Abstract
• Background and Aims Populus euphratica is a light-demanding species ecologically characterized as a pioneer. It grows in shelter belts along riversides, being part of the natural desert forest ecosystems in China and Middle Eastern countries. It is able to survive extreme temperatures, drought and salt stress, marking itself out as an important plant species to study the mechanisms responsible for survival of woody plants under heat stress.
• Methods Heat effects were evaluated through electrolyte leakage on leaf discs, and LT50 was determined to occur above 50 °C. Protein accumulation profiles of leaves from young plants submitted to 42/37 °C for 3 d in a phytotron were determined through 2D-PAGE, and a total of 45 % of up- and downregulated proteins were detected. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF)/TOF analysis, combined with searches in different databases, enabled the identification of 82 % of the selected spots.
• Key Results Short-term upregulated proteins are related to membrane destabilization and cytoskeleton restructuring, sulfur assimilation, thiamine and hydrophobic amino acid biosynthesis, and protein stability. Long-term upregulated proteins are involved in redox homeostasis and photosynthesis. Late downregulated proteins are involved mainly in carbon metabolism.
• Conclusions Moderate heat response involves proteins related to lipid biogenesis, cytoskeleton structure, sulfate assimilation, thiamine and hydrophobic amino acid biosynthesis, and nuclear transport. Photostasis is achieved through carbon metabolism adjustment, a decrease of photosystem II (PSII) abundance and an increase of PSI contribution to photosynthetic linear electron flow. Thioredoxin h may have a special role in this process in P. euphratica upon moderate heat exposure.
Keywords: Populus euphratica, moderate heat stress, mass spectrometry, proteome profiling, carbon metabolism
INTRODUCTION
Changes in environmental temperature induce stress in most crop plants, especially in those not adapted to face extreme temperatures. Most crops cultivated worldwide are exposed to severe heat stress during their life cycle, suffering a reduction in yield and quality of fruits (Maestri et al., 2002). Accelerated global warming is a growing concern as it represents an increase in the average temperature with which worldwide crops have to cope. Efforts to improve crop performance upon exposure to high temperatures have been focused on plant transformation with genes offering enhanced thermal tolerance (Grover et al., 2000; Sharkey, 2000; Iba, 2002; Wang et al., 2003). However, heat effects are still not as well understood as other abiotic stresses, such as cold or high-intensity light. In recent years, however, knowledge of the molecular mechanisms behind plants' responses to heat stress has grown substantially. Nowadays, it is clear that heat induces signalling cascades, in prokaryotic and eukaryotic cells, which trigger the transcription of a specific set of genes through the activation of several transcription factors. High temperature in eukaryotes induces nuclear import and binding of heat shock factor 1 (HSF1) trimers to heat shock promoter elements. HSFs promote transcription of these genes against depletion of constitutive genes (Mishra et al., 2002; Bharti et al., 2004; Port et al., 2004). The result is the increase of proteins involved in proteolysis and chaperone activity (Mathew et al., 1998). Changes in the redox state of the chloroplast electron transport chain or in pools of photosynthesis-coupled redox-active compounds (thioredoxin, glutathione) exert regulation over both plastid- and nuclear-encoded chloroplast-expressed proteins (Dat et al., 2000). Hydrogen peroxide, mainly resulting from the activity of NADPH oxidases, is rapidly accumulated upon high temperature, among other stresses (Laloi et al., 2004), and may act as an important signalling molecule (Foyer et al., 2005). A group of proteins, part of the plant antioxidant system, are rapidly activated in response to oxidative stress generated by heat, including superoxide dismutases, catalases and peroxidases. Modulation of the heat stress response is also dependent on cellular control of degradation and maintenance of quality of proteomes by the ubiquitin–proteasome system (Mathew and Morimoto, 1998; Mathew et al., 1998). This system, which is involved on the regulation of transcription factors (Ingvardsen et al., 2001), removes denaturated and misfolded proteins through proteolysis of soluble cytosolic and nuclear proteins.
Plant species of particular interest for the study of thermal stress tolerance or resistance are those highly adapted to survive in extreme environments (Robertson et al., 1994; Lund et al., 1998; Skylas et al., 2002; Wang et al., 2003; Wullschleger and Difazio, 2003) since they may retain regulatory mechanisms enabling their survival. The dissection of such mechanisms may reveal a set of genes, and their products, that may contribute to genetic improvement for thermal stress tolerance in other plants, such as economically important cultivars (Bohnert et al., 2000, 2001; Hanson and Tabita, 2003). This is the case with Populus euphratica, a light-demanding species ecologically characterized as a pioneer that grows during the summertime in shelter belts along riversides (Shiji et al., 1996), as part of the natural desert forest ecosystems in China and in Middle Eastern countries (Youlin et al., 2001). Euphrates poplar is highly adapted to salt stress, extreme temperatures and drought (Ma et al., 1997; Gu et al., 1999, 2004b). Hence, P. euphratica may be considered an important plant species to study the events responsible for woody plants survival under heat stress.
Most components of the heat stress response mechanism in plants can be identified through high-throughput transcriptomic and proteomic analysis (Thiellement et al., 2002; Hanson and Tabita, 2003; Gu et al., 2004a). Proteomic studies are currently performed through a combination of 2D-PAGE gels and mass spectrometry analysis. Protein separation in 2D gels may be followed by tryptic in gel digestion and mass spectrometry analysis of the resulting peptides. The original proteins can be identified with mass spectrometry and database searching through the use of either peptide mass fingerprinting or tandem mass spectrometry information, using a bottom-up strategy. These protein identification approaches are dependent on databases of already known peptide or nucleotide sequences. As genomic sequencing projects are relatively recent, only a few genomes are available in public databases (Liska and Shevchenko, 2003). Populus is a genus that already has a broad collection of expressed sequence tags (ESTs) available to public use and has a completely sequenced genome from Populus trichocharpa. The aim of our study was to analyse leaf proteomes of P. euphratica and identify up- and downregulated proteins through the use of different databases, aiming to contribute to the knowledge of the molecular mechanisms underlying Euphrates poplar tolerance/resistance to high temperatures.
MATERIALS AND METHODS
Plant propagation and hydroponic culture
Plants of Populus euphratica Oliv. maintained under greenhouse conditions were used, after entering dormancy, to prepare grafts. Rooting was performed by immersion of grafts in 500 µg L−1 1-naphthaleneacetic acid (NAA) solution for 48 h under greenhouse conditions. Rooted grafts were transferred to cuvettes of 5 × 5 × 8 cm, filled with river sand, and covered with plastic to maintain high humidity. After 3 weeks in the greenhouse (rooting ratio of 80 %), grafts were transferred to hydroponic culture in a phytotron, to mimic their natural habitat. Hydroponic culture was necessary to maintain the growth rate of P. euphratica plantlets in the absence of natural sunlight. The nutrient solution, containing 8·3 mm Ca2+, 3·44 mm K+, 1 mm Mg2+, 8·3 mm NO3–, 1·84 mm H2PO4–, 1 mm SO42–, 37·8 μm Fe·EDTA, 0·32 μm Cu2+, 0·8 μm Zn2+, 0·4 μm Mn2+, 50 μm H3BO3 and 0·082 μm MoO42–, was set to pH 5·5–5·8. Temperature and relative humidity (RH) in the Fitoclima 700 EDTU phytotron (Aralab, S. Domingos de Rana, Portugal) were set as 30 °C and 80 % for 16 h of light (350 μE m−2 s−1) and as 20 °C and 95 % for 8 h of darkness, corresponding approximately to the season photoperiod. Forty-eight plants were selected for sampling during heat treatments.
Cell membrane thermostability
Fifteen mature leaves were taken from young plants growing under greenhouse conditions and washed with distilled water to remove contaminants. Leaf discs of 3 mm diameter were prepared starting from total leaf width (average of 4 mm, including midrib) and kept in culture vials, each containing 15 discs prepared from five leaves. Leaf disc-containing vials were closed and submerged in a water bath (Thermomix BU coupled to Frigomix U, B. Braun, Melsungen AG, Germany) at each of the selected experimental temperatures (25, 40, 45, 50 and 55 °C). Each experimental temperature effect on leaf discs was assessed with nine replicates from three independent experiments. After 30 min, vials were withdrawn from the water bath and leaf discs were immediately immersed in sterile de-ionized water and then incubated for 2 h at room temperature under agitation at 30 r.p.m. on an Agitorb 300E (Aralab, S. Domingos de Rana, Portugal). Electrolyte leakage, from control and heat treatments, was immediately measured at +18 °C with a CDM 83 conductivity meter (Radiometer, Copenhagen, Denmark), using potassium hydroxide at 1 m for calibration. Maximum conductivity was measured after autoclaving for 15 min at 1·1 Pa and 121 °C, and after cooling at room temperature overnight under agitation. Significant membrane damage was considered to occur at the temperature at which the calculated percentage damage would exceed 50 % (lethal temperature, LT50). Electrolyte leakage (EL), directly proportional to cell membrane damage, was calculated using the equation EL(%) = [(Cx − Ci)/Cm] × 100, where C is water conductivity under control conditions (i), experimental high temperature (x) and at maximum conductivity (m; after autoclaving).
Heat treatment design
For the heat experiment, it was necessary to mimic greenhouse conditions inside a controlled environment cabinet or phytotron (Fitoclima 700 EDTU). Control conditions were set close to the season climatic parameters, allowing small deviations to the adaptation status of P. euphratica growth under greenhouse conditions. Daily cycles of temperature and RH were monitored and plotted to enable proper design of the experiment. Table 1 presents the established phytotron conditions for the heat experiment regarding RH, temperature and photoperiod. RH was set to 80 % during the light period. Since P. euphratica culture was carried out under hydroponic conditions, a high RH was chosen to allow higher thermal conductance between air and the plant body and to ensure proximity between these two values. Control samples were collected on the day before starting the heat treatment, after submitting P. euphratica plants to 30 °C for 6 h, under phytotron full light intensity (350 µmol photons m−2 s−1). Heat treatment started with a temperature increase from 20 to 42 °C for 5 h on the first day, corresponding to an increase of 4·2 °C h−1. This gradual increase in temperature during the heat treatment generates a lower inhibition of the photosynthetic machinery, compared with a rapid temperature increase, and allows an adaptation process (Law and Crafts-Brander, 1999). In this way, heat shock was induced and maintained for the remaining treatment. However, since transition from day to night implies a natural reduction in environmental temperature, a slight decrease in temperature (5 °C) on the transition from light to dark was introduced. Hence, the maximum temperature in the light period, set to 42 °C, was decreased to 37 °C for 5 h (–1 °C h−1) and maintained for the following 8 h of darkness. The total experiment lasted for 4 d and sampling was always performed at the end of the 6 h segment for control (30 °C) and heat stress (42 °C) conditions on day 1 (6 h), day 2 (30 h) and day 3 (54 h). Samples consisted of ten fully mature leaves taken from each plant of a total of 12 plants used for each experimental condition. Continuous light intensity was set for the whole light period in the culture chamber (except for 30 min of transition at the beginning of the first segment) in order to minimize light-induced oscillations of protein accumulation profiles. All the samples were taken at exactly the same time point each day so that protein accumulation profiles dependent on circadian control would not mask heat-induced accumulation profiles, producing misleading differences detected on protein profiles.
Table 1.
Experimental design programmed in the phytotron regarding photoperiod, temperature and relative humidity for sample collection
| Parameters | Control | Day 1 | Day 2 | Day 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sd (h) | 5 | 6 | 5 | 8 | 5 | 6 | 5 | 8 | 5 | 6 | 5 | 8 | 5 | 6 |
| Tb (°C) | 20 | 30 | 30 | 20 | 20 | 42 | 42 | 37 | 37 | 42 | 42 | 37 | 37 | 42 |
| Te (°C) | 30 | 30 | 20 | 20 | 42 | 42 | 37 | 37 | 42 | 42 | 37 | 37 | 42 | 42 |
| RHb (%) | 95 | 80 | 80 | 95 | 95 | 80 | 80 | 95 | 95 | 80 | 80 | 95 | 95 | 80 |
| RHe (%) | 80 | 80 | 95 | 95 | 80 | 80 | 95 | 95 | 80 | 80 | 95 | 95 | 80 | 80 |
Daily cycles in the phytotron were set as four segments per day. Sd(h), segment duration in h; for each segment, the temperature and relative humidity are presented. Tb (°C), temperature at the beginning of the segment in°C; Te (°C), temperature at the end of the segment in°C; RHb (%), percentage relative humidity at the beginning of the segment; RHe (%), percentage relative humidity at the end of the segment.
Protein precipitation
Mature leaves were used for protein precipitation. Pooled samples, representing 120 leaves—ten leaves from each of 12 plants—were ground in liquid nitrogen and approx. 1·5 g of powder was suspended in ethanol at four times the sample volume. After 1 h at −20 °C, the same volume of cold acetone was added and proteins were allowed to precipitate overnight, at −20 °C. Proteins were collected through centrifugation at 26 000 g (–10 °C; 15 min), followed by the addition of nine times the sample volume of a solution of ethanol : acetone : triple distilled water 4 : 4 : 1 (v/v/v). Proteins were then re-suspended and reprecipitated for 6 h at −20 °C. Following centrifugation at 26 000 g (–10 °C, 40 min), two more washing steps were performed as before and the final pellet was dried overnight at room temperature.
Protein solubilization and IEF
Proteins were solubilized by adding 3·5 mL of lysis buffer [7 m urea, 1·9 m thiourea, 1 % (v/v) of Pharmalyte 3–10 (Amersham Pharmacia Biotech, Uppsala, Sweden), 2 % (w/v) CHAPS and 0·4 % (w/v) dithiothreitol (DTT)] to the dry pellet. Proteins were allowed to solubilize for 24 h at room temperature (20 °C) and protein quantification was performed with Bradford standard assay. IPG strips of 18 cm, linear pH 4–7 (Amersham Pharmacia Biotech, Uppsala, Sweden), were rehydrated overnight in lysis buffer containing 200 µg of protein for each gel. IPG strips were transferred to a Multiphor II isoelectric focusing (IEF) unit and IEF was performed at 20 °C, 5 mA and 5 W to a total of 16 000 Vh. IPG strips were shaken for 15 min in equilibration buffer [2 % (w/v) SDS, 10 % (v/v) glycerol, 50 mm Tris–HCl pH 6·8 and 5 % (v/v) 2-mercaptoethanol] and stored at −70 °C until use.
2D-PAGE
IPG strips were thawed and re-equilibrated for 15 min using fresh equilibration buffer, and immediately loaded onto 20 × 18·5 × 0·1 cm, 12·5 % polyacrylamide gels (acrylamide to bisacrylamide ratio of 200 : 1) without SDS. Electrophoresis was performed in recirculating running buffer overnight at 20 °C, under constant current settings.
Staining, scanning and image analysis
Two-dimensional polyacrylamide gels were fixed and stained with Sypro Ruby. Gel images were obtained with a cooled CCD camera system and analysed with BioImage 2-D Analyzer software (Version 6·1). Protein expression was determined through the sum of all the pixel values within the boundary of each spot expressed as a percentage (integrated optical density percentage, IOD%) of the sum of IOD values for all the detected spots. All the detected spots were submitted to matching. After automated detection, spots were edited manually, matched, and the IOD% values were exported and analysed with Microsoft Excel.
Spot excision and protein digestion
Spots of interest were manually excised from 2D gels with a scalpel, washed with 70 μL of deionized water followed by acetonitrile 100 % (90 μL, 15 min; 30 μL, 2 min). The spots were dehydrated in a vacuum centrifuge and rehydrated with a solution of 67 ng of trypsin in 50 mm NH4HCO3, at 4 °C. After 20 min, 30 μL of 50 mm NH4HCO3 were added and digestion proceeded at 37 °C overnight, followed by storage at −20 °C until use.
Sample preparation and mass spectrometry
Desalting was performed on custom-made reverse-phase microcolumns, prepared with R2 resin (Perseptive Biosystems Inc., Framingham, MA, USA) as described elsewhere (Gobom et al., 1999). Peptide solution, obtained from digestion of each spot, was loaded onto the microcolumn, followed by washing with 10 μL of 1 % trifluoroacetic acid (TFA). Bound peptides were eluted with 0·8 μL of matrix solution [α-cyano-4-hydrocynnamic acid (5 g L−1) in 70 % acetonitrile/0·1 % TFA] directly onto the matrix-assisted laser desorption ionization (MALDI) target plate. Peptide mass spectra were acquired in positive reflector mode on a 4700 Proteomics Analyzer MALDI-time of flight (TOF)/TOF (Applied Biosystems, Foster City, CA, USA) using 20 kV of acceleration voltage. Each spectrum was obtained with a total of 1000–1200 laser shots and was externally calibrated using peptides derived by tryptic digestion of lactoglobulin. Further processing and interpretation of the MS spectra was performed with m/z software (Genomic Solutions Inc., Ann Harbor, MI, USA). Tandem mass spectra were acquired using the same instrument in MS/MS positive mode. Further processing and interpretation of the MS/MS spectra were performed using Data Explorer (version 4·4, Applied Biosystems). All MS/MS data from each individual spot were merged into a single file before search.
Database search
Peptide mass maps and sequences obtained by tandem mass spectrometry were searched against all Viridiplantae entries of NCBInr and against a poplar EST database (Shevchenko et al., 1997) from Umea Plant Science Centre (Umea, Sweden) using the Mascot search engine 2·0 (Matrix Science Ltd, London, UK) (Perkins, 1999). Proteins identified in both ways were always manually checked to exclude false-positive hits. Search parameters were carbonyl propionamidation (cystein) as fixed modifications, methionine oxidation as variable modifications, peptide mass tolerance of 70 ppm at the most and a general fragment mass tolerance of 0·25 Da (up to 0·8 Da when necessary). According to the search engine, a score of 65 represents a significant identification (P > 0·05) when the database is restricted to the Viridiplantae taxonomy (NCBInr 20041113).
RESULTS
Euphrates poplar has a high cell membrane thermostability
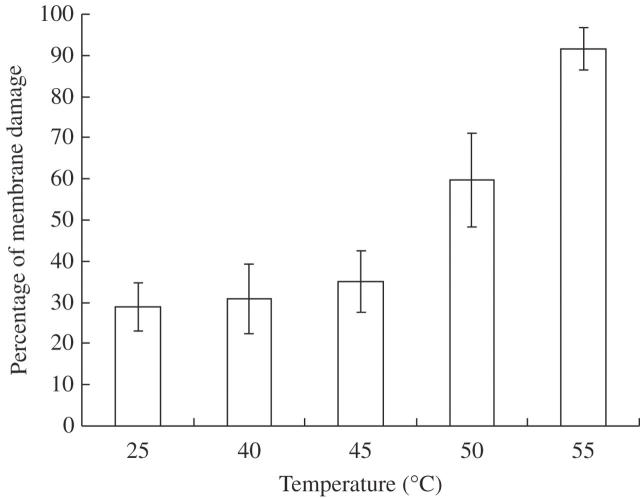
Under greenhouse conditions, P. euphratica plants are sensitive to daylight intensity. Stress signals include, in these cases, stem darkening, changing from light pink to magenta from basis to apex, and leaves darkening to dark green. The internodes stop elongation and the apical meristem loses morphogenic activity. Exposure of P. euphratica plants to 42 °C for 54 h did not affect their survival. Heat-induced changes on stem coloration were reversed after recovery from heat stress. Apical dominance was re-aquired and initial flushing of axillary buds was interrupted. Cell membrane thermostability was estimated in order to choose a non-lethal, nor sublethal, temperature for the heat treatment. Cell membrane thermostability can be estimated through electrolyte leakage following exposure to physical stress factors, such as heat or cold, as has been used in soybean (Glycine max), sorghum (Sorghum bicolor) and melon (Cucumis melo). It has also been used in cool season crops such as wheat (Triticum aestivum) and Kentucky bluegrass (Poa pratensis) (see Ismail and Hall, 1999 and references therein) and to assess osmotic stress tolerance in transgenic plants (Abe et al., 2003). The evaluation of cellular membrane thermostability through analysis of membrane electrolyte leakage revealed a small increase of cell membrane ion permeability up to 45 °C, presenting thereafter a higher increase up to 55 °C (Fig. 1). The lethal temperature (LT50) was determined to be between 50 and 55 °C, since membrane damage at these temperatures due only to heat was estimated to be 31 and 63 %, respectively. A temperature of 42 °C was selected as the maximum temperature to which to expose P. euphratica plants, since this should be non-lethal.
Fig. 2.

Position of identified spots from 2D 12·5 % polyacrylamide gels. From left to right, pI 4–7 linear range. Arrowheads indicate the spots identified by mass spectrometry, together with the respective spot number presented in Table 2.
Fig. 1.
Percentage of membrane damage of leaf cells from plants of P. euphratica submitted to temperatures up to 55 °C. Columns represent the average percentage membrane damage, calculated from the results of three independent experiments. The y-axis represents the total percentage of electrolyte leakage and the x-axis represents the temperature, in °C, to which leaf discs were exposed.
Moderate heat exposure induces growth arrest in P. euphratica
Euphrates poplar presents active growth under high temperature in its natural habitat, as long as it has access to the water table. It is possible to find this species as part of Chinese and Middle Eastern desert communities, distributed along riversides and following the track of the underground water. Therefore, it is not surprising that, even with a high salt concentration in the soil, or with atmospheric temperatures as high as 50 °C, it does not show a significant drought stress in its natural habitat (Brosche et al., 2005). When working with P. euphratica, we must use an experimental design where water availability and high light quality requirements are provided for this species, in order to represent true natural conditions. The system described in this work enabled those requirements to be guaranteed, compensating for low-quality light through direct nutrient supply to the root system. Macroscopic evaluation of the effects of temperature increase on P. euphratica plants was performed during the heat experiment. A slight curling of the younger leaves (generally the third and fourth node below the apex) and a change in stem coloration (from light pink to light green) were observed after 30 h of exposure to 42 °C. After 24 h of recovery from heat stress, P. euphratica axillary buds begun to flush. Although this initial flush was sustained for 4–6 d, it never gave rise to new shoots because apical meristem started developing again and regained dominance during that period. Plants recovering for 8–10 d again showed their light pink stem coloration; the young leaves were no longer curled and no other stress symptoms were observed. Observations of P. euphratica plants during the heat treatment and recovery reported here confirmed our prediction from electrolyte leakage results.
Protein identification by homology in a woody plant species
Proteomic and genomic studies in trees have been difficult to accomplish due to several problems related to genome sizes and recalcitrance to in vitro manipulation (Canovas et al., 2004), but the completion of genome sequencing of P. trichocharpa provided public availability of a precious information resource, as it is now possible to obtain more accurate data on gene and protein sequences on woody plant species. Therefore, protein identification by homology through cross-species search (within woody plant species) may achieve a higher level of accuracy. The number of identifications achieved for P. euphratica proteins in the work reported here was particularly high, considering that this study was performed in a woody plant species without an annotated genome. The combination of two different approaches (protein identification based on cross-species sequence homology among Viridiplantae and protein identification based on an EST collection of a closely related species, P. trichocarpa) contributed to these results, together with good quality spectra and database researching. In the case of citrate synthase identification by homology search, some of the most intense peaks matched SwissProt/TrEMBL accession no. O24259. This was considered sufficient to accept this entry as the closest homologue, although a statistically non-significant MASCOT algorithm score has been obtained (Table 2). The sequence tag MASCOT scores were obtained using the most powerful search tool (Sequence Query on http://www.matrixscience.com/search_form_select.html), through the use of a combination of molecular weight and sequence data information (Mann and Wilm, 1994). Using this combination of data, it became possible to achieve the best homology match because several peptide sequences from the same protein increase the information available and allow higher accuracy of matches compared with the use of single sequence data.
Image analysis of leaf proteomes allowed detection and quantification of 1355 spots, of which 653 showed IOD% changes of more than ±0·5-fold when compared with control values. Overall upregulation was observed for 19·9 % of the spots, while 16·4 % showed downregulation. Moreover, 2·1 % of the spots showed an accumulation profile classified as random. Therefore, 55 % of the 1355 spots have been classified as constant, showing changes of IOD% in the range of less than ±0·5-fold of control values. The up- or downregulated spots were grouped according to their accumulation profile as a function of time (Table 3). No statistical test or algorithm was applied to define these groups or to select the spots in each of them. Mass spectrometry analysis was performed for approx. 10 % of the spots of each profile group, selected based on their quality, to allow coverage of all groups. It was possible to identify protein spots for only nine of the 13 delimited groups, and proteins of four groups could not be identified (Table 3). MALDI-MS and MALDI-MS/MS analysis (Table 2) allowed significant homology matches to 51 out of 62 spots (82·3 % success rate) to be obtained. Thus, it became possible to achieve the best homology match as several peptide sequences for the same protein increase the information and allow higher accuracy of matches compared with the use of single sequence data. All proteins reported here were submitted to the UniProt via SPIN tool and were accepted after UniProt revision (accession numbers in Table 2). Twenty-one identifications were determined using a private Populus EST database, from which 17 identifications were obtained due to matches to homologous proteins from other plant species (Habermann et al., 2004) and four identifications were based only on EST database information. Eighteen of the 51 spots analysed matched ribulose-1,5-biphosphate carboxylase-oxygenase (Rubisco) entries and most of them showed upregulation (data not shown).
Table 2.
Protein spots from 2D gels identified through peptide mass fingerprinting and tandem mass spectrometry after tryptic digestion
| Spot no. | IOD % change (-fold) |
Peptide mass fingerprint (score, e-value); peptide sequences (score/e–value) | Homologue protein | Species | SwissProt/TrEMBL accession no. (UniProt accession no.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 h | 30 h | 54 h | ||||||||||||
| Amino acid metabolism | ||||||||||||||
| 310 | +1·6 | 0·0 | –0·2 | VSLAGHDEYIVR (64/4·8 × 10 −5) | Putative ketol-acid reductoisomerase | Oryza sativa (japonica cultivar group) | REFSEQ: NM_192395 | |||||||
| FYEKEGLPAFPMoxGK (65/4·4 × 10−5) | Ketol-acid reductoisomerase, chloroplast precursor | Spinacea oleracea | Q01292 (P84534) | |||||||||||
| FYEKEGLPAFPMoxGK (63/1 × 10 −4) | BLAST of EST: Q01292 | |||||||||||||
| 335 | 0·0 | –0·1 | –0·6 | TEFGPSQPFKGAK (48/9·1 × 10 −3) | Adenosylhomocysteinase* | P68172 (P84534) | ||||||||
| 338 | +1·1 | –0·1 | –0·7 | AEFGPSQPFKGAK (50/2·7 × 10 −3) | Adenosylhomocysteinase | Catharanthus roseus | P35007 (P84532) | |||||||
| AEFGPSQPFKGAK (50/5·9 × 10 −3) | BLAST of EST: P35007 | |||||||||||||
| 352 | –0·3 | –0·4 | –0·7 | AEFGPSQPFKGAK (40/0·021) | Adenosylhomocysteinase | Catharanthus roseus | P35007 (P84532) | |||||||
| AEFGPSQPFKGAK (40/0·046) | BLAST of EST: P35007 (P84532) | |||||||||||||
| 941 | –0·0 | –0·2 | –0·6 | 8 (61/0·13) | O-Acetylserine (thiol) lyase | Populus alba × P. tremula | Q6V3A7 (P84538) | |||||||
| LIVAIFPSFGER (64) | ||||||||||||||
| TPNSYILQQFENPANPK (102/6·1 × 10 −10) | ||||||||||||||
| 1526 | 0·0 | +1·3 | –0·6 | 7 (60/0·16) | Arginine decarboxylase | Capsella bursa-pastoris | O81178 | |||||||
| YLFAGVVDGR (82/1·3 × 10 −5); GVTGFGFDLVR + G→S [+30·01] (65/8 × 10−4) | Methionine synthase | Catharanthus roseus | Q42699 | |||||||||||
| Carbon metabolism | ||||||||||||||
| 183 | –0·2 | +1·1 | –0·6 | 10 (76/3·8 × 10−3) | NADP-dependent malic enzyme | Populus trichocarpa | P34105 (P84539) | |||||||
| GLIYPPLSNIR (31/0·042) | ||||||||||||||
| SIQVIVVTDGER (24/0·47) | ||||||||||||||
| 11 (77/2 × 10−3) | BLAST of EST: P34105 | |||||||||||||
| 214 | –0·2 | –0·3 | –0·6 | 8 (66/0·045) | Putative 2,3-bisphosphoglycerate- independent phosphoglycerate mutase* | Oryza sativa | REFSEQ: NM_191088 | |||||||
| 222 | –0·7 | –0·3 | –0·2 | ALPTYTPESPADATR (60/2·3 × 10−3) | Transketolase | Solanum tuberosum | Q43848 (P84540) | |||||||
| 8 (73/4·5 × 10−3) | BLAST of EST: transketolase | Craterostigma plantagineum | BLAST of EST: Q42676 | |||||||||||
| 286 | –0·1 | 0·0 | –0·7 | 6 (70/8·9 × 10−3) | BLAST of EST: dihydrolipoamide dehydrogenase* | Bruguiera gymnorrhiza | BLAST of EST: Q93WQ1 | |||||||
| 327 | –0·2 | +1·5 | –0·3 | 8 (83/8·6 × 10−4) | Enolase | Brassica rapa | Q6W7E8 | |||||||
| AAVPSGASTGVYEALELR (74/9·1 × 10−6) | Enolase 2 | Zea mays | P42895 (P84541) | |||||||||||
| FRAPVEPY (17/5·5) | ||||||||||||||
| 519 | –0·4 | –0·2 | –0·6 | 6 (54/0·68) | Citrate (si)-synthase, mitochondrial | Populus balsamifera | O24259 (P84543) | |||||||
| YYTVLFGVSR (46/0·049) | ||||||||||||||
| ALGLPLERPK (38/0·32) | ||||||||||||||
| 558 | –0·7 | –0·6 | –0·4 | 7 (48/2·7) | Transketolase, chloroplast | Craterostigma plantagineum | Q42676 | |||||||
| ALPTYTPESPADATR (41/0·018) | Solanum tuberosum | Q43848 (P84540) | ||||||||||||
| 9 (72/0·006) | BLAST of EST: transketolase | Craterostigma plantagineum | BLAST of EST: Q42676 | |||||||||||
| ALPTYTPESPADATR (41/0·03) | ||||||||||||||
| 553 | +1·2 | +1·5 | –0·4 | AVSLVLPQLK (62/5·9 × 10−4) | Glyceraldehyde-3-phosphate dehydrogenase B* | Pisum sativum | P12859 (P84544) | |||||||
| 1040 | –0·1 | +1·7 | –0·1 | VGKFPLLANSR (45/0·04) | Putative dihydrolipoamide dehydrogenase precursor* | Oryza sativa | REFSEQ: NM_183836 (P84545) | |||||||
| Fatty acid synthesis | ||||||||||||||
| 431 | +2·1 | –0·3 | –0·1 | ITAYLPSGGPFVR (78/2·3 × 10−6) | Biotin carboxylase | Brassica napus | Q93Y50 (P84546) | |||||||
| LLEEAPSPALTPELR (52/8 × 10−4) | ||||||||||||||
| ILVANRGEIAVR (37/6·5 × 10−3) | ||||||||||||||
| Sulfate assimilation | ||||||||||||||
| 449 | +1·8 | 0·0 | –0·4 | KADAVFAFQLR (40/0·008) | ATP sulfurylase* | Brassica oleracea var. botrytis | Q9SBL0 (P84547) | |||||||
| Vitamin biosynthesis | ||||||||||||||
| 916 | +3·2 | 0·0 | –0·9 | 5 (48/2·7) | Thiazole synthase, chloroplast | Alnus glutinosa | Q38709 | |||||||
| LFNAVAAEDLIVKGGR (83/4·8 × 10−7) | BLAST of EST: Q38709 (P84548) | |||||||||||||
| LFNAVAAEDLIVK (69/1·5 × 10−5) | ||||||||||||||
| FQPIKESIVSR (49/1·5 × 10−3) | ||||||||||||||
| EIVPGMIVTGMEVAEIDGAPR 2 oxidation (M) (26/0·77) | ||||||||||||||
| Cytoskeleton | ||||||||||||||
| 486 | +1·7 | +1·1 | –0·4 | 10 (81/1·3 × 10−3) | Tubulin α-1 chain | Anemia phyllitidis | P33623 | |||||||
| AIFVDLEPTVIDEVR (90/6·6 × 10−8) | Oryza sativa | P28752 (P84549) | ||||||||||||
| SLDIERPTYTNLNR (49/1·8 × 10−3) | ||||||||||||||
| FDGAINVDVTEFQTNLVPYPR (36/0·03) | ||||||||||||||
| 10 (86/2·5 × 10−4) | BLAST of EST: tubulin α-3/α-5 chain | Arabidopsis thaliana | BLAST of EST: REFSEQ: NM_121982 | |||||||||||
| Signal transduction | ||||||||||||||
| 997 | +1·6 | –0·3 | +1·1 | ALPNQQTVDYPSFK N-acetyl (protein) [+42·01] (64/1·4 × 10−4) | GTP-binding nuclear protein RAN/TC4 | Vicia faba | P38548 (P84557) | |||||||
| SNYNFEKPFLYLAR (53/4·6 × 10−4) | BLAST of EST: P38548 | |||||||||||||
| SNYNFEKPFLYLAR (53/1·7 × 10−3) | ||||||||||||||
| Transcription/translation | ||||||||||||||
| 1342 | –0·6 | –0·4 | –0·3 | LGAEISSLTLEEAR (61/2·8 × 10−4) | BLAST of EST: 50S ribosomal protein L12-1* | Arabidopsis thaliana | BLAST of EST: REFSEQ: NM_113699 (P84558) | |||||||
| 4341 | –0·5 | –0·4 | –0·3 | QIIEANLALR (47/1·4 × 10−3) | ATP synthase ɛ chain | Androya decaryi | Q8MF84 (P84559) | |||||||
| QIIEANLALRR (42/6·3 × 10−3) | ||||||||||||||
| GFGFVTFGNEK (82/2·7 × 10−6) | BLAST of EST: glycine-rich RNA-binding protein | Euphorbia esula | BLAST of EST: O48567 (P84560) | |||||||||||
| Photosynthesis | ||||||||||||||
| 724 | –0·2 | –0·3 | –0·6 | GFGILDVGYR (55/2·9 × 10−4) | PSII stability/assembly factor, chloroplast | Arabidopsis thaliana | REFSEQ: NM_122218 (P84561) | |||||||
| GTGITEEFEEVPVQSR (27/0·33) | ||||||||||||||
| 1418 | +2·8 | +2·6 | +4·4 | EAPVGFTPPELDPSTPSPIFG -GSTGGLLR (105/5·3 × 10−9) | BLAST of EST: PSI-D (PSI 20 kDa subunit)* | Cucumis sativus | BLAST of EST: P32869 (P84563) | |||||||
| Redox homeostasis | ||||||||||||||
| 3885 | +2·5 | +2·6 | +2·0 | MIAPIFAELAK (52/1·1 × 10−3) | Thioredoxin h | Populus tremula × P. tremuloides | Q8S3L3 (P84564) | |||||||
| TVGADKDGLPTLVAK (53/5·1 × 10−4) | ||||||||||||||
| MIAPIFAELAK (52/2·4 × 10−3) | BLAST of EST: Q8S3L3 | |||||||||||||
| TVGADKDGLPTLVAK (53/1·3 × 10−3) | ||||||||||||||
| Protein metabolism | ||||||||||||||
| 74 | –0·3 | –0·4 | –0·5 | 21 (151) | OSJNBa0039C07·4 protein | Oryza sativa | Q7F9I1 | |||||||
| GSGFVAVEIPFTPR (56/4·2 × 10−4) | Chloroplast ATP-dependent Clp protease ATP-binding subunit ClpA homologue CD4B | Lycopersicon esculentum | P31542 (P84565) | |||||||||||
| VLELSLEEAR (18/2·2) | ||||||||||||||
| VLENLGADPSNIR (70/1·6 × 10−5) | ||||||||||||||
| FLPDKAIDLIDEAGSR (119/2·1 × 10−10) | ||||||||||||||
| VLELSLEEAR (22/2·2) | Chloroplast ATP-dependent Clp protease ATP-binding subunit ClpA homologue CD4A | Lycopersicon esculentum | P31541 | |||||||||||
| FLPDKAIDLIDEAGSR (89/2·1 × 10−10) | ||||||||||||||
| FQPVKVPEPSVDETIQILK (65/1·5 × 10−4) | ||||||||||||||
| 76 | –0·2 | –0·1 | –0·5 | 25 (180) | ATP-dependent Clp protease ATP-binding subunit/ClpC | Arabidopsis thaliana | REFSEQRELEASE: NM_124471 | |||||||
| VLENLGADPSNIR (63/9·7 × 10−5) | Chloroplast ATP-dependent Clp protease ATP-binding subunit ClpA homologue CD4B | Lycorpersicon esculentum | P31542 (P84565) | |||||||||||
| GSGFVAVEIPFTPR (55/6·3 × 10−4) | ||||||||||||||
| VLELSLEEAR (41/0·015) | ||||||||||||||
| 159 | +1·5 | +1·2 | –0·2 | VFISDDFDGELFPR (93/7·7 × 10−8) | Putative heat shock protein (strong similarity to HSP90) | Arabidopsis thaliana | REFSEQ: NM_179601 (P84577) | |||||||
| FLSVTEPSLLGDGGDLEIR G→A [+14·02] (76/4·2 × 10−6) | ||||||||||||||
| GVVDSDDLPLNVSR (65/3·8 × 10−5) | ||||||||||||||
| 242 | –0·5 | –0·6 | –0·6 | 12 (91/1·2 × 10−4) | FtsH-like protein Pftf | Nicotiana tabacum | Q9ZP50 (P84578) | |||||||
| FLEYLDKDR (26/6) | ||||||||||||||
| VRVQLPGLSQELLQK (18/31) | ||||||||||||||
| TPGFSGADLANLLNEAAILAGR (93/5·4 × 10−4) | ||||||||||||||
| TPGFSGADLANLLNEAAILAGRR (19) | ||||||||||||||
| 8 (81/6 × 10−4) | BLAST of EST: Q9ZP50 | |||||||||||||
| TPGFSGADLANLLNEAAILAGR (93/4 × 10−8) | ||||||||||||||
| TPGFSGADLANLLNEAAILAGRR (19/13) | ||||||||||||||
| 939 | –0·6 | –0·1 | –0·4 | IVDTFPGQSIDFFGALR (37/0·037) | Rubisco activase | Cucumis sativus | Q01587 (P84562) | |||||||
| LVDTFPGQSIDFFGALR (37/0·066) | BLAST of EST: Q01587 | |||||||||||||
| EGPPTFEQPAMTIEK (25/1) | ||||||||||||||
| 1138 | +1·5 | +1·3 | –0·2 | VAEAEEKTAGGLLLTETTK T→A [–30·01] (105/3·5 × 10−9) | Chloroplast protein Cpn 10 | Arabidopsis thaliana | O65282 (P84579) | |||||||
| EKPSIGTVIAVGPGSLDEEGKITP E→V [–29·97] (80/1·2 × 10−6) | ||||||||||||||
| YTSIKPLGDR (55/1·6 × 10−4) | ||||||||||||||
| 1558 | +1·6 | +2·3 | +1·8 | VVAAGANPVLITR (47/6·9 × 10−4) | Chaperonin 60 β subunit (Rubisco subunit binding-protein, β subunit) | Solanum tuberosum | P93570 (P84581) | |||||||
| DLVNVLEDAIR (27/1) | ||||||||||||||
| VVAAGANPVLITR (73/5·3 × 10−5) | BLAST of EST: P93570 | |||||||||||||
| ATP synthesis | ||||||||||||||
| 1461 | +3·9 | +6·9 | +6·3 | 6 (62/0·055) | BLAST of EST: plastid ATP synthase CF1 α chain | Nicotiana tabacum | BLAST of EST: P00823 (P84582) | |||||||
| TQFQEIISSTK (51/2·8 × 10−3) | ||||||||||||||
| KFLVELR (22/0·71) | ||||||||||||||
| 3570 | +1·6 | +1·6 | –0·3 | 9 (60/0·15) | Mitochondrial F1 ATP synthase β subunit | Arabidopsis thaliana | P83483 | |||||||
| GQPVLNTGSPITVPVGR (17/3·2) | BLAST of EST: mitochondrial F1 ATP synthase β subunit | Arabidopsis thaliana | BLAST of EST: P83483 | |||||||||||
| VVDLLAPYQR (33/0·09) | ||||||||||||||
| QISELGIYPAVDPLDSTSR (50/3·1 × 10−3) | ||||||||||||||
| 4341 | –0·5 | –0·4 | –0·3 | QIIEANLALR (47/1·4 × 10−3) | Chloroplast ATP synthase ɛ chain | Androya decaryi | Q8MF84 (P84581) | |||||||
| QIIEANLALRR (42/6·3 × 10−3) | ||||||||||||||
| GFGFVTFGNEK (82/2·7 × 10−6) | Glycine-rich RNA-binding protein | Euphorbia esula | BLAST of EST: O48567 | |||||||||||
| Proteins of unknown function | ||||||||||||||
| 1453 | +1·1 | +1·9 | +1·2 | IINDFTNLVNQVEPLK (105/2·9 × 10−9) | BLAST of EST: stable protein 1 | Populus tremula | BLAST of EST: Q9AR79 (P84580) | |||||||
| HIVFVR (44/2·1 × 10−3) | Wound-responsive mRNAs | P. trichocarpa × P. deltoides | Q42482 | |||||||||||
| MIMDYYLF (41/5·9 × 10−3) | ||||||||||||||
The ESTs-based identifications were always accompanied by a BLAST search (Zhang and Madden, 1997) against publicly available databases to obtain information based on homologous protein function. All the identifications were manually evaluated and found to be reliable, and all the sequences obtained by tandem mass spectra have been submitted to UniProt via SPIN tool, and their accession numbers are also presented in the table. *Indicates the updated homology matches through new mascot searches in NCBInr giving strong evidence for the presented protein identity.
Proteins showing short-term upregulation (U6, U30 and U54)
Ketol-acid reductoisomerase, biotin carboxylase, α-tubulin, GTP-binding nuclear protein RAN, heat stress protein 90 (HSP90), chaperonin 10 (CPN10) and ATP sulfurylase (ATPS) were upregulated early upon heat stress in P. euphratica, returning later to control values (group U6, Table 3). The fact that α-tubulin was upregulated early in heat-stressed P. euphratica suggests that the short-term heat stress response leads to cytoskeleton remodelling. Since the first committed step of fatty acid synthesis is catalysed by biotin carboxylase, as part of the acetyl-CoA carboxylase multicomponent enzyme, the early upregulation of biotin carboxylase in P. euphratica suggests an increase in fatty acid synthesis in the first hours of heat stress exposure, most probably to face membrane instability. Membrane fluidity changes, which influence tensile forces, and cytoskeleton organization (Sangwan et al., 2002) have been reported previously in response to environmental stimuli (Volkmann and Baluska, 1999). Cytosolic Ca2+ oscillations upon cold exposure lead to Ca2+ binding to tubulin molecules, which destabilize microtubules (MTs) (Mazars et al., 1997). MTs disassemble upon low temperatures and modulate the sensitivity of cold-sensitive calcium channels (Mazars et al., 1997), for which membrane fluidity changes have been reported to be essential (Sangwan et al., 2002). As could be predicted by electrolyte leakage analysis in P. euphratica, cell membrane stability would not be greatly affected by exposure to 42 °C, which is supported by the return of biotin carboxylase abundance levels to the control range during this experiment. GTP-binding nuclear protein RAN was upregulated after 6 h of heat stress, later returning to control values. This suggests strong transient nucleocytoplasmic interactions as GTPase RAN proteins are involved in protein import into the nucleus and RNA export from the nucleus, in chromatin condensation and in cell cycle control (Kahana and Cleveland, 1999; Stochaj and Rother, 1999; Yang, 2002; Yamazaki et al., 2004). ATPS was upregulated early in heat-stressed P. euphratica and is involved in sulfate pathway in plants. It catalyses the activation of sulfate through binding to AMP and forming 5′-adenylylsulfate (APS). In P. euphratica under high temperature, the upregulation of ATPS suggests an increase of APS synthesis and, indirectly, also of cysteine synthesis. As the final product of the sulfate reduction pathway, cysteine acts as a sulfur donor to methionine (Droux, 2004) and it is part of glutathione (GSH), which removes toxic metabolites from the cell while maintaining the reduced form of sulphydryl groups. Environmental conditions that induce GSH synthesis require higher sulfur assimilation into cysteine. In Arabidopsis thaliana under heavy metal stress, the high cysteine biosynthesis rate has been related to the synthesis of GSH and phytochelatins, as part of the plant detoxification mechanism (Dominguez-Solis et al., 2001). In the same way, ketol-acid reductoisomerase was upregulated after 6 h of heat stress, being involved in the biosynthesis pathway of the amino acids valine, leucine and isoleucine, which are hydrophobic amino acids. Ketol-acid reductoisomerase is under the control of the rpoH homologue of Escherichia coli and was found to be upregulated upon heat stress in Agrobacterium tumefaciens (Rosen et al., 2002). The rpoH gene codes for δ32, one of the transcription factors controlling the heat shock regulon in E. coli, which is responsible for transcription of all major assimilation HSPs. In Avicennia marina, which is a mangrove with high salinity tolerance, the stress responsiveness of the ketol-acid reductoisomerase-encoding gene has been reported for the first time (Tanaka et al., 2002). Increased hydrophobic amino acid synthesis may be related to de novo protein synthesis in a more oxidative environment, which may favour isoforms with a higher percentage of these amino acids.
Table 3.
Classification of spots according to their IOD% changes of ±0.5 in comparison with control values
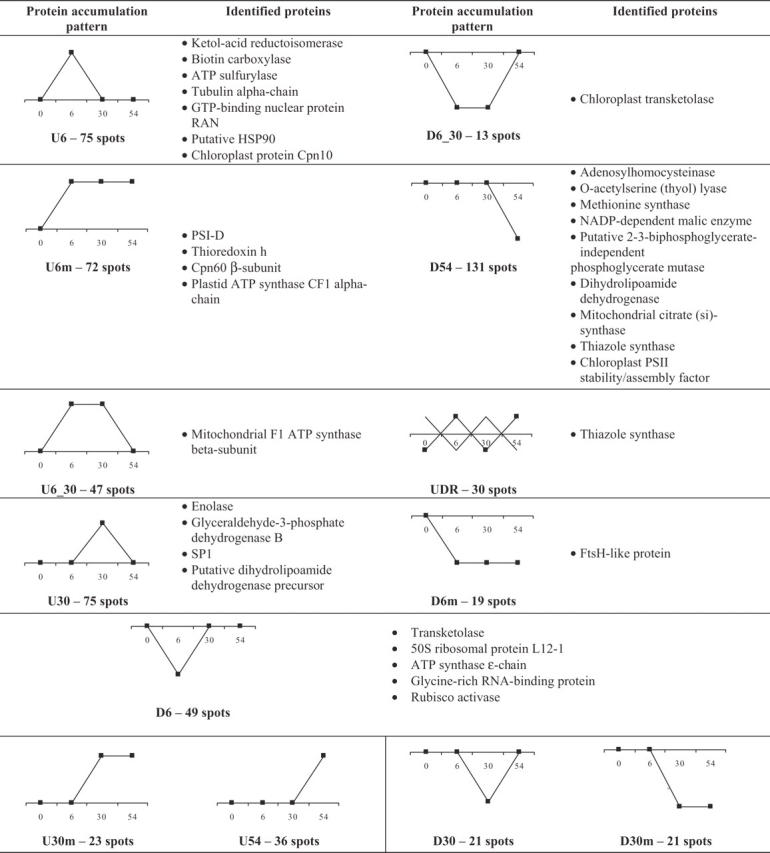
Graphical representation: the x-axis represents experimental time points in h and the y-axis represents changes in spot intensity incomparison with control, with no attributed scale. Thus, the control value is represented by zero and the arbitrary variations of +1 or −1 signify up- or downregulation, respectively. Group nomenclature: up/down only at 6 h (U6/D6); up/down only at 30 h (U30/D30); up/down only at 54 h (U54/D54); up/down at 6 h and maintained thereafter (U6m/D6m); up/down at 30 h and maintained thereafter (U30m/D30m); up/down only at 6 h and 30 h (U6_30/D6_30); up/down at 6 h with sucessive inversions in the following time points (UDR, where R stands for random).
Enolase and glyceraldehyde-3-phosphate dehydrogenase (GDPDH), which are integral enzymes of the glycolytic pathway, stable protein 1 (SP1) and a putative dihydrolipoamide dehydrogenase (DLDH) precursor have shown short-term upregulation in P. euphratica (group U30, Table 2). Enolase transcripts were reported to be induced upon different abiotic stresses, such as water stress in Lycopersicon esculentum (Van der Straeten et al., 1991), and in Zea mays (Riccardi et al., 1998), in response to heat shock, salt stress and abscisic acid (ABA) treatment in ice plant (Forsthoefel et al., 1995) and during fruit ripening in tomato (Van der Straeten et al., 1991). Enolase and GDPDH upregulation suggest early acceleration of the glycolytic pathway upon exposure to high temperature, with a consequent increase in pyruvate production, followed by a decrease. This seems to be supported by the late downregulation of putative 2-3-bisphosphoglycerate-independent phosphoglycerate mutase, which is the enzyme responsible for the conversion of 2-phosphoglycerate into 3-phosphoglycerate in the glycolytic pathway, using its own phosphoryl group to carry out that conversion. Bridging the accumulation profile of enolase, GDPDH and 2-3-biphosphoglycerate-independent phosphoglycerate mutase is the accumulation profile of a putative DLDH precursor. Its accumulation levels increased in P. euphratica after 30 h of heat stress but then decreased after 54 h. As part of the pyruvate dehydrogenase (PDH) complex, as its E3 subunit, DLDH acts in the final step of pyruvate conversion into acetyl-CoA, where it re-oxidizes the dihydrolipoamide moiety using NAD+. Its increase, followed by a decrease in abundance, suggests a transient increase in thiamine biosynthesis and a further reduction in its availability. This is also in agreement with the late downregulation profile determined for thiazole synthase, which is involved in thiamine biosynthesis in mitochondria and chloroplasts. Thiamine is a cofactor for two enzymatic complexes, PDH and α-ketoglutarate dehydrogenase in the tricarboxylic acid (TCA) cycle (Belanger et al., 1995), and PDH may have been upregulated early upon heat exposure, as described before.
SP1 is transiently upregulated in P. euphratica submitted to heat stress (group U30, Table 2). SP1 is a hydrophilic, homo-oligomeric protein composed of 12 subunits, each of 12·4 kDa, and is highly thermostable under extreme conditions (Wang et al., 2002). The SP1 protein characteristics of, simultaneously, an LEA protein and an sHSP, suggest that SP1 may be part of a new protein family due to its unique thermostability and distinct function in stress protection (Dgany et al., 2004). In fact, there is already a patent application for its use in industrial processes that require thermal stabilization of other proteins under extreme conditions (patent WO2004022697). SP1 is constitutively expressed in aspen plants and accumulates upon salt, cold, heat and desiccation stress, and during stress recovery (Wang et al., 2002; Renaut et al., 2004), and it acts as a chaperone to stabilize other proteins. Previous reports state that SP1 accumulation is particularly significant after heat stress is removed and when plants enter the recovery period (Wang et al., 2002). The common accumulation profile between SP1 and the glycolytic pathway enzymes found in this work suggests some sort of interaction with proteins related to carbon flow pathways or with other proteins that, indirectly, intervene in those pathways.
Proteins showing long-term upregulation (U6 m, U30 m and U6_30)
Photosystem I subunit D (PSI-D), thioredoxin h, chaperonin 60 β-subunit (CPN60-β) and ATP synthase CF1 α-chain were upregulated in the long term in heat-stressed P. euphratica.
CPN60-β was found to maintain high levels of accumulation, in contrast to HSP90 or CPN10 proteins. The major role of CPN60-β is in assisting in protein folding and preventing unfolding (Horwich et al., 2001), which is very similar to the function of other stress proteins, such as HSP90. CPN60 is composed of two subunits, α and β, it exists in chloroplasts and mitochondria, and it is involved in protein folding, together with CPN10, in an ATP-dependent manner. CPN60 seems to be an obligatory molecular chaperone in the folding/assembly pathway of Rubisco in higher plants. The folding reaction assisted by CPN60 develops through a binding–release cycle with CPN10, which alternates with a binding–release cycle with the unfolded protein substrate (Lund, 2005). Another chaperone molecule, HSP90, showed early upregulation, suggesting that, together with co-chaperones, HSP90 intervenes in the folding of newly synthesized proteins as well as stabilizing and refolding denatured proteins upon stress. The majority of HSP90 target proteins, among the several hundred already identified in eukaryotic cells (Sreedhar et al., 2004), are molecules involved in signal transduction.
Prolonged accumulation of thioredoxin h upon heat stress in P. euphratica may be related to its role in the oxidative stress response. Thioredoxin h is part of the thioredoxin antioxidant system in plants, acting after the oxidative stress generated in the electron transport chain through heat exposure, which induces redox changes in different compartments (Laloi et al., 2004). Thioredoxin h is able specifically to reduce small proteins containing intramolecular disulfide bonds (Besse and Buchanan, 1997). It has been shown to accumulate in salt-stressed Hordeum vulgare roots (Ueda et al., 2002) and, in Populus tremula × P. tremuloides, it is able to reduce peroxiredoxin Q, among other molecules which GSH is not able to reduce completely (Rouhier et al., 2004). Thioredoxin is able to regulate biosynthesis of the hydrophobic amino acids leucine, valine and isoleucine in Chlamydomonas reinhardtii (Lemaire et al., 2004). Valine and isoleucine share ketol-acid reductoisomerase in their biosynthesis, and this protein was, in fact, the single amino acid biosynthesis-related enzyme detected as upregulated in P. euphratica upon moderate heat exposure.
Long-term upregulation of the PSI-D subunit upon heat exposure of P. euphratica suggests the search for maintenance of structural stability of PSI. The PSI-D N-terminus allows stable binding of PSI subunit C and subunit E to the PSI complex, also contributing to ferredoxin docking (Andersen et al., 1992). Low amounts of PSI-D in A. thaliana were related to a decrease of all the other subunits of PSI, suggesting that the absence of PSI-D leads to incorrect PSI assembly and to its degradation (Haldrup et al., 2003). PSI-D accumulation, in the work here presented, suggests that PSI enhanced stability and minimized degradation. Similarly, chloroplastidial F1 α- (CF1-α) and mitochondrial F1 β-subunits were continuously upregulated in P. euphratica upon 42 °C exposure, although the CF1 α-subunit showed a higher extent of upregulation. It has been shown that it is the interaction between α, β and γ ATP synthase subunits that makes up the necessary force to stabilize CF1 under thermal denaturation (Wang et al., 1993). Wang et al. (1993) propose that the CF1 α-subunit has an organizing function in the assembly of the multisubunit enzyme ATP synthase upon thermal denaturation, as it contains non-catalytic sites where ATP can bind and stabilize ATP synthase CF1. The CF1 α–βcomplex is the ATP synthase minimum catalytic core (Avital et al., 1991), suggesting that upregulation of CF1 α- and β- subunits in P. euphratica is related to their central role in the enzyme complex. It has been proposed previously that ATP synthase ɛ-subunit may have a minor influence on ATP synthase thermal stability, as suggested by some authors (Wang et al., 1993), in contrast to α- and β-subunits.
Proteins showing short-term downregulation (D6, D30 and D54)
Proteins which were downregulated early at 42 °C in P. euphratica included transketolase, 50S ribosomal L12-1 protein, ATP synthase ɛ-subunit, glycine-rich RNA-binding protein and Rubisco activase. Downregulation of the 50S ribosomal L12-1 protein suggests that, at moderately high temperature, protein synthesis might have been affected early on, although a later increase in levels suggests a recovery of protein synthesis. The 50S ribosomal L12 protein seems to be the binding site for several factors involved in protein synthesis, with an essential role in accurate translation (Munchbach et al., 1999). A similar regulatory mechanism has been reported in E. coli, where ribosomes might be sensors for the heat shock response (VanBogelen et al., 1990). Glycine-rich RNA-binding proteins, which bind to RNA, may help in protecting these molecules at non-optimal temperature. A recent study reports the temperature-regulated expression of two distinct genes that code for two glycine-rich RNA-binding proteins in Dunaliella salina (Zchut et al., 2003). Rubisco activase, which is highly sensitive to high temperature, was detected as being downregulated immediately after the beginning of heat exposure, later returning to control range values. As part of the AAA+ family, its function comprises the regeneration of carbamylated active sites in Rubisco, maintaining Rubisco activity through ATP hydrolysis. The ability of Rubisco activase to promote activation, or to maintain the Rubisco active state in in vitro conditions, decreased above 30 °C in cotton and tobacco leaves (Crafts-Brandner and Salvucci, 2000). As the ATPase activity of Rubisco activase is not directly involved in activase association with the Rubisco enzyme, and as it increases with temperature up to 42 °C, the ability of activase to promote Rubisco activation at moderately high temperature was reported as being related to instability of the quaternary structure of activase under those conditions. In the same way, transketolase was also detected as being downregulated after 6 h of heat exposure. A homologue of a chloroplastid transketolase isoform was also identified as being downregulated, but this returned to control range values after only 54 h of heat exposure. Transketolases are key enzymes in the reductive and oxidative pentose phosphate pathways, as they are responsible for the synthesis of sugar phosphate intermediates, and their downregulation in P. euphratica shown in this work indicates a transient reduction of carbon flux in those pathways.
Late downregulated proteins included proteins related to sulfate assimilation. Although early upregulation of ATPS suggests enhanced sulfate assimilation early upon high temperature exposure in P. euphratica, an inhibition may have occurred in later stages, as suggested by the OAS-TL and methionine synthase accumulation profiles. OAS-TL catalyses the last step of the sulfate reduction pathway, converting O-acetylserine into cysteine through incorporation of the sulfur atom. Methionine, which results from homocysteine conversion catalysed by methionine synthase, is the immediate precursor of S-adenosyl-methionine, which plays a crucial role in the biosynthesis of ethylene and polyamines, being these compounds involved in the plant abiotic stress response (Capell et al., 2004).
Proteins showing long-term downregulation (D6 m and D30 m)
In this category only FtsH-like protein was identified. FtsH proteins are chaperone metalloproteases of the AAA+ family. Downregulation of P. euphratica FtsH during the whole heat experiment suggests a possible reduction in proteolysis of specific proteins that may become necessary during the heat exposure such as, for instance, homologues of E. coli σ factor, which control heat stress response in those bacteria. FtsH proteases of E. coli are partially responsible for the degradation of heat stress transcription factor δ32 (Shotland et al., 1997; Fischer et al., 2002), which accumulates upon FtsH depletion or upon exposure to high temperature. It has been suggested that E. coli FtsH may also have a non-proteolytic role since its absence causes abnormal orientation of some proteins of the plasma membrane (Langer, 2000). In silico analysis of the peptide sequences determined for P. euphratica using Clustal W (Thompson et al., 1994) allows confirmation that there is no signal peptide and, therefore, the FtsH-homologue protein found in P. euphratica is not a precursor but one of its isozymes. A recent report suggests that, unlike what was expected previously, the relative importance of different chloroplast FtsH isozymes is determined by their abundance, and not necessarily by different specific functions or specialized expression under certain conditions (Sinvany-Villalobo et al., 2004). This brings about the observation of a downregulation of the identified FtsH homologue in this work that, although not possible to locate in the chloroplast, may be related to heat stress response triggering.
DISCUSSION
In this work, we have analysed leaf proteomes of P. euphratica upon moderate heat stress. The main effects of the mimicked abiotic stress were detected as changes in the abundance of proteins involved in photosynthesis and carbon metabolism, suggesting a tight connection between those processes on reaching photostasis in P. euphratica. As enzymes related to lipid biogenesis, cytoskeleton structure, sulfate assimilation, thiamine and hydrophobic amino acid biosynthesis, and nuclear transport have been detected as being upregulated early in the short term in our experiment, signalling upon short-term moderate heat exposure in P. euphratica may involve structures and processes where these enzymes intervene. Simultaneously, there should have been an immediate inhibition of the communication between the pentose phosphate pathway and glycolysis, accomplished through transient downregulation of transketolase and Rubisco activase. A change in the cellular redox status was expected in P. euphratica leaves upon moderate heat, and redox homeostasis-related enzymes were expected to be upregulated. However, thioredoxin h was the only antioxidant enzyme found. Thioredoxins h comprise a large and diverse group of protein disulfide reductases, but the function of each thioredoxin h isoform is still unclear although the role of these enzymes in chloroplast redox homeostasis is well known (Foyer et al., 2005; Gelhaye et al., 2005). It is noteworthy that thioredoxin h has been detected as early long-term upregulated and that most proteins identified in this work have already been suggested as probable thioredoxin h chloroplast targets (Gelhaye et al., 2005). Recently, Lemaire et al. (2004) reported the identification of a thioredoxin-linked nuclear transport factor, RAN, in C. reinhardtii, the first ever found in a photosynthetic organism. Thioredoxin h may have a particular role in signalling related to photostasis in response to heat stress in P. euphratica, as in the redox regulation of the activity of several chloroplast enzymes.
Photostasis of P. euphratica
Changes in environmental temperature are primarily reflected in photosynthesis, which triggers a response aimed at reaching the best possible performance in the new situation. For this, a balance is sought between absorbed light energy, carbon assimilation and consumption in metabolic sinks. Overall plant growth is temperature dependent, while primary steps of photosynthesis are temperature independent. Photosynthetic non-biochemical processes are much quicker (10−15–10−12 s) than biochemical reactions catalysed by plant enzymes (10−3–100 s) (Ensminger et al., 2006). Upon a thermal shift, photosynthetic biochemical processes, affected to a much larger extent, induce a negative feedback control on non-biochemical photosynthetic processes (Fey et al., 2005), probably using singlet oxygen species generated on thylakoid membranes. This photostasis depends on an efficient sensory system to perceive cellular energetic status, for which the primary component is the plastoquinone (PQ) pool. Under moderate heat exposure, there is an increase of thylakoid proton conductance; linear electron flow is also affected and may be partially replaced by cyclic electron flow around PSI. According to Joliot et al. (2002), it is controlled by PSI assembly in supercomplexes, which depends on the ATP concentration. These authors suggest that an increase in ATP concentration, promoted by cyclic electron flow, would increase PSI availability to integrate linear electron flow and would decrease cyclic electron flow itself. The way in which the redox state of the PQ pool regulates plant acclimatory responses to light and temperature shifts is still not understood, although an organized signalling cascade has been suggested (Fey et al., 2005). PSII is highly heat susceptible, whereas PSI is relatively heat stable; thus, cyclic electron flow around PSI may help in reducing PSII activity and production of reactive oxygen species (ROS) under moderate heat stress. The late downregulation of PSII stability/assembly factor and the early long-term upregulation of PSI-D detected in P. euphratica suggest a strong involvement of PSI in thylakoid membrane electron flow. State transition is one of the strategies that plants use to cope with an excess of energy in photosystems. Photosynthetic linear electron flow is enhanced upon moderate heat exposure unless CO2 availability suffers a reduction. This enhancement was not the case in the experiment reported here in P. euphratica, raising the possibility of the existence of a transient cyclic electron flow. A possible downregulation of linear electron flow could be related, not to PSII damage under moderate heat stress, but to photostasis balance through adjustment of carbon metabolism. Plant cells can maintain photostasis under cold exposure through several mechanisms, the increase of electron sink capacity being one of these mechanisms, through upregulation of CO2 assimilation and carbon metabolism (Ensminger et al., 2006). The results reported here show that some carbon metabolism-related enzymes of P. euphratica are upregulated only after plants are maintained under moderate heat exposure (37 °C) during the night. Photostasis after 30 h of heat exposure may have induced emission of retrograde signals and changed expression of nuclear-encoded plastid proteins, to match the functional status of plastids under light conditions. In P. euphratica, the identified carbon metabolism-related enzymes have decreased in abundance after a second cycle of dark period exposure to moderate heat stress, returning to control range values; this fact probably represents shifts in photostasis. Equilibrium recovery between carbon sources and sinks may have been reached by P. euphratica plants at that time. NADP-dependent malic enzyme has been related to lower stomatal conductance when overexpressed in tobacco (Laporte et al., 2002). Its late downregulation in P. euphratica may be related to a decrease in carbon metabolism, with possible consequences for stomatal conductance. This late decrease in carbon metabolism is reflected in the late downregulated enzymes to an abundance level below that of the control, thus suggesting that carbon metabolism develops at a lower rate after 54 h of moderate heat exposure, in comparison with control conditions. Photostasis may have been reached through a reduction in steady-state molecular oxygen evolution, with a consequent decrease of CO2 assimilation, possibly limited by the number of stable PSII complexes in thylakoid membranes. Reduction of Krebs cycle and glycolysis, thiamine synthesis, acetyl coenzyme-A and methionine synthesis, malate conversion into pyruvate and sulfur assimilation in P. euphratica plants after 54 h of exposure to moderate heat suggests a general reduction in overall metabolism which may represent an achievement of equilibrium and, thus, an adaptation to the imposed conditions. This slower growth rate or plant development arrest, even if transient, observed in the P. euphratica plants used in the experiment reported here, may reflect this general reduction on the overall plant metabolism.
Finally, it would be interesting to confirm if the photostasis maintenance strategy of P. euphratica, under prolonged exposure to moderate heat stress, includes a partial replacement of linear electron flow by cyclic electron flow, as reported for other plants under heat stress, drought stress or for cyanobacteria upon salt exposure (Hibino et al., 1996; Sudhir et al., 2005). Confirmation of this photosynthetic shift would enable a better understanding of chloroplast metabolic regulation, through energetic imbalance, with plant cell redox regulation of carbon metabolism under moderate high temperature.
Acknowledgments
The authors wish to acknowledge the technical assistance of Andrea Lorentzen (Protein Research Group), the contribution of Christian Ahrens to the accomplishment of this collaboration and the assistance of Ruisheng Gu in plant material propagation. Funding for instrumentation from the Danish Research Agency to the Danish Biotechnology Instrument Center is acknowledged. The authors also thank the Portuguese Foundation for Science and Technology for financial support in the production of this work and for supporting S.F. (PhD fellowship SFRH/BD/6444/2001).
LITERATURE CITED
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2003. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B, Scheller HV, Moller BL. 1992. The PSI-E subunit of photosystem I binds ferredoxin:NADP+ oxidoreductase. FEBS Letters 311: 169–73. [DOI] [PubMed] [Google Scholar]
- Avital S, Gromet-Elhanan Z. 1991. Extraction and purification of the beta subunit and an active alpha beta-core complex from the spinach chloroplast CFoF1-ATP synthase. Journal of Biological Chemistry 266: 7067–7072. [PubMed] [Google Scholar]
- Belanger FC, Leustek T, Chu B, Kriz AL. 1995. Evidence for the thiamine biosynthetic pathway in higher-plant plastids and its developmental regulation. Plant Molecular Biology 29: 809–21. [DOI] [PubMed] [Google Scholar]
- Besse I, Buchanan BB. 1997. Thioredoxin-linked plant and animal processes: the new generation. Botanical Bulletin of Academia Sinica 38: 1–11. [Google Scholar]
- Bharti K, von Koskull-Doring P, Bharti S, Kumar P, Tintschl-Korbitzer A, Treuter E, Nover L. 2004. Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell 16: 1521–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Cushman JC. 2000. The ice plant cometh: lessons in abiotic stress tolerance. Journal of Plant Growth Regulation 19: 334–346. [Google Scholar]
- Bohnert HJ, Ayoubi P, Borchert C, Bressan RA, Burnap RL, Cushman JC, et al.2001. A genomics approach towards salt stress tolerance. Plant Physiology and Biochemistry 39: 295–311. [Google Scholar]
- Brosche M, Vinocur B, Alatalo ER, Lamminmaki A, Teichmann T, Ottow EA, et al.2005. Gene expression and metabolite profiling of Populus euphratica growing in the Negev desert. Genome Biology 6 R101. [DOI] [PMC free article] [PubMed]
- Canovas FM, Dumas-Gaudot E, Recorbet G, Jorrin J, Mock HP, Rossignol, M. 2004. Plant proteome analysis. Proteomics 4: 285–298. [DOI] [PubMed] [Google Scholar]
- Capell T, Bassie L, Christou, P. 2004. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proceedings of the National Academy of Sciences of the USA 101: 9909–9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2000. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proceedings of the National Academy of Sciences of the USA 97: 13430–13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F. 2000. Dual action of the active oxygen species during plant stress responses. Cellular and Molecular Life Sciences 57: 779–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dgany O, Gonzalez A, Sofer O, Wang W, Zolotnitsky G, Wolf A, et al. 2004. The structural basis of the thermostability of SP1, a novel plant (Populus tremula) boiling stable protein. Journal of Biological Chemistry 279: 51516–51523. [DOI] [PubMed] [Google Scholar]
- Dominguez-Solis J, Gutierrez-Alcala G, Vega J, Romero L, Gotor C. 2001. The cytosolic o-acetylserine(thiol)lyase gene is regulated by heavy metals and can function in cadmium tolerance. Journal of Biological Chemistry 276: 31474–31474. [DOI] [PubMed] [Google Scholar]
- Droux M. 2004. Sulfur assimilation and the role of sulfur in plant metabolism: a survey. Photosynthesis Research 79: 331–348. [DOI] [PubMed] [Google Scholar]
- Ensminger I, Busch F, Huner N. 2006. Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiologia Plantarum 126: 28–44. [Google Scholar]
- Fey V, Wagner R, Brautigam K, Pfannschmidt T. 2005. Photosynthetic redox control of nuclear gene expression. Journal of Experimental Botany 56: 1491–1498. [DOI] [PubMed] [Google Scholar]
- Fischer B, Rummel G, Aldridge P, Jenal U. 2002. The FtsH protease is involved in development, stress response and heat shock control in Caulobacter crescentus. Molecular Microbiology 44: 461–478. [DOI] [PubMed] [Google Scholar]
- Forsthoefel NR, Cushman MA, Cushman JC. 1995. Posttranscriptional and posttranslational control of enolase expression in the facultative crassulacean acid metabolism plant Mesembryanthemum crystallinum L. Plant Physiology 108: 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Noctor G. 2005. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment 28: 1056–1071. [Google Scholar]
- Gelhaye E, Rouhier N, Navrot N, Jacquot JP. 2005. The plant thioredoxin system. Cell and Molecular Life Sciences 62: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobom J, Nordhoff E, Mirgorodskaya E, Ekman R, Roepstorff P. 1999. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. Journal of Mass Spectrometry 34: 105–116. [DOI] [PubMed] [Google Scholar]
- Grover A, Agarwal M, Katiyar-Agarwal S, Sahi C, Agarwal S. 2000. Production of high temperature tolerant transgenic plants through manipulation of membrane lipids. Current Science 79: 557–559. [Google Scholar]
- Gu RS, Jiang XN, Guo ZC. 1999. Structure characteristics associated with salt tolerance of Populus euphratica. Acta Botanica Sinica 41: 576–579. [Google Scholar]
- Gu, RS, Fonseca S, Puskas LG, Hackler L, Zvara A, Dudits D, Pais MS. 2004a. Transcript identification and profiling during salt stress and recovery of Populus euphratica. Tree Physiology 24: 265–276. [DOI] [PubMed] [Google Scholar]
- Gu RS, Liu QL, Pei D, Jiang XN. 2004b. Understanding saline and osmotic tolerance of Populus euphratica suspended cells. Plant Cell Tissue and Organ Culture 78: 261–265. [Google Scholar]
- Habermann B, Oegema J, Sunyaev S, Shevchenko A. 2004. The power and the limitations of cross-species protein identification by mass spectrometry-driven sequence similarity searches. Molecular and Cellular Proteomics 3: 238–249. [DOI] [PubMed] [Google Scholar]
- Haldrup A, Lunde C, Scheller HV. 2003. Arabidopsis thaliana plants lacking the PSI-D subunit of photosystem I suffer severe photoinhibition, have unstable photosystem I complexes, and altered redox homeostasis in the chloroplast stroma. Journal of Biological Chemistry 278: 33276–33283. [DOI] [PubMed] [Google Scholar]
- Hanson TE, Tabita FR. 2003. Insights into the stress response and sulfur metabolism revealed by proteome analysis of a Chlorobium tepidum mutant lacking the rubisco-like protein. Photosynthesis Research 78: 231–248. [DOI] [PubMed] [Google Scholar]
- Hibino T, Lee B, Rai A, Ishikawa H, Kojima H, Tawada M, et al. 1996. Salt enhances photosystem I content and cyclic electron flow via NAD(P)H dehydrogenase in the halotolerant cyanobacterium Aphanothece halophytica. Australian Journal of Plant Physiology 23: 321–330. [Google Scholar]
- Horwich AL, Fenton WA, Rapoport TA. 2001. Protein folding taking shape. Workshop on molecular chaperones. EMBO Reports 2: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba K. 2002. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review of Plant Biology 53: 225–245. [DOI] [PubMed] [Google Scholar]
- Ingvardsen C, Veierskov B. 2001. Ubiquitin- and proteasome-dependent proteolysis in plants. Physiologia Plantarum 112: 451–459. [DOI] [PubMed] [Google Scholar]
- Ismail A, Hall, A. 1999. Reproductive-stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop Science 39: 1762–1768. [Google Scholar]
- Joliot P, Joliot A. 2002. Cyclic electron transfer in plant leaf. Proceedings of National Academy of Sciences of the USA 99: 10209–10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana JA, Cleveland DW. 1999. Beyond nuclear transport. RAN-GTP as a determinant of spindle assembly. Journal of Cell Biology 146: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A. 2004. Reactive oxygen signalling: the latest news. Current Opinion in Plant Biology 7: 323–328. [DOI] [PubMed] [Google Scholar]
- Langer T. 2000. AAA proteases: cellular machines for degrading membrane proteins. Trends in Biochemical Science 25: 247–251. [DOI] [PubMed] [Google Scholar]
- Laporte MM, Shen B, Tarczynski MC. 2002. Engineering for drought avoidance: expression of maize NADP-malic enzyme in tobacco results in altered stomatal function. Journal of Experimental Botany 53: 699–705. [DOI] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ. 1999. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiology 120: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire SD, Guillon B, Le Marechal P, Keryer E, Miginiac-Maslow M, Decottignies P. 2004. New thioredoxin targets in the unicellular photosynthetic eukaryote Chlamydomonas reinhardtii. Proceedings of National Academy of Sciences of the USA 101: 7475–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska AJ, Shevchenko A. 2003. Expanding the organismal scope of proteomics: cross-species protein identification by mass spectrometry and its implications. Proteomics 3: 19–28. [DOI] [PubMed] [Google Scholar]
- Lund AA, Blum PH, Bhattramakki D, Elthon TE. 1998. Heat-stress response of maize mitochondria. Plant Physiology 116: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. 2005. The chaperonin cycle and protein folding. BioEssays 16: 229–231. [DOI] [PubMed] [Google Scholar]
- Ma HC, Fung L, Wang SS, Altman A, Hutterman A. 1997. Photosynthetic response of Populus euphratica to salt stress. Forest Ecology and Management 93: 55–61. [Google Scholar]
- Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N. 2002. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Molecular Biology 48: 667–681. [DOI] [PubMed] [Google Scholar]
- Mann M, Wilm M. 1994. Error tolerant identification of peptides in sequence databases by peptide sequence tags. Analytical Chemistry 66: 4390–4399. [DOI] [PubMed] [Google Scholar]
- Mathew A, Morimoto RI. 1998. Role of the heat-shock response in the life and death of proteins. Stress of Life 851: 99–111. [DOI] [PubMed] [Google Scholar]
- Mathew A, Mathur SK, Morimoto RI. 1998. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin–proteasome pathway. Molecular and Cellular Biology 18: 5091–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazars C, Thion L, Thuleau P, Graziana A, Knight MR, Moreau M, Ranjeva R. 1997. Organization of cytoskeleton controls the changes in cytosolic calcium of cold-shocked Nicotiana plumbaginifolia protoplasts. Cell Calcium 22: 413–420. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD. 2002. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes and Development 16: 1555–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munchbach M, Dainese P, Staudenmann W, Narberhaus F, James P. 1999. Proteome analysis of heat shock protein expression in Bradyrhizobium japonicum. European Journal of Biochemistry 264: 39–48. [DOI] [PubMed] [Google Scholar]
- Perkins DP, Creasy DM, Cottrell JS. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567. [DOI] [PubMed] [Google Scholar]
- Port M, Tripp J, Zielinski D, Weber C, Heerklotz D, Winkelhaus S, et al.2004. Role of Hsp17·4-cii as coregulator and cytoplasmic retention factor of tomato heat stress transcription factor HsfA2(1). Plant Physiology 135: 1457–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaut J, Lutts S, Hoffmann L, Hausman JF. 2004. Responses of poplar to chilling temperatures: proteomic and physiological aspects. Plant Biology 6: 81–90. [DOI] [PubMed] [Google Scholar]
- Riccardi F, Gazeau P, de Vienne D, Zivy M. 1998. Protein changes in response to progressive water deficit in maize. Quantitative variation and polypeptide identification. Plant Physiology 117: 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AJ, Ishikawa M, Gusta LV, Mackenzie SL. 1994. Abscisic acid-induced heat tolerance in bromus-inermis leyss cell-suspension cultures—heat-stable, abscisic acid-responsive polypeptides in combination with sucrose confer enhanced thermostability. Plant Physiology 105: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen R, Buttner K, Becher D, Nakahigashi K, Yura T, Hecker M, Ron EZ. 2002. Heat shock proteome of Agrobacterium tumefaciens: evidence for new control systems. Journal of Bacteriology 184: 1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N, Gelhaye E, Gualberto JM, Jordy MN, De Fay E, Hirasawa M, et al. 2004. Poplar peroxiredoxin Q. A thioredoxin-linked chloroplast antioxidant functional in pathogen defense. Plant Physiology 134: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS. 2002. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant Journal 31: 629–638. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. 2000. Perspectives: plant biology. Some like it hot. Science 287: 435, 437. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Mann M. 1997. Peptide sequencing by mass spectrometry for homology searches and cloning of genes. Journal of Protein Chemistry 16: 481–490. [DOI] [PubMed] [Google Scholar]
- Shiji W, Bingao C, Huqun L. 1996. Euphrates poplar forest. Beijing: China Environmental Science Press.
- Shotland Y, Koby S, Teff D, Mansur N, Oren DA, Tatematsu K, et al. 1997. Proteolysis of the phage lambda CII regulatory protein by FtsH (HflB) of Escherichia coli. Molecular Microbiology 24: 1303–1310. [DOI] [PubMed] [Google Scholar]
- Sinvany-Villalobo G, Davydov O, Ben-Ari G, Zaltsman A, Raskind A, Adam Z. 2004. Expression in multigene families. Analysis of chloroplast and mitochondrial proteases. Plant Physiology 135: 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skylas DJ, Cordwell SJ, Hains PG, Larsen MR, Basseal DJ, Walsh BJ, et al. 2002. Heat shock of wheat during grain filling: proteins associated with heat-tolerance. Journal of Cereal Science 35: 175–188. [Google Scholar]
- Sreedhar AS, Kalmar E, Csermely P, Shen YF. 2004. Hsp90 isoforms: functions, expression and clinical importance. FEBS Letters 562: 11–15. [DOI] [PubMed] [Google Scholar]
- Stochaj U, Rother K. 1999. Nucleocytoplasmic trafficking of proteins: with or without RAN? BioEssays 21: 579–589. [Google Scholar]
- Sudhir P, Pogoryelov D, Kovacs L, Garab G, Murthy S. 2005. The effects of salt stress on photosynthetic electron transport and thylakoid membrane proteins in the cyanobacterium Spirulina platensis. Journal of Biochemistry and Molecular Biology 38: 481–485. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ikeda K, Ono M, Miyasaka H. 2002. Isolation of several anti-stress genes from a mangrove plant Avicennia marina. World Journal of Microbiology & Biotechnology 18: 801–804. [Google Scholar]
- Thiellement H, Zivy M, Plomion C. 2002. Combining proteomic and genetic studies in plants. Journal of Chromatography B 782: 137–149. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Shi WM, Nakamura T, Takabe T. 2002. Analysis of salt-inducible genes in barley roots by differential display. Journal of Plant Research 115: 119–130. [DOI] [PubMed] [Google Scholar]
- VanBogelen RA, Neidhardt FC. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proceedings of the National Academy of Sciences of the USA 87: 5589–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Straeten D, Rodrigues-Pousada RA, Goodman HM, Van Montagu M. 1991. Plant enolase: gene structure, expression, and evolution. Plant Cell 3: 719–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann D, Baluska F. 1999. Actin cytoskeleton in plants: from transport networks to signaling networks. Microscopy Research and Technique 47: 135–154. [DOI] [PubMed] [Google Scholar]
- Wang WX, Pelah D, Alergand T, Shoseyov O, Altman A. 2002. Characterization of SP1, a stress-responsive, boiling-soluble, homo-oligomeric protein from aspen. Plant Physiology 130: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WX, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1–14. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Freire E, McCarty RE. 1993. Influence of nucleotide binding site occupancy on the thermal stability of the F1 portion of the chloroplast ATP synthase. Journal of Biological Chemistry 268: 20785–20790. [PubMed] [Google Scholar]
- Wullschleger SD, Difazio SP. 2003. Emerging use of gene expression microarrays in plant physiology. Comparative and Functional Genomics 4: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Motohashi K, Kasama T, Hara Y, Hisabori T. 2004. Target proteins of the cytosolic thioredoxins in Arabidopsis thaliana. Plant and Cell Physiology 45: 18–27. [DOI] [PubMed] [Google Scholar]
- Yang Z. 2002. Small GTPases: versatile signaling switches in plants. Plant Cell 14: S375–S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youlin Y, Squires V, Qi L. 2001. Global alarm: dust and sandstorms from the world's drylands. Bangkok: United Nations.
- Zchut S, Weiss M, Pick U. 2003. Temperature-regulated expression of a glycine-rich RNA-binding protein in the halotolerant alga Dunaliella salina. Journal of Plant Physiology 160: 1375–1384. [DOI] [PubMed] [Google Scholar]
- Zhang J, Madden TL. 1997. Powerblast: a new network blast application for interactive or automated sequence analysis and annotation. Genome Research 7: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]