Abstract
• Background and Aims Pollen and seed dispersal in herbaceous insect-pollinated plants are often restricted, inducing strong population structure. To what extent this influences mating within and among patches is poorly understood. This study investigates the influence of population structure on pollen performance using controlled pollinations and genetic markers.
• Methods Population structure was investigated in a patchily distributed population of gynodioecious Silene vulgaris in Switzerland using polymorphic microsatellite markers. Experimental pollinations were performed on 21 hermaphrodite recipients using pollen donors at three spatial scales: (a) self-pollination; (b) within-patch cross-pollinations; and (c) between-patch cross-pollinations. Pollen performance was then compared with respect to crossing distance.
• Key Results The population of S. vulgaris was characterized by a high degree of genetic sub-structure, with neighbouring plants more related to one another than to distant individuals. Inbreeding probably results from both selfing and biparental inbreeding. Pollen performance increased with distance between mates. Between-patch pollen performed significantly better than both self- and within-patch pollen donors. However, no significant difference was detected between self- and within-patch pollen donors.
• Conclusions The results suggest that population structure in animal-pollinated plants is likely to influence mating patterns by favouring cross-pollinations between unrelated plants. However, the extent to which this mechanism could be effective as a pre-zygotic barrier preventing inbred mating depends on the patterns of pollinator foraging and their influence on pollen dispersal.
Keywords: Population structure, microsatellites, pollen performance, gynodioecy, Silene vulgaris
INTRODUCTION
Restricted pollen and seed dispersal results in significant population structure in many flowering plants. However, the extent to which population structure influences mating patterns and plant fitness is poorly understood. There is considerable evidence that the distance between mates can influence their female fertility (Waser and Price, 1983; Levin, 1984; Schemske and Pautler, 1984; Delph, 2004; Robertson and Ulappa, 2004), but whether this reflects pre-zygotic pollen tube growth and/or post-zygotic abortion of developing seeds is usually not clear. Proximity-dependent influences on pollen germination and/or pollen tube growth have rarely been assessed, and the results that are available have often been mixed (Levin, 1989; Waser and Price, 1991; Souto et al., 2002). Moreover, few studies have simultaneously investigated evidence for population structure using genetic markers and the performance of crosses at different spatial scales (but see Souto et al., 2002).
Gynodioecy is a sexual system in which hermaphrodites and females co-occur within populations. Sex ratios vary considerably among gynodioecious populations and also at a local scale within populations (Couvet et al., 1990; Delph, 1990; McCauley et al., 2000; Laporte et al., 2001). Because sex expression is generally nucleocytoplasmic in gynodioecious populations (Saumitou-Laprade et al., 1994; Charlesworth and Laporte, 1998), variation in sex ratios is often the consequence of significant spatial structure among sex-determining genes (Frank, 1989; Gigord et al., 1998). Indeed, strong population structure has been demonstrated using neutral genetic markers in several gynodioecious species (McCauley, 1998; Laporte et al., 2001). In populations with spatial structure, mating events are more likely to occur between nearby related individuals, and thus many progeny are inbred. Inbreeding depression is the principal hypothesis explaining the maintenance of females and has been shown to be important in several gynodioecious species (Shykoff, 1988; Kohn and Biardi, 1995; Sakai et al., 1997; Mutikainen and Delph, 1998; Thompson and Tarayre, 2000; Koelewijn, 2004). Fine scale population structure, sex ratio variation and inbreeding depression should therefore result in fitness benefits when mating events occur between distant pollen donors.
This study investigated whether proximity-dependent pollen performance is evident in the insect-pollinated, perennial, gynodioecious herb Silene vulgaris (Caryophyllaceae). A natural population in Switzerland of 83 individuals distributed in five distinct patches with varying sex ratios was used to assess fine scale genetic structure using microsatellites (Juillet et al., 2003). The in vivo pollen performance was compared from three classes of controlled pollination conducted on hermaphrodite plants transplanted from this population into a glasshouse. The experimental pollinations were: (a) self-pollination; (b) within-patch pollinations (crossing distance <5 m); and (c) between-patch pollination (crossing distance >11 m). We predicted considerable population structure resulting from inbreeding in local patches of S. vulgaris and an increase in pollen performance with increasing distance between mates.
MATERIALS AND METHODS
Study species
Silene vulgaris (Moench) Garke is a widespread species native to Europe but introduced to other parts of the world: North America, Asia and North Africa (Dulberg and Horovitz, 1984). In Switzerland, populations can be found at low altitudes along roadsides and up to an altitude of 2500 m in mountain grasslands. Populations generally consist of female and hermaphrodite individuals with sex ratio variation evident at fine spatial scales. Hermaphrodites are self-compatible and strongly protandrous: in natural populations, pollen is generally removed before stigma receptivity. Autonomous self-pollination is therefore infrequent, as reported by Pettersson (1992) who found <5 % seed set resulting from intra-floral selfing. However, hermaphrodites have the potential for self-fertilization through geitonogamous pollen transfer since (a) the number of flowers open daily can be large (up to 100 per day); and (b) pollinators (Noctuidae moths) forage within restricted areas and often within a plant (Pettersson, 1991, 1992; M. Glaettli, unpubl. data.). Fruits are capsules containing up to 100 seeds that disperse through gravity. Thus, restricted pollen and seed dispersal is likely to result in a high level of relatedness among neighbouring individuals (McCauley, 1998).
Study population, sampling and growth conditions
The study population is located in Les Mosses (Switzerland, 138°2′6″N, 573°44′45·6″E) at an altitude of 1430 m and was surveyed in August 2002. It consisted of 83 flowering plants, patchily distributed within an area of approx. 3000 m2. For each individual, its distance to a fixed point and to two other individuals was measured to map the location of all individuals. Five patches were obvious at first sight and were spatially defined. All individuals belonging to a patch were separated by <5 m. In contrast, individuals in different patches were separated by 11–85 m. Each patch contained a different number of individuals and sex ratio (Table 1). In August 2002, ramets (a mixture of vegetative and flowering ramets for each plant) were transplanted from each individual into pots (Ø 15 cm) containing 50 % peat and 50 % clay soil. The transplanted ramets were taken back to a glasshouse of the University of Lausanne and kept in controlled conditions (15 h light/9 h dark, 18–20 °C, 50–60 % relative humidity, no fertilizer). After a few days, new ramets started to grow and old ones (i.e. those grown in the field) were excised to synchronize flowering among individuals.
Table 1.
Population characteristics
| Patch | Total | Undet | H | F | Sex ratio | FIS |
|---|---|---|---|---|---|---|
| Route | 23 | 4 | 2 | 17 | 0·90 | 0·315 |
| Sapin | 15 | 6 | 8 | 1 | 0·11 | 0·326 |
| Enclos | 9 | 4 | 4 | 1 | 0·20 | 0·310 |
| PR1 | 7 | 2 | 3 | 2 | 0·40 | 0·594 |
| PR2 | 29 | 8 | 18 | 3 | 0·14 | 0·352 |
| All patches | 83 | 24 | 35 | 24 | 0·41 | 0·353 |
The number of hermaphrodites (H) and females (F) and the sex ratio were recorded as the proportion of females [F/(F+H)]. Individuals in the vegetative state for which sex could not be determined (Undet) were also recorded. The total number of plants in each patch was the sum of females, hermaphrodites and undetermined individuals (Total). FIS, the inbreeding coefficient is indicated for each patch.
Microsatellite analysis
DNA was extracted from dry leaves using a FastDNA kit (Qbiogene®). All 83 individuals were used to conduct genetic analysis using four highly polymorphic microsatellite loci (A5, A11, B29 and G3), and the experimental procedure developed by Juillet et al. (2003) was followed. A5 and A11 loci were enriched from a CA library and their size ranged from 150 to 245 and from 156 to 219, respectively. B29 and G3 loci were GA repeats and their size ranged from 133 to 177 and from 136 to 223, respectively (Juillet et al., 2003).
The population structure was analysed by computing f and θ (Weir and Cockerham, 1984), analogous to Wright's FIS and FST, and the significance of both fixation indices was tested for with permutation tests (1000) using Fstat 2.9.3.2 (Goudet, 1995). Using the entire study population as a reference, pair-wise coefficients of relatedness (r) between each pair of mates were computed with Kinship 1.3 (Queller and Goodnight, 1989).
Pollination experiment
Experimental design and procedure
A pollination experiment was carried out using 21 recipient hermaphrodite plants. Recipient flowers were emasculated immediately after anthesis, before styles extended beyond the calyx. After emasculation, single flowers were bagged with transparent philatelist envelopes for 4 d until stigmas had expanded. Hermaphroditic flowers become receptive on day four (1 d after anthers have wilted) and stay receptive for approx. 3 d. Each flower was pollinated on the first day of stigma receptivity. Three types of pollen were used on each recipient plant: (a) self-pollen (self); (b) pollen from a nearby plant, occurring in the same patch as the recipient plant (within-patch); and (c) pollen from a plant belonging to another patch (between-patch). The closest pollen-producing neighbour in the field was chosen for the within-patch pollination treatment (range: 0·5–5 m around the recipient plant); and for the between-patch treatment, a sire was randomly chosen among the flowering plants belonging to a different patch. To facilitate pollen tube discrimination, small amounts of pollen (5–80 pollen grains) were deposited on each stigma. Since pollen tubes of different origin cannot be distinguished in a style, each of the three pollen treatments was applied to different flowers, resulting in pollination of three flowers per recipient plant. Since each flower bears three styles, each pollination treatment was replicated three times within a flower. The order of pollination treatment within a recipient plant was randomized to avoid flower position effects but also because the occurrence of three open flowers with receptive stigmas on the same day was infrequent.
Pollen tubes were allowed to grow for 4 h and then the styles were removed including a small portion of the ovary before fixation in FAA solution (40 % formaldehyde, glacial acetic acid and 70 % ethanol at the ratios 1 : 1 : 18) for 24 h. Subsequently, the styles were stored in 70 % ethanol at 4 °C. They were softened for 3·5 h in 4 n NaOH and washed overnight in tap water. The styles were then stained using 1·5 mL of 0·1 % aniline blue in phosphate buffer (HK2PO4, H2KPO4 in H2O) pH 7·8 for 4 h. Finally, the styles were squashed on a microscope slide and the pollen tubes examined by epifluorescence microscopy [Leitz®-Diaplan microscope with a 4′,6-diamidino-2-phenylindole (DAPI) filter and Leica®-300F digital camera].
In the related Dianthus chinensis, Aizen et al. (1990) found that faster rates of pollen tube growth correlated with longer styles and thus suggested that the growth of pollen tubes should be scaled to the length of the style in which they grow. The length of each style was therefore measured using an ocular micrometer. The number of pollen grains attached to each stigma (PG) was counted to control for variation in pollen load, since large pollen loads can increase the pollen germination rate and influence pollen tube growth rates (Waser and Price, 1991). Two sections were then defined which where each 0·9 mm long and the whole width of the style was included. The number of pollen tubes traversing each section S1 and S2 was then examined (Fig. 1). Section S1 and S2 were placed 4 and 8·9 mm (approximately one-third and two-thirds of the style) below the stigma, respectively (Fig.1).
Fig. 1.
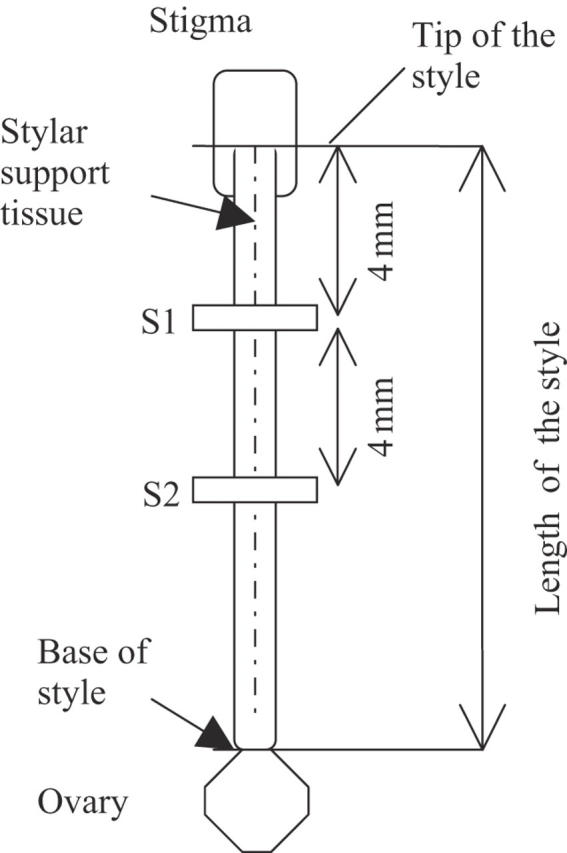
Measuring procedure: pollen grains attached to stigma are counted directly on a microscope to control for differences in pollen load. The length of the style is estimated as the length of the stylar support tissue and measured with an ocular micrometer. Sections S1 and S2 (respectively 4 and 8·9 mm below the stigma) were 0·9 mm large and included the whole width of the style. The number of pollen tubes in each of the two sections was recorded to estimate pollen performances among the self, within-patch and between-patch pollination treatments. Measures were made using a Leitz®-Diaplan microscope with DAPI filter and a Leica®-300F digital camera.
Counting pollen tubes
Since pollen tubes are not always easily distinguishable, the number of callose plugs was used to estimate the number of pollen tubes. The number of callose plugs in a given section is often used as a reliable indicator of pollen tube number (e.g. Weller and Ornduff, 1989; Aizen et al., 1990; Snow and Spira, 1991a, b). Measurements in which individual pollen tubes were distinguishable (n = 82) indicated that this was the case for S. vulgaris. The callose plug per pollen tube ratio (CP/PT) was constant (1·03 ± 0·03). Thus, when pollen tubes were difficult to see, the number of callose plugs (CP) in given section (S) was used to estimate the number of pollen tubes (PT) by the linear proportion PTS = αCPS. Regressions made on sections S1 and S2 separately were not significantly different. Thus, a single regression was used to estimate the number of pollen tubes from the number of callose plugs (n = 82, α = 0·972 ± 0·028, P < 0·001; R2 = 0·93).
Measures of pollen performance
Pollen performance was estimated in two ways: ‘early pollen performance’ (PT1) is the number of pollen tubes observed in section S1; and ‘late pollen performance’ (PT2) is the number of pollen tubes traversing section S2.
Statistical analyses
(REML) mixed model analyses of covariance (ANCOVAs) were performed to analyse pollen performance. Pollination treatment was introduced as a fixed effect repeated in a recipient plant which was random. Two covariates: style length and pollen load (PG) were also used to analyse ‘early pollen performance’ (PT1); and style length and PT1 to analyse ‘late pollen performance’ (PT2). To fulfil ANCOVA conditions, PT1 was log-transformed and a boxcox transformation was applied (λ = 0·33) for PT2. Pairs of pollination treatments were further compared using Bonferroni corrections to see which treatments differed significantly from each other. The statistical package R (version 2.1.0; R Development Core Team, 2005) was used to perform all statistical analyses.
RESULTS
Population structure
Microsatellites were highly polymorphic in the population of S. vulgaris with between 15 and 47 alleles per locus. The population exhibited a large overall inbreeding coefficient (FIS = 0·353) with significant structure among patches (FST = 0·013). Both fixation indices were found to be highly significant (P < 0·005), indicating non-random mating in the population. Pollen performance was correlated neither with the proportion of alleles shared by the mates, nor with relatedness (r). Although not significantly different, the relatedness of ‘within-patch’ mates (0·042 ± 0·17) was on average ten times higher than the relatedness of ‘between-patch’ mates (0·0043 ± 0·1). FIS per patch was not correlated with sex ratio where many females may reduce opportunities for selfing (Spearman, n = 5, P > 0·05), nor with patch size (Pearson, n = 5, P > 0·05).
Pollen performance
For ‘early pollen performance’ (PT1), the average number of pollen tubes reaching S1 after 4 h of growth (± s.e.) was 11·9 (± 3·7, n = 43) for self-pollen, 19·5 (± 2·6, n = 41) for within-patch pollen and 21·3 (± 3·1, n = 44) for between-patch pollen. As predicted, the number of self-pollen tubes in section S1 was lower than for within-patch pollen, which in turn was lower than for between-patch pollen; however, only self- and between-patch pollination treatments were significantly different. (Table 2, Fig. 2A).
Table 2.
Analysis of covariance (F-values) for ‘early pollen performance’ (PT1) and ‘late pollen performance’ (PT2)
| Source of variation |
|||||
|---|---|---|---|---|---|
| Style length |
Pollen load |
Pollen tubes in S1 |
Treatment |
||
| d.f.D | d.f.N = 1 | d.f.N = 1 | d.f.N = 1 | d.f.N = 2 | |
| No. of pollen tubes in S1 (PT1) | 100 | 0·02 | 81·17** | – | 13·09** |
| No. of pollen tubes in S2 (PT2) | 100 | 0·25 | – | 79·08** | 11·95** |
Covariates are style length and pollen load or pollen tubes in section S1 (PT1), for ‘early’ and ‘late’ pollen performance, respectively. Pollination treatment was a fixed effect repeated in the recipient which was random. PT1 and PT2 were respectively log and boxcox transformed.
*P < 0·01.
Fig. 2.
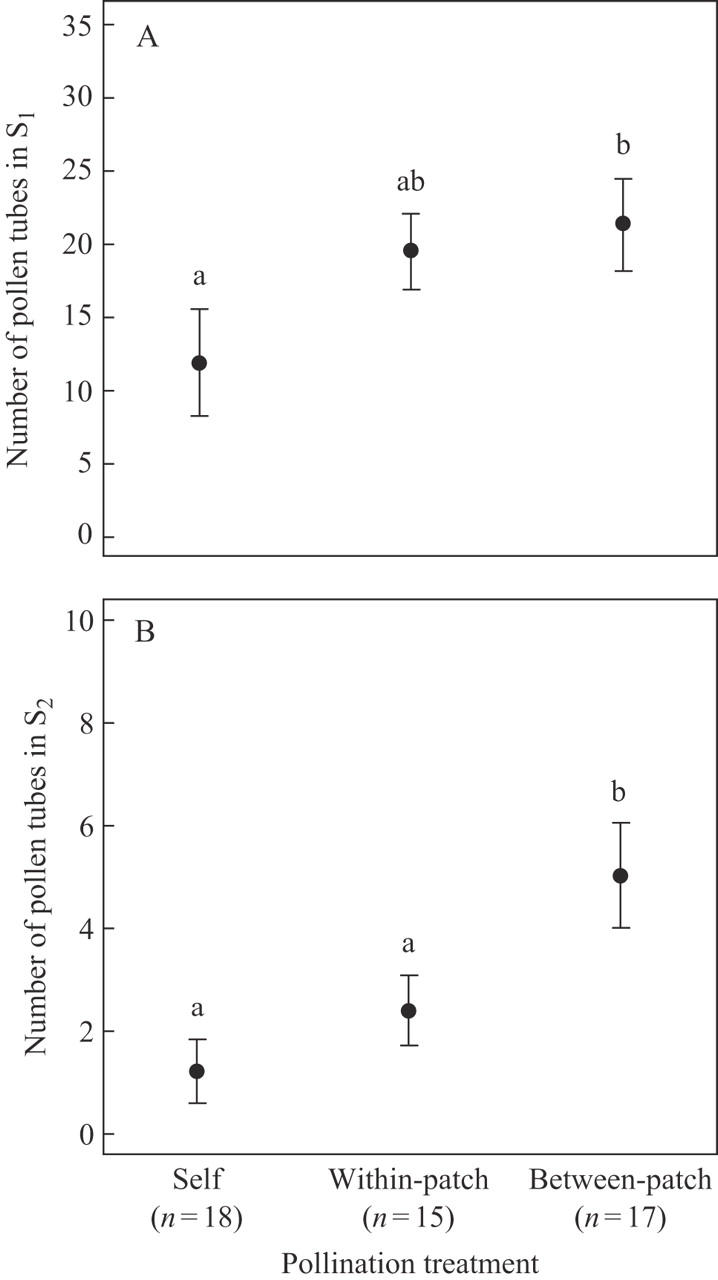
Observed means (± s.e.) of pollen performance measured in stylar section S1 (A) and S2 (B). Pollination treatment compares self, within-patch or between-patch pollen donors. Contrasts between pairs of treatments were compared: a,b indices indicate significant differences among treatments after Bonferroni corrections.
For ‘late pollen performance’ (PT2), the mean number of pollen tubes (± s.e.) that grew through S2 was 1·2 (± 0·6, n = 43) for self-pollen, 2·4 (± 0·7, n = 41) for within-patch pollen and 5·0 (± 1·0, n = 44) for between-patch pollen (Fig. 2B). Once again, the patterns of pollen performance followed our expectations: the number of pollen tubes for between-patch pollen donors was larger than for within-patch, which in turn was larger than the number of self-pollen tubes reaching section S2. There was a significant pollination treatment effect (P < 0·01, Table 2), which was due to a difference between the between-patch pollination treatments and the other self- and within-patch pollination treatments (Fig. 2B). No significant difference was detected between the within-patch and self-pollination treatment. The covariate pollen load or PT1 for ‘early’ and ‘late pollen performance’, respectively, was positively and significantly correlated with the number of pollen tubes going through each section. However, there was no significant interaction between covariates (style length and pollen load or PT1) and pollination treatment, indicating that covariates did not alter the outcomes of pollination treatments.
DISCUSSION
The results reported here provide evidence for proximity-dependent pollen performance in hermaphrodite plants of gynodioecious S. vulgaris. The number of pollen tubes reaching section S2, close to the ovary, was significantly larger for between-patch pollen donors than for self- and within-patch pollen donors. Moreover, pollen performance of within-patch pollen donors tended to be higher than that of self-pollen donors, although this trend was not significant (Fig. 2). These results suggest that the distance between mates is likely to influence pollen performance under field conditions. As significant population structure was found between patches of S. vulgaris, pollen performance seems likely to depend on the genetic similarity between mates. Such effects could potentially act as a pre-zygotic barrier limiting the intensity of selfing and bi-parental inbreeding. Mechanisms such as gametophytic selection (Mulcahy, 1979) and/or maternal mate choice (Waser and Price, 1993) have been invoked to explain the non-random genetic composition of offspring. However, direct competition experiments using mixed pollen loads and genetic markers remain to be explored to confirm the occurrence of such a pre-zygotic barrier (Snow and Spira, 1991a).
Population structure
Heterozygote deficiency due to non-random mating and significant structure were found among patches in our study population. The significant FST indicates that individuals co-occurring within a patch are more related to each other than individuals belonging to different patches. Similarly, the significant FIS indicates that inbreeding is substantial in these patches. Observations of pollinator foraging behaviour by Lepidoptera on S. vulgaris (Pettersson, 1991, 1992) have indicated restricted pollen dispersal within plants and patches. Pettersson (1992) used dye particles to estimate pollen dispersal in S. vulgaris and found that approx. 60 % of the dye particles were deposited within source individuals. The majority of the remaining dye particles were found within 5 m around the plant, although some occasionally travelled >15 m. This suggests that most pollen is dispersed within a very short distance, thus promoting selfing through geitonogamy and bi-parental mating, as indicated by the large FIS value found in this study.
Similarly, fruit set in females of S. vulgaris decreased with increasing distance from pollen sources, whereas hermaphrodite seed set was unaltered (Taylor et al., 1999). Females were already suffering from pollen limitation when isolated at 20 m from the pollen pool; however, the proportion of capsules and seeds per capsules produced was constant when isolated at distance of 20, 60 or 80 m. This confirms that pollen dispersal in S. vulgaris is generally restricted and that hermaphrodites can potentially ‘escape’ isolation through self-fertilization. Because inbred S. vulgaris plants suffer from inbreeding depression (Jolls and Chenier, 1989; Pettersson, 1992; Emery and McCauley, 2002; Glaettli, 2004), pre-zygotic mechanisms reducing the intensity of self-fertilization and/or bi-parental inbreeding such as differential pollen performance could be of functional significance when reproductive success is not pollen limited.
The advantage of unrelated pollen may be lowered in pollen-limited populations since each pollen grain should be able to find a mate. In a gynodioecious species, only strongly female-biased isolated populations could suffer from pollen limitation (McCauley and Brock, 1998). Although the sex ratio varies from 10 to 100 % hermaphrodites, female-biased sex ratios are not very common in natural populations of S. vulgaris. Only 10 % (two out of 20) of the populations in North America and 8 % (three out of 40) in the Western Swiss Alps were composed of 50 % females or more (McCauley et al., 2000; M. Glaettli, unpubl. res.). Thus, populations suffering from pollen limitation seem to be not frequent enough to break down the potential pre-zygotic mechanism in S. vulgaris.
Proximity-dependent pollen performance
The present in vivo measures of pollen performance revealed that between-patch pollen was significantly larger than for self-pollen donors in both sections (S1 and S2). Moreover, between-patch pollen performance was significantly larger than within-patch pollen performance in section S2 (Fig. 2). In a preliminary study of eight females from the present study population, a parallel although non-significant trend was detected (M. Glaettli, unpubl. res.). Values for ‘late pollen performance’ (PT2) increased with distance, with values of 6·5 ± 1·3 and 7·2 ± 1·8 for within- and between-patch pollination treatments, respectively. Either differential pollen tube growth or pollen tube attrition could explain these differences in pollen performance (Aizen et al., 1990; Cruzan and Barrett, 1996). However, the experiment performed here does not allow discrimination between these two processes.
The potential increase in pollen performance in matings between unrelated individuals is interesting (a) in a metapopulation scenario and (b) in the context of gynodioecy. In a metapopulation, a single or a few immigration events might replenish genetic variation, reduce inbreeding depression and increase the persistence of a given population or patch; a process known as ‘genetic rescue’ (Madsen, 1999; Richards, 2000; Ingvarsson, 2001). When adding the complexity of gynodioecy, fine scale sex ratio variation can result in pollen limitation in patches with strongly female-biased sex ratios (McCauley and Brock, 1998; Taylor et al., 1999). The few hermaphrodites in these patches will achieve high male reproductive success by fertilizing the seeds of all surrounding female plants. However, the progeny of female S. vulgaris are likely to be female (Charlesworth and Laporte, 1998) and sex ratios will become even more female biased. The female reproductive success of hermaphrodites is likely to be reduced since their seeds will suffer from inbreeding depression (Pettersson, 1992; Emery and McCauley, 2002). Moreover, the progeny from self-fertilized hermaphrodites generally show a female-biased sex ratio relative to progeny from cross-fertilization (Emery and McCauley, 2002; Glaettli, 2004; Bailey and McCauley, 2005). Thus, the higher pollen performance of distant cross-pollen could not only increase the fitness of individuals but could also prevent sex ratios from becoming too strongly biased, reducing the possibilities of patch extinction.
Differential pollen performance could act as a pre-zygotic barrier reducing the intensity of selfing and bi-parental inbreeding in S. vulgaris. Direct competition using mixed pollen loads has revealed that differential pollen tube growth of self- and cross-pollen is related to seed siring success in Hibiscus moschetus (Snow and Spira, 1991a), Betula pendula (Pasonen et al., 1999) and Picea abies (Aronen et al., 2002). These authors demonstrated that pollen performance is likely to be a relevant estimate of seed siring success, although other factors can influence seed paternity (see Melser et al., 1997). Within-style interactions of different pollen donors, interaction between the pollen and the style, and post-zygotic events such as selective seed abortion have all been shown to influence the outcome of pollen competition experiments (Cruzan, 1990; Herrero and Hormaza, 1996; Hormaza and Herrero, 1996). To confirm the occurrence of differential pollen performance leading to variation in siring success in S. vulgaris, future studies of pollen-tube growth, mixed pollinations, and the use of genetic markers will be required.
Acknowledgments
We thank Chantal Décaillet for field work, Rose-Marie Hofer for microscopy instruction, Nicolas Juillet for genetic analysis, Lynda F. Delph and Nickolas M. Waser, for methodology advice, and Spencer C. H. Barrett, Giorgina Bernasconi, Luc D. B. Gigord, Susan R. Kephart, David E. McCauley and Christian Parisod for comments on the manuscript. This work is part of the PhD requirements for M.G. which was financed by grant no. 3100-05945·98 to J.G. Further financial support was provided by the Société Académique Vaudoise (grant to M.G.), by the Roche Research Foundation (grant to M.G.) and by the Swiss National Science Foundation (grant no. PBLAA-109434 to M.G.).
LITERATURE CITED
- Aizen MA, Searcy KB, Mulcahy DL. 1990. Among-flower and within-flower comparisons of pollen-tube growth following self-pollinations and cross-pollinations in Dianthus chinensis (Caryophyllaceae). American Journal of Botany 77: 671–676. [Google Scholar]
- Aronen T, Nikkanen T, Harju A, Tiimonen H, Häggman H. 2002. Pollen competition and seed-siring success in Picea abies. Theoretical and Applied Genetics 104: 638–642. [DOI] [PubMed] [Google Scholar]
- Bailey MF, McCauley DE. 2005. Offspring sex ratio under inbreeding and outbreeding in a gynodioecious plant. Evolution 59: 287–295. [PubMed] [Google Scholar]
- Charlesworth D, Laporte V. 1998. The male-sterility polymorphism of Silene vulgaris: analysis of genetic data from two populations and comparison with Thymus vulgaris. Genetics 150: 1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvet D, Atlan A, Belhassen E, Gliddon C, Gouyon PH, Kjellberg F. 1990. Co-evolution between two symbionts: the case of cytoplasmic male-sterility in higher plants. In: Futuyama D, Antonovics J, eds. Oxford surveys in evolutionary biology, Vol. 7. Oxford: Oxford University Press, 225–247.
- Cruzan MB. 1990. Pollen–pollen and pollen–style interactions during pollen-tube growth in Erythronium grandiflorum (Liliaceae). American Journal of Botany 77: 116–122. [Google Scholar]
- Cruzan MB, Barrett SCH. 1996. Postpollination mechanisms influencing mating patterns and fecundity: an example from Eichhornia paniculata. American Naturalist 147: 576–598. [Google Scholar]
- Delph LF. 1990. Sex-ratio variation in the gynodioecious shrub Hebe strictissima (Scrophulariaceae). Evolution 44: 134–142. [DOI] [PubMed] [Google Scholar]
- Delph LF. 2004. Testing for sex differences in biparental inbreeding and its consequences in a gynodioecious species. American Journal of Botany 91: 45–51. [DOI] [PubMed] [Google Scholar]
- Dulberg R, Horovitz A. 1984. Gender polymorphism in flowers of Silene vulgaris (Moench) Garke (Caryophyllaceae). Botanical Journal of the Linnean Society 89: 101–117. [Google Scholar]
- Emery SN, McCauley DE. 2002. Consequences of inbreeding for offspring fitness and gender in Silene vulgaris, a gynodioecious plant. Journal of Evolutionary Biology 15: 1057–1066. [Google Scholar]
- Frank S. 1989. The evolutionary dynamics of cytoplasmic male sterility. American Naturalist 133: 345–376. [Google Scholar]
- Gigord LBD, Lavignes C, Shykoff JA, Atlan A. 1998. No evidence for local adaption between cytoplasmic male sterility and nuclear restorer genes in the gynodioecious species Thymus vulgaris L. Heredity 81: 156–163. [Google Scholar]
- Glaettli M. 2004. Mechanisms involved in the maintenance of inbreeding depression in gynodioecious Silene vulgaris (Caryophyllaceae): an experimental investigation. PhD Thesis, University of Lausanne, Switzerland.
- Goudet J. 1995. FSTAT (Version 1·2): a computer program to calculate F-statistics. Journal of Heredity 86: 485–486. [Google Scholar]
- Herrero M, Hormaza JI. 1996. Pistil strategies controlling pollen-tube growth. Sexual Plant Reproduction 9: 343–347. [Google Scholar]
- Hormaza JI, Herrero M. 1996. Dynamics of pollen-tube growth under different competition regimes. Sexual Plant Reproduction 9: 153–160. [Google Scholar]
- Ingvarsson PK. 2001. Restoration of genetic variation lost—the genetic rescue hypothesis. Trends in Ecology and Evolution 16: 62–63. [DOI] [PubMed] [Google Scholar]
- Jolls CL, Chenier TC. 1989. Gynodioecy in Silene vulgaris (Caryophyllaceae): progeny success, experimental design and maternal effects. American Journal of Botany 76: 1360–1367. [Google Scholar]
- Juillet N, Freymond H, Degen L, Goudet J. 2003. Isolation and characterization of highly polymorphic microsatellite loci in the bladder campion, Silene vulgaris (Caryophyllaceae). Molecular Ecology Notes 3: 358–359. [Google Scholar]
- Koelewijn HP. 2004. Sibling competition, size variation and frequency-dependent outcrossing advantage in Plantago coronopus. Evolutionary Ecology 18: 51–74. [Google Scholar]
- Kohn JR, Biardi JE. 1995. Outcrossing rates and inferred levels of inbreeding depression in gynodioecious Cucurbita foetidissima (Cucurbitaceae). Heredity 75: 77–83. [Google Scholar]
- Laporte V, Viard F, Bena G, Valero M, Cuguen J. 2001. The spatial structure of sexual and cytonuclear polymorphism in the gynodioecious Beta vulgaris ssp. maritima: I. At a local scale. Genetics 157: 1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA. 1984. Inbreeding depression and proximity-dependent crossing success in Phlox drummondii. Evolution 38:116–127. [DOI] [PubMed] [Google Scholar]
- Levin DA. 1989. Proximity-dependent cross compatibility in Phlox. Evolution 43: 1114–1116. [DOI] [PubMed] [Google Scholar]
- Madsen T. 1999. Conservation biology—restoration of an inbred adder population. Nature 402: 34–35. [Google Scholar]
- McCauley DE. 1998. The genetic structure of a gynodioecious plant: nuclear and cytoplasmic genes. Evolution 52: 255–260. [DOI] [PubMed] [Google Scholar]
- McCauley DE, Brock MT. 1998. Frequency-dependent fitness in Silene vulgaris, a gynodioecious plant. Evolution 52: 30–36. [DOI] [PubMed] [Google Scholar]
- McCauley DE, Olson MS, Emery SN, Taylor DR. 2000. Population structure influences sex ratio evolution in a gynodioecious plant. American Naturalist 155: 814–819. [DOI] [PubMed] [Google Scholar]
- Melser C, Rademaker MCJ, Klinkhamer PGL. 1997. Selection on pollen donors by Echium vulgare (Boraginaceae). Sexual Plant Reproduction 10: 305–312. [Google Scholar]
- Mulcahy DL. 1979. The rise of angiosperms: a genecological factor. Science 206: 20–23. [DOI] [PubMed] [Google Scholar]
- Mutikainen P, Delph LF. 1998. Inbreeding depression in gynodioecious Lobelia siphilitica: among-family differences override between-morph differences. Evolution 52: 1572–1582. [DOI] [PubMed] [Google Scholar]
- Pasonen HL, Pulkkinen O, Kapyla M, Blom A. 1999. Pollen-tube growth rate and seed-siring success among Betula pendula clones. New Phytologist 143: 243–251. [Google Scholar]
- Pettersson MW. 1991. Pollination by a guild of fluctuating moth populations: option for unspecialization in Silene vulgaris. Journal of Ecology 79: 591–604. [Google Scholar]
- Pettersson MW. 1992. Advantages of being a specialist female in gynodioecious Silene vulgaris (Caryophyllaceae). American Journal of Botany 79: 1389–1395. [Google Scholar]
- Queller DC, Goodnight KF. 1989. Estimating relatedness using genetic markers. Evolution 43: 258–275. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2005. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org.
- Robertson IC, Ulappa AC. 2004. Distance between pollen donor and recipient influences fruiting success in slickspot peppergrass, Lepidium papilliferum. Canadian Journal of Botany 82: 1705–1710. [Google Scholar]
- Sakai AK, Weller SG, Chen M-L, Chou S-Y, Tasanont C. 1997. Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates and resource allocation in Schiedea adamantis (Caryophyllaceae). Evolution 51: 724–736. [DOI] [PubMed] [Google Scholar]
- Saumitou-Laprade P, Cuguen J, Vernet P. 1994. Cytoplasmic male-sterility in plants—molecular evidence and the nucleocytoplasmic conflict. Trends in Ecology and Evolution 9: 431–435. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Pautler LP. 1984. The effect of pollen composition on fitness components in a neotropical herb. Oecologia 62: 31–36. [DOI] [PubMed] [Google Scholar]
- Shykoff JA. 1988. Maintenance of gynodioecy in Silene acaulis (Caryophyllaceae): stage-specific fecundity and viability selection. American Journal of Botany 75: 844–850. [Google Scholar]
- Snow AA, Spira TP. 1991a. Differential pollen-tube growth rates and nonrandom fertilization in Hibiscus moscheutos (Malvaceae). American Journal of Botany 78: 1419–1426. [Google Scholar]
- Snow AA, Spira TP. 1991b. Pollen vigor and the potential for sexual selection in plants. Nature 352: 796–797. [Google Scholar]
- Souto CP, Aizen MA, Premoli AC. 2002. Effects of crossing distance and genetic relatedness on pollen performance in Alstroemeria aurea (Alstroemeriaceae). American Journal of Botany 89: 427–432. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Trimble S, McCauley DE. 1999. Ecological genetics of gynodioecy in Silene vulgaris: relative fitness of females and hermaphrodites during the colonization process. Evolution 53: 745–751. [DOI] [PubMed] [Google Scholar]
- Thompson J, Tarayre M. 2000. Exploring the genetic basis and proximate causes of female fertility advantage in gynodioecious Thymus vulgaris. Evolution 54: 1510–1520. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. 1983. Optimal and actual outcrossing in plants, and the nature of plant–pollinator interaction. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. Scientific Academic Editions, New York, 341–259.
- Waser NM, Price MV. 1991. Outcrossing distance effects in Delphinium nelsonii: pollen loads, pollen-tubes and seed set. Ecology 72: 171–179. [Google Scholar]
- Waser NM, Price MV. 1993. Crossing distance effects on prezygotic performance in plants: an argument for female choice. Oikos 68: 303–308. [Google Scholar]
- Weir BS, Cockerham CC. 1984. Estimating F-statistics for the analysis of population-structure. Evolution 38: 1358–1370. [DOI] [PubMed] [Google Scholar]
- Weller SG, Ornduff R. 1989. Incompatibility in Amsinckia grandiflora (Boraginaceae)—distribution of callose plugs and pollen-tubes following intermorph and intramorph crosses. American Journal of Botany 76: 277–282. [Google Scholar]


