Abstract
• Background and Aims Germination studies of species from fire-prone habitats are often focused on the role that fire plays in breaking dormancy. However, for some plant groups in these habitats, such as the genus Leucopogon (Ericaceae), dormancy of fresh seeds is not broken by fire cues. In the field, these same species display a flush of seedling emergence post-fire. Dormancy and germination mechanisms therefore appear complex and mostly unknown. This study aimed to identify these mechanisms by establishing dormancy class and testing the effects of a set of typical germination cues, including those directly related to fire and entirely independent of fire.
• Methods To classify dormancy, we assessed seed permeability and embryo morphology, and conducted germination experiments at seasonal temperatures in incubators. To test the effects of fire cues on germination, factorial combinations of smoke, heat and dark treatments were applied. Ageing treatments, using burial and seasonal incubation, were also tested. Germination phenology was established.
• Key Results Seeds were dormant at release and had underdeveloped embryos. Primary dormancy of the study species was classified as morphophysiological. Seasonal temperature changes overcame primary dormancy and controlled timing of germination. Fire cues did not break primary dormancy, but there was a trend for smoke to enhance germination once this dormancy was overcome.
• Conclusions Despite the fact that fire is a predominant disturbance and that many species display a flush of emergence post-fire, seasonal temperatures broke the primary physiological dormancy of the study species. It is important to distinguish between fire being responsible for breaking dormancy and solely having a role in enhancing levels of post-fire germination for seeds in which dormancy has been overcome by other factors. Biogeographical evidence suggests that morphological and physiological factors, and therefore seasonal temperatures, are likely to be important in controlling the dormancy and patterns of post-fire germination of many species in fire-prone regions.
Keywords: Morphophysiological dormancy, fire, embryo morphology, dormancy classification, germination, seasonal temperature, south-eastern Australia, Leucopogon exolasius, Leucopogon setiger, Leucopogon esquamatus, Ericaceae, Epacridaceae
INTRODUCTION
Dormancy in plant propagules can restrict germination and seedling emergence to times when there are suitable environmental conditions (Baskin and Baskin, 1998; Bell, 1999). The germination of many species from fire-prone habitats is related, either directly or indirectly, to fire. Germination cues that are directly related to fire include heat shock (Keeley, 1987; Auld and O'Connell, 1991; Bell et al., 1993), smoke or smoke products (de Lange and Boucher, 1990; Dixon et al., 1995; Keeley and Fotheringham, 1997) and combinations of these (Keith, 1997; Kenny, 2000; Thomas et al., 2003). Indirect cues include light and ambient temperature conditions (Bond and van Wilgen, 1996) that are altered as a result of the canopy and leaf litter being burned and removed. These cues can lead to a pulse of seedling emergence in the post-fire environment, where there are increased resources available as well as reduced competition (Gill, 1981; Keeley, 1991; Whelan, 1995; Bell, 1999).
There is still limited understanding of the range of dormancy mechanisms operating in fire-prone regions around the world. This is partly due to the large number of species that display obvious and rapid germination responses to fire characteristics, such as heat and smoke (Auld and O'Connell, 1991; Keeley, 1991; Brown, 1993; Dixon et al., 1995; Whelan, 1995; Bond and van Wilgen, 1996). As a result, the majority of seed germination studies have concentrated on the extent to which dormancy is broken by fire rather than exploring all the mechanisms that might be responsible for breaking dormancy (Keeley, 1995). Seed dormancy is defined as a characteristic of the seed rather than of its environment (Vleeshouwers et al., 1995). Overcoming primary dormancy is therefore distinct from stimulating germination from a seed bank (Thompson et al., 2003). Factors required to break primary dormancy may be completely unrelated to fire; however, fire may provide subsequent germination cues and suitable environmental conditions (Bell, 1999).
There is growing evidence that many species in fire-prone environments do not have primary dormancy broken by fire. Germination can be enhanced after fire in some species, but in others it can be diminished or unchanged (Keeley, 1987; Davis et al., 1989). Laboratory studies support this, because a significant number of species show no response to heat or smoke treatments (Dixon et al., 1995; Roche et al., 1997; Keeley and Fotheringham, 1998; Clarke et al., 2000; van Staden et al., 2000). Deciphering which cues are controlling primary dormancy in these species may be more difficult than with species that respond directly to fire cues.
Seed dormancy classification, using a system such as that outlined by Baskin and Baskin (2004), distinguishes between physical, morphological and/or physiological mechanisms as controls on dormancy and can help to identify the most likely factors involved in overcoming dormancy. Stratification at seasonal temperatures is the main known requirement for overcoming both morphological and physiological factors, whereas heat shock or scarification can overcome physical dormancy. To classify seed dormancy, information on permeability of seeds, embryo morphology and germination response at different seasonal temperatures, among other things, is required (Baskin and Baskin, 2003). Classification can help to gain an understanding of a species' dormancy by enabling comparisons with other species within the same dormancy class.
Several studies have suggested that morphological and/or physiological factors play a role in controlling dormancy of species from fire-prone regions (Schatral et al., 1997; Keeley and Fotheringham, 1998; Bell, 1999; Tieu and Egerton-Warburton, 2000; Allan et al., 2004). However, the role of fire as a cue for breaking primary dormancy involving morphological and physiological mechanisms is yet to be clarified.
In Australia's fire-prone habitats, the predominance of fire as a disturbance appears to focus most study effort on the role that fire plays in breaking dormancy, and less on other mechanisms. Many common species from temperate understorey vegetation, including several members of the Ericaceae, Rutaceae and Dilleniaceae, have unknown dormancy mechanisms (Fox et al., 1987; Dixon et al., 1995; Auld, 2001; Baskin and Baskin, 2003; Allan et al., 2004), although it is likely that many of these species have some form of physiological dormancy (Ooi, in press). Some progress has been made in unravelling dormancy cues of individual species from these groups, including Leucopogon conostephioides and L. melaleucoides (Tieu et al., 2001; O'Brien and Johnston, 2004) and several Hibbertia species (Schatral et al., 1997; Allan et al., 2004). Nevertheless, freshly dispersed seeds of the majority of species from these groups tested in laboratory germination trials tend not to respond to direct fire cues (Dixon et al., 1995; Roche et al., 1997; Clarke et al., 2000; Tieu et al., 2001), and in many cases do not germinate at all. Considerable germination in response to fire has been observed for the same species either in situ or after burial (Dixon et al., 1995; Enright et al., 1997; Roche et al., 1997; Tieu et al., 2001; Rokich et al., 2002; Wills and Read, 2002; Ooi et al., 2004a). During burial, seeds experience daily and seasonal temperature fluctuations, physical deterioration of seed structures and physiological changes over time (Baskin and Baskin, 1998). This suggests that a sequence of processes may be required. The first set of processes are required to break primary dormancy, with subsequent processes then promoting increased levels of germination post-fire.
In this study, three Leucopogon (Ericaceae) species from south-eastern Australia were used to investigate dormancy mechanisms and timing of germination, and the potential role that burial, seasonal temperature regimes, dark and fire cues play in controlling these mechanisms. Leucopogon is the largest genus within the sub-family Styphelioideae, (formerly recognized as Epacridaceae) with approx. 230 species, and is part of the largest tribe, Styphelieae. Dormancy mechanisms for this genus (and tribe) appear complex, and seeds have been described as difficult to germinate (Dixon et al., 1995). Unlike many other species within the same plant communities, Leucopogon displays a seasonal emergence pattern from a soil-stored seed bank, with peak densities recorded in autumn and winter (Ooi et al., 2004a). There is no distinct rainfall season, but seedlings emerge at the same time of year, irrespective of rainfall or the timing of a fire event. Nevertheless, there is increased germination post-fire. Subsequently, the post-fire flush of emergence can be delayed in relation to many other co-occurring species (Ooi et al., 2004a).
The specific aims are to answer the following questions. (a) Are seeds of the study species dormant at the time of release from the parent plant? (b) What classification of seed dormancy do the three Leucopogon species have? (c) Is dormancy broken during burial? What factors promote loss of dormancy? (d) Do fire cues break primary dormancy? What are the relative effects of fire cues on both fresh seeds and buried seeds? (e) What drives the seasonal emergence pattern displayed by Leucopogon species in the field?
MATERIALS AND METHODS
Study species and seed collection
The three study species are all obligate seeders (Ooi et al., 2006) with soil-stored seed banks (Ooi et al., 2004a). Leucopogon exolasius is an erect shrub that grows to approx. 2 m, and is listed as ‘vulnerable’ both under the national Australian Environment Protection and Biodiversity Conservation Act (1999), and under the state of New South Wales (NSW) Threatened Species Conservation Act (1995). The species is endemic to the southern Sydney region of NSW, Australia and occurs in woodlands. Leucopogon setiger, also an erect shrub that grows to approx. 2 m, is more widespread, extending from the central western slopes of NSW to the coast (distributional range >450 km). It is found in woodlands and open forest. Leucopogon esquamatus is a slender shrub, which grows to approx. 1 m. It is also widespread, occurring from the coast to the mountains (as for L. setiger), but extending south into the states of Victoria and Tasmania (distributional range >1000 km). It occurs in heath, woodlands and open forests.
Leucopogon fruit are drupes. Fresh exocarps of both L. exolasius and L. setiger are fleshy, while the endocarp is hard and lignified. Leucopogon esquamatus exocarp is papery and the endocarp comparatively more fibrous. Seeds are held inside the fruit. Drupes ripen in summer (generally November–December) and were collected between 1999/2000 and 2003/2004 from within Royal (34°03′S, 151°03′;E) and Heathcote (34°07′S, 150°58′E) National Parks in the southern Sydney region, and at Garigal (33°46′S, 151°14′E) National Park in northern Sydney. Before the commencement of experiments, flesh was removed from the collected drupes of each species by soaking in water for several days. After drying, a minimum of 40 drupes were used to estimate mean weight. Drupes were stored in envelopes at room temperature in the laboratory (approx. 22 °C) prior to commencement of experiments.
The climate in the Sydney region is temperate with no dry season (using the Köppen classification system) (Stern et al., 2000). Average annual rainfall for the area is approx. 1050 mm. It is distributed relatively evenly throughout the year, with the four highest monthly averages occurring in January, March, June and November. Average temperatures (maximum/minimum) are approx. 27/18 °C and 16/6 °C in summer and winter, respectively. Climate data were obtained from the Lucas Heights weather station, a few kilometres from the southern collection sites.
Seed embryo morphology and growth
Twenty-five embryos from freshly dispersed seeds were used for each of the three Leucopogon species to investigate embryo morphology. Drupes were scarified using a scalpel to nick the pericarp at the bract end, exposing seed tissues. After soaking for at least 48 h, seeds were removed from the drupes by cutting away the pericarp with a scalpel. Once removed, embryos were separated from endosperm using a scalpel and fine forceps. Embryos could often be squeezed from the seed by applying a small amount of pressure with the forceps. Seed and embryo lengths were measured using a dissecting microscope fitted with a stage micrometer. Embryo length to seed length ratios (E : S) were calculated.
To ascertain whether embryos grow prior to germination, the same methods of measurement were employed. Twenty fresh seeds of each species were placed in Petri dishes lined with filter paper and moistened with distilled water. These were then stratified for 24 weeks in incubators at warm (28/18 °C), cold (16/6 °C) or cold followed by warm temperatures, before embryos were excised and measured. Seeds subjected to burial treatments (described below in Experiment 3) were also used to measure embryo growth. A minimum of 20 embryos were measured immediately after retrieval from the soil. A similar number were measured after the buried seeds had been subsequently incubated for 24 weeks at warm temperatures.
Imbibition and viability
To see whether freshly matured drupes could imbibe water, three replicates of five fruits for each species were placed on moist filter paper. At 0, 1, 3, 5, 12 and 96 h, each replicate batch was weighed after excess water was removed from the drupe surface with blotting paper. Further observations of excised seeds were made before and after imbibition to assess the relative softness of the endosperm. This was evaluated to indicate whether water was penetrating through the endocarp and into the seed.
For each species, collected drupes were pooled across sites. To test the viability of each pooled lot, a cut test was conducted using a minimum of three replicates of 20 drupes. Seeds that were firm and contained healthy looking white endosperm were considered viable. Viability was high (60–92 %) for all three species. The cut test provides an accurate assessment of viability for these species (Ooi et al., 2004b). Each drupe usually contained a single viable seed, although approx. 20 % of L. esquamatus drupes contained two viable seeds. However, the term ‘seed’ will be used from here on to describe the whole dispersal unit.
Germination experiments
Between 60 and 120 seeds, depending on availability, divided into three replicates were used for each treatment during all germination experiments. During experiments, seeds were placed on three layers of moistened filter paper in 9 cm Petri dishes in light- and temperature-controlled germination cabinets. Incubators were set at 12 h/12 h light/dark and maximum/minimum temperature cycles. Seeds were checked weekly and watered with distilled water as required. Germination was scored on the emergence of the radicle and expressed as a proportion of total viable seed.
Experiment 1: assessing seed dormancy
The definition of a dormant seed is one that does not have the capacity to germinate under combinations of normal environmental factors (such as temperature and light/dark) that are otherwise favourable for its germination (Baskin and Baskin, 2004). To assess whether seeds of the study species have a primary dormancy (i.e. dormant at the time of primary dispersal), freshly dispersed seeds were tested for their germination response to seasonal temperatures (three levels: winter, spring/autumn and summer) and light (two levels: light, dark). For each species, seeds were equally divided into three groups of six replicate dishes. Half of the six dishes were assigned to a dark treatment and wrapped in two layers of aluminium foil. Each group of six dishes was then placed at each of three seasonal temperature settings in three incubators. The incubators were set at temperatures approximating either (a) winter (16/6 °C); (b) spring/autumn (20/10 °C); or (c) summer (28/18 °C) conditions. Germination was monitored for 30 weeks. When checking germination for the dark treatment, seeds were exposed to dim lighting for <20 s.
Experiment 2: single season temperatures—fire cues and scarification
To assess the effect of fire cues on primary dormancy at any one of the three separate season temperatures, factorial combinations of smoke (two levels: smoked, unsmoked) and heat shock (two levels: heated, unheated) were applied to replicate dishes of seeds in experiments conducted soon after seed collection. Incubator settings were the same as described above. Additionally, when sufficient seed was available, scarification was included as an independent treatment to assess whether there was a physical component to primary seed dormancy (Table 1). Smoke was applied to both the seeds and filter paper (used as the substrate during the experiments) for approx. 10 min, using a beekeeper smoker, burning a combination of dry and fresh vegetation material collected from the study sites. This period of time is reported to enhance germination in a number of species in the region (Morris, 2000; Thomas et al., 2003). Smoked filter paper maintains good levels of smoke derivatives (Keith, 1997) and smoke cues have been reported as being able to persist in the soil for long periods (van Staden et al., 2000). Heat treatments were set at 90 °C for 10 min in an oven. This duration and temperature have been reported to enhance germination in a wide range of species (Auld and O'Connell, 1991; Keith, 1997). For each species, each replicate smoke and heat treatment was applied separately to avoid psuedoreplication (Morrison and Morris, 2000). For smoke × heat treatments, the heat treatment was applied first. Seeds were scarified by nicking the seed coat with a scalpel.
Table 1.
Treatments used for the initial dormancy experiments on fresh seeds at the three different incubation temperatures for 30 weeks
|
L. exolasius |
L. setiger |
L. esquamatus |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | 16/6 °C | 20/10 °C | 28/18 °C | 16/6 °C | 20/10 °C | 28/18 °C | 16/6 °C | 20/10 °C | 28/18 °C |
| Control | x | x | x | x | x | x | x | x | x |
| Smoke | x | x | x | x | x | x | x | ||
| Heat | x | x | x | x | x | x | x | ||
| Smoke + heat | x | x | x | x | x | x | x | x | |
| Dark | x* | x | x* | x* | x | x* | x* | x | |
| Scarified | x* | x* | x* | x* | x* | x* | |||
*These treatments were only followed for 15 weeks.
Experiment 3: single season temperatures—burial
To assess whether primary dormancy is broken during burial, experiments were conducted at spring/autumn (20/10 °C) and summer (28/18 °C) alternating temperatures, using seeds retrieved after 18 months burial in situ. Seeds stored dry in envelopes in the laboratory for 18 months were used as a control. Insufficient seeds were available to run the experiment also at winter temperatures (16/6 °C). Buried seeds used in the experiments had been placed in soil-filled mesh bags and buried within the top 5 cm of the soil at the seed collection sites soon after seed release. Mesh size retained the seeds but allowed moisture to pass through. Bags were retrieved approx. 18 months after burial, and had experienced the late summer period, followed by a winter, summer, winter cycle and retrieved as the next summer approached. Seeds were sifted out, washed in distilled water and their viability was assessed before they were used in the experiments.
Experiment 4: seasonal temperature regimes—germination phenology and fire cues
These experiments were designed to study the germination phenology of the study species and enable comparisons with temporal emergence patterns observed in the field (Ooi et al., 2004a). In addition, the effects of fire cues and darkness, when combined with a seasonal temperature regime, on dormancy and germination timing were determined. The design included temperature variation (two levels: unvarying control at 20/10 °C and varying temperatures following the seasonal pattern), factorial combinations of smoke, heat and dark (L. exolasius and L. esquamatus), or smoke and heat only (L. setiger), and scarification (Table 2).
Table 2.
Treatments applied for experiments on fresh seeds conducted at the seasonal temperature regime
|
L. exolasius |
L. setiger |
L. esquamatus |
||||
|---|---|---|---|---|---|---|
| Treatment | Seasonal | 20/10 °C | Seasonal | 20/10 °C | Seasonal | 20/10 °C |
| Control | x | x | x | x | x | x |
| Smoke | x | x | x | x | x | x |
| Heat | x | x | x | x | x | x |
| Dark | x | x | ||||
| Smoke + heat | x | x | x | x | x | x |
| Smoke + dark | x | x | ||||
| Heat + dark | x | x | ||||
| Smoke + heat + dark | x | x | ||||
| Scarified | x | x | x | x | x | x |
Changes between each temperature level in the incubators followed a time line similar to natural changes occurring in the field, from summer then autumn, winter and then onto the following summer, over a 60 week period. Experiments run at 20/10 °C were used as a control.
Incubators were set to mimic seasonal changes in temperature in the study region. Four temperature levels were chosen, reflecting monthly averages in the field. These temperatures were 28/18 °C (December, January, February), 25/15 °C (October and March), 20/10 °C (April, May, September) and 16/6 °C (June, July, August). Changes between each temperature level in the incubator followed a time line similar to natural changes. For example, incubators set at 28/18 °C for 3 months, then changed to 25/15 °C for 1 month, 20/10 °C for the next 2 months and 16/6 °C for 3 months, mimicked a seasonal pattern in the field from summer through to winter. Fire cues were applied as described earlier. Each experiment was started at warm temperatures, with seeds therefore undergoing a warm, cold, warm, cold cycle (i.e. following a pattern of summer, autumn, winter, spring, summer temperatures, and so on). The time period applied to mimic the first summer was shorter than subsequent summer temperature periods, reflecting the shorter time following seed maturation during the first summer in the field. Smoke and heat shock were applied to seeds after this first summer treatment, which consisted of 4 weeks at 25 °C followed by 6 weeks at 20/10 °C. All species placed in the incubator at a constant temperature regime of 20/10 °C (equivalent to April/May or September) for 60 weeks were treated with factorial combinations of smoke and heat only (Table 2).
Experiment 5: seed ageing processes
This experiment aimed to determine which aspect of the ageing process was responsible for breaking primary dormancy by comparing seeds previously buried (therefore undergoing seasonal changes, as well as the physical deterioration and leaching that occurs in situ) with seeds aged in an incubator set at a seasonal regime (therefore undergoing a seasonal temperature treatment only). Dry-stored seeds were used as a control for all species. For L. exolasius, it was also possible to test whether there were any different ageing effects between a single season temperature and an annual seasonal temperature regime, by including seeds previously incubated for 60 weeks at 20/10 °C in the comparison.
Seeds from the burial treatment had been buried for 15 months in situ using methods described earlier. Previously incubated seeds (seeds from the above germination timing experiment) had undergone 60 weeks (approx. 15 months) at temperatures mimicking seasonal changes. Dry-stored seeds were aged for 18 months in envelopes at room temperature (approx. 22 °C). It was assumed that the effect of an extra 3 months storage for dry-stored seeds was minimal. After the ageing treatments were completed, replicate dishes for all three species were placed in the incubator. Incubator temperatures were initially set at 28/18 °C and moved through the seasonal regime over 30 weeks.
Experiment 6: fire response of aged seeds
This experiment aimed to assess the effects of fire treatments on aged seeds. Seeds aged by 15 months burial (as described above) were used and treated with factorial combinations of smoke and heat shock cues (L. setiger and L. esquamatus), or with smoke treatments only applied to L. exolasius. Replicate dishes for all three species were then placed in the incubator, as described above, and monitored for 30 weeks.
Analyses
The final percentage germination was expressed as a proportion of seeds assessed as viable at the beginning of the trials. All data were assessed for homogeneity of variance using Levene's test and arcsine transformed if required, to meet the assumptions for parametric analysis. For each species, experiments were designed to analyse germination data using a three-factor orthogonal analysis of variance (ANOVA), with smoke, heat and dark as factors. For experiments where a dark treatment was not applied, data were analysed using a two-factor ANOVA and a one-factor ANOVA used with smoke-only experiments. The effects of seed ageing experiments were also analysed using a one-factor ANOVA, with ageing as the factor. Multiple comparisons were made using the Student–Newman–Kuels (SNK) test, with appropriate adjustments made for multiple factor ANOVAs with significant or non-significant interactions (Underwood, 1997). All graphs are presented using untransformed data.
Temporal germination patterns for all experiments using the seasonal temperature regime were plotted. For each species, total numbers of germinants were pooled and the proportions germinating at each time period calculated. For comparisons, proportional data were standardized by categorizing into four time periods, which were equivalent to temperatures occurring in December–February (summer), March–May (autumn), June–August (winter) and September–November (spring).
RESULTS
Embryo morphology and growth and seed imbibition
All species contained differentiated embryos that are underdeveloped and linear. These were positioned at the base of the seed, surrounded by endosperm. E : S ratios were approximately one-third for each species (Table 3). Embryos of freshly dispersed L. exolasius and L. setiger seeds had not grown after any of the stratification treatments. Leucopogon esquamatus mean embryo length increased from 0·76 mm [±0·021 (s.e.)] to 1·03 mm (±0·025) after 24 weeks warm stratification. A total of 23 % had germinated and several embryos had grown to the full length of the seed. Measurements of embryos after 18 months burial revealed that embryo lengths had not changed for any species. Too few of the previously buried L. esquamatus seeds remained ungerminated for assessment after 24 weeks warm incubation. However, L. exolasius mean embryo length had increased from 0·95 mm (±0·034) to 1·40 mm (±0·162). Several L. exolasius embryos had grown to the full length of the seed.
Table 3.
Morphological details of the three study species (mean ± s.e.)
| Species | Fruit weight (mg) | Seed length (mm) | Embryo length (mm) | E : S ratio | Embryo type |
|---|---|---|---|---|---|
| Leucopogon exolasius | 13·1 ± 0·37 | 3·07 ± 0·034 | 0·95 ± 0·034 | 0·31 | Underdeveloped linear |
| Leucopogon setiger | 10·9 ± 1·70 | 3·00 ± 0·033 | 1·14 ± 0·029 | 0·38 | Underdeveloped linear |
| Leucopogon esquamatus | 4·9 ± 0·16 | 2·41 ± 0·035 | 0·76 ± 0·021 | 0·32 | Underdeveloped linear |
Fresh drupes of all species were able to imbibe water. Leucopogon exolasius, L. setiger and L. esquamatus total weights increased by 15, 17 and 33 %, respectively. Approximately 50 % of this weight increase occurred within 3 h for all species. Endosperm structure had weakened considerably after imbibition, indicating that water had penetrated past the pericarp and into the endosperm.
Experiment 1: assessing seed dormancy
Leucopogon exolasius and L. setiger seeds did not germinate within 30 weeks at any of the single diurnal temperatures. Fresh seeds of these species therefore have a primary dormancy at release. No germination was recorded for fresh L. esquamatus seeds during 30 weeks at 20/10 or 16/6 °C. However, approx. 14 % germinated at 28/18 °C (12 h/12 h light/dark) and the majority of these germinated between 8 and 12 weeks into the experiment (data not shown). The majority of fresh L. esquamatus seeds are therefore dormant at seed release, but a small proportion can germinate after several months at warm temperatures. None of the seeds kept in darkness germinated.
Experiments 2 and 3: single season temperatures—fire cues, scarification and burial
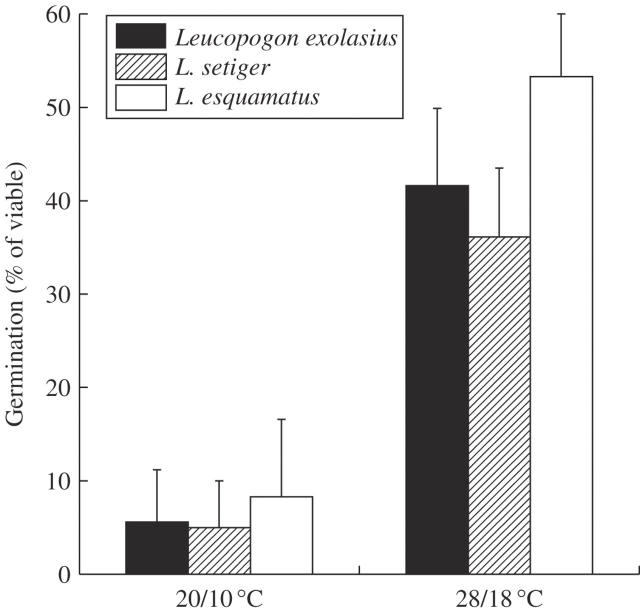
No seeds treated with fire cues or scarification germinated at single season temperatures (Experiment 2). Seeds that had been previously buried for 18 months germinated to relatively high proportions, with more seeds germinating at 28/18 °C than at 20/10 °C (Experiment 3, Fig. 1). No seeds dry-stored for 18 months in the laboratory germinated during Experiment 3 (data not shown).
Fig. 1.
Experiment 3: mean percentage germination of previously buried seeds after 30 weeks at two different temperatures for Leucopogon exolasius, L. setiger and L. esquamatus. Error bars represent the s.e.m.
Experiment 4: seasonal temperature regimes—germination timing and fire cues
Fire cues
Seeds kept in the unvarying temperature regime (20/10 °C) for 60 weeks did not germinate. Under the seasonal temperature regime, too few L. setiger seeds germinated to conduct any statistical analyses. There was a significant, and negative effect of heat as a main effect on germination of L. exolasius (F1,16 = 5·56, P = 0·031) and L. esquamatus (F1,16 = 11·19, P = 0·004). Total L. exolasius germination was quite low (<10 %) whilst L. esquamatus germination was relatively high, with >30 % germination recorded for untreated and smoke-treated seeds (data not shown). Scarification did not significantly increase germination for any species.
Germination phenology
Due to the relatively small numbers of germinants, analyses of germination phenology for each species were conducted using pooled data from all treatments. No germination of L. exolasius or L. setiger occurred during the first cycle of warm temperatures. Approximately 89 % of L. exolasius and 100 % of L. setiger seeds germinated in the autumn equivalent period, between weeks 38 and 48, after the second cycle of warm temperatures (Fig. 2). The rest germinated at times equivalent to late summer. Leucopogon esquamatus seeds germinated in two pulses, after both warm cycle periods (Fig. 2). Of the total, 32 % germinated in the first pulse and 68 % in the second. No germination was recorded for 25 weeks between the two pulses (equivalent to the end of winter, spring and summer periods). Approximately 85 % of seeds that germinated did so in the autumn equivalent period. For all three species, almost no germination occurred after the onset of 16/6 °C winter equivalent temperatures, around week 50 (Fig. 2), even though a large number of viable seeds were still present.
Fig. 2.
Experiment 4: temporal patterns of germination of fresh seeds during 60 weeks under the seasonal temperature regime for Leucopogon exolasius, L. setiger and L. esquamatus. Data are the cumulative percentage, each week, of the total number of seeds germinated.
Experiment 5: seed ageing processes
Treatments significantly affected germination for L. exolasius (F3,8 = 23·34, P < 0·001), L. setiger (F2,6 =107·48, P < 0·001) and L. esquamatus (F2,6 = 6·91, P = 0·028).Seasonal incubation resulted in similar (two species) or greater (one species) germination than burial, whilst dry storage resulted in either zero or slight germination (Fig. 3). Seasonal incubation and burial also resulted in greater germination than incubation at a single season temperature for L. exolasius (Fig. 3A).
Fig. 3.
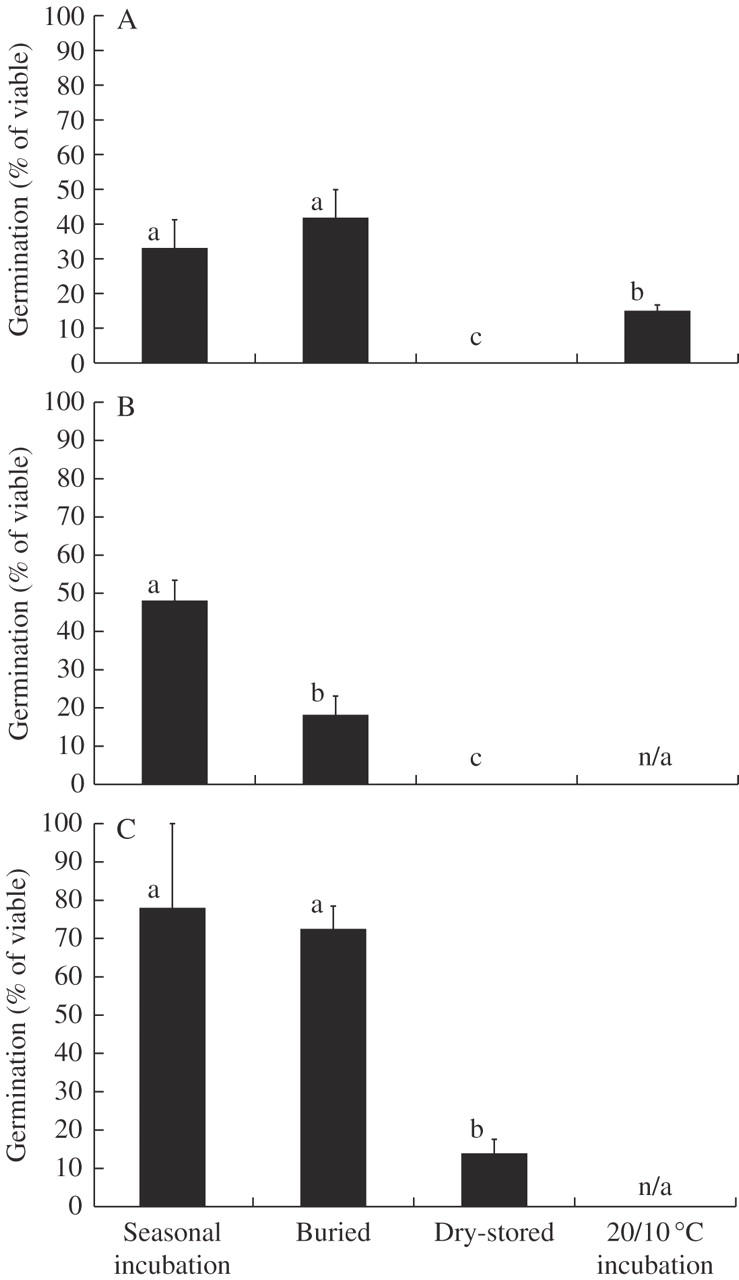
Experiment 5: effect of different ageing treatments on mean percentage germination for (A) Leucopogon exolasius, (B) L. setiger and (C) L. esquamatus after 30 weeks under the seasonal temperature regime, starting at warm temperatures. Error bars represent the s.e.m. Means with the same letter are not significantly different (SNK test, P > 0.05) (n/a denotes treatment not applied).
Experiment 6: fire response of aged seeds
The effects of applied fire treatments on seeds previously aged by 15 months burial varied considerably. Smoke, the only treatment applied to L. exolasius, increased germination totals from 41 to 60 %, but this increase was not significant (Fig. 4A). For L. setiger, there was a significant interaction between smoke and heat (F1,8 = 13·22, P = 0·007). Smoke increased germination for unheated seeds, but this effect disappeared for heated seeds (Fig. 4B). For all treatments, L. esquamatus germinated to between 72 and 87 % (Fig. 4C).
Fig. 4.
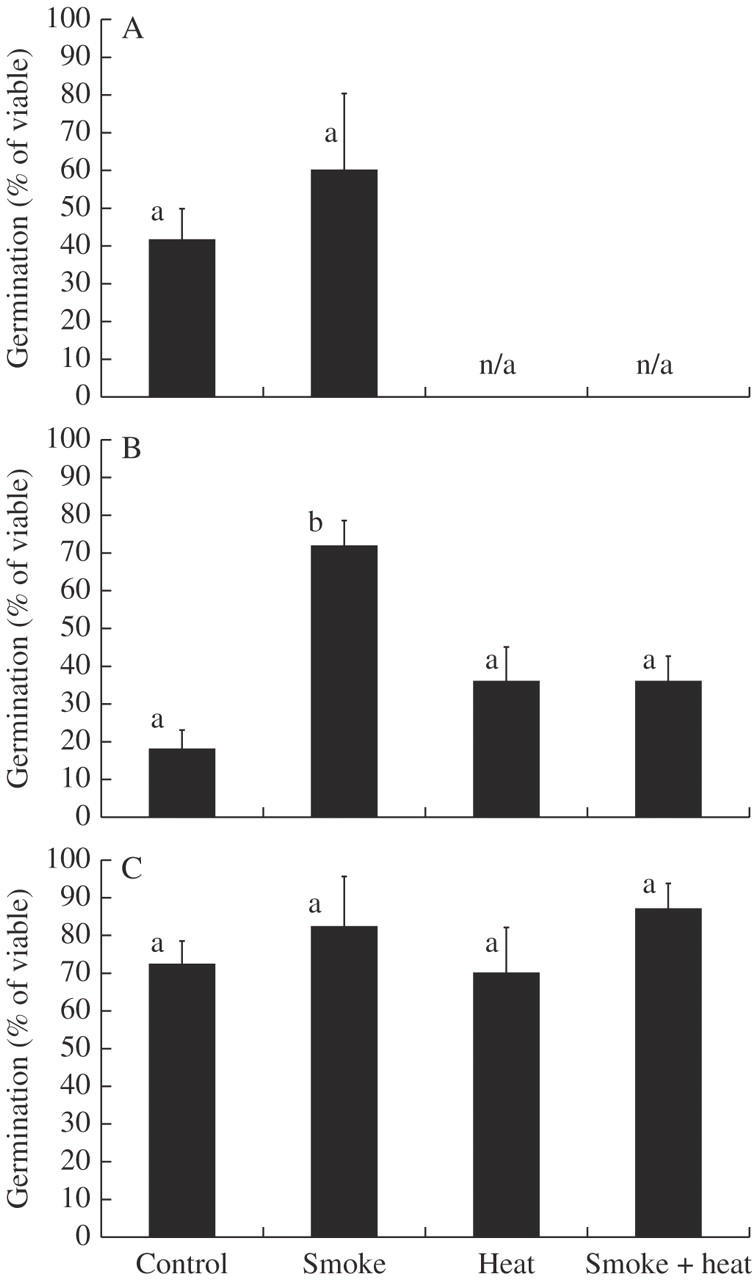
Experiment 6: treatment effects on seeds, previously aged by 18 months burial, then treated with smoke and/or heat. Columns represent mean percentage germination after 30 weeks under the seasonal temperature regime, starting at warm temperatures for (A) Leucopogon exolasius, (B) L. setiger and (C) L. esquamatus (n/a denotes treatment not applied).
DISCUSSION
Fresh seeds of the Leucopogon species studied are dormant at release and have underdeveloped linear embryos. Seeds with underdeveloped embryos that have the ability to grow, that do not germinate after testing for several weeks at temperatures that typically occur in their environment, have a primary dormancy that is a combination of morphological and physiological factors (Baskin and Baskin, 1998). The primary dormancy of all three species investigated is therefore classified as morphophysiological (Baskin and Baskin, 2004). Morphological dormancy is imposed by the need for embryos to grow to a species-specific length before germination can take place. This growth occurs at particular seasonal temperatures (Baskin and Baskin, 1998). In the case of the Leucopogon species, embryos of two of the species were able to grow at warm temperatures, and several seeds observed after incubation had embryos which had extended to the full length of the seed. Embryos must therefore triple in size before germination occurs. Physiological dormancy is caused by physiological inhibition mechanisms within the embryo or structures surrounding the embryo. Physiological dormancy can be broken before, after or concurrently with morphological dormancy and is dependent on stratification at warm or cold temperatures, or some combination of these, experienced within the habitat (Baskin and Baskin, 1998). In the field, stratification occurs at seasonal temperatures.
This study supports the conventional concept that stratification at seasonal temperatures is the principal mechanism for breaking morphophysiological dormancy (Baskin and Baskin, 1998). It has been hypothesized that other factors that occur during burial, such as physical deterioration of the seed coat or general ageing, are important requirements for Leucopogon germination (Roche et al., 1997; Tieu and Egerton-Warburton, 2000). However, in this study, seeds subjected to temperatures equivalent to seasonal changes germinated to at least the same levels as the buried seeds (Experiment 5). Incubator-aged seeds are subjected to temperature changes only and do not undergo the same physical deterioration. Results from other assessments, such as the ability of fresh seeds to imbibe water and the lack of increase in germination from scarified treatments, also suggest that seasonal temperatures are more important than reducing mechanical constraints for breaking dormancy. In addition, seeds dry-stored at room temperature for 18 months, or incubated at a single temperature setting (Experiment 5), produced much lower levels of germination, emphasizing that fluctuating temperatures are the cue rather than simply an ageing process.
The distinction between whether fire cues break primary dormancy or increase subsequent germination after primary dormancy is broken is an important one, as it clarifies the sequence of processes occurring naturally in the seed bank (Baskin and Baskin, 2003). The fire cues, smoke and heat shock, did not break primary morphophysiological dormancy of freshly dispersed seeds of the Leucopogon species studied, and very low levels of germination were recorded both at single-season temperatures (Experiment 2) and at seasonally varying temperatures (Experiment 4). Combined with evidence from other studies (Keeley, 1987; Dixon et al., 1995; Keeley and Fotheringham, 1998; Clarke et al., 2000), it is considered unlikely that fire cues overcome this type of dormancy in species from other fire-prone habitats. However, once primary dormancy was broken in our study (during burial), there was a trend for smoke to enhance germination for two of the study species. Although not dependent entirely upon smoke (untreated seeds from all experiments germinated to between 20 and 80 %), germination of L. exolasius and L. setiger seeds was increased by between 10 and 55 % above control treatments.
One possible explanation for these results is that smoke may have a positive effect on seeds that remain morphologically dormant, after physiological dormancy has been removed during burial or stratification. Embryo lengths of the study species remained unchanged after burial but grew in length and germinated once placed at warm incubation temperatures in light. Smoke increases the sensitivity of seeds to the hormones that promote embryo growth (and therefore overcome morphological dormancy) (van Staden et al., 2000; Schwachtje and Baldwin, 2004) and this could increase the proportion of seeds within a seed lot that germinate. Identifying the order in which morphophysiological factors are broken would help to validate this hypothesis.
Factors other than smoke may also promote embryo growth and germination from a persistent seed bank. Leucopogon germination pulses significantly in the first germination season after fire, and observations suggest that they are possibly gap recruiters (Ooi et al., 2004a). Gaps are created after fire by the removal of canopies and litter (Keeley, 1995; Bond and van Wilgen, 1996). Light and increased temperature amplitude both provide mechanisms that could promote a flush of seedlings, either post-fire or in gaps. A light requirement is an important mechanism adopted to detect gaps, particularly by small-seeded species, from a variety of habitats (Pons, 1992); however, its importance in regulating dormancy and germination in Australian fire-prone habitats is not well understood (Bell et al., 1993; Clarke et al., 2000). Daily temperature amplitude can increase significantly after the removal of aboveground canopy and litter (Auld and Bradstock, 1996). Further investigations into the effect of smoke, light and daily temperature amplitude on Leucopogon germination are needed to gain a fuller understanding of recruitment dynamics.
In the field, seasonal emergence from a persistent soil seed bank has been recorded for all three species, even though the region does not have seasonal rainfall patterns (Ooi et al., 2004a, 2006). These patterns emphasize the importance of seasonal temperatures, not only for breaking primary dormancy, but also for determining germination timing via subsequent conditional dormancy. The majority of germination of seasonally incubated seeds was timed to the period equivalent to autumn, possibly because immature embryos required a long period of time at warm temperatures for growth, after physiological dormancy was overcome. Leucopogon seedling emergence occurs primarily in autumn and winter in the field (Ooi et al., 2004a). Allowing for the lag time between radicle protrusion and seedling emergence of 3–4 weeks (M. K. J. Ooi, pers. obs.), results from the germination trials and seedling emergence patterns in the field correlate well.
Although the three Leucopogon species studied are the first in south-eastern Australia to be classified as having a physiological dormancy component, there is strong biogeographical evidence to suggest that morphological and physiological factors, and therefore seasonal temperatures, are likely to control the dormancy of many species from this fire-prone region (Baskin and Baskin, 1998; Ooi, in press). It is therefore likely that a suite of dormancy mechanisms exists in fire-prone regions generally (Bell, 1999), and a greater understanding of all of these can contribute to a better understanding and management of these ecosystems. A relatively recent increase in the practise and scrutiny of practical management applications, such as habitat restoration and native species horticulture, has focused the need for this understanding.
Acknowledgments
We thank Todd Minchinton, Charles Morris and two anonymous referees for providing helpful comments on the original manuscript, and Andrew Denham for assistance in the field. Financial support is gratefully acknowledged from an Australian Research Council APAI Scholarship. This is contribution number 270 from the Ecology and Genetics Group at the University of Wollongong.
LITERATURE CITED
- Allan SM, Adkins SW, Preston CA, Bellairs SM. 2004. Improved germination of the Australian natives: Hibbertia commutata, Hibbertia amplexicaulis (Dilleniaceae), Chameascilla corymbosa (Liliaceae) and Leucopogon nutans (Epacridaceae). Australian Journal of Botany 52: 345–351. [Google Scholar]
- Auld TD. 2001. The ecology of the Rutaceae in the Sydney region of south-eastern Australia: poorly known ecology of a neglected family. Cunninghamia 7: 213–239. [Google Scholar]
- Auld TD, Bradstock RA. 1996. Soil temperatures after the passage of a fire: do they influence the germination of buried seeds? Australian Journal of Ecology 21: 106–109. [Google Scholar]
- Auld TD, O'Connell MA. 1991. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Australian Journal of Ecology 16: 53–70. [Google Scholar]
- Baskin CC, Baskin JM. 1998. Seeds: ecology, biogeography and evolution of dormancy and germination. San Diego: Academic Press.
- Baskin CC, Baskin JM. 2003. Overview and recommendations for future research priorities on native seed dormancy and germination of Australian plants. Australian Plant Conservation 11: 2–9. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Bell DT. 1999. Turner review No. 1: the process of germination in Australian species. Australian Journal of Botany 47: 475–517. [Google Scholar]
- Bell DT, Plummer JA, Taylor SK. 1993. Seed germination ecology in south-western Western Australia. Botanical Review 59: 24–73. [Google Scholar]
- Bond WJ, van Wilgen BW. 1996. Fire and plants. London: Chapman and Hall.
- Brown NAC. 1993. Promotion of germination of fynbos seeds by plant-derived smoke. New Phytologist 123: 575–583. [DOI] [PubMed] [Google Scholar]
- Clarke PJ, Davison EA, Fulloon L. 2000. Germination and dormancy of grassy woodland and forest species: effects of smoke, heat, darkness and cold. Australian Journal of Botany 48: 687–700. [Google Scholar]
- Davis FW, Borchert MI, Odion C. 1989. Establishment of microscale vegetation pattern in maritime chaparral after fire. Vegetatio 84: 53–67. [Google Scholar]
- Dixon KW, Roche S, Pate JS. 1995. The promotive effect of smoke derived from burnt vegetation on seed germination of western Australian plants. Oecologia 101: 185–192. [DOI] [PubMed] [Google Scholar]
- Enright NJ, Goldblum D, Ata P, Ashton DH. 1997. The independent effects of heat, smoke and ash on emergence of seedlings from the soil seed bank of a heathy Eucalyptus woodland in Grampians (Gariwerd) National Park, western Victoria. Australian Journal of Ecology 22: 81–88. [Google Scholar]
- Fox J, Dixon B, Monk D. 1987. Germination in other plant families. In: Langkamp PJ, ed. Germination of Australian native plant seed. Melbourne: Inkata Press, 211–223.
- Gill AM. 1981. Adaptive responses of Australian vascular plant species to fire. In: Gill AM, Groves R, Noble I, eds. Fire and the Australian biota. Canberra: Australian Academy of Science, 243–271.
- Keeley JE. 1987. Role of fire in seed germination of woody taxa in California chaparral. Ecology 68: 434–443. [Google Scholar]
- Keeley JE. 1991. Seed germination and life history syndromes in the California chaparral. Botanical Review 57: 81–116. [Google Scholar]
- Keeley JE. 1995. Seed-germination patterns in fire-prone Mediterranean-climate regions. In: Kalin Arroyo MT, Zedler PH, Fox MD, eds. Ecology and biogeography of Mediterranean ecosystems in Chile, California, and Australia. New York: Springer-Verlag, 239–273.
- Keeley JE, Fotheringham CJ. 1997. Trace gas emissions and smoke-induced seed germination. Science 276: 1248–1250. [Google Scholar]
- Keeley JE, Fotheringham CJ. 1998. Smoke-induced seed germination in California chaparral. Ecology 79: 2320–2336. [Google Scholar]
- Keith DA. 1997. Combined effects of heat shock, smoke and darkness on germination of Epacris stuartii Stapf., an endangered fire-prone Australian shrub. Oecologia 112: 340–344. [DOI] [PubMed] [Google Scholar]
- Kenny BJ. 2000. Influence of multiple fire-related germination cues on three Sydney Grevillea (Proteaceae) species. Austral Ecology 25: 664–669. [Google Scholar]
- de Lange JH, Boucher C. 1990. Autecological studies on Audouinia capitata (Bruniaceae). 1. Plant derived smoke as a seed germination cue. South African Journal of Botany 55: 700–703. [Google Scholar]
- Morris EC. 2000. Germination response of seven east Australian Grevillea species (Proteaceae) to smoke, heat exposure and scarification. Australian Journal of Botany 48: 179–189. [Google Scholar]
- Morrison D, Morris EC. 2000. Pseudoreplication in experimental designs for the manipulation of seed germination experiments. Austral Ecology 25: 179–189. [Google Scholar]
- O'Brien SD, Johnston ME. 2004. Seed viability and dormancy mechanisms of Leucopogon melaleucoides Cunn. ex DC. (Epacridaceae). Seed Science and Technology 32: 5–10. [Google Scholar]
- Ooi MKJ. In press. Dormancy classification and potential dormancy-breaking cues for shrub species from fire-prone south-eastern Australia. In: Adkins SW, Navie SC, Ashmore S, eds. Seed science research: advances and applications. Wallingford, UK: CAB International.
- Ooi MKJ, Auld TD, Whelan RJ. 2004a. Delayed post-fire seedling emergence linked to season: a case study with Leucopogon (Epacridaceae). Plant Ecology 174: 183–196. [Google Scholar]
- Ooi MKJ, Auld TD, Whelan RJ. 2004b. Comparison of the cut and tetrazolium tests for assessing seed viability: a study using Australian native Leucopogon species. Ecological Management and Restoration 5: 141–143. [Google Scholar]
- Ooi MKJ, Whelan RJ, Auld TD. 2006. Persistence of obligate-seeding species at the population scale: the effects of fire intensity, fire patchiness and long fire-free intervals. International Journal of Wildland Fire 15: 261–269.
- Pons TL. 1992. Seed responses to light. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities. Wallingford: CAB International, 259–284.
- Roche S, Dixon KW, Pate JS. 1997. Seed aging and smoke: partner cues in the amelioration of seed dormancy in selected Australian native species. Australian Journal of Botany 45: 783–815. [Google Scholar]
- Rokich DP, Dixon KW, Sivasithamparam K, Meney KA. 2002. Smoke, mulch, and seed broadcasting effects on woodland restoration in Western Australia. Restoration Ecology 10: 185–194. [Google Scholar]
- Schatral A, Osborne JM, Fox JED. 1997. Dormancy in seeds of Hibbertia cuneiformis and H. huegelii (Dilleniaceae). Australian Journal of Botany 45: 1045–1053. [Google Scholar]
- Schwachtje J, Baldwin IT. 2004. Smoke exposure alters endogenous gibberellin and abscisic acid pools and gibberellin sensitivity while eliciting germination in the post-fire annual, Nocotiana attenuata. Seed Science Research 14: 51–60. [Google Scholar]
- van Staden J, Brown NAC, Jäger AK, Johnson TA. 2000. Smoke as a germination cue. Plant Species Biology 15: 167–178. [Google Scholar]
- Stern H, de Hoedt G, Ernst J. 2000. Objective classification of Australian climates. Australian Meteorological Magazine 49: 87–96. [Google Scholar]
- Thomas PB, Morris EC, Auld TD. 2003. Interactive effects of heat shock and smoke on germination of nine species forming soil seed banks within the Sydney region. Austral Ecology 28: 674–683. [Google Scholar]
- Thompson K, Ceriani RM, Bakker JP, Bekker RM. 2003. Are seed dormancy and persistence in the soil related? Seed Science Research 13: 97–100. [Google Scholar]
- Tieu A, Egerton-Warburton LM. 2000. Contrasting seed morphology dynamics in relation to the alleviation of dormancy with soil storage. Canadian Journal of Botany 78: 1187–1198. [Google Scholar]
- Tieu A, Dixon KW, Meney KA, Sivasithamparam K. 2001. Interaction of burial and smoke on germination patterns in seeds of selected Australian native plants. Seed Science Research 11: 69–76. [Google Scholar]
- Underwood AJ. 1997. Experiments in ecology. Cambridge: Cambridge University Press.
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031–1037. [Google Scholar]
- Wills TJ, Read J. 2002. Effects of heat and smoke on germination of soil-stored seed in a south-eastern Australian sand heathland. Australian Journal of Botany 50: 197–206. [Google Scholar]
- Whelan RJ. 1995. The ecology of fire. Cambridge: Cambridge University Press.