Abstract
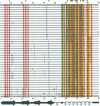
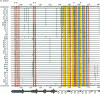
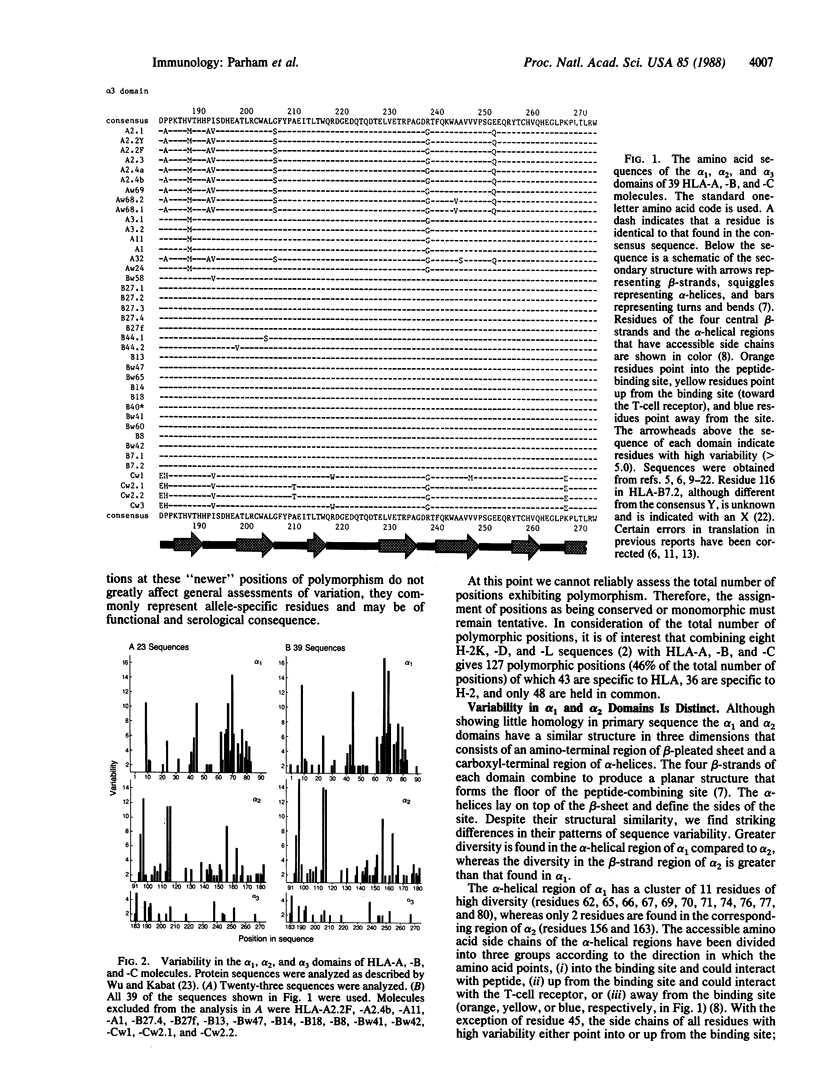
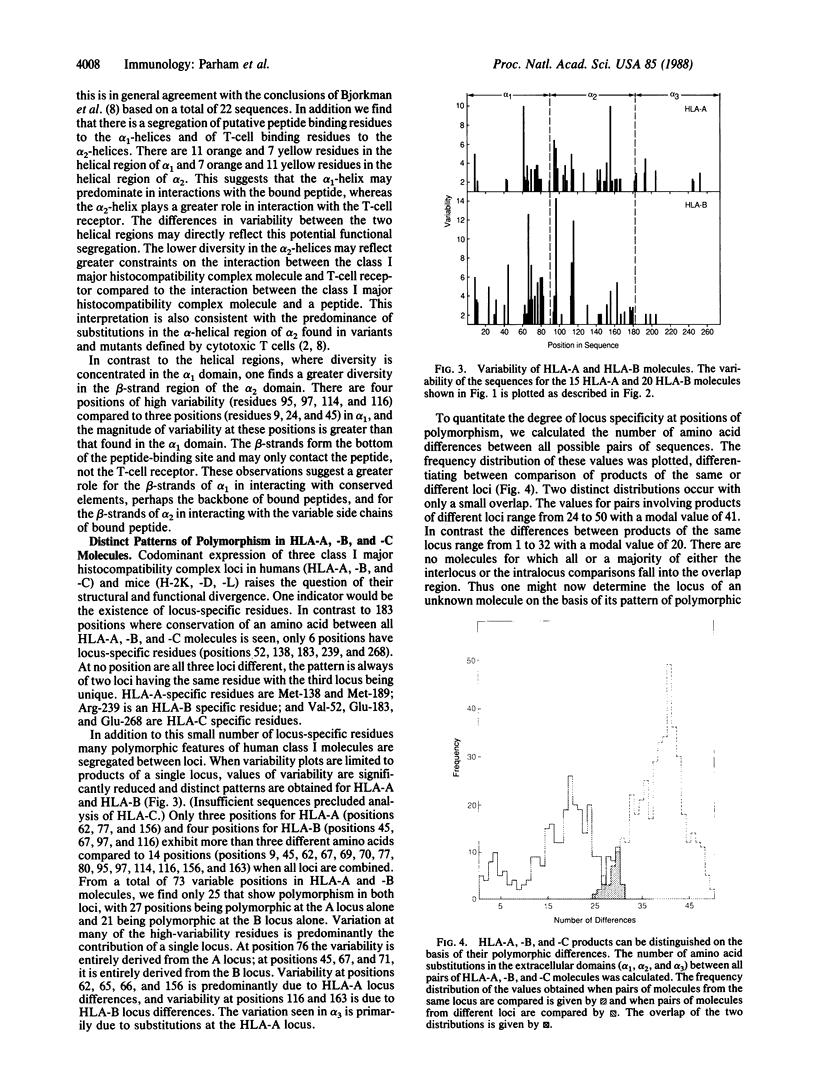
Diversity in 39 HLA-A, -B, and -C molecules is derived from 20 amino acid positions of high variability and 71 positions of low variability. Variation in the structurally homologous alpha 1 and alpha 2 domains is distinct and may correlate with partial segregation of peptide and T-cell receptor binding functions. Comparison of 15 HLA-A with 20 HLA-B molecules reveals considerable locus-specific character, due primarily to differences at polymorphic residues. The results indicate that genetic exchange between alleles of the same locus has been a more important mechanism in the generation of HLA-A, -B, and -C diversity than genetic exchange events between alleles of different loci.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C. O., Mathis D. J., Kanter M. R., Williams V. E., 2nd, McDevitt H. O. Regions of allelic hypervariability in the murine A alpha immune response gene. Cell. 1983 Aug;34(1):169–177. doi: 10.1016/0092-8674(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Cowan E. P., Jelachich M. L., Biddison W. E., Coligan J. E. DNA sequence of HLA-A11: remarkable homology with HLA-A3 allows identification of residues involved in epitopes recognized by antibodies and T cells. Immunogenetics. 1987;25(4):241–250. doi: 10.1007/BF00404694. [DOI] [PubMed] [Google Scholar]
- Davis M. M. Molecular genetics of the T cell-receptor beta chain. Annu Rev Immunol. 1985;3:537–560. doi: 10.1146/annurev.iy.03.040185.002541. [DOI] [PubMed] [Google Scholar]
- Doménech N., Ezquerra A., Castaño R., López de Castro J. A. Structural analysis of HLA-A2.4 functional variant KNE. Implications for the mapping of HLA-A2-specific T-cell epitopes. Immunogenetics. 1988;27(3):196–202. doi: 10.1007/BF00346586. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Güssow D., Rein R. S., Meijer I., de Hoog W., Seemann G. H., Hochstenbach F. M., Ploegh H. L. Isolation, expression, and the primary structure of HLA-Cw1 and HLA-Cw2 genes: evolutionary aspects. Immunogenetics. 1987;25(5):313–322. doi: 10.1007/BF00404424. [DOI] [PubMed] [Google Scholar]
- Holmes N., Ennis P., Wan A. M., Denney D. W., Parham P. Multiple genetic mechanisms have contributed to the generation of the HLA-A2/A28 family of class I MHC molecules. J Immunol. 1987 Aug 1;139(3):936–941. [PubMed] [Google Scholar]
- Holmes N., Parham P. Exon shuffling in vivo can generate novel HLA class I molecules. EMBO J. 1985 Nov;4(11):2849–2854. doi: 10.1002/j.1460-2075.1985.tb04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. Gene conversion in MHC genes. Transplantation. 1984 Oct;38(4):327–329. doi: 10.1097/00007890-198410000-00002. [DOI] [PubMed] [Google Scholar]
- Klein J. H-2 mutations: their genetics and effect on immune functions. Adv Immunol. 1978;26:55–146. doi: 10.1016/s0065-2776(08)60229-1. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Geraghty D., Orr H. T., Shimizu Y., DeMars R. Organization of the human class I major histocompatibility complex genes. Immunol Res. 1987;6(1-2):1–10. doi: 10.1007/BF02918100. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Sidwell B., DeMars R., Orr H. T. Isolation of HLA locus-specific DNA probes from the 3'-untranslated region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5175–5178. doi: 10.1073/pnas.81.16.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottmann A. H., Seemann G. H., Guessow H. D., Roos M. H. DNA sequence of the coding region of the HLA-B44 gene. Immunogenetics. 1986;23(6):396–400. doi: 10.1007/BF00372673. [DOI] [PubMed] [Google Scholar]
- Maloy W. L. Comparison of the primary structure of class I molecules. Immunol Res. 1987;6(1-2):11–29. doi: 10.1007/BF02918101. [DOI] [PubMed] [Google Scholar]
- N'Guyen C., Sodoyer R., Trucy J., Strachan T., Jordan B. R. The HLA-AW24 gene: sequence, surroundings and comparison with the HLA-A2 and HLA-A3 genes. Immunogenetics. 1985;21(5):479–489. doi: 10.1007/BF00430931. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Orr H. T., López de Castro J. A., Lancet D., Strominger J. L. Complete amino acid sequence of a papain-solubilized human histocompatibility antigen, HLA-B7. 2. Sequence determination and search for homologies. Biochemistry. 1979 Dec 11;18(25):5711–5720. doi: 10.1021/bi00592a030. [DOI] [PubMed] [Google Scholar]
- Pease L. R. Diversity in H-2 genes encoding antigen-presenting molecules is generated by interactions between members of the major histocompatibility complex gene family. Transplantation. 1985 Mar;39(3):227–231. doi: 10.1097/00007890-198503000-00001. [DOI] [PubMed] [Google Scholar]
- Rojo S., Aparicio P., Choo S. Y., Hansen J. A., López de Castro J. A. Structural analysis of an HLA-B27 population variant, B27f. Multiple patterns of amino acid changes within a single polypeptide segment generate polymorphism in HLA-B27. J Immunol. 1987 Aug 1;139(3):831–836. [PubMed] [Google Scholar]
- Seemann G. H., Rein R. S., Brown C. S., Ploegh H. L. Gene conversion-like mechanisms may generate polymorphism in human class I genes. EMBO J. 1986 Mar;5(3):547–552. doi: 10.1002/j.1460-2075.1986.tb04245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan T., Sodoyer R., Damotte M., Jordan B. R. Complete nucleotide sequence of a functional class I HLA gene, HLA-A3: implications for the evolution of HLA genes. EMBO J. 1984 Apr;3(4):887–894. doi: 10.1002/j.1460-2075.1984.tb01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Krangel M. S., Spits H., de Vries J., Strominger J. L. Structural analysis of an HLA-B7 antigen variant detected by cytotoxic T lymphocytes. J Immunol. 1984 Aug;133(2):816–821. [PubMed] [Google Scholar]
- Wan A. M., Ennis P., Parham P., Holmes N. The primary structure of HLA-A32 suggests a region involved in formation of the Bw4/Bw6 epitopes. J Immunol. 1986 Dec 1;137(11):3671–3674. [PubMed] [Google Scholar]
- Ways J. P., Coppin H. L., Parham P. The complete primary structure of HLA-Bw58. J Biol Chem. 1985 Oct 5;260(22):11924–11933. [PubMed] [Google Scholar]
- Ways J. P., Lawlor D. A., Wan A. M., Parham P. A transposable epitope of HLA-B7, B40 molecules. Immunogenetics. 1987;25(5):323–328. doi: 10.1007/BF00404425. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmour J., Ennis P. D., Parham P., Dupont B. Comparison of the structure of HLA-Bw47 to HLA-B13 and its relationship to 21-hydroxylase deficiency. Immunogenetics. 1988;27(4):281–287. doi: 10.1007/BF00376123. [DOI] [PubMed] [Google Scholar]