Abstract
• Background and Aims Various alien species have been introduced to the Ogasawara Islands (Japan). A survey was made investigating whether the native pollination systems fit an ‘island syndrome’ (biasing the flora to dioecy, with subdued, inconspicuous flowers) and whether alien species have disrupted the native pollination network.
• Methods Flower visitors and floral traits were determined in the field (12 islands) and from the literature. Associations among floral traits such as sexual expression, flower colour and flower shape were tested.
• Key Results Among the 269 native flowering plants, 74·7 % are hermaphroditic, 13·0 % are dioecious and 7·1 % are monoecious. Classification by flower colour revealed that 36·0 % were white, 21·6 % green and 13·8 % yellow. Woody species (trees and shrubs) comprised 36·5 % of the flora and were associated with dioecy and white flowers. Solitary, endemic small bees were the dominant flower visitors and visited 66·7 % of the observed species on satellite islands where the native pollination networks are preserved. In contrast to the situation on the satellite islands, introduced honeybees were the most dominant pollinator (visiting 60·1 % of observed species) on the two main islands, Chichi-jima and Haha-jima, and had spread to satellite islands near Chichi-jima Island.
• Conclusions The island syndrome for pollination systems in the Ogasawara Islands was evident in a high percentage of dioecious species, the subdued colour of the native flora and solitary flower visitors on satellite islands. The shape and colour adaptations of several flowers suggested native pollination niches for long-proboscis moths and carpenter bees. However, the domination and expansion of introduced honeybees have the potential for disruption of the native pollination network in the two main, and several satellite, islands of the Ogasawara Islands.
Keywords: Dioecy, flower traits, introduced honeybees, island syndrome, oceanic island, pollination niche
INTRODUCTION
Pollination disruption is a serious problem in biodiversity hotspots (Vamosi et al., 2006). In addition, island ecosystems are vulnerable to invasion by alien species. Recently, disruption of pollination networks on several islands has been demonstrated (Kearns et al., 1998; Kato et al., 1999; Cox and Elmqvist, 2000; Hansen et al., 2002; Olesen et al., 2002). Alien pollinators have disturbed pollination systems (Traveset and Richardson, 2006) through resource competition with native pollinators (Roubik, 1978; Buchmann, 1996; Dupont et al., 2004; Paini and Roberts, 2005) and through the reduction of plant reproductive success (Vaughton, 1996; Gross and MacKay, 1998; Paton, 2000; Carmo and Franceschinelli, 2004). Pollination biology at the community level has been studied in the following island groups: the Galapagos (McMullen, 1987, 1993), Juan Fernández (Bernardello et al., 2001), Canary Islands (Dupont et al., 2003) and the Azorean Flores Islands and Mauritian Île aux Aigrettes (Olesen et al., 2002). However, the pattern of disruption of the pollination network and its causes and effects on plant reproductive success are still poorly understood for many oceanic islands.
The ecosystems of oceanic islands can be very different from those of nearby continents, as a result of immigration history and the radiation of species on the islands. For example, loss of dispersability, increased dioecy, gigantism of body size and other changes as described below are common on oceanic islands (Carlquist, 1974; Brown and Lomolino, 1998; Whittaker, 1998). The unique phenomena that occur on remote islands are collectively referred to as the ‘island syndrome’ (Whittaker, 1998) or ‘island rule’ (Brown and Lomolino, 1998).
Flowers and pollinators can exhibit some characteristics of the island syndrome, such as being small, inconspicuous in colour and having an accessible flower shape, and opportunistic generalist pollinators or wind pollination are also observed (Godley, 1979; Olesen, 1985; Barrett, 1996; Bernardello et al., 2001; Olesen et al., 2002; Carpenter et al., 2003). The main pollinators on oceanic islands are typically generalists such as solitary bees and flies, whereas butterflies and social bees are largely absent. A bias of floral sex expression toward dioecy and small inconspicuous flowers may be associated with the presence of opportunistic pollinators on islands (Bawa, 1982; Baker and Cox, 1984; Sakai et al., 1995a, b; Barrett, 1996). Wind pollination may be associated with the less-diverse insect fauna than on the mainland (Janzen, 1973; Andrews, 1979; Spears, 1987).
While generalist flowers and generalist insects comprise the majority in island biota, pollination networks often exhibit unique interactions because of high rates of endemism in island biotas. In addition, extreme flower characteristics have occasionally co-evolved with endemic specialist pollinators under a stable environment with an impoverished species composition on an island (Nilsson et al., 1985). Specialist pollination on islands is also well known in New Zealand (Lloyd, 1985; Ecroyd, 1996), New Caledonia (Kawakita and Kato, 2004) and the Canary Islands (Dupont and Skov, 2004). In island ecosystems, interactions of both endemic plants and endemic pollinators may play an important role in community structure, stability and diversity.
The Ogasawara Islands are a group of volcanic oceanic islands in Japan that contain highly endemic biota (Ono, 1998; Shimizu, 2003). The honeybee was introduced to Chichi-jima Island for bee-keeping in 1880 (Funakoshi, 1990; Hara, 1996). Although Kato et al. (1999) noted that introduced honeybees (Apis mellifera) may have a potentially great impact on the Ogasawara Islands, the native plant–pollinator interactions of these islands have not been well studied. Basic information on the native plant–pollinator interactions is needed to understand how biological invasions disturb the native pollination system on these islands. Thus, an investigation was made of the floral traits and pollinators of 269 native plant species in 80 families. In this paper, the pollination system of the native flora is described and an analysis is made of floral traits (sexual expression, flower colour and flower shape) in order to determine whether it fits an island syndrome. In addition, the impact of alien pollinators that disturb the native plant–pollinator relationships on these islands is discussed.
METHODS
Study site
The Ogasawara (Bonin) Islands are of relatively recent volcanic origin, and are located 1000 km south of the Japanese mainland between 27°44′N and 24°14′N, and near 140°12′ E. The island group includes about 50 small islands (Fig. 1). The Paleo Izu-Ogasawara Arc was formed on the Philippine Sea Plate 45 million years ago and the present Izu-Ogasawara Arc developed 15 million years ago (Taira, 1994). The present Ogasawara Islands may have been present above sea-level for at least several million years (Kaizuka, 1977). The largest island, Chichi-jima, is only 24·0 km2 in size, with its highest point 317 m above sea level; Haha-jima Island is next largest, with an area of 20·8 km2 and a maximum elevation of 453 m. People live on these two largest islands. The other islands are smaller than 8 km2 and are uninhabited. These islands have a subtropical monsoon climate, with a long, dry summer and a mild winter. Average temperature is 23·2 °C and annual precipitation averages 1292 mm on Chichi-jima Island (Toyoda, 2003). Because the annual precipitation is low, dry forest and shrubland dominate most of the islands, with the exception of the mountain range of Haha-jima Island, where only mesic cloud forest develops (Mueller-Dombois and Fosberg, 1998; Toyoda, 2003). Under this subtropical climate and vegetation, the biota contains a high percentage of endemic and rare species (Shimizu, 2003). To provide a better understanding of this biota, a census route was selected to cover all the main vegetation types and to observe rare species found in this small area (Table 1).
Fig. 1.
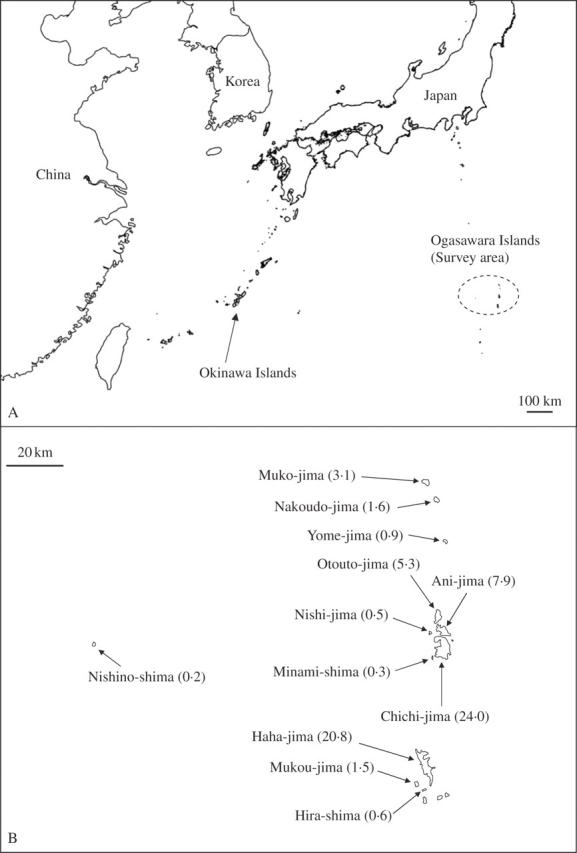
(A) Location of the Ogasawara Islands. (B) Distribution of main and satellite islands. Numbers within parenthesis in (B) are the area of the island (km2).
Table 1.
Survey area, number of surveys, length of census route, vegetation type and altitude ranges of the area in which each flower census was performed
| Area | Number of surveys | Length of census route (km) | Vegetation1 | Altitude (m) |
|---|---|---|---|---|
| Chichi-jima Island | ||||
| Nagasaki | 18 | 0·5 | S | 50–160 |
| Asahiyama | 20 | 1·3 | D, R | 205–265 |
| Asahidaira | 9 | 0·2 | S, R | 210–230 |
| Yoakedaira | 3 | 0·9 | D, S | 190–220 |
| Higashidaira | 20 | 1·1 | D, S | 210–250 |
| Hatsuneura | 19 | 1·0 | D, S | 200–240 |
| Chuousan | 13 | 0·4 | D, R | 260–315 |
| Shigureyama | 2 | 1·2 | D, R | 170–235 |
| Kitafukurozawa | 15 | 1·0 | C, H | 0–15 |
| Sakaiura | 4 | 1·1 | C, H | 20–45 |
| Maihama | 12 | 0·2 | C, H | 0–5 |
| Kasayama | 10 | 0·2 | S, R | 230–285 |
| Haha-jima Island | ||||
| Sekimon | 14 | 2·2 | M | 260–400 |
| Sakaigatake | 16 | 1·4 | M, S | 265–440 |
| Nagahama | 6 | 0·8 | M, C | 125–230 |
| Yashihama | 4 | 0·5 | C | 0–20 |
| Chibusayama | 7 | 4·2 | M, D | 20–460 |
| Higashiyama | 3 | 2·5 | D, R | 50–290 |
| Kuwanokiyama | 17 | 0·7 | M | 220–255 |
| Nishiura | 8 | 0·9 | D | 30–110 |
| Omotohama | 14 | 1·0 | D, C | 0–55 |
| Minamisaki | 2 | 1·6 | D, C | 0–85 |
| Nakanotaira | 13 | 0·8 | D, H | 60–90 |
| Satellite islands | ||||
| Ani-jima | 6 | 1·4 | D, S, C, R | 0–180 |
| Otouto-jima | 3 | 1·2 | D, C, R | 0–45 |
| Minami-shima | 2 | 0·6 | R, C | 0–40 |
| Nishi-jima | 1 | 0·7 | D, C, R | 0–60 |
| Muko-jima | 1 | 2·1 | R | 0–60 |
| Yome-jima | 2 | 1·3 | R | 0–85 |
| Nakoudo-jima | 1 | 0·8 | R | 0–60 |
| Nishino-shima | 1 | 0·7 | R | 0–25 |
| Mukou-jima | 8 | 1·5 | D, C, R | 0–100 |
| Hira-shima | 3 | 0·8 | D, C, R | 0–55 |
1 M = mesic forest; D = dry forest; S = shrubland; C = coastal vegetation; R = rocky grassland; H = inhabited area.
Data collection
Floral traits were classified based on several features (see Table 2). Floral traits (flower size, colour, shape, habit, longevity, number of flowers per inflorescence and number of inflorescence per individual) and flower visitors were observed on Chichi-jima, Haha-jima, Ani-jima, Otouto-jima, Mukou-jima, Hira-shima, Muko-jima, Yome-jima, Nakoudo-jima, Minami-shima, Nishi-jima and Nishino-shima Islands. The field survey was conducted for 3–30 d per trip in March, July and November 2001; January, May, September and December 2002; March, April, August and October 2003; February, April, June and November 2004; and January, April, May, August and November 2005. If flowers were found whilst following the census route of Table 1, they were monitored for the presence of visitors for 10 min. After making this observation, a record was made of the flower colour, shape, number of flowers per inflorescence, flower size, flower habit and nectar volume in species for which these parameters had not already been recorded. Then, the insect visitors remaining on the flowers were collected and the intact inflorescences were marked in order to observe the flowering phenology every day so that flower longevity could be recorded. If no flower visitor was oberved over 10 min, the observation time was occasionally extended up to 30 min. This sampling method has been shown to be appropriate for detecting overall patterns in the pollinator community (Kato et al., 1993; Kato and Kawakita, 2004). More than five individuals were recorded on 70·2 % (73 out of 104) of the observed species.
Table 2.
Classification of the flower characteristics in the survey: trait number, abbreviation of species features used in the Appendix and the unification of features for statistical analysis
| Number | Traits | Feature (Abbreviation) | Unified features |
|---|---|---|---|
| 1 | Type | E, endemic; I, indigenous | |
| 2 | RDB category1 | Ex, extinct; C, threatened (critically endangered); E, threatened (endangered); V, threatened (vulnerable); L, low risk (near threatened); D, data deficient | |
| 3 | Population on islands | A, abundant; C, common; Rw, rare and wide distribution; Rn, rare and narrow distribution | |
| 4 | Life form | T, tree; S, shrub; P, perennial herb; A, annual; V, vine | W (wood, T + S + V); H (herb, A + P + V) |
| 5 | Sex | H, hermaphrodite; Mo, monoecy; D, dioecy; Am, andromonoecy; Gm, gynomonoecy; Mix, mixed | M (monoecy, Mo + Am + Gm + Mix); H (H); D (D) |
| 6 | Flower habit | S, canopy surface; U, forest understory; O, open space; H, hydrophyte | S (S); U (U); O (O + H) |
| 7 | Flower shape | Be, bell; Br, brush; D, dish; Fi, fig; Fu, funnel; I, inconspicuous (with no optical attraction); T, tube; Z, zygomorphic | I (Br + Fi + I); D (D); T (Be + Fu + T); Z (Z) |
| 8 | Flower color | Bl, blue; Bk, black; Br, brown; G, green; P, pink; R, red; V, violet; W, white; Y, yellow*; calyx, stamen, stigma, bract, inflorescence; no mark, corolla | S (subdued; Bk + Br + G); V (vivid, Bl + P + R + V); W (W); Y (Y) |
| 9 | Flower longevity | S, short (<2 d); M, medium (2–4 d); L, long (4–6 d); Vl: very long (>6 d); –, no available information; no mark, observed data (average) | |
| 10 | Flower size | Vs, very small (<5 mm in diameter); S, small (5–10 mm); M, medium (10–30 mm); L, large (>30 mm) | L (L); S (M + S); Vs (Vs) |
| 11 | Flowers/inflorescence | 1, 1–10; 10, 11–100; 100, 101–1000; 1000, more than 1001 | 1 (1); 10 (10); 100 (100 + 1000) |
| 12 | Inflorescences/plant | 1, 1–10; 10, 11–100; 100, 101–1000; 1000, 1001–10000; 10000, more than 10001 | |
| 13 | Pollination | A, anemophily; H, hydrophily; I, insect; V, vertebrate; *, specialist | A (abiotic; A + H); B (biotic; I + V) |
| 14 | Observed visitors | Hb, honeybees; Xb, Xylocopa bees; Eb, endemic small bees; Be, other bees; F, flies; Bt, beetles; Bu, butterflies; Bi, birds; M, moths; A, ants; T, thrips; -, observed but no visitors; ( ), night |
1 RDB = Red Data Book (Environmental Agency of Japan, 2000).
* From literature only.
Flower visitors were also observed using an unattended digital video camera (Sony DCR-TRV50, Tokyo, Japan) for 80 min per trial. The video camera offered the advantage of allowing observation of visits by birds, which avoid humans. The camera's zoom and distance from the plant were adjusted so that the infloresence filled the screen, thus facilitating identification of visitors. Flower visitors were classified into 12 taxonomic groups: honeybees; carpenter bees; solitary, endemic small bees; other hymenopterans; flies (dipterans); beetles; butterflies; birds; moths; ants; thrips; and other visitors (including bugs, grasshoppers, crickets, cockroaches, geckos, lizards and hermit crabs). Solitary, endemic small bees included eight species that were listed as endemic bees by Kato et al. (1999); the larger, and also endemic, carpenter bee Xylocopa ogasawarensis being recorded separately. Diurnal observations were performed between 0600 h and 1800 h. During these periods, observervations were made by watching about 10 inflorescences at a time, with a range of 1–30 inflorescences depending on the species traits and visitation frequency. When visitation rates were high, fewer inflorescences were observed in order to minimize counting errors. The digital video camera was also used to observe 1–3 inflorescences (usually one inflorescence) per trial, the low number being because of the camera's narrow view. To calculate the visitation frequency per flower, the number of inflorescences within the observation range and the number of flowers per inflorescences were recorded. When there were no or few visitors during the day, the flowers were also observed at night using the camera's ‘night-shot’ function, which could record at low-light conditions (Fig. 2N).
Fig. 2.

Examples of the wide range of characteristics for flowers endemic to the Ogasawara Islands. (A) Alpinia boninensis (Zingiberaceae) has large, white flowers pollinated by the endemic Xylocopa ogasawarensis (Anthophoridae, Hymenoptera) in the forest understorey. (B) Bulbophyllum boninensis (Orchidaceae) has a few medium-sized, yellow flowers and may be pollinated by specialist moth or by X. ogasawarensis in an understorey habitat. (C) Eulophia toyosimae (Orchidaceae) and (D) Sciaphila okabeana (Triuridaceae) are also rare understorey herbs and saprophytes. (E) Scutellaria longituba (Labiatae) has a very long flower tube compared with that of other Scutellaria species, but lepidopteran pollinators have not been observed. (F) A highly endangered tree, Claoxylon centinarium (Euphorbiaceae), has small, subdued flowers that are mainly visited by flies. (G) Santalum boninense (Santalaceae) has small, white, bell-shaped flowers but sets few fruits despite frequent visit by honeybees. (H) Vaccinium boninense (Ericaceae) selects for certain pollinators by means of the narrow floral mouth. (I) Wikstroemia pseudoretusa (Thymelaeaceae) and (J) Dendrocacalia crepididifolia (Compositae) have evolved to dioecy in the Ogasawara Islands. (K) Myrsine maximowiczii (Myrsinaceae) and (L) Boninia glabra (Rutaceae) have many small, white flowers, as is also the case for many endemic woody species. (M) Lobelia boninensis (Campanulaceae) has a monocarpic life cycle and the shape of its flowers is suited for pollination by X. ogasawarensis (arrow). (N) Stachyurus macrocarpus (Stachyuraceae) is also a highly endangered shrub, and is pollinated by nocturnal moths (recorded by the video camera's ‘night shot’ function). (O) Ligustrum micranthum (Oleaceae) and (P) Machilus boninensis (Lauraceae) are pollinated by flies. Introduced honeybees visit many endemic species including endangered species such as (Q) Crepidiastrum grandicollum (Compositae) and sometimes act as nectar robbers for species such as (R) Hedyotis grayi (Rubiaceae).
The amount of nectar per flower was measured for 56 native species during daytime (1–26 flowers per species) using a microsyringe (Hamilton 7000·5 KH, Reno, NV, USA) with 0·1 μL resolution. This measurement was conducted during either one or two of the route censuses. Since the flowers used for measurement were open to visitors, the maximum value measured during the course of the trials was defined as the potential amount of nectar for the species. If I could not detect any more nectar during five absorption trials, then I judged all nectar was measured at that flower. The sugar concentration of the nectar was also measured using a pocket refractometer (Bellingham & Stanley Ltd., Kent, UK). If the nectar volume of an individual flower was not enough to measure, additional nectar was collected from other flowers within same individual.
Literature survey
For the species that did not produce flowers during the research visits to the islands, a literature survey was conducted. An analysis was made of 269 plant species (120 endemic and 149 indigenous) in terms of their flower characteristics. In this paper, ‘endemic’ means species found only on the Ogasawara Islands and ‘indigenous’ means native species that are also distributed in other regions. Toyoda (2003) was referred to for a list of flora and floral traits, and Satake et al. (1982, 1989), Hatsushima and Nakajima (1979) and Environmental Agency of Japan (2000) were examined for additional information on floral traits. In addition to the field observations of flower visitors, referrence was made to Kato (1992), Tanaka (1993, 1994, 1995), Abe et al. (1994), Yokoyama and Iwatsuki (1997) and Goto and Washitani (2001). The analysis of visitation frequency was based only original field data from the current study, but the data presented in the Appendix also include previous records from the literature.
Among the Compositae, the flowerhead was treated as the unit of visitation in terms of flower size and numbers of flowers and inflorescences (see classification presented in Table 2). The flower shape was considered to be a ‘dish’ in all Compositae. In the Graminaceae and Cyperaceae, the numbers of flowers and inflorescences were represented by the number of florets per panicle and the number of panicles per individual, respectively. In dioecious species, the size of the flower and the numbers of flowers and inflorescences were based on those of the male plant because the larger floral display of males was likely to associate with pollinator visitations. Flower colours were recorded for the most visually attractive parts within flowers (e.g. corolla, calyx, bracts). Flower size equalled the mean diameter of an individual flower. However, if the vertical length was longer than the diameter in tube- or funnel-shaped flowers, the flower size was represented by its vertical length on the assumption that pollinators are most likely to be attracted based on the maximum dimension. Where flowers could not be found for an individual species, the literature was referred to for flowering habit (54 of 269 native species).
Data analysis
Traits were analysed at the generic level as well as the species level, in order to partially correct for the fact that several congeneric species within the same lineage are not statistically independent of one another. To evaluate the association among the various traits, a test was made of the number of species and genera that possessed a given feature using a statistical expectation; the hypothesis was tested that there was no association among features. Features that were present in only a few species were combined for the purpose of significance tests (see Table 2). When features were combined, vine species were classified both to woody and to herb life forms according to Satake et al. (1982, 1989). The association among features was tested for different traits as a function of sex expression, flower shape and flower colour, which contain characteristics of the island syndrome (dioecy, accessible shape and subdued colour) and are recognized as important factors in plant–pollinator systems. Genera comprised of species with multiple features (31 genera) were omitted, and 161 genera were used for contingency analysis. Two-way contingency tables were analysed by G-test using the JMP 6 (SAS, Cary, NC, USA). Conservative rejection level was P = 0·05/247 = 0·0002 because 247 independent tests were possible for the combinations using 8 traits (sex expression, flower colour, flower shape, flower size, inflorescence size, flower habit, life form, and pollination type).
To standardize the data and facilitate comparisons, records of visitation frequency (both by direct observation and using the video camera) were transformed into the number of visits per flower per 12 h (Y) in daytime using the following equation:
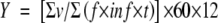 |
where, v is the number of flowers visited within an observed inflorescence, f is the average number of open flowers per inflorescence when observed, inf is the number of inflorescences observed, and t is the observation time (min.).
RESULTS
Floral composition
Among the 269 native species of flowering plants (80 families, 192 genera), 51 species (19·0 %) and 31 genera (16·1 %) were trees, 47 (17·5 %) and 26 (13·5 %) were shrubs, 106 (39·4 %) and 72 (37·5 %) were perennial herbs, 42 (15·6 %) and 30 (15·6 %) were annual herbs and 23 (8·6 %) and 15 (7·8 %) were vines. Insect-pollinated species totalled 196 (72·9 %), followed by 70 wind-pollinated species (25·9 %), two vertebrate-pollinated species (0·7 %) and one water-pollinated species (0·4 %). Most of the wind-pollinated species (87·1 %) were members of the Gramineae and Cyperaceae.
Of the 269 species, 120 (44·6 %) are endemic and 101 (37·4 %) are designated as endangered by the Red Data Book (Environmental Agency of Japan, 2000), of which the families with the most number of species were the Orchidaceae (10 species), Compositae (7), Gramineae and Cyperaceae (6 each) and Rubiaceae (5). Most flowers bloomed under exposed (bright) conditions—on the crown surface of a tree or shrub for 48 species (30 genera) and on open ground for 184 species (126 genera). Flowering on the forest floor or the ground beneath other vegetation was less common (36 species and 24 genera), and included all Orchidaceae species (e.g. Fig. 2B, C).
The most common form of sex expression was hermaphroditic (201 species, 74·7 %, and 144 genera, 75·0 %), followed by dioecious (35 species, 13·0 %, and 21 genera, 10·9 %) and monoecious (19 species, 7·1 %, and 14 genera, 7·3 %). The dominant flower colour was white (97 species, 36·0 %, and 67 genera, 34·9 %), followed by green (58 species, 21·6 %, and 34 genera, 17·7 %) and yellow (37 species, 13·8 %, and 25 genera, 13·0 %). The most common flower shape was a dish (113 species, 42·0 %, and 78 genera, 40·6 %), followed by ‘inconspicuous’ (with no optical petal or attraction; 79 species, 29·4 %, and 51 genera, 26·6 %). Flower size (as defined in Table 2) was dominated by very small (106 species, 39·4 %, and 70 genera, 36·5 %), followed by small, medium and large (S, M, L) with a gradually decreasing number of species and genera. Thus, the majority of the flora was composed of small flowers with subdued colour and accessible shape. Flower longevity was recorded for 64 species, of which 24 species (37·5 %) were classified as medium (M in Table 2), followed by short (S, 20 species, 31·3 %). The greatest longevity was 19·6 d (N = 22), for the flower of Calanthe hattorii (Orchidaceae).
Association among traits
Sex expression was significantly associated with life form at both species and genera level, but was not associated with other traits (Table 3). Dioecy was 2·3-fold (in species; 2·4-fold in genera) more frequent than the expectation value in woody life forms, 1·8- (2·2-) fold in small flowers and 2·7- (2·7-) fold for a habitat at the canopy surface. The association between dioecy and small flowers and accessible flower shape was not significant based on the conservative rejection level (Table 3).
Table 3.
Association of floral characteristics (rows) with sex expression (columns). The observed/expected number of species (genera in brackets) for each feature are shown for each cell
| Traits and features | Dioecy | Hermaphrodite | Monoecy | d.f. | G | P |
|---|---|---|---|---|---|---|
| Pollination | 2 | 9·11 (4·6) | 0·0103 (0·1002) | |||
| Abiotic | 4/10 (3/4) | 53/53 (25/29) | 14/9 (10/6) | |||
| Biotic | 32/26 (12/11) | 147/147 (97/93) | 19/24 (14/18) | |||
| Life form | 2 | 50·95 (22·43) | <0·0001 (<0·0001) | |||
| Herb | 4/22 (3/10) | 144/123 (90/78) | 17/20 (10/15) | |||
| Wood | 32/14 (12/5) | 56/77 (32/44) | 16/13 (14/9) | |||
| Flower colour | 6 | 4·9 (6·42) | 0·5564 (0·3774) | |||
| Subdued | 9/12 (6/5) | 70/67 (36/38) | 11/11 (8/7) | |||
| Vivid | 4/6 (1/3) | 35/33 (27/23) | 6/6 (3/5) | |||
| White | 18/13 (4/5) | 66/72 (44/45) | 13/12 (11/9) | |||
| Yellow | 5/5 (4/2) | 29/28 (15/16) | 3/5 (2/3) | |||
| Flower shape | 6 | 19·72 (22·53) | 0·0031 (0·001) | |||
| Dish | 16/15 (6/6) | 84/84 (48/48) | 13/14 (9/9) | |||
| Inconspicuous | 12/11 (8/5) | 56/63 (28/37) | 17/10 (13/7) | |||
| Tube | 8/7 (1/3) | 38/36 (28/23) | 3/6 (2/5) | |||
| Zygomorphic | 0/3 (0/2) | 22/16 (18/14) | 0/3 (0/3) | |||
| Flower size | 4 | 21·14 (24·25) | 0·0003 (<0·0001) | |||
| Large | 7/11 (0/5) | 75/63 (47/38) | 3/10 (3/7) | |||
| Small | 18/10 (11/5) | 47/58 (32/39) | 13/10 (8/8) | |||
| Very small | 11/14 (4/6) | 78/79 (43/45) | 17/13 (13/9) | |||
| Habit | 4 | 35·56 (17·2) | <0·0001 (0·0018) | |||
| Open | 16/25 (9/10) | 156/138 (91/84) | 13/23 (11/17) | |||
| Surface | 16/6 (6/3) | 22/36 (14/22) | 10/6 (9/4) | |||
| Understorey | 4/5 (0/2) | 22/27 (17/16) | 10/4 (4/3) | |||
| Inflorescence size | 4 | 7·36 (9·67) | 0·1183 (0·0464) | |||
| 1 | 7/8 (2/3) | 49/44 (34/28) | 3/7 (1/6) | |||
| 10 | 15/18 (8/8) | 102/102 (60/62) | 20/17 (14/12) | |||
| 100 | 14/10 (5/4) | 49/54 (28/32) | 10/9 (9/6) |
N = 269 species (161 genera) for all comparisons. A conservative rejection level is P = 0·05/247 = 0·0002.
Flower shape was associated with pollination, flower colour, flower size and habit (Table 4). Dish-shaped flowers were 1·3- (species; 1·2 genera) fold more frequent than expectation in a woody life form, 1·3- (1·3-) fold in biotic pollination, 1·5- (1·3-) fold in small flowers and 1·4- (1·4-) fold in white flowers found at the crown surface (1·6-fold and 1·5-fold for species and genera, respectively). On the other hand, tube-shapes flowers were characterized by large (1·8- and 1·4-fold) and vividly coloured flowers (2·0- and 1·5-fold) found in the understorey (1·6- and 1·8-fold). Similarly, zygomorphic flowers were characterized by being large (2·4- and 2·2-fold) with biotic pollination (1·4- and 1·3-fold) found in the forest understorey or other shaded habitats (3·7- and 4·0-fold).
Table 4.
Association of floral characteristics (rows) with flower shape (columns). The observed/expected number of species (genera in brackets) for each feature are shown for each cell
| Traits and features | Dish | Inconspicuous | Tube | Zygomorphic | df | G | P |
|---|---|---|---|---|---|---|---|
| Pollination | 3 | 219·84 (111·1) | <0·0001 (<0·0001) | ||||
| Abiotic | 1/30 (1/15) | 70/22 (37/12) | 0/13 (0/7) | 0/6 (0/4) | |||
| Biotic | 112/83 (62/48) | 15/63 (12/37) | 49/36 (31/24) | 22/16 (18/14) | |||
| Life form | 3 | 29·19 (5·9) | <0·0001 (0·115) | ||||
| Herb | 55/69 (35/40) | 67/52 (34/31) | 24/30 (19/20) | 19/13 (15/12) | |||
| Wood | 58/44 (28/23) | 18/33 (15/18) | 25/19 (12/11) | 3/9 (3/6) | |||
| Sex expression | 6 | 19·72 (22·53) | 0·0031 (0·001) | ||||
| Dioecy | 16/15 (6/6) | 12/11 (8/5) | 8/7 (1/3) | 0/3 (0/2) | |||
| Hermaphrodite | 84/84 (48/48) | 56/63 (28/37) | 38/36 (28/23) | 22/16 (18/14) | |||
| Monoecy | 13/14 (9/9) | 17/10 (13/7) | 3/6 (2/5) | 0/3 (0/3) | |||
| Flower colour | 9 | 141·77 (64·41) | <0·0001 (<0·0001) | ||||
| Subdued | 15/38 (8/20) | 69/28 (36/15) | 3/16 (3/10) | 3/7 (3/6) | |||
| Vivid | 21/19 (15/12) | 2/14 (2/9) | 16/8 (9/6) | 6/4 (5/3) | |||
| White | 59/41 (32/23) | 7/31 (6/18) | 23/18 (15/11) | 8/8 (6/7) | |||
| Yellow | 18/16 (8/8) | 7/12 (5/6) | 7/7 (4/4) | 5/3 (4/2) | |||
| Flower size | 6 | 166·75 (85·74) | <0·0001 (<0·0001) | ||||
| Large | 41/36 (23/20) | 0/27 (0/15) | 27/15 (14/10) | 17/7 (13/6) | |||
| Small | 49/33 (25/20) | 10/25 (9/16) | 14/14 (12/10) | 5/9 (5/6) | |||
| Very small | 23/45 (15/23) | 75/33 (40/18) | 8/19 (5/12) | 0/9 (0/7) | |||
| Habit | 6 | 42·84 (33·34) | <0·0001 (<0·0001) | ||||
| Open | 74/78 (45/43) | 66/58 (34/34) | 34/34 (22/21) | 11/15 (10/12) | |||
| Surface | 32/20 (17/11) | 12/15 (10/9) | 4/9 (2/6) | 0/4 (0/3) | |||
| Understory | 7/15 (1/8) | 7/11 (5/6) | 11/7 (7/4) | 11/3 (8/2) | |||
| Inflorescence size | 6 | 44·7 (23·42) | <0·0001 (0·0007) | ||||
| 1 | 34/25 (20/14) | 6/19 (4/11) | 15/11 (9/7) | 4/5 (4/4) | |||
| 10 | 56/58 (28/32) | 37/43 (23/25) | 27/25 (18/16) | 17/11 (13/9) | |||
| 100 | 23/31 (15/16) | 42/23 (22/13) | 7/13 (4/8) | 1/6 (1/5) |
N = 269 species (161 genera) for all comparisons. A conservative rejection level is P = 0·05/247 = 0·0002.
Flower colour was associated with pollination, life form, flower shape and flower size (Table 5). A subdued colour was characterized by abiotic pollination (2·6 and 2·8-fold), a herbaceous life form (1·3- and 1·3-fold), and very small (2·0- and 1·9-fold) and inconspicuous flowers (2·5- and 2·4-fold). White flowers were 1·6- (1·6-) fold more frequent in a woody life form and for growth at both the canopy surface (1·7- and 1·5-fold) and in the forest understorey (1·3- and 1·4-fold).
Table 5.
Association of floral characteristics (rows) with flower colour (columns). The observed/expected number of species (genera in brackets) for each feature are shown for each cell
| Traits and features | Subdued | Vivid | White | Yellow | d.f. | G | P |
|---|---|---|---|---|---|---|---|
| Pollination | 3 | 136·79 (71·26) | <0·0001 (<0·0001) | ||||
| Abiotic | 63/24 (33/12) | 1/12 (1/7) | 4/26 (3/14) | 3/10 (1/5) | |||
| Biotic | 27/66 (17/38) | 44/33 (30/24) | 93/71 (56/45) | 34/27 (20/16) | |||
| Life form | 3 | 45·44 (21·98) | <0·0001 (<0·0001) | ||||
| Herb | 74/55 (42/32) | 31/28 (22/20) | 35/59 (25/38) | 25/23 (14/13) | |||
| Wood | 16/35 (8/18) | 14/17 (9/11) | 62/38 (34/21) | 12/14 (7/8) | |||
| Sex expression | 6 | 4·9 (6·42) | 0·5564 (0·377) | ||||
| Dioecy | 9/12 (6/5) | 4/6 (1/3) | 18/13 (4/5) | 5/5 (4/2) | |||
| Hermaphrodite | 70/67 (36/38) | 35/33 (27/23) | 66/72 (44/45) | 29/28 (15/16) | |||
| Monoecy | 11/11 (8/7) | 6/6 (3/4) | 13/12 (11/9) | 3/5 (2/3) | |||
| Flower shape | 9 | 141·77 (64·41) | <0·0001 (<0·0001) | ||||
| Dish | 15/38 (8/20) | 21/19 (15/12) | 59/41 (32/23) | 18/16 (8/8) | |||
| Inconspicuous | 69/28 (36/15) | 2/14 (2/9) | 7/31 (6/18) | 7/12 (5/6) | |||
| Tube | 3/16 (3/10) | 16/8 (9/6) | 23/18 (15/11) | 7/7 (4/4) | |||
| Zygomorphic | 3/7 (3/6) | 6/4 (5/3) | 8/8 (6/7) | 5/3 (4/2) | |||
| Flower size | 6 | 96·65 (40·81) | <0·0001 (<0·0001) | ||||
| Large | 4/28 (4/16) | 18/14 (12/10) | 45/31 (24/18) | 18/12 (10/7) | |||
| Small | 16/26 (10/16) | 16/13 (12/10) | 34/28 (22/19) | 12/11 (7/7) | |||
| Very small | 70/35 (36/19) | 11/18 (7/12) | 18/38 (13/22) | 7/15 (4/8) | |||
| Habit | 6 | 21·17 (14·84) | 0·0017 (0·022) | ||||
| Open | 70/62 (38/34) | 36/31 (27/21) | 51/67 (31/41) | 28/25 (15/14) | |||
| Surface | 11/16 (6/9) | 3/8 (3/6) | 29/17 (17/11) | 5/7 (3/4) | |||
| Understory | 9/12 (6/7) | 6/6 (1/4) | 17/13 (11/8) | 4/5 (3/3) | |||
| Inflorescence size | 6 | 34·47 (21·62) | <0·0001 (0·001) | ||||
| 1 | 8/20 (5/11) | 18/10 (13/7) | 16/21 (9/14) | 17/8 (10/5) | |||
| 10 | 47/46 (28/25) | 20/23 (14/16) | 56/49 (34/30) | 14/19 (6/11) | |||
| 100 | 35/24 (17/13) | 7/12 (4/8) | 25/26 (16/15) | 6/10 (5/5) |
N = 269 species (161 genera) for all comparisons. A conservative rejection level is P = 0·05/247 = 0·0002.
Biotic pollination and niche
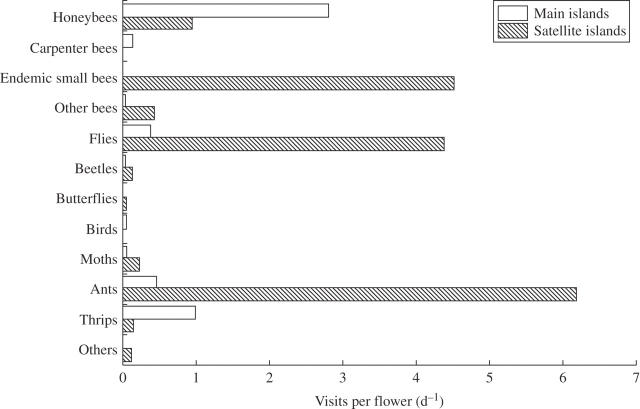
Among the Ogasawara flora, 198 species (73·6 %) were pollinated by animals, and these plant species included a range of floral shapes, colour, sex expression and life forms. The visitation rate was observed in the field survey for 103 native plant species and flower visitors were found on 92 of them (89·3 %). Based on the field data and the results of the literature search, 99 of the native plants either received or have been reported as receiving visits from pollinators, including honeybees (68 species), flies (54), ants (46), endemic small bees (30), other bees (26), thrips (21), moths (19), carpenter bees (12), beetles (11), butterflies (8), birds (7), bugs (5), bats (3), geckos (3), cockroaches (3), lizards (2), grasshoppers (2), mayflies (1), grasshoppers (1), spiders (1), crickets (1) and hermit crabs (1). The observed frequencies showed that the primary flower visitors on the two main Ogasawara Islands were the introduced honeybee (Apis mellifera; Fig. 2Q), an endemic carpenter bee (Xylocopa ogasawarensis; Fig. 2M) and moths (Fig. 2N); on the satellite islands there were several solitary endemic bees, an endemic carpenter bee, flies (Fig. 2O, P) and moths. In the field observations, the above-mentioned endemic small bees were observed on the flowers of 66·7 % (28 of 42) of the species on the satellite islands, despite the shorter observation times (102 h 40 min) spent than on the two main islands (271 h 50 min). Among the satellite islands, Nishino-shima Island had no bee species, most likely because the island has only emerged from the sea recently (Abe, 2006). However, endemic small bees visited flowers frequently on the other satellite islands. In addition, flies, ants and other bees increased the visitor diversity; thus, the native pollination network remained largely intact on the satellite islands (Fig. 3). Among the eight species of endemic small bee, Ceratina boninensis (Anthophoridae), and Hylaeus ikedai and H. incomitatus (both Colletidae), were dominant, with less frequent visits by Heriades fulvohispidus and Megachile asahinai (both Megachilidae), Hylaeus boninensis and H. yasumatsui (both Colletidae) and Lithurge ogasawarensis (Megachilidae).
Fig. 3.
Visitation rates for native flowers on the Ogasawara Islands. Main = Chichi-jima Island and Haha-jima Island. Satellite = all other islands (see Table 1). Visitation rate is the number of flowers visited per day averaged across all species.
Alien pollinators such as honeybees, wasps (Delta pyriforme), swallowtail butterflies (Papilio xuthus) and Japanese white-eye (Zosterops japonica) were frequently observed on Chichi-jima and Haha-jima Islands. Introduced honeybees were observed visiting 56·7 % (51 out of 90) of the native species on the two main islands, and were also observed on several satellite islands (Ani-jima, Otouto-jima and Minami-shima). Honeybees even visited wind-pollinated species such as Scirpus ternatanus (Cyperaceae) and Trema orientalis (Ulmaceae), although it is unknown how much they contribute to their reproductive success.
An endemic carpenter bee, X. ogasawarensis, was the largest of the endemic bees, and visited Alpinia bilamellata, A. boninensis (Fig. 2A), Calophyllum inophyllum, Clematis terniflora var. boninensis, Hedyotis grayi, Hibiscus glaber, Ipomoea pes-caprae, Lobelia boninensis (Fig. 2 M), Melastoma tetramerum, M. tetramerum var. pentapetalum, Metrosideros boninensis, Scaevola frutescens and Schima mertensiana. These flowers are medium-to-large in size, secrete nectar deep at the bottom of the flower and their apertures are sufficient to allow access to the carpenter bees. In addition, some of these plants flower in the understorey (shaded) habitats. Among these, L. boninensis, A. boninensis and A. bilamellata have zygomorphic flowers with sticky stigmata and anthers above, and this configuration ensures contact with back of X. ogasawarensis foraging for nectar at the bottom of flowers. Pollination of these flowers by carpenter bees seems to represent a unique niche in the Ogasawara Islands.
Bird visitation was observed in Calophyllum inophyllum, Freycinetia boninensis, Metrosideros boninensis, Morinda citrifolia, Rhaphiolepis umbellata, Satakentia liukiuensis, and Scaevola frutescens. Metrosideros boninensis has typical bird-pollinated flowers, characterized by being large and red with a lot of nectar secretion. Bird visitors were Zosterops japonicus, Apalopteron familiare and Hypsipetes amaurotis. Visitation by bats has been previously recorded in Freycinetia boninensis, Livistona boninensis and Schima mertensiana, but the frequency of bat pollination may be low in the Ogasawara Islands as no bat pollination was observed in this survey. Various nocturnal visitors were observed, including moths and beetles in S. mertensiana (see Appendix).
Flowers in the forest understorey were not visited by dominant pollinators such as honeybees and solitary endemic small bees, but were visited by minor pollinators such as endemic carpenter bees, flies, moths and small beetles. The most common colour in the forest understorey (36 species) was white (17 species, 47·2 %), but this association with flower habit was not significant (Table 5) because of the wide variety of colours: green (7 species), yellow (4 species) and violet (4 species).
Nectar volumes were recorded for 58 species, and maximum volumes ranged from 0·00 to 16·70 μL. Many flowers had a small nectar volume and 20 of these species (34·5 %) produced no nectar. The only species that secreted more than 5 μL of nectar were Gardenia boninensis, Schima mertensiana and Metrosideros boninensis (Table 6). Endemic species with deep flowers were characterized by high flower longevity, but nectar volume varied widely, ranging from 16·7 μL (Gardenia boninensis) to 0·1 μL (Platanthera boninensis). However, examples with no nectar were also detected: Lysimachia rubida (flower depth = 11·3 mm,N = 6; flower longevity = 4·7 d, N = 10), Pittosporum boninense (depth = 10·0 mm, N = 10; longevity = 2·9 d,N = 21), P. beecheyi (depth = 13·0 mm, N = 8; longevity = 4·4 d, N = 29).
Table 6.
Species list of nectar volume (where >0·1 μL detected), sugar concentration, flower longevity and flower depth.
| Species (family) | N | Nectar volume (μL) | Sugar concentration (%) | N | Flower longevity (d) | N | Flower depth (mm) |
|---|---|---|---|---|---|---|---|
| Gardenia boninensis (Rubiaceae)* | 2 | 16·7 | 27·5 | 3 | 5·7 | 6 | 44·8 |
| Schima mertensiana (Theaceae)* | 7 | 8·0 | – | 37 | 3·4 | 5 | 15·6 |
| Metrosideros boninensis (Myrtaceae)* | 6 | 7·2 | 12·3 | 23 | 2·6 | 8 | 17·5 |
| Rhaphiolepis umbellata (Rosaceae) | 15 | 4·2 | 35·6 | 38 | 3·1 | 10 | 8·1 |
| Morinda citrifolia (Rubiaceae) | 4 | 3·3 | 33·3 | 7 | 1·0 | 9 | 12·5 |
| Scutellaria longituba (Labiatae)* | 6 | 2·5 | 17·0 | 27 | 4·1 | 13 | 43·4 |
| Mucuna gigantea (Leguminosae) | 1 | 1·8 | 36·0 | – | – | 1 | 33·5 |
| Hernandia nymphaefolia (Hernandiaceae) | 5 | 1·8 | – | 41 | 1·5 | 1 | 5·5 |
| Hibiscus glaber (Malvaceae)* | 10 | 1·5 | – | 22 | 1·4 | 3 | 31·1 |
| Pittosporum chichijimense (Pittosporaceae)* | 3 | 1·4 | – | – | – | 3 | 11·2 |
| Lobelia boninensis (Campanulaceae)* | 6 | 1·2 | – | 20 | 2·0 | 7 | 8·4 |
| Photinia wrightiana (Rosaceae) | 5 | 1·2 | 32·5 | – | – | – | – |
| Santalum boninense (Santalaceae)* | 9 | 1·1 | 55·0 | 113 | 1·2 | 7 | 2·9 |
| Elaeagnus rotundata (Elaeagnaceae)* | 6 | 1·0 | – | 12 | 1·8 | 10 | 4·8 |
| Scaevola frutescens (Goodeniaceae) | 10 | 0·7 | – | 28 | 3·6 | 5 | 10·8 |
| Sophora tomentosa (Leguminosae) | 5 | 0·6 | 55·3 | 7 | 3·0 | 5 | 14·9 |
| Elaeocarpus photiniaefolius (Elaeocarpaceae)* | 4 | 0·6 | – | 18 | 6·2 | 4 | 8·4 |
| Psychotria homalosperma (Rubiaceae)* | 5 | 0·5 | – | – | – | 5 | 12·3 |
| Alpinia bilamellata (Zingiberaceae)* | 2 | 0·5 | – | 22 | 1·6 | 2 | 9·6 |
| Vaccinium boninense (Ericaceae)* | 4 | 0·5 | – | 39 | 5·2 | 10 | 10·2 |
| Vitex rotundifolia (Verbenaceae) | 29 | 0·5 | – | 16 | 1·2 | 11 | 7·2 |
| Terminalia catappa (Combretaceae) | 8 | 0·5 | – | 53 | 1·5 | 9 | 0·7 |
| Syzygium cleyeraefolium var. microphyllum | 18 | 0·4 | – | 28 | 2·0 | 7 | 2·6 |
| Tarenna subsessilis (Rubiaceae)* | 5 | 0·4 | – | 53 | 4·2 | 5 | 4·6 |
| Planchonella obovata (Sapotaceae) | 5 | 0·4 | – | – | – | 11 | 3·1 |
| Ipomoea pes-caprae (Convolvulaceae) | 12 | 0·4 | – | 20 | 1·0 | 8 | 35·5 |
| Wikstroemia pseudoretusa (Thymelaeaceae)* | 11 | 0·3 | – | 24 | 5·4 | 9 | 6·9 |
| Ilex mertensii (Aquifoliaceae)* | 10 | 0·2 | – | 8 | 4·4 | 4 | 0·0 |
| Hibiscus tiliaceus (Malvaceae) | 2 | 0·2 | – | 8 | 1·0 | 4 | 57·9 |
| Geniostoma glabrum (Loganiaceae)* | 6 | 0·1 | – | 119 | 2·4 | 5 | 1·5 |
| Platanthera boninensis (Orchidaceae)* | 11 | 0·1 | – | – | – | 11 | 16·9 |
| Ixeris longirostra (Compositae)* | 5 | 0·1 | – | 12 | 1·7 | 5 | 4·1 |
| Satakentia liukiuensis (Palmae)* | 5 | 0·1 | – | – | – | – | – |
| Ochrosia nakaiana (Apocynaceae)* | 4 | 0·1 | – | 118 | 1·1 | 2 | 4·3 |
| Osmanthus insularis (Oleaceae) | 5 | 0·1 | – | 15 | 2·9 | 7 | 2·7 |
Nectar theft (i.e. extracting nectar without pollinating) by honeybees was observed in Hibiscus glaber and Hedyotis grayi (Fig. 2R), and theft by endemic carpenter bees was also seen, with the corollas of Vaccinium boninense being pierced (Fig. 2H). Honeybees were the only visitors other than ants observed for the extrafloral nectaries on Hibiscus tiliaceus, Planchonella obovata and Ochrosia nakaiana.
DISCUSSION
Island syndrome
The species and generic composition of the native Ogasawara flora was characterized by plants with small flowers and subdued colours, as is the case on other oceanic islands (Carlquist, 1974; Webb and Kelly, 1993; Barrett, 1996; Bernardello et al., 2001). In addition, native visitors observed on satellite islands were solitary and small generalists except for the carpenter bee. Thus, the ‘island syndrome’ for pollination and floral traits was generally supported in the Ogasawara Islands. The proportion of dioecy (13 %) was lower than that of New Zealand (18 %, Lloyd, 1985) and La Reunion (15–20 %, Humeau et al., 2003), but was similar to that of Hawaii (15 %, Sakai et al., 1995b), Juan Fernández (9 %, Bernardello et al., 2001), Guam (13 %, Stone, 1970) and Tonga (16 %, Yuncker, 1959), and was higher than that of continental floras such as the British Isles (3 %, Clapham et al., 1962), Portugal (2 %, Pires de Lima, 1947), the Carolinas (4 %, Conn et al., 1980) and California (3 %, Freeman et al., 1980). Frequent visitation by small, solitary insects such as endemic small bees and flies on the satellite islands suggests a selective advantage for subdued colours and accessible structures rather than for showy colours and rich floral rewards. Among these generalist visitors, Hylaeus bees are expected to act as faithful and effective pollinators, based on pollen analysis from bees' nests in Australia (Paini and Roberts, 2005).
Wind pollination is likely to offer advantages in an island environment that includes a relatively impoverished pollinator fauna and frequently intense winds (Whitehead, 1969; Carlquist, 1974; Barrett, 1996; Bernardello et al., 2001). Thus, entomophilous flowers sometimes evolve into anemophilous flowers (Cox, 1991; Anderson et al., 2000). However, the proportion of wind-pollinated species (25·9 %) among the native Ogasawara flora seems to be lower than that of Juan Fernández (4 7 %, Bernardello et al., 2001). Instead, the Ogasawara flora is dominated by entomophilous flowers that may have successfully colonized the islands by establishing interactions with several endemic small bees, flies and short-proboscis moths. Three entomophilous endemic genera (Callicarpa, Dendrocacalia and Wikstroemia) have evolved dioecy on the Ogasawara Islands (Kawakubo, 1990; Kato and Nagamasu, 1995; Sugawara et al., 2004), which suggests the presence of a selection force in this environment of largely opportunistic pollination. The presence of a generalist pollinator fauna in the absence of social bees seems to be advantageous for the reproduction of dioecious plants with small and numerous flowers (Beach, 1981; Charlesworth, 1993) because of its opportunistic visitation and low discrimination ability between floral sexes. Associations between dioecy and a woody life form, white flowers and small flowers in a woody life form, and accessible flower shape and subdued flower colour were formed by the interactions of these generalist visitors with the native flora of the Ogasawara Islands.
Pollination niche
Although the Ogasawara flora exhibited the island syndrome characteristic of domination by small flowers with subdued colour and accessible shapes, unique pollination niches adapted to more specialized pollinators were recognized in some species. In addition to the three endemic Ficus species, birds, long-proboscis moths and carpenter bees were likely to associate with minority traits of flowers (vivid colour, large size and deep shape), which were exceptions to the island syndrome character.
In this study, 14·4 % of observed plant species had no floral visitors. Thus, their potential pollinators must be hypothesized based on floral traits, the regional biota capable of pollinating these flowers and information from the literature. For example, moths may be the primary pollinators of Gardenia boninensis on the Ogasawara Islands because there are no birds with sufficiently long bills to reach the bottom of the long, narrow flower tubes of this species (tube diameter = 3·1–3·7 mm, N = 6). Other Gardenia species have moth-pollinated flowers that attract their pollinators by producing volatile linalool (Raguso and Pichersky, 1999). Although the volatile chemicals of G. boninensis are still unclear, the strong fragrance of this flower also supports the syndrome of nocturnal pollination by moths. In addition to this species, Scutellaria longituba, Psychotria homalosperma and Calanthe hoshii were characterized by long, narrow-tubed, white flowers, and are probably pollinated by hawk-moths since there are such species in the insect fauna (Kato, 1991).
Floral characteristics such as a tube or bell shape, a strong scent, white flowers and a long life span [seen in flowers such as Lysimachia rubida, Platanthera boninensis, Tarenna subsessilis, Vaccinium boninense (Fig. 2H), and three Pittosporum species] are also typical of a moth-pollination syndrome, and in this study they were observed being visited by short-proboscis moths at night. Flowers shaped like a tube or a narrow bell, such as those of Elaeagnus rotundata and Wikstroemia pseudoretusa (Fig. 2I), also limit access to rewards by visitors, and this suggests a niche that involves pollination by lepidopterans and endemic carpenter bees.
Endemic carpenter bees probably act as the primary pollinator for large and zygomorphic flowers in the Ogasawara pollination community. For example, the carpenter bee was observed to visit two endemic Melastoma (see Appendix). Pollen presentation in Melastoma and Solanum species is generally adapted to buzz pollination (Buchmann, 1983; Gross, 1993; Clausing and Renner, 2001). The presence of these genera in the native flora implies the existence of a narrow pollination niche that includes this endemic carpenter bee, even though its visitation was infrequent.
Visitation to endemic orchids was very rare and flower visitors were only observed in Eulophia toyoshimae (Fig. 2C; beetles and ants), Goodyera boninensis (honeybees), Malaxis hahajimensis (small bees and flies) and Platanthera boninensis (moths). Since honeybees usually do not visit dark environments (Kato, 1998; Rincon et al., 1999), the pollinator composition of the forest floor tends to be unique (Herrera, 1997; Moore, 1997). Because all orchids in the Ogasawara Islands inhabit the forest floor, the main pollinators are unlikely to be honeybees and endemic small bees, but rather carpenter bees, flies and nocturnal moths. Honeybee visitation on G. boninensis has been observed in exceptional circumstances after leaves were stripped from trees by a typhoon in a Bischofia javanica forest. Many orchids have adapted to specialist pollinators and tend to develop a specific pollination niche within the floral community (Paxton and Tengó, 2001; Schiestl et al., 2003; Gravendeel et al., 2004; Johnson and Brown, 2004). A long floral life span (>7 d) of orchids suggests that pollination depends on infrequent pollinators in the Ogasawara Islands. The flowers of an understorey orchid, Platanthera boninensis have short spurs (16·9 mm, N = 11) and were visited by small noctuid moths, which suggests that they are adapted for pollination by short-proboscis moths as found for other Platanthera species (Maad, 2000; Plepys et al., 2002).
In this study, nocturnal pollination was mainly sought in the species that did not appear to receive any visitors during the day. Thus, it is likely that there are more nocturnally pollinated flowers than were detected here and that moths have played an important role as partners in adaptation with native Ogasawara flowers.
The results of this study confirm that the native flora and visitor fauna of the Ogasawara Islands show many of the characteristics of the ‘island syndrome’, including an adaptation for opportunistic pollinators as well as the presence of partially specialized pollination niches. However, introduced honeybees have invaded extensively into the pollination network on Chichi-jima and Haha-jima Islands. Domination by introduced honeybees and a decline of native pollinators will change not only plant reproductive success but also the future pollinator-mediated selection for native Ogasawara flowers.
Acknowledgments
The Ministry of the Environment (‘Global environment research fund’ F-051) and the Ministry of Education, Culture, Sports, Science and Technology (‘Grant-in-aid for scientific research’ No. 15770018) funded this study. I am grateful to Takaya Yasui, Yoshio Hoshi, Yuka Kato and Katsuyuki Wada for help in the field. The comments of Shun'ichi Makino, Kaoru Niiyama, Don Levin, Anna Traveset, Jens M. Olesen, David Inouye and an anonymous reviewer are gratefully acknowledged.
APPENDIX
List of native flowering plants on the Ogasawara Islands and their characteristics. The meanings of abbreviations are given in Table 2. Families are according to Melchior (1964).
Table 7.
Table A1.
| Plant |
Floral traits |
Display |
Pollination |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Cupressaceae | Juniperus taxifolia | E | V | Rw | S | D | O | I | Br | – | S | 10 | 100 | A | |
| Ulmaceae | Celtis boninensis | E | – | C | T | Mo | S | I | Y | – | Vs | 10 | 10000 | A | |
| Trema orientalis | I | – | C | T | Mix | S | I | W | M | Vs | 10 | 10000 | A | Hb*,– | |
| Moraceae | Ficus boninsimae | E | – | C | T | D | S | Fi | G | – | Vs | 1* | 100 | I* | Be* |
| F. iidana | E | C | Rn | T | D | S | Fi | G | – | Vs | 1* | 1000 | I* | Be* | |
| F. nishimurae | E | E | C | S | D | S | Fi | G | – | Vs | 1* | 100 | I* | Be* | |
| Morus boninensis | E | C | Rw | T | D | S | Br | G | – | S | 10 | 10000 | A | ||
| Urticaceae | Boehmeria boninensis | E | – | C | S | Mo | O | I | R | – | Vs | 100 | 1 | A | |
| Procris boninensis | E | C | Rn | P | Mo | U | I | W | – | Vs | 10 | 10 | A | – | |
| Santalaceae | Santalum boninense | E | E | Rn | S | H | O | Be | W | S | S | 10 | 100 | I | Hb,(M),(cockroach) |
| Loranthaceae | Korthalsella japonica | I | – | C | S* | Mo | S | I | G | – | Vs | 10 | 1 | I | |
| Polygonaceae | Persicaria hydropiper | I | – | C | A | H | O | Be | G | – | Vs | 10 | 10 | I | |
| Rumex japonicus | I | – | C | P | H | O | Be | G | – | Vs | 100 | 1 | I | ||
| Nyctaginaceae | Boerhavia diffusa | I | – | C | P | H | O | T | P | – | S | 10 | 10 | I | – |
| Pisonia umbellifera | I | – | C | T | D | S | D | W | – | S | 10 | 1000 | I | ||
| Molluginaceae | Sesuvium portulacastrum | I | – | C | P | H | O | D | P | – | M | 1 | 10 | I | |
| Aizoaceae | Tetragonia tetragonioides | I | – | C | P | H | O | D | Y | – | Vs | 1 | 10 | I | |
| Portulacaceae | Talinum crassifolium | I | – | Rw | A | H | O | D | W | – | S | 1 | 10 | I | |
| Caryophyllaceae | Cerastium holosteoides var. hallaisanense | I | – | C | A | H | O | D | W | – | M | 1 | 10 | I | |
| Drymaria cordata var. pacifica | I | – | C | A | H | O | D | W | – | S | 1 | 10 | I | ||
| Sagina maxima | I | – | C | A | H | O | D | W | – | S | 1 | 10 | I | ||
| Stellaria alsine var. undulata | I | – | C | A | H | O | D | W | – | M | 1 | 10 | I | ||
| S. media | I | – | C | A | H | O | D | W | – | M | 1 | 10 | I | ||
| Amaranthaceae | Achyranthes obtusifolia | I | – | C | P | H | O | I | G | – | S | 10 | 10 | I | |
| Lauraceae | Cassytha filiformis | I | – | C | V | H | O | D | W | – | Vs | 10 | 100 | I | |
| Cinnamomum pseudopedunculatum | E | – | Rw | T | H | S | D | G | M | S | 10 | 100 | I | ||
| Machilus boninensis | E | – | C | T | H | S | D | G | S | S | 10 | 100 | I | F,T | |
| M. kobu | E | – | C | T | H | S | D | G | – | S | 10 | 100 | I | ||
| M. pseudokobu | E | E | Rw | T | H | S | D | G | – | S | 10 | 100 | I | ||
| M. japonicus var. kusanoi | I | – | Rw | T | H | S | D | G | – | S | 10 | 100 | I | ||
| Neolitsea aurata | I | – | C | T | D | S | D | Y | L | S | 10 | 100 | I | –,(M) | |
| N. boninensis | E | – | C | T | D | S | D | W | – | S | 10 | 1000 | I | ||
| Hernandiaceae | Hernandia nymphaeifolia | I | – | A | T | Gm | S | D | W | S | M | 10 | 1000 | I | Hb*,F |
| Ranunculaceae | Clematis boninensis | E | V | C | V | H | O | D | W | L | L | 10 | 10 | I | Hb,Xb,F,(–) |
| Piperaceae | Peperomia boninsimensis | E | V | Rn | P | H | U | I | W | – | Vs | 100 | 10 | I | |
| Piper kadsura | I | – | Rn | V | D | O | I | W | – | Vs | 100 | 100 | A | ||
| P. postelsianum | E | C | Rn | P | D | O | I | W | – | Vs | 100 | 100 | A | ||
| Theaceae | Eurya boninensis | E | E | Rn | S | D | O | D | W | – | S | 10 | 1000 | I | Hb,(M),(A) |
| Schima mertensiana | E | A | T | H | S | D | W | M | L | 10 | 100 | I | Hb, Xb, Eb, Be*, Bu*, M, A, T, grasshopper, lizard, (M), (Bt), (grasshopper), (mayfly), (spider), (bat)*, (gecko) | ||
| Guttiferae | Calophyllum inophyllum | I | A | T | H | S | D | W | M | M | 10 | 1000 | I | Hb,Xb,Bi,(A) | |
| Papaveraceae | Corydalis heterocarpa var. brachystyla | I | C | A | H | U | T | Y | – | M | 10 | 10 | I | – | |
| Cruciferae | Capsella bursa–pastoris | I | C | A | H | O | D | W | – | Vs | 10 | 10 | I | Hb | |
| Hamamelidaceae | Distylium leidotum | E | A | T | Am | S | I | R | – | S | 100 | 100 | I | ||
| Crassulaceae | Sedum boninense | E | V | Rn | P | H | O | D | Y | – | S | 1 | 10 | I | – |
| Pittosporaceae | Pittosporum beecheyi | E | V | Rw | S | D | U | Be | W | L | M | 1 | 100 | I | F,M,A,(M),(A) |
| P. boninense | E | C | T | D | S | Be | W | M | M | 10 | 100 | I | Hb,F,T,(F),(M),(A) | ||
| P. chichijimense | E | C | Rn | S | D | U | Be | W | – | M | 1 | 100 | I | T | |
| P. parvifolium | E | C | Rn | S | D | O | Be | W | – | M | 1 | 100 | I | ||
| Rosaceae | Osteomeles boninensis | E | V | C | S | H | O | D | W | M | M | 10 | 100 | I | Hb,Eb,F,A |
| O. lanata | E | V | C | S | H | O | D | W | – | M | 10 | 100 | I | Hb | |
| Photinia wrightiana | I | V | C | T | H | S | D | W | M | S | 10 | 100 | I | Hb | |
| Rhaphiolepis indica var. umbellata | I | A | T | H | S | D | W | M | M | 10 | 1000 | I | Hb,Eb,F,Bi | ||
| Rubus nakaii | E | C | Rw | S | H | O | D | W | – | L | 1 | 100 | I | ||
| R. nishimuranus | I | C | S | H | O | D | W | M | L | 1 | 100 | I | – | ||
| Leguminosae | Caesalpinia bonduc | I | Rw | V | H | O | D | Y | – | S | 10 | 10 | I | ||
| C. globulorum | I | Rw | V | H | O | D | Y | – | M | 10 | 100 | I | |||
| Canavalia lineata | I | C | V | H | O | Z | P | – | L | 10 | 10 | I | Hb,Eb,F,Bu,A,T | ||
| C. occidentalis | I | Rw | A | H | O | Z | Y | – | M | 1 | 10 | I | |||
| Mucuna gigantea | I | L | Rw | V | H | U | Z | G | – | L | 10 | 100 | V | – | |
| Sophora tomentosa | I | V | Rw | S | H | O | Z | Y | M | M | 10 | 100 | I | Hb | |
| Vigna marina | I | C | V | H | O | Z | Y | – | M | 10 | 100 | I | |||
| Oxalidaceae | Oxalis corniculata var. trichocaulon | I | C | P | H | O | Fu | Y | – | S | 1 | 10 | I | Hb,F | |
| Euphorbiaceae | Claoxylon centenarium | E | C | Rn | T | Mo | U | Br | W | – | Vs | 10 | 100 | I | Be,F,A |
| Drypetes integerrima | E | V | Rw | T | D | S | I | Y | – | S | 10 | 100 | I | ||
| Euphorbia pilulifera var. glaberrima | E | D | C | A | H | O | D | G | – | Vs | 10 | 10 | I | ||
| Phyllanthus debilis | I | C | A | Mo | O | D | R | – | Vs | 10 | 10 | I | |||
| Rutaceae | Boninia glabra | E | C | T | D | U | D | W | M | S | 100 | 100 | I | Hb,F,Bt,A,T,(A) | |
| B. grisea | E | C | T | D | S | D | W | – | S | 100 | 100 | I | Hb,Eb,Be,F,A,T,bug | ||
| B. grisea var. crassifolia | E | E | Rn | S | D | O | D | W | – | S | 100 | 100 | I | ||
| Euodia nishimurae | E | C | Rw | T | D | S | D | W | M | S | 100 | 100 | I | Hb,F | |
| Zanthoxylum ailanthoides var. boninshimae | E | C | T | D | S | D | W | – | S | 100 | 100 | I | Hb* | ||
| Z. beecheyanum | I | Rw | S | D | O | I | G | M | S | 10 | 100 | I | Hb,F,A | ||
| Meliaceae | Melia azedarach | I | C | T | H | S | D | V | – | M | 100 | 1000 | I | ||
| Sapindaceae | Dodonaea viscosa | I | C | S | Mo | O | D | G | – | M | 10 | 100 | I | Hb,F,A,bug | |
| Sapindus mukorossi | I | C | T | Mo | S | D | W | – | S | 100 | 100 | I | |||
| Aquifoliaceae | Ilex beecheyi | E | C | C | T | D | S | D | W | – | M | 10 | 100 | I | |
| I. matanoana | E | V | Rw | S | D | O | D | W | L | M | 10 | 100 | I | Hb,Eb,F,A | |
| I. mertensii | E | V | C | T | D | S | D | W | – | M | 10 | 100 | I | Hb,F | |
| Celastraceae | Euonymus boninensis | E | V | Rw | S | H | O | D | W | S | M | 10 | 100 | I | F |
| Vitaceae | Cayratia japonica | I | C | V | H | O | D | G | – | Vs | 100 | 10 | I | ||
| Elaeocarpaceae | Elaeocarpus photiniifolius | E | A | T | H | S | D | W | L | M | 10 | 1000 | I | Hb,F | |
| Malvaceae | Hibiscus glaber | E | A | T | H | S | Be | Y | S | L | 10 | 1000 | I | Hb,Xb,Eb,Be*,F, Bt,Bu*,A | |
| H. tiliaceus | I | C | T | H | O | Be | Y | S | L | 10 | 100 | I | A | ||
| Malva pusilla | I | Rw | P | H | O | D | P | – | M | 1 | 10 | I | |||
| Sida acuta | I | Rw | P | H | O | D | Y | – | M | 1 | 10 | I | |||
| Thymelaceae | Wikstroemia pseudoretusa | E | V | C | S | D | O | T | Y | L | S | 1 | 100 | I | Hb,A,T,(–) |
| Elaeagnaceae | Elaeagnus rotundata | E | C | S | H | O | T | W | M | S | 1 | 100 | I | Hb,F,T,(–) | |
| Stachyuraceae | Stachyurus macrocarpus | E | C | Rn | S | Mix | U | Be | W | Vl | S | 10 | 100 | I | F,(F),(M) |
| S. macrocarpus var. prunifolius | E | C | Rn | S | Mix | U | Be | W | – | S | 10 | 100 | I | ||
| Cucurbitaceae | Trichosanthes boninensis | E | C | Rw | V | Mo | O | T | W | – | M | 10 | 10 | I | |
| Myrtaceae | Metrosideros boninensis | E | E | Rw | T | H | S | D | R | M | L | 10 | 1000 | V | Hb,Xb,Bi,lizard |
| Syzygium cleyerifolium | E | V | C | S | H | O | D | W | – | S | 10 | 100 | I | Eb,Be,F,A | |
| S. cleyerifolium var. microphyllum | E | C | T | H | S | D | W | M | M | 10 | 1000 | I | Hb,Eb,Be,F,Bt,A,T, bug,cricket | ||
| Melastomataceae | Melastoma tetramerum | E | C | Rn | S | H | O | D | P | S | L | 1 | 100 | I | Xb,F,A |
| M. tetramerum var. pentapetalum | E | V | Rw | S | H | O | D | P | S | L | 1 | 100 | I | Xb,A,T | |
| Combretaceae | Terminalia catappa | I | A | T | Gm | S | D | W | S | S | 100 | 100 | I | Hb,Be,F | |
| Onagraceae | Ludwigia hyssopifolia | I | C | A | H | O | D | Y | – | S | 1 | 10 | I | ||
| L. octovalvis | I | C | P | H | O | D | Y | – | M | 1 | 10 | I | |||
| Araliaceae | Fatsia oligocarpella | E | V | Rw | T | D | S | Br | G | M | S | 100 | 10 | I | Hb,F |
| Umbelliferae | Centella asiatica | I | C | P | H | O | D | V | – | Vs | 1 | 100 | I | ||
| Hydrocotyle maritima | I | C | P | H | O | D | G | – | Vs | 1 | 100 | I | |||
| H. sibthorpioides | I | C | P | H | O | D | W | – | Vs | 10 | 100 | I | |||
| Peucedanum boninense | I | L | Rw | P | H | O | D | W | – | Vs | 1000 | 1 | I | Hb* | |
| Ericaceae | Rhododendron boninense | E | C | Rn | S | H | O | Fu | W | – | L | 10 | 100 | I | |
| Vaccinium boninense | E | V | C | S | H | O | Be | W | L | M | 10 | 100 | I | Eb,M,A,T,(M) | |
| Myrsinaceae | Ardisia sieboldii | I | A | T | H | S | D | W | S | M | 100 | 100 | I | Hb*,F,T,(M), (cockroach), (gecko) | |
| Myrsine maximowiczii | E | V | Rw | T | M | S | D | W | L | S | 100 | 100 | I | A | |
| M. okabeana | E | E | Rn | T | M | S | D | W | – | S | 100 | 100 | I | ||
| Primulaceae | Lysimachia japonica | I | C | P | H | O | D | Y | – | S | 1 | 10 | I | ||
| L. rubida | E | Rw | P | H | O | Be | W | L | M | 10 | 1 | I | Eb,F,A,(M) | ||
| Plumbaginaceae | Limonium wrightii | I | V | Rn | P | H | O | T | R | – | Vs | 10 | 10 | I | Eb,F,A,T |
| Sapotaceae | Planchonella boninensis | E | C | Rn | T | D | S | D | W | – | Vs | 100 | 100 | I | |
| P. obovata | I | A | T | H | S | D | W | – | Vs | 100 | 100 | I | Hb,Bt | ||
| P. obovata var. dubia | I | V | Rn | S | H | O | D | W | – | Vs | 100 | 100 | I | ||
| Symplocaceae | Symplocos boninensis | E | V | Rn | T | H | S | D | W | – | M | 10 | 100 | I | |
| S. kawakamii | E | C | Rn | S | H | O | D | W | – | M | 10 | 100 | I | Hb,Be,bug | |
| S. pergracilis | E | C | Rn | S | H | O | D | W | – | M | 10 | 100 | I | Hb,F,(M) | |
| Oleaceae | Ligustrum micranthum | E | C | S | H | O | D | W | S | S | 100 | 100 | I | Hb,Eb,F,A,T* | |
| Osmanthus insularis | I | C | T | H | S | D | W | M | S | 10 | 100 | I | Hb,A,T,(gecko) | ||
| Loganiaceae | Geniostoma glabrum | E | V | C | T | H | S | Be | W | M | Vs | 10 | 100 | I | Hb,Be,A,T |
| Apocynaceae | Ochrosia nakaiana | E | C | T | H | S | T | W | S | S | 100 | 100 | I | Hb,Be,F,(M),(Bt),(A) | |
| Trachelospermum asiaticum | I | C | V | H | O | T | W | M | M | 10 | 100 | I | –,(M),(cockroach) | ||
| Rubiaceae | Galium spurium var. echinospermon | I | C | A | H | O | D | W | – | Vs | 10 | 10 | I | ||
| Gardenia boninensis | E | V | C | S | H | O | T | W | L | L | 1 | 100 | I | Eb,F,A,T,(–) | |
| Hedyotis leptopetala | E | L | C | S | H | O | T | P | L | M | 10 | 100 | I | Hb,Xb,Eb,Be*,F,M,A | |
| H. hookeri | E | V | Rw | S | H | O | T | P | – | M | 10 | 100 | I | Eb,F,A | |
| Morinda boninensis | E | C | V | H | O | D | G | – | S | 10 | 100 | I | |||
| M. boninensis var. hahajimensis | E | V | C | V | H | O | D | G | – | S | 10 | 100 | I | Eb,Be,A | |
| M. citrifolia | I | Rw | S | H | O | T | W | S | M | 10 | 100 | I | Hb,Eb,Bu,Bi,A | ||
| Paederia scandens | I | C | V | H | O | T | V | – | S | 10 | 100 | I | Hb,Be | ||
| P. scandens var. maritima | I | C | V | H | O | T | V | – | S | 10 | 100 | I | |||
| Psychotria boninensis | E | C | V | H | S | D | W | – | S | 10 | 100 | I | |||
| P. homalosperma | E | V | C | T | H | O | T | W | – | M | 10 | 100 | I | Hb,Eb,Be*,Bt,(–) | |
| Tarenna subsessilis | E | C | S | H | U | T | W | L | M | 100 | 10 | I | Hb,Be,(M),(A) | ||
| Convolvulaceae | Evolvulus alsinoides | I | Rw | P | H | O | T | V | – | S | 1 | 10 | I | ||
| Ipomoea gracilis | I | Rn | V | H | O | Fu | P | – | L | 1 | 100 | I | |||
| I. pes–caprae subsp. brasiliensis | I | C | V | H | O | Fu | P | S | L | 1 | 100 | I | Hb,Xb*,Eb,Be,F*,Bt | ||
| I. tuba | I | Rn | V | H | O | Fu | Y | – | L | 1 | 100 | I | |||
| Stictocardia tiliifolia | I | Rw | V | H | O | Fu | P | – | L | 1 | 100 | I | |||
| Boraginaceae | Argusia argentea | I | C | S | Gm | O | D | W | S | S | 100 | 10 | I | Hb,Eb,Be,F | |
| Bothriospermum tenellum | I | C | A | H | O | D | Bl | – | Vs | 10 | 10 | I | |||
| Heliotropium ovalifolium var. depressum | I | C | P | H | O | T | V | – | Vs | 100 | 1 | I | |||
| Verbenaceae | Callicarpa glabra | E | C | Rn | S | D | U | T | P | – | Vs | 100 | 10 | I | – |
| C. nishimurae | E | C | Rn | S | D | O | T | P | – | Vs | 100 | 10 | I | ||
| C. subpubescens | E | C | T | D | O | T | P | S | Vs | 100 | 100 | I | Hb,Eb* | ||
| Vitex rotundifolia | I | C | S | H | O | Z | V | S | M | 10 | 100 | I | Hb,Eb,Be,F,A*,T,(Bt), (hermit crab) | ||
| Labiatae | Ajuga boninsimae | E | C | Rn | P | H | O | Z | W | – | S | 10 | 1 | I | |
| Scutellaria longituba | E | L | Rw | P | H | U | T | W | L | L | 10 | 1 | I | Hb,(–) | |
| Solanaceae | Lycium sandwicense | I | Rw | S | H | O | D | V | – | S | 10 | 10 | I | ||
| Solanum biflorum var. glabrum | I | C | Rn | P | H | O | D | W | – | M | 1 | 10 | I | – | |
| S. nigrum | I | C | A | H | O | D | W | – | S | 1 | 10 | I | Hb | ||
| Tubocapsicum boninense | I | Rn | P | H | O | D | W | – | S | 1 | 10 | I | |||
| Scrophulariaceae | Vandellia anagallis | I | Rw | A | H | O | Z | P | – | S | 1 | 10 | I | ||
| Veronica javanica | I | C | A | H | O | Z | Bl | – | S | 10 | 1 | I | |||
| Orobanchaceae | Aeginetia indica | I | Rw | A | H | O | Be | P | – | M | 1 | 1 | I | – | |
| Orobanche boninsimae | E | Rw | A | H | U | Be | Y | – | M | 1 | 1 | I | (–) | ||
| Myoporaceae | Myoporum boninense | E | Rw | P | H | O | Be | W | – | S | 10 | 1 | I | Hb,Eb,A,T | |
| Caprifoliaceae | Sambucus javanica var. formosana | I | C | P | H | O | D | W | – | Vs | 100 | 1 | I | Hb,F | |
| Viburnum boninsimense | E | V | Rw | S | H | U | D | W | – | Vs | 100 | 10 | I | ||
| Campanulaceae | Lobelia boninensis | E | V | Rw | P | H | O | Z | W | M | M | 100 | 1 | I | Xb,Eb,Be,F,Bu,A |
| Wahlenbergia marginata | I | Rn | P | H | O | Fu | V | – | S | 1 | 10 | I | |||
| Goodeniaceae | Scaevola sericea | I | A | S | H | O | Z | W | M | M | 10 | 100 | I | Hb,Xb,Eb,Be*,F,Bi,A | |
| Compositae | Artemisia princeps | I | C | P | Gm | O | D | G | – | S | 100 | 10 | A | ||
| Cirsium boninense | E | V | Rn | P | H | O | D | W | – | L | 10 | 1 | I | – | |
| C. toyoshimae | E | Ex | – | P | H | O | D | P | – | L | 10 | 1 | I | ||
| Crepidiastrum ameristophyllum | E | C | Rn | S | H | O | D | W | L | S | 10 | 10 | I | Hb,F*,Bu*,M* | |
| C. grandicollum | E | E | Rn | P | H | O | D | Y | M | Vs | 10 | 1 | I | Hb,A | |
| C. linguifolium | E | C | Rn | S | H | O | D | W | M | S | 10 | 1 | I | Hb,Eb,F,Bt,Bu*,M,A* | |
| Dendrocacalia crepididifolia | E | V | Rn | T | D | O | D | P | Vl | Vs | 10 | 100 | I | Hb,F,Bu | |
| Eclipta prostrata | I | C | A | H | O | D | W | – | S | 1 | 10 | I | |||
| Ixeris debilis | I | C | P | H | O | D | Y | – | M | 1 | 10 | I | |||
| I. longirostra | E | V | Rn | P | H | O | D | Y | S | S | 1 | 1 | I | Eb,F,M | |
| I. stolonifera | I | C | P | H | O | D | Y | – | M | 1 | 10 | I | |||
| Kalimeris indica | I | Rw | P | H | O | D | Bl | – | M | 1 | 100 | I | |||
| Lactuca indica f. indivisa | I | C | P | H | O | D | Y | – | L | 100 | 1 | I | |||
| Solidago altissima | I | Rw | P | Gm | O | D | Y | – | Vs | 100 | 1 | I | |||
| Sonchus oleraceus | I | C | A | H | O | D | Y | – | M | 1 | 1 | I | Hb,F,T | ||
| Wedelia chinensis | I | C | P | H | O | D | Y | – | M | 1 | 10 | I | |||
| Youngia japonica | I | C | A | H | O | D | Y | – | S | 10 | 1 | I | Hb | ||
| Potamogetonaceae | Ruppia maritima | I | E | Rn | P | H | H | I | G | – | Vs | 1 | 10 | H | |
| Triuridaceae | Sciaphila japonica | I | E | Rn | A | Mo | U | D | V | – | S | 1 | 1 | I | F |
| S. okabeana | E | V | Rn | A | Mo | U | D | V | – | S | 1 | 1 | I | ||
| S. tosaensis | I | V | Rn | A | Mo | U | D | V | – | S | 10 | 1 | I | ||
| Liliaceae | Dianella ensifolia | I | C | P | H | O | D | V | – | S | 10 | 1 | I | ||
| Smilax china var. yanagitae | I | C | V | D | O | D | G | – | S | 10 | 100 | I | |||
| Amaryllidaceae | Crinum asiaticum var. sinicum | I | C | P | H | O | T | W | – | L | 10 | 1 | I | ||
| Dioscoreaceae | Dioscorea bulbifera | I | C | V | D | O | D | G | – | Vs | 10 | 100 | I | ||
| Commelinaceae | Commelina benghalensis | I | Rw | A | H | O | D | Bl | – | S | 1 | 1 | I | Hb* | |
| Murdannia angustifolia | I | C | A | H | O | D | P | – | S | 1 | 1 | I | |||
| Gramineae | Aristida boninensis | E | E | Rn | P | H | O | I | Br | – | Vs | 1 | 10 | A | |
| Arthraxon hispidus | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| Bothriochloa parviflora | I | C | P | H | O | I | Br | – | Vs | 100 | 1 | A | |||
| Chloris dolichostachys | I | C | A | H | O | I | Br | – | Vs | 100 | 1 | A | |||
| Chrysopogon aciculatus | I | C | P | H | O | I | G | – | Vs | 10 | 100 | A | |||
| Cymbopogon. tortilis var. goeringii | I | C | P | Gm | O | I | Br | – | S | 10 | 10 | A | |||
| Cyrtococcum patens | I | C | P | H | O | I | G | – | Vs | 10 | 10 | A | |||
| Digitaria ciliaris | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| D. microbachne | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| D. platycarpha | E | E | Rw | P | H | O | I | G | – | Vs | 10 | 10 | A | ||
| D. pruriens | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| Echinochloa colona | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| E. crus–galli var. caudata | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| E. crus–galli var. formosensis | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| Eleusine indica | I | C | A | H | O | I | G | – | Vs | 100 | 1 | A | |||
| Eragrostis amabilis | I | C | A | H | O | I | Br | – | Vs | 100 | 1 | A | |||
| Eragrostis bulbillifera | I | Rn | P | H | O | I | G | – | Vs | 100 | 1 | A | |||
| Imperata cylindrica | I | C | P | H | O | I | Br | – | Vs | 100 | 1 | A | |||
| Ischaemum ischaemoides | E | E | Rw | P | H | O | I | Br | – | Vs | 10 | 1 | A | ||
| Leptochloa panicea | I | C | A | H | O | I | Br | – | Vs | 1000 | 1 | A | |||
| Lepturus repens | I | V | Rw | P | H | O | I | Br | – | Vs | 10 | 100 | A | ||
| Miscanthus boninensis | E | L | C | P | H | O | I | Br | – | Vs | 1000 | 10 | A | ||
| Oplismenus compositus | I | C | P | H | U | I | G | – | Vs | 10 | 1 | A | |||
| Paspalidium tuyamae | E | E | Rn | P | H | O | I | G | – | Vs | 10 | 1 | A | ||
| Paspalum scrobiculatum | I | C | P | H | O | I | G | – | Vs | 100 | 1 | A | |||
| Pennisetum purpureum | I | C | P | H | O | I | G | – | Vs | 100 | 10 | A | |||
| P. sordidum | I | C | P | H | O | I | Br | – | Vs | 100 | 10 | A | |||
| Poa annua | I | C | A | H | O | I | G | – | Vs | 10 | 1 | A | |||
| Sporobolus diander | I | C | P | H | O | I | G | – | Vs | 100 | 10 | A | |||
| S. virginicus | I | C | P | H | O | I | G | – | Vs | 100 | 10 | A | |||
| Thuarea involuta | I | Rw | P | Gm | O | I | G | – | Vs | 1 | 100 | A | |||
| Zoysia. matrella | I | C | P | H | O | I | G | – | Vs | 10 | 100 | A | |||
| Z. tenuifolia | I | C | P | H | O | I | G | – | Vs | 10 | 100 | A | |||
| Palmae | Clinostigma savoryanum | E | V | Rw | T | Mo | S | I | W | – | Vs | 1000 | 1 | I | Hb,Eb,Be,F,Bi,bug* |
| Livistona chinensis var. boninensis | E | L | C | T | H | S | I | Y | – | Vs | 1000 | 1 | I | Eb,Be,F,(bat)* | |
| Pandanaceae | Freycinetia boninensis | E | A | V | D | O | I | Y | – | S | 100 | 10 | I | F*,Bi*,A*,(bat)* | |
| Pandanus boninensis | E | L | C | S | D | O | I | Y | – | S | 100 | 1 | I | Hb,A | |
| Cyperaceae | Bulbostylis barbata | I | C | A | H | O | I | G | – | Vs | 10 | 10 | A | ||
| Carex oahuensis var. robusta | I | C | P | Mo | U | I | G | – | Vs | 10 | 10 | A | |||
| C. hattoriana | E | A | P | Mo | U | I | G | – | Vs | 10 | 10 | A | |||
| C. toyoshimae | E | V | Rn | P | Mo | U | I | G | – | Vs | 10 | 1 | A | ||
| Cladium chinense | I | C | P | H | O | I | Br | – | Vs | 100 | 100 | A | |||
| Cyperus brevifolius var. brevifolius | I | C | P | H | O | I | G | – | Vs | 100 | 100 | A | |||
| C. cyperinus | I | C | P | H | O | I | G | – | Vs | 100 | 100 | A | |||
| C. cyperoides | I | C | P | H | O | I | G | – | Vs | 100 | 10 | A | |||
| C. flavidus | I | C | A | H | O | I | Br | – | Vs | 100 | 100 | A | |||
| C. odoratus | I | C | P | H | O | I | Y | – | Vs | 100 | 10 | A | |||
| C. polystachyos | I | Rw | P | H | O | I | Br | – | Vs | 100 | 10 | A | |||
| C. rotundus | I | C | P | H | O | I | Br | – | Vs | 100 | 10 | A | |||
| Fimbristylis dichotoma | I | C | P | H | O | I | Br | – | Vs | 10 | 10 | A | |||
| F. ferruginea | I | C | P | H | O | I | Br | – | Vs | 10 | 10 | A | |||
| F. longispica var. boninensis | E | E | Rw | P | H | O | I | Br | – | Vs | 10 | 10 | A | ||
| F. longispica var. hahajimensis | E | C | Rw | P | H | O | I | Br | – | Vs | 10 | 10 | A | ||
| F. miliacea | I | C | P | H | O | I | Br | – | Vs | 100 | 10 | A | |||
| Gahnia aspera | I | C | P | Am | O | I | Bk | – | Vs | 10 | 1 | A | |||
| Machaerina glomerata | I | C | P | Am | O | I | Y | – | Vs | 10 | 10 | A | |||
| M. nipponensis | I | C | P | Am | O | I | Br | – | Vs | 10 | 10 | A | |||
| Rhynchospora boninensis | E | D | C | P | H | O | I | Br | – | Vs | 100 | 10 | A | ||
| R. chinensis var. curvoaristata | E | V | Rn | P | H | O | I | Br | – | Vs | 10 | 10 | A | ||
| R. rubra | I | C | P | H | O | I | Br | – | Vs | 100 | 10 | A | |||
| Schoenus brevifolius | I | C | P | H | O | I | Br | – | Vs | 10 | 10 | A | |||
| Scirpus grossus | I | V | Rn | P | H | O | I | Br | – | Vs | 10 | 10 | A | ||
| S. ternatanus | I | C | P | H | O | I | Br | – | Vs | 100 | 10 | A | Hb* | ||
| S. triqueter | I | C | P | H | O | I | Br | – | Vs | 10 | 10 | A | |||
| Scleria levis | I | Rw | P | Mo | O | I | G | – | Vs | 10 | 10 | A | |||
| Zingiberaceae | Alpinia bilamellata | E | C | Rw | P | H | O | Z | P | S | M | 10 | 10 | I | Hb |
| A. boninsimensis | E | E | Rn | P | H | U | Z | W | S | L | 10 | 10 | I | Xb,F,A,grasshopper | |
| Orchidaceae | Bulbophyllum boninense | E | E | Rw | P | H | U | Z | Y | – | L | 1 | 1 | I | – |
| Calanthe hattorii | E | C | Rw | P | H | U | Z | Y | Vl | M | 10 | 1 | I | A,(A) | |
| C. hoshii | E | C | Rn | P | H | U | Z | W | – | M | 10 | 1 | I | A,(–) | |
| Corymborkis subdensa | E | E | Rw | P | H | U | Z | W | – | M | 10 | 1 | I | ||
| Eulophia toyoshimae | E | Rw | P | H | U | Z | Br | Vl | M | 10 | 1 | I | Bt,A,(Bt),(A) | ||
| Gastrodia boninensis | E | Rn | P | H | U | Be | Br | – | M | 10 | 1 | I | (–) | ||
| Goodyera boninensis | E | C | P | H | U | Be | W | Vl | S | 10 | 1 | I | Hb,F,(–) | ||
| Liparis hostaefolia | E | C | Rn | P | H | U | Z | P | – | M | 10 | 1 | I | ||
| Luisia boninensis | E | E | Rn | P | H | U | Z | G | – | S | 1 | 1 | I | ||
| Malaxis boninensis | E | C | Rn | P | H | U | D | G | – | Vs | 10 | 1 | I | ||
| M. hahajimennsis | E | C | Rn | P | H | U | D | V | – | Vs | 10 | 1 | I | Be,F,(–) | |
| Platanthera boninensis | E | V | Rw | P | H | U | Z | W | – | M | 10 | 1 | I | –,(M) | |
| Zeuxine boninensis | E | Ex | – | P | H | U | Z | W | – | S | 10 | 1 | I | ||
LITERATURE CITED
- Abe M, Maeda K, Ishii N, Sano H. 1994. Distribution, food habit and territory of Pteropus pselaphon. Ogasawara Kenkyu Nenpo 18: 4–43. (in Japanese) [Google Scholar]
- Abe T. 2006. Colonization of Nishino-shima Island by plants and arthropods, 31 years after eruption. Pacific Science 60: 355–365. [Google Scholar]
- Anderson GJ, Bernardello G, Lopez P, Stuessy TF, Crawford DJ. 2000. Dioecy and wind pollination in Pernettya rigida (Ericaceae) of the Juan Fernández Islands. Botanical Journal of the Linnean Society 132: 121–141. [Google Scholar]
- Andrews RM. 1979. Evolution of life histories: a comparison of Anolis lizards from matched island and mainland habitats. Breviora 454: 1–51. [Google Scholar]
- Baker HG, Cox PA. 1984. Further thoughts on dioecism and islands. Annals of the Missouri Botanical Garden 71: 244–253. [Google Scholar]
- Barrett SCH. 1996. The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society of London B 351: 725–733. [Google Scholar]
- Bawa KS. 1982. Outcrossing and the incidence of dioecism in island floras. American Naturalist 119: 866–871. [Google Scholar]
- Beach JH. 1981. Pollinator foraging and the evolution of dioecy. American Naturalist 118: 572–577. [Google Scholar]
- Bernardello G, Anderson GJ, Stuessy TF, Crawford DJ. 2001. A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernández Islands (Chile). Botanical Review 67: 255–308. [Google Scholar]
- Brown JH, Lomolino MV. 1998. Biogeography, 2nd edn. Sunderland, MA: Sinauer.
- Buchmann SL. 1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Scientific and Academic Editions, 73–113.
- Buchmann SL. 1996. Competition between honey bees and native bees in the Sonoran Desert and global bee conservation issues. In: Matheson A, Buchmann SL, O'Toole C, Westrich P, Williams IH, eds. The conservation of bees. London: Academic Press, 125–142.
- Carlquist S. 1974. Island biology. New York: Columbia University Press.
- Carmo RM, Franceschinelli EV. 2004. Introduced honeybees (Apis mellifera) reduce pollination success without affecting the floral resource taken by native pollinators. Biotropica 36: 371–376. [Google Scholar]
- Carpenter RJ, Read J, Jaffrè T. 2003. Reproductive traits of tropical rain-forest trees in New Caledonia. Journal of Tropical Ecology 19: 351–365. [Google Scholar]
- Charlesworth D. 1993. Why are unisexual flowers associated with wind pollination and unspecialized pollinators? American Naturalist 141: 481–490. [Google Scholar]
- Clapham ER, Tutin TG, Warburg EF. 1962. Floral of the British Isles. Edition 2. London: Cambridge University Press.
- Clausing G, Renner SS. 2001. Molecular phylogenetics of Melastomataceae and Memecylaceae: implications for character evolution. American Journal of Botany 88: 486–498. [PubMed] [Google Scholar]
- Conn JS, Wentworth TR, Blum U. 1980. Patterns of dioecy in the floral of the Carolinas. American Midland Naturalist 103: 310–315. [Google Scholar]
- Cox PA. 1991. Abiotic pollination: an evolutionary escape for animal-pollinated angiosperms. Philosophical Transactions of the Royal Society of London B 333: 217–224. [Google Scholar]
- Cox PA, Elmqvist T. 2000. Pollinator extinction in the Pacific islands. Conservation Biology 14: 1237–1239. [Google Scholar]
- Dupont YL, Skov C. 2004. Influence of geographical distribution and floral traits on species richness of bees (Hymenoptera: Apoidea) visiting Echium species (Boraginaceae) of the Canary Islands. International Journal of Plant Sciences 165: 337–386. [Google Scholar]
- Dupont YL, Hansen DM, Olesen JM. 2003. Structure of a plant-pollinator network in the high altitude sub-alpine desert of Tenerife, Canary Islands. Ecography 28: 301–310. [Google Scholar]
- Dupont YL, Hansen DM, Valido A, Olesen JM. 2004. Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biological Conservation 118: 301–311. [Google Scholar]
- Ecroyd, C. 1996. The ecology of Dactylanthus taylorii and threats to its survival. New Zealand Journal of Ecology 20: 81–100. [Google Scholar]
- Environmental Agency of Japan. 2000. Threatened wildlife of Japan. Red data book, 2nd edn. Volume 8, Vascular plants. Tokyo: Japan Wildlife Research Center. (in Japanese)
- Funakoshi M. 1990. Formation of Leucaena glauca forests in the Ogasawara Islands 4. Introduction and spread. Ogasawara Kenkyu Nenpo 14: 21–51. (in Japanese) [Google Scholar]
- Freeman DC, Harper KT, Ostler, WK. 1980. Ecology of plant dioecy in the intermountain region of western North America. Oecologia 44: 410–417. [DOI] [PubMed] [Google Scholar]
- Godley EJ. 1979. Flower biology in New Zealand. New Zealand Journal of Botany 17: 441–466. [Google Scholar]
- Goto A, Washitani I. 2001. Present ecological status of endangered plants endemic to the Bonin Islands, Crepidiastrum linguifolium and Crepidiastrum ameristophyllum, and proposition of conservation measures. Japanese Journal of Conservation Ecology 6: 1–20. (in Japanese) [Google Scholar]
- Gravendeel B, Smithson A, Slik FJW, Schuiteman A. 2004. Epiphytism and pollinator specialization: drivers for orchid diversity? Philosophical Transactions of the Royal Society of London B 359: 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CL. 1993. The breeding system and pollinators of Melastoma affine (Melastomataceae): a pioneer shrub in tropical Australia. Biotropica 25: 468–474. [Google Scholar]
- Gross CL, MacKay D. 1998. Honeybees reduced fitness in the pioneer shrub Melastoma affine (Melastomataceae). Biological Conservation 86: 169–178. [Google Scholar]
- Hansen DM, Olesen JM, Jones CG. 2002. Trees, birds and bees in Mauritius: exploitative competition between introduced honey bees and endemic nectarivorous birds? Journal of Biogeography 29: 721–734. [Google Scholar]
- Hara M. 1996. European honeybee and Japanese honeybee. Tokyo: Bunka Publisher Co. Ltd. (in Japanese)
- Hatsushima S, Nakajima K. 1979. Flowers of the Ryukyu Islands. Tokyo: Kodansha Co. Ltd. (in Japanese)
- Herrera CM. 1997. Thermal biology and foraging responses of insect pollinators to the forest floor irradiance mosaic. Oikos 78: 601–611. [Google Scholar]
- Humeau L, Pailler T, Thompson JD. 2003. Flower size dimorphism in diclinous plants native to La Réunion Island. Plant Systematics and Evolution 240: 163–173. [Google Scholar]
- Janzen DH. 1973. Sweep samples of tropical foliage insects: effects of seasons, vegetation types, elevation, time of day, and insularity. Ecology 54: 687–708. [Google Scholar]
- Johnson SD, Brown M. 2004. Transfer of pollinaria on birds' feet: a new pollination system in orchids. Plant Systematics and Evolution 244: 181–188. [Google Scholar]
- Kaizuka S. 1977. Geology and geography of Ogasawara. Ogasawara Kenkyu Nenpo 1: 29–34. (in Japanese) [Google Scholar]
- Kato M. 1991. A list of insects from the Ogasawara Islands. Ogasawara Research 18: 32–59. (in Japanese) [Google Scholar]
- Kato M. 1992. Endangered bee fauna and its floral hosts in the Ogasawara Islands. Japanese Journal of Entomology 60: 487–494. [Google Scholar]
- Kato M. 1998. Plant–pollinator interactions in the understory of a lowland mixed dipterocarp forest in Sarawak. American Journal of Botany 83: 732–743. [Google Scholar]
- Kato M, Kawakita A. 2004. Plant–pollinator interactions in New Caledonia influenced by introduced honey bees. American Journal of Botany 91: 1814–1827. [DOI] [PubMed] [Google Scholar]
- Kato M, Nagamasu H. 1995. Dioecy in the endemic genus Dendrocacalia (Compositae) on the Bonin (Ogasawara) Islands. Journal of Plant Research 108: 443–450. [Google Scholar]
- Kato M, Matsumoto M, Kato T. 1993. Flowering phenology and anthophilous insect community in the cool-temperate subalpine forests and meadows at Mt. Kushigata in the central part of Japan. Contributions from Biological Laboratry, Kyoto University 28: 119–172. [Google Scholar]
- Kato M, Shibata A, Yasui T, Nagamasu H. 1999. Impact of introduced honeybees, Apis mellifera, upon native bee communities in the Bonin (Ogasawara) Islands. Research on Population Ecology 41: 217–228. [Google Scholar]
- Kawakita A, Kato M. 2004. Evolution of obligate pollination mutualism in New Caledonian Phyllanthus (Euphorbiaceae). American Journal of Botany 91: 410–415. [DOI] [PubMed] [Google Scholar]
- Kawakubo N. 1990. Dioecism of the genus Callicarpa (Verbenaceae) in the Bonin (Ogasawara) Islands. Botanical Magazine, Tokyo 103: 57–66. [Google Scholar]
- Kearns CA, Inouye DW, Waser NM. 1998. Endangered mutualisms: the conservation of plant–pollinator interactions. Annual Review of Ecology and Systematics 29: 83–112. [Google Scholar]
- Lloyd DG. 1985. Progress in understanding the natural history in New Zealand plants. New Zealand Journal of Botany 23: 707–722. [Google Scholar]
- Maad J. 2000. Phenotypic selection in hawkmoth-pollinated Platanthera bifolia: targets and fitness surfaces. Evolution 54: 112–123. [DOI] [PubMed] [Google Scholar]
- McMullen CK. 1987. Breeding systems of selected Galápagos Islands angiosperms. American Journal of Botany 74: 1694–1705. [Google Scholar]
- McMullen CK. 1993. Flower-visiting insects of the Galapagos Islands. Pan-Pacific Entomology 69: 95–106. [Google Scholar]
- Melchior H. 1964. A. Engler's Syllabus der Pflanzenfamilien 12. auflage. Bd. 2. Angiospermen, Berlin: Gebrüder Borntraeger.
- Moore PD. 1997. Insect pollinators see the light. Nature 387: 759–760. [Google Scholar]
- Mueller-Dombois D, Fosberg FR. 1998. Vegetation of the tropical Pacific islands. New York: Springer.
- Nilsson LA, Jonsson L, Rason L, Randrainjohany E. 1985. Monophily and pollination mechanisms in Angraecum arachnites Schltr. (Orchidaceae) in a guild of long-tongued hawk-moths (Sphingidae) in Madagascar. Biological Journal of the Linnean Society 26: 1–19. [Google Scholar]
- Olesen JM. 1985. The Macaronesian bird–flower element and its relation to bird and bee opportunists. Botanical Journal of the Linnean Society 91: 395–414. [Google Scholar]
- Olesen JM, Eskildsen LI, Venkatasamy S. 2002. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Diversity and Distributions 8: 181–192. [Google Scholar]
- Ono M. 1998. Conservation of the endemic vascular plant species of the Bonin (Ogasawara) Islands. In: Stuessy TF, Ono M, eds. Evolution and speciation of island plants. Cambridge: Cambridge University Press, 169–180.
- Paini DR, Roberts JD. 2005. Commercial honey bees (Apis mellifera) reduce the fecundity of an Australian native bee (Hylaeus alcyoneus). Biological Conservation 123: 103–112. [Google Scholar]
- Paton DC. 2000. Disruption of bird–plant pollination systems in southern Australia. Conservation Biology 14: 1232–1234. [Google Scholar]
- Paxton RJ, Tengó J. 2001. Doubly duped males: the sweet and sour of the orchid's bouquet. Trends in Ecology and Evolution 16: 167–169. [DOI] [PubMed] [Google Scholar]
- Pires de Lima A. 1947. Flora Portuguesa by G. Sampaio. Edition 2. Porto: Imprensa Moderna.
- Plepys D, Ibarra F, Löfstedt C. 2002. Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae). Oikos 99: 69–74. [Google Scholar]
- Raguso RA, Pichersky E. 1999. A day in the life of a linalool: chemical communication in a plant-pollinator system. Part 1: Linalool biosynthesis in flowering plants. Plant Species Biology 14: 95–120. [Google Scholar]
- Rincon M, Roubik DW, Finegan B, Delgado D, Zamora N. 1999. Understory bees and floral resources in logged and silviculturally treated Costa Rican rainforest plots. Journal of the Kansas Entomological Society 72: 379–393. [Google Scholar]
- Roubik DW. 1978. Competitive interactions between neotropical pollinators and Africanized honey bees. Science 201: 1030–1032. [DOI] [PubMed] [Google Scholar]
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. 1995a. Biogeographical and ecological correlates of dioecy in the Hawaiian Flora. Ecology 76: 2530–2543. [Google Scholar]
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. 1995b. Origins of dioecy in the Hawaiian Flora. Ecology 76: 2517–2529. [Google Scholar]
- Satake Y, Ohwi J, Kitamura S, Watari S, Tominari T. 1982. Wild flowers of Japan: Herbaceous plants I, II, III. Tokyo: Heibonsha Ltd., Publishers. (in Japanese)
- Satake Y, Hara H, Watari S, Tominari T. 1989. Wild flowers of Japan: Woody plants I, II. Tokyo: Heibonsha Ltd., Publishers. (in Japanese)
- Schiestl FP, Peakall R, Mant JG, Ibarra F, Schulz C, Frankie S, Francke W. 2003. The chemistry of sexual deception in an orchid-wasp pollination system. Science 302: 437–438. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. 2003. The nature of Ogasawara and its conservation. Global Environmental Reserch 7: 3–14. [Google Scholar]
- Spears Jr, EE. 1987. Island and mainland pollination ecology of Centrosema virginianum and Opuntia stricta. Journal of Ecology 75: 351–362. [Google Scholar]
- Stone BL. 1970. The flora of Guam. Micronesica 6: 1–659. [Google Scholar]
- Sugawara T, Watanabe K, Kato H, Yasuda K. 2004. Dioecy in Wikstroemia pseudoretusa (Thymelaeaceae) endemic to the Bonin (Ogasawara) Islands. Acta Phytotaxonomy and Geobotany 55: 55–61. [Google Scholar]
- Taira A. 1994. Subduction of plate causes growth of continent. Nikkei Science 24: 56–70. (in Japanese) [Google Scholar]
- Tanaka H. 1993. Pollination ecology in Ogasawara Islands (1). Ogasawara Kenkyu Nenpo 17: 25–38. (in Japanese) [Google Scholar]
- Tanaka H. 1994. Pollination ecology in Ogasawara Islands (2). Ogasawara Kenkyu Nenpo 18: 44–53. (in Japanese) [Google Scholar]
- Tanaka H. 1995. Pollination ecology in Ogasawara Islands (3). Ogasawara Kenkyu Nenpo 19: 19–29. (in Japanese) [Google Scholar]
- Toyoda T. 2003. Flora of Bonin Islands, 2nd edn. Kamakura: Aboc-sha Co., Ltd. (in Japanese)
- Traveset A, Richardson DM. 2006. Biological invasions as disruptors of plant reproductive mutualisms. Trends in Ecology and Evolution 21: 208–216. [DOI] [PubMed]
- Vamosi JC, Knight TM, Steets JA, Mazer SJ, Burd M, Ashman TL. 2006. Pollination decays in biodiversity hotspots. Proceedings of the National Academy of Sciences of the USA 103: 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughton G. 1996. Pollination disruption by European honeybees in the Australian bird-pollinated shrub Grevillea barklyana (Proteaceae). Plant Systematics and Evolution 200: 89–100. [Google Scholar]
- Webb CJ, Kelly D. 1993. The reproductive biology of the New Zealand flora. Trends in Ecology and Evolution 8: 442–447. [DOI] [PubMed] [Google Scholar]
- Whitehead D. 1969. Wind pollination in the angiosperms. Evolution 23: 28–35. [DOI] [PubMed] [Google Scholar]
- Whittaker RJ. 1998. Island biogeography—ecology, evolution and conservation. Oxford: Oxford University Press.
- Yokoyama J, Iwatsuki K. 1997. A faunal survey of fig-wasps (Chalcidoidea: Hymenoptera) distributed in Japan and their associations with figs (Ficus: Moraceae). Entomological Science 1: 37–46. [Google Scholar]
- Yuncker TG. 1959. Plants of Tonga. Honolulu: Bernice P. Bishop Museum.