Abstract
Hepatitis C is a major cause of chronic liver disease. It has been recognized as a global health problem because of the progression to cirrhosis and hepatocellular cancer. Chronic hepatitis C is usually asymptomatic but can cause considerable liver damage before its recognition. This review discusses the natural history, clinical features, diagnosis, therapy, treatment responses and the side effects associated with the treatment of hepatitis C.
Keywords: natural history, therapy, genotype, peginterferon, ribavirin
Introduction
About 170 million people in the world are infected with hepatitis C virus (HCV). Since the discovery of HCV in 1989 (Alter et al, 1999), the number of acute HCV cases has fallen by more than 80% (Wasley and Alter, 2000). However, hepatitis C is still a major health burden because 60–80% of infected people progress to chronic infection (Di Bisceglie, 2000). HCV is a single-stranded RNA virus belonging to the Flaviviridae family (Lindenbach and Rice, 2005). The major routes of transmission are injection drug use, blood transfusion, hemodialysis, organ transplantation and less frequently sexual intercourse. Six major genotypes (1–6) of HCV have been identified, and they have varying geographical distribution. Genotypes 1, 2 and 3 are distributed worldwide with genotype 1 accounting for 40–80% of all cases. Genotype 4 is found in the Middle East and Egypt, genotype 5 in South Africa and genotype 6 in South East Asia (Wasley and Alter, 2000).
Natural history
Hepatitis C virus infection can present as acute or chronic hepatitis. Acute hepatitis usually is asymptomatic and rarely leads to hepatic failure. Symptomatic acute HCV has a mild clinical course with <25% of patients presenting with jaundice. About 60–80% people with acute infection develop chronic infection (Hoofnagle, 2002). The rate of spontaneous viral clearance in patients with chronic HCV is very low. Approximately one fifth (20–30%) of patients with chronic HCV develop cirrhosis over a time period of 10–30 years (Thomas and Seeff, 2005). Several factors may determine the rapidity of disease progression. Some of the well-characterized factors are HCV acquisition at an advanced age (>40–55 years), male sex, HIV co-infection, higher body mass index, presence of hepatic steatosis and consumption of alcohol. Patients with cirrhosis can decompensate with complications. Decompensated cirrhosis results in portal hypertension, which can lead to ascites, spontaneous bacterial peritonitis, esophageal varices, neurological complications such as hepatic encephalopathy and hepatic coma, and hepatorenal syndrome leading to renal failure. Chronic infection can also be associated with extra-hepatic manifestations such as cryoglobulinemia, porphyria cutanea tarda, arthralgia, membranoproliferative glomerulonephritis, Sjogren’s syndrome, Raynaud’s syndrome, idiopathic thrombocytopenic purpura and non-Hodgkin’s lymphoma (Liang et al, 2000). Among those with cirrhosis, 1–4% per year develop hepatocellular carcinoma (Fattovich et al, 2004). Deaths associated with chronic HCV are usually a result of the complications of decompensated cirrhosis and hepatocellular carcinoma. Survival rates decline rapidly with the onset of decompensation. The 5-year survival rate for patients with compensated cirrhosis is as high as 90% as compared to 50% for those with decompensated cirrhosis (Fattovich et al, 1997; Hu and Tong, 1999).
Clinical features and diagnostic evaluation
Most patients with chronic HCV are asymptomatic or may present with nonspecific symptoms such as fatigue or malaise. Some of them may have arthralgia and myalgia. Patients with decompensated disease may display peripheral manifestations of cirrhosis, such as palmar erythema, spider nevi, Dupuytren’s contracture, gynaecomastia, parotid enlargement, temporal muscle wasting, ascites, hepatosplenomegaly or testicular atrophy. The diagnosis of HCV is made by the presence of anti-HCV antibody and HCV RNA in the blood. Further evaluation includes genotyping and quantifying HCV viral level, which usually is in the range of 0.2–5 million IU ml−1. Basic laboratory tests like liver function tests, prothrombin time and hepatitis B as well as HIV serologies should be performed. Liver biopsy is often useful in making the correct diagnosis and determining the severity of inflammation and stage of fibrosis (Kleiner, 2005).
Treatment
Treatment considerations for hepatitis C are based on the presentation of the disease (acute vs chronic), genotype, laboratory values, presence of co-infection (HIV, hepatitis B) and co-morbidities. The main goal of treatment of HCV is to achieve sustained virologic response (SVR), defined as the absence of HCV RNA in serum at least 6 months after the discontinuation of therapy. The cost of a 48-week treatment ranges from $30 000 to $40 000 not including the clinic visits during ongoing treatment (Hoofnagle and Seeff, 2006; Shah and Wong, 2006). Hence, every patient should be carefully evaluated with respect to treatment indications, associated co-morbidities, adherence to treatment and reliability of follow-up.
Chronic hepatitis C
Treatment of chronic hepatitis C in adults is recommended for those who have detectable HCV RNA levels, elevated aminotransferase (ALT) levels, liver biopsy findings suggestive of progressive liver disease and the absence of any serious co-morbid conditions or contraindications as listed in Table 1 (Strader et al, 2004; Dienstag and McHutchison, 2006). ALT levels however, do not always correlate with disease severity and hence, treatment should not be denied in those with normal ALT levels (Bacon, 2002).
Table 1.
Contraindications to treatment with peginterferon and ribavirin
Absolute contraindications
|
Relative contraindications
|
| Close monitoring and dose adjustment might be required in patients with anemia, thrombocytopenia or leucopenia. Patients with renal dysfunction might need adjustment of ribavirin dose. |
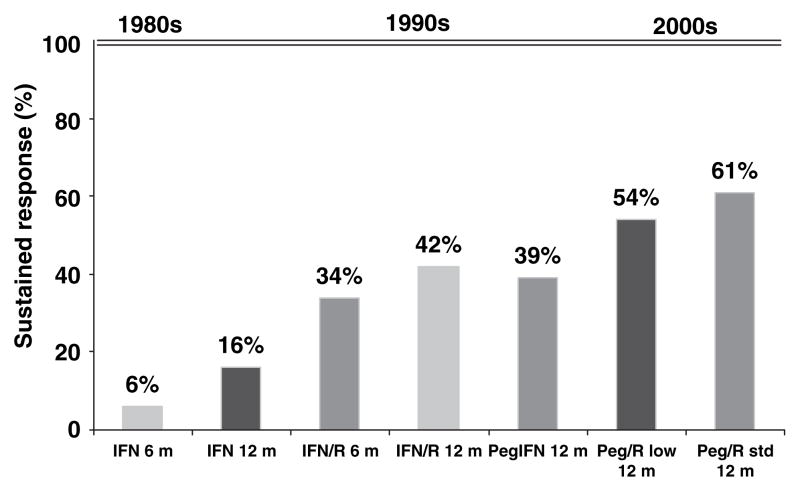
The therapy of hepatitis C has improved substantially over the years (Figure 1). The current recommended treatment of chronic HCV is the combination of peginterferon and ribavirin. α-Interferon was first shown to have a benefit in chronic hepatitis C infection in 1986 (Di Bisceglie and Hoofnagle, 2002). Five to fifteen percent of patients achieved SVR after a 6-to 12-month course of IFN-α. The overall response rates were substantially increased by the addition of the oral nucleoside analog ribavirin. IFN-α, by activating a variety of antiviral pathways, is active against many RNA viruses including HCV. The mechanism of action of ribavirin is not well understood. It has no direct antiviral activity and appears to enhance the antiviral effect of IFN-α in the treatment of hepatitis C, possible by a combination of mechanisms (Feld and Hoofnagle, 2005).
Figure 1.
Progress in therapy of hepatitis C. IFN, interferon; PegIFN, peginterferon; R, ribavirin; R low, low-dose ribavirin; R std, standard-dose ribavirin
Another significant advance in HCV therapy came with the development of long-acting pegylated interferon (peginterferon). A polyethylene glycol moiety is covalently attached to the IFN-α molecule resulting in a product that is still biologically active, but has a larger molecular mass, improved distribution and increased half-life. The combination of peginterferon and ribavirin has been shown to yield the highest response rates in three pivotal trials (Manns et al, 2001; Fried et al, 2002; Hadziyannis et al, 2004). With peginterferon and ribavirin, 70–80% of patients with genotype 2 or 3 infection and 42–45% of those with genotype 1 infection can achieve a SVR. These results were significantly better than those achieved with standard combination therapy or peginterferon monotherapy. The current recommended regimen is summarized in Table 2. There is no current established treatment for patients with genotype 4, 5 or 6. These patients are usually treated with the same regimen used for genotype 1 patients (El-Zayadi et al, 2005).
Table 2.
Current recommended therapy for chronic hepatitis C
| Genotype | Peginterferon dose (SQ) | Ribavirin dose (PO) | Duration of treatment (weeks) |
|---|---|---|---|
| 1 | Peginterferon α-2a: 180 μg week−1 | 1000 mg day−1 for body wt. ≤ 75 kg | 48 |
| Peginterferon α- 2b: 1.5 μg kg−1 week−1 | 1200 mg day−1 for body wt. > 75 kg | ||
| 2 or 3 | Same as above | 800 mg day−1 | 24 |
Acute hepatitis C
An optimal treatment regimen for acute hepatitis C has not yet been established. Genotype and HCV RNA levels do not seem to play a role in determining treatment outcomes (Alberti et al, 2002). Up to 50% of patients with acute HCV spontaneously clear the virus (Gerlach et al, 2003; Hofer et al, 2003). Hence, a delay of treatment for 8–12 weeks after the onset of acute hepatitis C has been suggested. A recent trial demonstrated >90% SVR rates in individuals with acute HCV when treatment with peginterferon was started within 12 weeks after onset of disease. Both IFN and peginterferon with or without the combination of ribavirin have been used in various studies with promising results. Studies with IFN-α have reported SVR rates of as high as 95% when patients are treated for 24 weeks (Kamal et al, 2006). Similarly, studies of peginterferon with or without ribavirin have reported SVR rates of 80–89% with 24 weeks of treatment (Kamal et al, 2004; Wiegand et al, 2006).
Treatment response
There are three types of response to therapy: SVR, relapse or breakthrough, and non-response. In patients with SVR, HCV RNA levels become undetectable in 4–24 weeks and remain negative for the entire duration of follow-up. ALT levels return to normal and liver histology shows improvement. In those with relapse (20%), the HCV RNA levels reappear after therapy is stopped, usually within a few weeks of the end of treatment. In those with breakthrough (10%), HCV RNA levels initially become undetectable but reappear during therapy. In patients with non-response, HCV RNA levels never become undetectable during treatment. In genotype 1 patients, it is unlikely to achieve SVR if they do not become HCV RNA negative by 24 weeks of therapy (Manns et al, 2001; Hadziyannis et al, 2004). Moreover, if HCV RNA levels do not decrease by 2 log10 IU ml−1 or more by 12 weeks of therapy, SVR is unlikely in >98% of cases and therefore therapy should be stopped (Davis, 2002; McHutchison et al, 2002).
Predictors of treatment response
Various factors have been associated with lower response rates in patients undergoing treatment with peginterferon and ribavirin. They are genotype 1, African American race, pretreatment HCV RNA levels of >800 000 IU ml−1, male sex, higher body mass index and advanced fibrosis (Feld and Hoofnagle, 2005; Hoofnagle and See., 2006). Hence, these factors should be reviewed when considering therapy.
Adverse effects of treatment
Treatment with peginterferon and ribavirin is associated with numerous adverse effects (Table 3). Most patients undergoing treatment experience side effects ranging from mild to severe. As a result, patient education of potential side effects as well as monthly visits for monitoring blood counts and symptoms should be emphasized before the initiation of therapy. The most common side effects of peginterferon are fatigue, muscle aches and psychological side effects such as depression, anxiety, irritability and sleep disturbance. These side effects are usually managed in an outpatient setting with patient education, antidepressants/anxiolytics, referral to a psychiatrist and the use of erythropoietin or granulocyte macrophage colony stimulating factor if needed. If side effects are not well controlled, dose reduction from 180 μg week−1 to 135 or 90 μg week−1 for peginterferon alfa-2a, and from 1.5 to 1.0 μg kg−1 week−1 for peginterferon alfa-2b as well as drug discontinuation should be considered (Fried, 2002).
Table 3.
Adverse effects
| Adverse effects of peginterferon | Comments |
|---|---|
| Influenza like symptoms: Fever | Typically after the first injection. |
| Gastrointestinal symptoms: Nausea | |
| Hair loss: | 20–25% of patients, usually temporary. |
| Bone marrow suppression: Leucopenia, thrombocytopenia | Typically 30–50% reduction in counts, may treat with G-CSF. |
| CNS toxicity: headache, depression, irritability, psychological changes | 10% of patients, use of anti-depressants, dose reduction if severe. |
| Seizures | 1–2% of patients, dose discontinuation. |
| Autoimmune thyroid disease: Hypothyroidism, hyperthyroidism | 1–2% of patients, treat if symptomatic. |
| Others: cardiac side effects, interstitial nephritis, vision and hearing disturbances, elevation in serum aminotransferases |
| Adverse effects of Ribavirin | Comments |
|---|---|
| Hemolytic anemia | Dose reduction if symptomatic or Hct <30%, dose discontinuation if Hct drops further, may use erythropoietin. |
| Lymphopenia | Lymphocyte counts may decrease by 10–15%. |
| Gout: Increase in uric acid | Discontinue if attack severe/prolonged. |
| Pruritis | 20% of patients, discontinue if severe. |
| Nasal stuffiness, sinusitis | 20% of patients, treat if symptomatic. |
| Teratogenicity | Discontinue if pregnancy test positive. |
| Others: Hepatic iron accumulation, cholelithiasis, retinal changes |
Hct, hematocrit; G-CSF, granulocyte-colony stimulating factor.
The most common side effect of ribavirin is hemolytic anemia, which usually warrants dose reduction. Around 1–2% of patients on combination therapy experience serious side effects such as myocardial infarction in patients with underlying coronary artery disease or stroke in patients with atherosclerotic disease. About 30–40% of patients on 48 weeks of treatment require dose reductions with early discontinuation in up to 20% of patients (Fried et al, 2002; Hadziyannis et al, 2004). Temporary discontinuation of ribavirin should also be considered in patients with severe anemia until the hematocrit returns to >30 either with or without the institution of erythropoietin.
Future advances in therapy
Many new approaches to therapy of chronic hepatitis C are being studied in clinical trials. Some of the recent advances have been made in the modification of the dose and duration of current recommended combination therapy. Ribavirin in high doses (1400–2400 mg) has been shown to achieve higher SVR rates in a small trial at the expense of a higher toxicity profile (Lindahl et al, 2005). New forms of IFN are being tested clinically. A new generation of small molecule inhibitors targeting the viral-encoded enzymes, such as the proteases and polymerases is being developed. Many of them are in late phase clinical trials (Pawlotsky, 2007). Although some of them are quite promising in suppressing HCV levels in HCV-infected people, drug resistant mutants emerge rapidly after the initiation of therapy. Therefore they would have to be used in combination with IFN-based therapy.
Conclusion
Treatment of hepatitis C has improved substantially in response rates across all genotypes. However, progress remains to be made to improve the SVR rates for genotype 1 patients as well as relapsers and non-responders. Until the above-discussed new agents pass rigorous scrutiny in clinical trials, the current combination therapy of peginterferon and ribavirin will remain the mainstay treatment of hepatitis C for the next 3–5 years.
References
- Alberti A, Boccato S, Vario A, Benvegnu L. Therapy of acute hepatitis C. Hepatology. 2002;36(5 Suppl 1):S195–S200. doi: 10.1053/jhep.2002.36808. [DOI] [PubMed] [Google Scholar]
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- Bacon BR. Treatment of patients with hepatitis C and normal serum aminotransferase levels. Hepatology. 2002;36(5 Suppl 1):S179–S184. doi: 10.1053/jhep.2002.36386. [DOI] [PubMed] [Google Scholar]
- Davis GL. Monitoring of viral levels during therapy of hepatitis C. Hepatology. 2002;36(5 Suppl 1):S145–S151. doi: 10.1053/jhep.2002.36798. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31:1014–1018. doi: 10.1053/he.2000.5762. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM, Hoofnagle JH. Optimal therapy of hepatitis C. Hepatology. 2002;36(5 Suppl 1):S121–S127. doi: 10.1053/jhep.2002.36228. [DOI] [PubMed] [Google Scholar]
- Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. quiz 14)17. [DOI] [PubMed] [Google Scholar]
- El-Zayadi AR, Attia M, Barakat EM, et al. Response of hepatitis C genotype-4 naive patients to 24 weeks of Peginterferon-alpha2b/ribavirin or induction-dose interferon-alpha2b/ribavirin/amantadine: a non-randomized controlled study. Am J Gastroenterol. 2005;100:2447–2452. doi: 10.1111/j.1572-0241.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 Suppl 1):S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- Hofer H, Watkins-Riedel T, Janata O, et al. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology. 2003;37:60–64. doi: 10.1053/jhep.2003.50019. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36(5 Suppl 1):S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, See LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311–1316. doi: 10.1002/hep.510290424. [DOI] [PubMed] [Google Scholar]
- Kamal SM, Ismail A, Graham CS, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology. 2004;39:1721–1731. doi: 10.1002/hep.20266. [DOI] [PubMed] [Google Scholar]
- Kamal SM, Fouly AE, Kamel RR, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology. 2006;130:632–638. doi: 10.1053/j.gastro.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Kleiner DE. The liver biopsy in chronic hepatitis C: a view from the other side of the microscope. Semin Liver Dis. 2005;25:52–64. doi: 10.1055/s-2005-864781. [DOI] [PubMed] [Google Scholar]
- Liang TJ, Rehermann B, See LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- Lindahl K, Stahle L, Bruchfeld A, Schvarcz R. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology. 2005;41:275–279. doi: 10.1002/hep.20563. [DOI] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM. Treatment of hepatitis C: don’t put all your eggs in one basket! Gastroenterology. 2007;132:1611–1615. doi: 10.1053/j.gastro.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Shah BB, Wong JB. The economics of hepatitis C virus. Clin Liver Dis. 2006;10:717–734. doi: 10.1016/j.cld.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Strader DB, Wright T, Thomas DL, See LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- Thomas DL, See LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398. vi. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- Wiegand J, Buggisch P, Boecher W, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250–256. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]