light intensity of 375 μmol m−2 s−1 and 65 % relative humidity.
Experimental treatments
Previous studies showed that there are a number of methods for inducing the formation of iron plaques, and different culture methods might result in different results (Greipsson, 1994; Ye et al., 1997; Zhang et al., 1999; Batty et al., 2000, 2002; Liu et al., 2004a, b; Chen et al., 2005). Thus two methods for inducing iron plaque were used to find the most suitable one. After 2 or 3 weeks, iron plaque was induced on the rice roots as follows.
Method I
Two-week-old seedlings grown in half-strength Kimura B nutrient solution were used to induce the formation of iron plaque by 30·0 mg L−1 Fe(II)-EDTA (+Fe) [the control (–Fe) was 1·12 mg L−1 Fe(II)-EDTA] in half-strength Kimura B nutrient solution (pH 4·5) in a 1 L pot for 6 d, during which the solutions were changed twice and the same nutrient solutions were supplemented every day to keep the culture solution at the same volume.
Method II
Three-week-old seedlings grown in half-strength Kimura B nutrient solution were chosen as the materials. Prior to induction of iron plaque, all seedlings were placed in 0·5 mm CaCl2 solution (pH 4·5) for 2 h to minimize any interference from other elements with Fe. They were then transferred to a freshly prepared 1 L solution containing 30·0 mg L−1 ferrous iron as FeSO4.7H2O (+Fe) or without ferrous iron (–Fe) for 12 h. The solution pH was adjusted to 4·5 or 5·0 with 0·1 m HCl or 0·1 m NaOH to determine a suitable pH value of the solutions for healthy root growth.
After iron plaque was induced, seedlings were placed in 0·5 mm CaCl2 solution (pH 4·5) overnight and then exposed to 0·5 mm CaCl2 solution (pH 4·5) with 50 μm AlCl3 (+Al) or without Al (–Al) as described by Ma et al. (2002). After 24 h of Al treatment, rice plant materials and root exudates were harvested as described below. Each experiment was repeated at least twice with three replicates each.
Scanning electron microscopy (SEM) and energy dispersive X-ray spectrometric microanalysis (EDAX)
Roots pre-treated with Fe(II)-EDTA or FeSO4 were harvested after Al treatment and were quick-frozen and freeze-dried. After coating with gold, root segments were investigated by scanning electron microscopy (SEM; Sirion 200, FEI, The Netherlands) and energy dispersive X-ray spectroscopy (EDAX; Genesis, EDAX Inc., USA).
Collection and analysis of root exudates
After 24 h exposure to 0·5 mm CaCl2 solution (pH 4·5) with 50 μm AlCl3 (+Al) or without Al (–Al), the rice was harvested and the solutions were collected and passed through a column (20 mL, Bio-RAD) filled with 5 mL of cation-exchange resin Amberlite IR-120 (H-form, 16–45 mesh), followed by a column filled with 2 g of anion-exchange resin AG 1-X8 (formate form, 100–200 mesh) for collection of root exudates. The organic acids retained in the anion-exchange resin were eluted with 2 m HCl, and the eluate was concentrated to dryness by a rotary evaporator at 40 °C. Then the residue was dissolved in 1 mL of ultra-pure water and detected by HPLC (LC-10AT VP, Shimadzu, Tokyo, Japan) equipped with a Shim-pack SCR-102H column (8·0 mm i.d. × 30 cm) according to Ma et al. (2002).
Extraction and determination of Fe and Al on the root surface
At harvest, the plant material was split into roots and shoots and rinsed thoroughly in distilled water. Then the iron plaque deposited on the root surface was extracted using the dithionite–citrate–carbonate (DCB) method of Taylor and Crowder (1983) and McLaughlin et al. (1985): The fresh roots were incubated for 3 h at 25 °C in 45 mL of a solution (pH 6·5) containing 0·27 m sodium citrate (Na3C6H5O7.2H2O) and 0·11 m sodium bicarbonate (NaHCO3), with the addition of 3·0 g of sodium dithionite (Na2S2O4). Na2S2O4 is a strong reducer in NaHCO3 solution, which reduces Fe3+ to Fe2+; while citrate in Na3C6H5O7.2H2O can form a complex with Al3+ and Fe2+. Thus they remove iron plaques on root surfaces. After filtration, the extraction solution was then analysed for Fe and Al as described below, and roots and shoots were oven dried at 75 °C for 3 d and weighed before digestion.
Analysis of tissue elements
At harvest (before DCB extraction), ten roots tips (0–1 cm) in each pot were randomly selected and excised with a razor and then placed in a plastic tube (1·5 mL) containing 1 mL of 2 m HCl for extraction of total Al and Fe in root tips (Ma et al., 2002). The tubes stood for at least 24 h with occasional shaking before determination of Al and Fe contents.
Dried plant materials were ground to a fine powder and digested with concentrated HNO3 (heavy-metal grade). The concentrations of Al in digested solutions, concentrations of Al and Fe in the DCB extracts and root tip extractions were determined by ICP-AES (IRIS-Advantage, Thermo Elemental, MA, USA), after an appropriate dilution.
Statistical analysis
Statistical analyses were carried out on plant biomass, concentrations of metals and organic acid data using a one-way analysis of variance (ANOVA) in the SPSS statistical package.
RESULTS
Formation of iron plaque on rice root surfaces
Morphology of iron plaque on rice roots
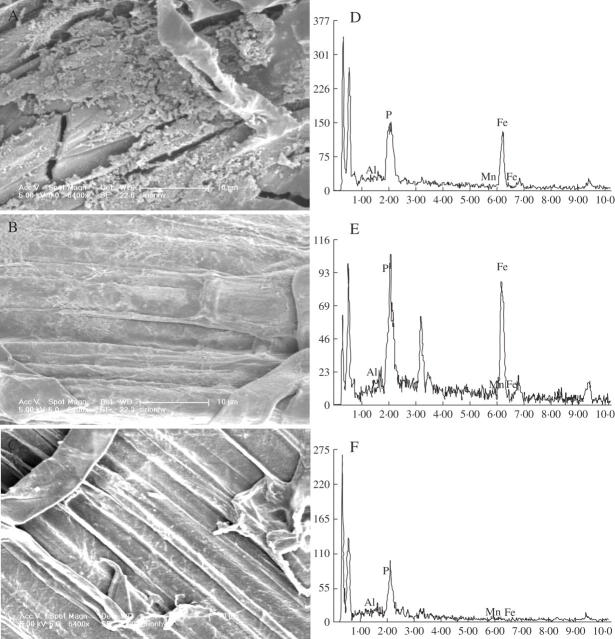
After a 6 d pre-treatment with Fe(II)-EDTA in nutrient solution (Method I), rice roots showed normal growth with good root development. Thin yellowish coatings appeared, indicating the formation of iron plaque, and SEM-EDAX studies also demonstrated that Fe(II)-EDTA induced the uneven distribution of iron plaque with a porous structure on the root surfaces (Fig. 1A, D). For Method II, after 12 h of pre-treatment with FeSO4 in the water solution, plaque deposits were clearly visible as a dense and massive reddish coating on the root surfaces (Fig. 1B, E). There were visible differences between micrographs of precipitates formed in FeSO4 solution and in Fe(II)-EDTA (Fig. 1A, B), but data from EDAX showed no significant differences for the key elements in iron plaque such as Fe and P (Fig. 1D, E). The roots with –Fe treatment remained white, indicating the absence of iron plaque (Fig. 1C, F). However, unlike the treatment with Fe(II)-EDTA, the root tips treated with FeSO4 appeared bulgy and approx. 0–5 mm from the tip turned a brown colour, which showed that the root tips were injured.
Fig. 1.
Scanning electron micrographs (A, B and C) and energy-dispersive X-ray analysis (EDAX) (D, E and F) of precipitates formed on rice (Koshihikari) roots in hydroponic culture supplemented with iron or aluminium. (A) Rice was grown in Kimura B nutrient solution (pH 4·5) containing 30 mg L−1Fe(II)-EDTA for 6 d (Method I). (B) Rice was pre-treated with a solution (pH 4·5) containing 30·0 mg L−1 FeSO4 for 12 h (Method II). (C) Rice was not pre-treated with iron but was exposed for 24 h to 0·5 mm CaCl2 solution (pH 4·5) containing 50 μm AlCl3. (D), (E) and (F) correspond to (A), (B) and (C), respectively. These micrographs are representative of the general morphology and image.
Fe and Al contents in iron plaques of rice roots
The amounts of Fe on the root surfaces extracted by DCB under both methods increased remarkably after the induction of iron plaque. There was more Fe in Method I than in Method II (Fig. 2A). The Al concentrations in DCB extracts from both methods showed that there was significant enrichment of Al after Fe pre-treatment and there was more Al in Method I than in Method II (Fig. 2B).
Fig. 2.
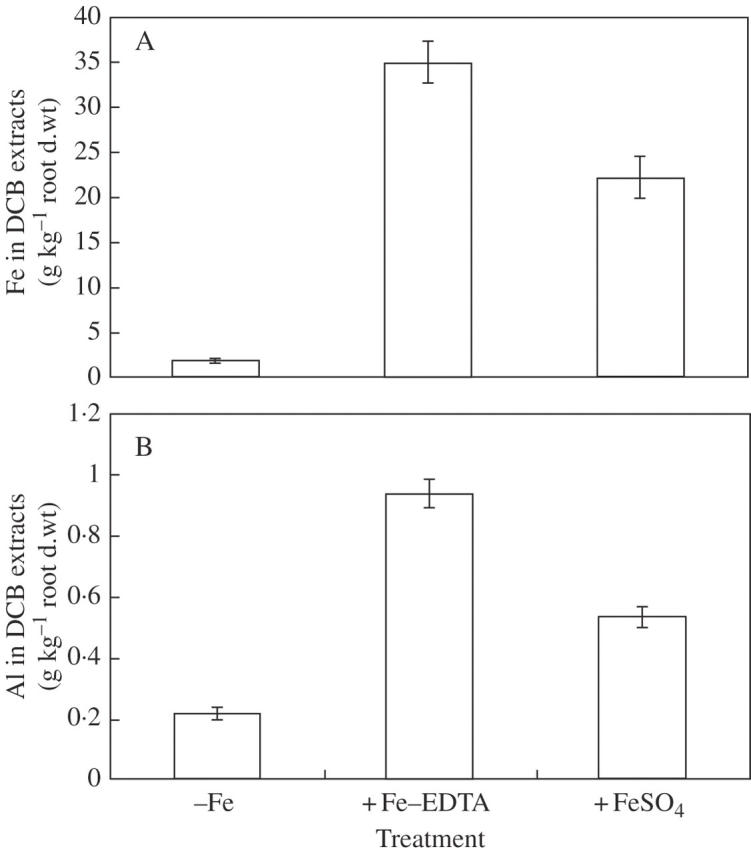
Fe (A) and Al (B) concentrations in dithionite–citrate–bicarbonate (DCB) extracts. Two-week-old seedlings were pre-treated on the root surfaces with 30 mg L−1 Fe(II)-EDTA [the control (–Fe) was1·12 mg L−1 Fe(II)-EDTA] in Kimura B nutrient solution (pH 4·5) for 6 d (Method I) or 3-week-old seedlings were pre-treated with water solution (pH 4·5) containing 30·0 mg L−1 FeSO4 for 12 h (Method II). Error bars represent ± s.e. (n = 3).
Effect of pH on the formation of iron plaque and Al adsorption
The pH of the environment was regarded as one of the important factors in the formation of iron plaque and controlling uptake of metals (Batty et al., 2000). In order to find out the reason for the root damage, a different pH regime of the treatment solution was set. The results showed that there were no visible differences in the degree of damage between pH 4·5 and 5·0 of the pre-treatment solution, i.e. the pH value of the solution should not be mainly responsible for the damage of root tips. The pH also had no significant effect on the formation of iron plaque and the adsorption of Al (data not shown).
Based on the information obtained thus far, Method I was chosen in this study and the following results were all based on the results using Method I.
Effect of iron plaque on the growth of rice seedlings
Throughout the experiment of Method I, rice seedlings showed normal growth, and no visible difference during growth was observed between +Fe and –Fe treatments. The dry weights of shoots and roots of rice seedlings were not significantly different between the presence and absence of iron plaques (Table 1), indicating that iron plaques had no significant effects on rice growth.
Table 1.
Biomass (dry weight) of shoots and roots of 3-week-old rice (Oryza sativa) seedlings
| Treatment | Shoot (g) | Root (g) |
|---|---|---|
| –Fe | 1·68 ± 0·07a | 0·31 ± 0·02a |
| +Fe | 1·48 ± 0·08a | 0·27 ± 0·01a |
Seedlings (5 d old) were transplanted to a 1 L pot containing half-strength Kimura B nutrient solution. After 2 weeks, the half-strength Kimura B nutrient solution containing 30·0 mg L−1 Fe(II)-EDTA was used to induce the formation of iron plaque for 6 d. Data are means (per 15 plants values) ± s.e. of three replicates. Means with the same superscript within columns do not differ significantly at P = 0·05 using one-way ANOVA.
Effects of iron plaque on Al accumulation in roots and translocation from roots to shoots
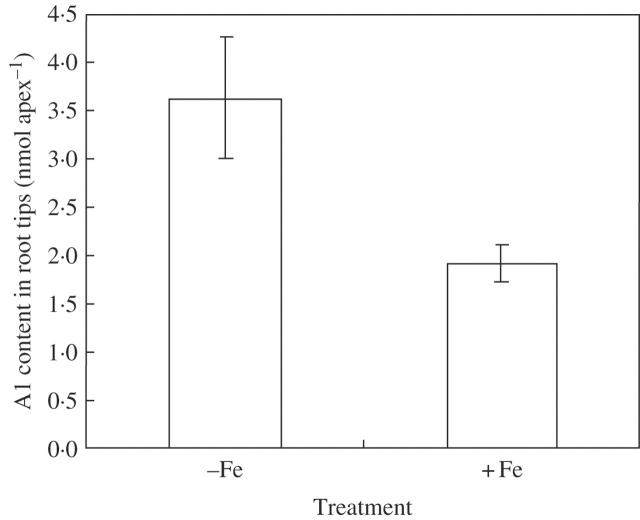
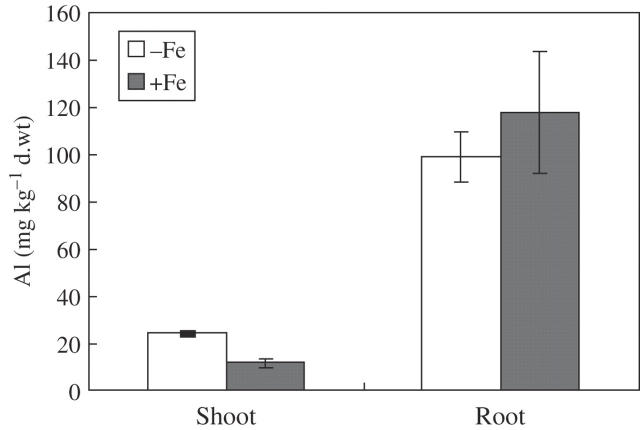
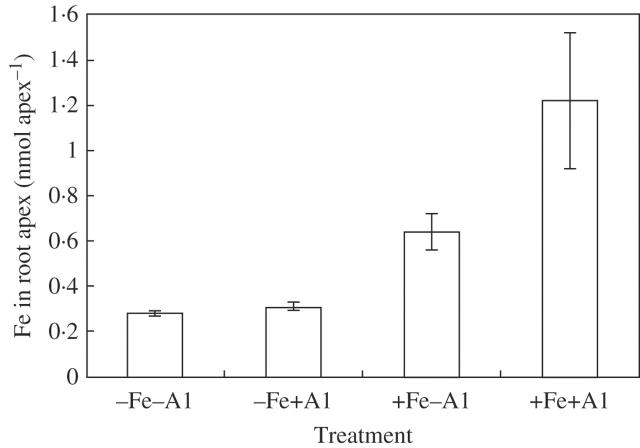
Rice accumulated less Al in root tips in the presence of iron plaque than in the absence of iron plaque (Fig. 3). Al contents in roots (the remaining Al after DCB extraction) showed no significant difference between treatments with and without iron plaque, while Al in shoots was lower with iron plaque although Al contents were much higher in roots than in shoots (Fig. 4).
Fig. 3.
Aluminium (Al) content in the root apex of rice (Koshihikari). When the seedlings [pre-treated with Fe(II)-EDTA] were harvested, root apices (1 cm) were excised and extracted with 2 m HCl. The Al concentration was detected by ICP-AES. Error bars represent ± s.e. (n = 3).
Fig. 4.
Effect of iron plaque on the distribution of aluminium (Al) in whole roots and shoots. After extraction with DCB, the whole root and shoot were dried and digested with concentrated HNO3. Error bars represent ± s.e. (n = 3).
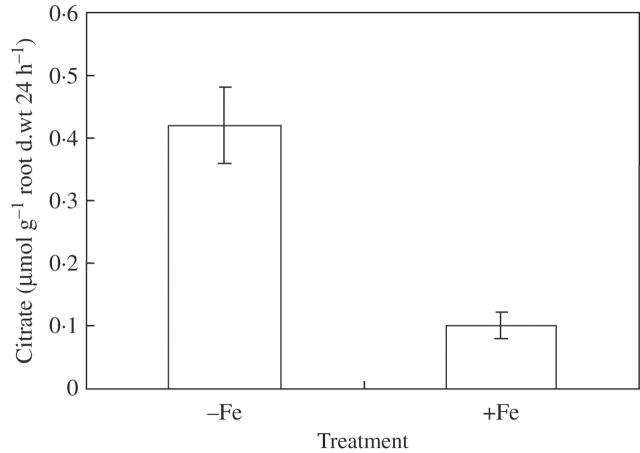
Citrate exudation from rice roots and Fe contents in rice root tips
Citrate secretion was not detected unless the rice roots were exposed to Al stress in treatments both with and without iron plaques; however, it was remarkably depressed when there were iron plaques on the root surface (Fig. 5).
Fig. 5.
Effect of iron plaque on the release of citrate from rice (Koshihikari) under aluminium (Al) stress. Fe(II)-EDTA (30·0 mg L−1) was used to pre-treat the root surfaces for 6 d, and then the seedlings were exposed for 24 h to 0·5 mm CaCl2 solution (pH 4·5) containing 50 μM AlCl3, and root exudate was collected. Error bars represent ± s.e. (n = 3).
Fe contents in rice root tips extracted by 2 m HCl were significantly higher in the +Fe treatment. Al treatment had no significant effect on Fe content in both –Fe and +Fe treatments (Fig. 6).
Fig. 6.
Iron (Fe) content in the root apex of rice (Koshihikari). When the seedlings were harvested, root apices (1 cm) were excised and extracted with 2 m HCl. The iron concentration was detected by ICP-AES. Error bars represent ± s.e. (n = 3).
DISCUSSION
Formation of iron plaque
The iron plaque on root surfaces is, in principle, facilitated by the release of oxygen and oxidants into the rhizosphere by aquatic plants; it could be induced in a number of ways in the field and laboratory depending on the purposes of the experiments. For example, in the laboratory, it can be induced by ferrous iron (FeSO4) solution (Greipsson, 1994, 1995; Liu et al., 2004a), by nutrient solution containing Fe(OH)3 or FeSO4 (Ye et al., 1997; Zhang et al., 1999) or by nutrient solution containing (NH4)2Fe(SO4)2, FeSO4 or Fe(II)-EDTA, but not containing phosphate (Batty et al., 2000, 2002; Liu et al., 2004b). Some were induced under field conditions such as rice grown in flooded soil (Chen et al., 1980a, b), J. bulbosus, the pioneer species of acid lakes (Chabbi, 1999), and Phragmites australis collected from a field contaminated by coal mine drainage (Batty et al., 2000). In the present study, 30·0 mg L−1 Fe(II)-EDTA in half-strength Kimura B nutrient solution (pH 4·5) containing 0·09 mm phosphate was employed to pre-treat the rice root for 6 d; Fe concentrations in DCB extracts were enhanced greatly (Fig. 2A), and results from SEM-EDAX (Fig. 1) also showed the formation of iron plaque on the root surface in rice. Thus, Fe(II)-EDTA in nutrient solution could also induce the formation of iron plaque compared with the common method using FeSO4, and the effects of the two methods of induction on the formation and Al sequestration were analogous, although fewer iron plaques formed on the root surfaces in Method II than that in Method I and less Al was deposited on the root surfaces (Fig. 2). Since the targeting area for Al3+ toxicity is the root tip (Ryan et al., 1993), it is crucial to keep the root tip healthy before it is subjected to Al stress; thus Method I was chosen to evaluate the effect of iron plaque on Al stress in rice in the present study.
A sink for Al in iron plaque
Iron plaque was regarded as a ‘buffer’ for arsenic (As) in the rhizosphere (Liu et al., 2004a) and it might sequestrate As and consequently reduce the translocation of As from roots to shoots (Liu et al., 2004b). In contrast, investigations into the growth of Typha latifolia demonstrated that roots with plaque adsorbed less nickel (Ni) (Ye et al., 1997), and less cadmium (Cd) (Ye et al., 1998) than those without. These discrepancies may be partly attributed to the differences in the culture methods, the metal/metalloid characters and the plant species. In our experiment, more Al was found on root surfaces with iron plaque than those without (Fig. 2B), indicating that Al might be sequestrated onto the root surfaces as a sink.
The chemical signature of coatings formed in the absence of Fe was dominated by Al and P (Fig. 1F) and, in the presence of Fe, the coatings comprised a combination of Fe, Al and P (Fig. 1D, E), suggesting that Fe, Al and P were co-precipitated. Batty et al. (2002) also found that Al was not adsorbed onto the surface of plaque but formed a separate phosphate deposit resembling the iron plaque in P. australis.
Al contents in rice root tips
The prime site of Al toxicity is the root tip (Ryan et al., 1993). So what is the response of the root tips in the presence of iron plaques under Al stress? Chen et al. (1980b) reported that Fe plaques tended to form on older root parts rather than the more active root tips and fresh root hairs of rice. Batty et al. (2002) observed using SEM that iron plaques started approx. 1 cm behind the P. australis root tip and gradually thinned with distance from this region to the tip. Our results regarding iron plaque determined by EDAX also showed that less Fe was present on root tips than on other regions (data not shown). However, the Fe contents in root tips extracted with 2 m HCl did increase in +Fe treatment compared with –Fe treatment (Fig. 6); this might lead to the pre-saturation of Al-binding sites in root tips with Fe ions, and consequently contribute to depress the accumulation of Al in the root tips (Fig. 3). This reduction of Al contents in root tips could protect the rice root tips from suffering Al stress to a certain extent.
Effects of iron plaque on Al translocation in rice
Rice is not an Al accumulator; much less Al was accumulated in shoots (Chen and Shen, Institute of Soil Science, CAS, China, unpubl. res.) compared with shoots of buckwheat (Shen and Ma, 2001). DCB-extractable Al on root surfaces was increased significantly with iron plaque (Fig. 2B), but Al in the roots (the remaining Al after DCB extraction) was not influenced by iron plaque (Fig. 4), which suggested that iron plaque could not prevent the Al from entering the roots. However, Al in the shoots was reduced with iron plaque (Fig. 4). Thus one of the key effects of iron plaques might be to hold back Al translocation from roots to shoots, though the underlying mechanisms remains to be elucidated. This effect is consistent with the results of Liu et al. (2004b) who studied the role of iron plaque on As uptake in rice.
Depression of citrate exudation
Secretion of organic acid from plant roots when subjected to Al stress is considered as an important mechanism for plants to achieve high tolerance to Al (Ma, 2000). Anion channels, a kind of membrane-bound transport proteins existing on the plasma membrane, mediate the secretion of organic acid anion, and Al activates these anion channels by interacting directly with the channel protein or with a specific receptor on the membrane (Ma et al., 2001). Less citrate was exuded from roots coated by iron plaque (Fig. 5). This might be explained partially by the high Fe contents in the root tip with iron plaque (Fig. 6), because competition between Al and Fe on the membrane of root tip cells may depress the ability for Al activation.
In conclusion, Fe(II)-EDTA in nutrient solution could also induce the formation of iron plaque on root surfaces of rice. Iron plaque on rice root surfaces can act as a sink for Al and sequester Al onto the root surfaces and reduce the translocation of Al from roots to shoots. Increasing Fe contents in root tips with Fe pre-treatment may pre-saturate the Al-binding sites and consequently depress the accumulation of Al in root tips. The Al-induced secretion of citrate could be greatly depressed with iron plaque. Further investigation is needed to see whether and how iron plaque on root surface contributes to Al tolerance in rice.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (40371072, 30571114), Natural Science Foundation of Jiangsu Province (BK2002150), and by CAS ‘100-talent Program’ and International Partnership Project (CXTD-Z2005-04). We thank Phil Brookes and Anna Swain from Rothamsted Research, UK for their critical reading of the manuscript.
LITERATURE CITED
- Batty LC, Baker AJM, Wheeler BD, Curtis CD. 2000. The effect of pH and plaque on the uptake of Cu and Mn in Phragmites australis (Cav.) Trin ex. Steudel. Annals of Botany 86: 647–653. [Google Scholar]
- Batty LC, Baker AJM, Wheeler BD. 2002. Aluminum and phosphate uptake by Phragmites australis: the role of Fe, Mn and Al root plaques. Annals of Botany 89: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbi A. 1999. Juncus bulbosus as a pioneer species in acidic lignite mining lakes: interactions, mechanism and survival strategies. New Phytologist 144: 133–142. [Google Scholar]
- Chen CC, Dixon JB, Turner FT. 1980a. Iron coatings on rice roots: mineralogy and quantity influencing factors. Soil Science Society of American Journal 44: 635–639. [Google Scholar]
- Chen CC, Dixon JB, Turner FT. 1980b. Iron coatings on rice roots: morphology and models of development. Soil Science Society of American Journal 44: 1113–1119. [Google Scholar]
- Chen Z, Zhu YG, Liu WJ, Meharg AA. 2005. Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytologist 165: 91–97. [DOI] [PubMed] [Google Scholar]
- Fageria NK, Carvalho JRP. 1982. Influence of aluminum in nutrient solutions on chemical composition in upland rice cultivars. Plant and Soil 69: 31–44.
- Foy CD. 1988. Plant adaptation to acid, aluminum-toxic soils. Communications in Soil Science and Plant Analysis 19: 959–987. [Google Scholar]
- Greipsson S. 1994. Effects of iron plaque on roots of rice on growth and metal concentration of seeds and plant tissues when cultivated in excess copper. Communications in Soil Science and Plant Analysis 25: 2761–2769. [Google Scholar]
- Greipsson S. 1995. Effect of iron plaque on roots of rice on growth of plants in excess zinc and accumulation of phosphorus in plants in excess copper or nickel. Journal of Plant Nutrition 18: 1659–1665. [Google Scholar]
- Hansel CM, Fendorf S, Sutton S, Newville M. 2001. Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environmental Science and Technology 35: 3863–3868. [DOI] [PubMed] [Google Scholar]
- International Rice Research Institute (IRRI). 1978. Aluminum toxicity. IRRI Annual Report for1977. Los Bavos, The Philippines.
- Kochian LV, Hoekenga OA, Pineros MA. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminium tolerance and phosphorus efficiency. Annual Review of Plant Biology 55: 459–493. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Zhu YG, Smith FA, Smith SE. 2004a. Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? Journal of Experimental Botany 55: 1707–1713. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Zhu YG, Smith FA, Smith SE. 2004b. Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? New Phytologist 162: 481–488. [Google Scholar]
- Ma JF. 2000. Role of organic acids in detoxification of aluminum in higher plants. Plant and Cell Physiology 41: 383–390. [DOI] [PubMed]
- Ma JF, Ryan PR, Delhaize E. 2001. Aluminium tolerance in plants and the complexing role of organic acids. Trends in Plant Science 16:273–278. [DOI] [PubMed]
- Ma JF, Shen RF, Zhao Z, Wissuwa M, Takeuchi Y, Ebitani T, Yano M. 2002. Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant and Cell Physiology 43: 652–659. [DOI] [PubMed]
- McLaughlin BE, Loon GW, Crowder AA. 1985. Comparison of selected washing treatments on Agrostis gigantean samples from mine tailings near Copper Cliff, Ontario, before analysis for Cu, Ni, Fe and K content. Plant and Soil 85: 433–436. [Google Scholar]
- Nguyen VT, Burow MD, Nguyen HT, Le BT, Le TD, Paterson AH. 2001. Molecular mapping of genes conferring aluminium tolerance in rice (Oryza sativa L.). Theoretical and Applied Genetics 102: 1002–1010. [Google Scholar]
- Nguyen VT, Nguyen BD, Sarkarung S, Martinez C, Paterson AH, Nguyen HT. 2002. Mapping of genes controlling aluminium tolerance in rice: comparison of different genetic backgrounds. Molecular Genetics and Genomics 267: 772–780. [DOI] [PubMed] [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV. 1993. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. Journal of Experimental Botany 44: 437–446. [Google Scholar]
- Shen RF, Ma JF. 2001. Distribution and mobility of aluminium in an Al-accumulating plant, Fagopyrum esculentum Moench. Journal of Experimental Botany 52: 1683–1687. [PubMed] [Google Scholar]
- Taylor GJ, Crowder AA. 1983. Use of DCB technique for extraction of hydrous iron oxides from roots of wetland plants. American Journal of Botany 70: 1254–1257.
- Vasconcelos SS, Jacob-Neto J, Rossiello ROP. 2002. Differential root responses to aluminum stress among Brazilian rice genotypes. Journal of Plant Nutrition 25: 655–669. [Google Scholar]
- Wagatsuma T, Kawashima T, Kawaraya K. 1988. Comparative stainability of plant root cells with basic dye (methylene blue) in association with aluminium tolerance. Communications in Soil Science and Plant Analysis 19: 1207–1215. [Google Scholar]
- Wu P, Liao CY, Hu B, Yi KK, Jin WZ, Ni JJ, He C. 2000. QTLs and epistasis for aluminium tolerance in rice (Oryza sativa L.) at different seeding stages. Theoretical and Applied Genetics 100: 1295–1303. [Google Scholar]
- Ye ZH, Baker AJM, Wong MH, Willis AJ. 1997. Copper and nickel uptake, accumulation and tolerance in Typha latifolia with and without iron plaque on the root surface. New Phytologist 136: 481–488. [DOI] [PubMed] [Google Scholar]
- Ye ZH, Baker AJM, Wong MH, Willis AJ. 1998. Zinc, lead and cadmium accumulation and tolerance in Typha latifolia as affected by iron plaque on the root surface. Aquatic Botany 61:55–67. [Google Scholar]
- Zhang XK, Zhang FS, Mao DR. 1999. Effect of iron plaque outside roots on nutrient uptake by rice (Oryza sativa L.): phosphorus uptake. Plant and Soil 209: 187–192. [Google Scholar]