Abstract
We report a case of visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected 37-year-old Thai fisherman who presented with nephritonephrotic syndrome, fever, anemia, and thrombocytopenia. Bone marrow biopsy revealed many amastigotes within macrophages. Kidney biopsy showed membranoproliferative glomerulonephritis. Polymerase chain reaction (PCR) and nucleotide sequence analysis of the internal transcribed spacer 1 of the small subunit ribosomal RNA gene in blood and kidney biopsy specimens showed Leishmania species previously described in a Thai patient with visceral leishmaniasis. Only four autochthonous cases of leishmaniasis have been reported in Thailand since 1996. To the best of our knowledge, this is the first report of autochthonous visceral leishmaniasis in an HIV-infected Thai. With an increasing number of patients with autochthonous leishmaniasis in association with the presence of potential vector, it remains to be determined whether this vector-borne disease will become an emerging infectious disease in Thailand.
Leishmaniasis is a vector-borne infection caused by an obligate intracellular protozoon, Leishmania sp., which is transmitted by phlebotomine sandflies.1–3 It occurs worldwide in tropical and subtropical regions including the Middle East, India, China, Africa, and southern and central America. Thailand is not a known endemic area for leishmaniasis. Most imported cases were reported between 1960 and 1986 in Thai workers returning from the Middle East.4,5 The first reported indigenous patient with leishmaniasis was a 3-year-old girl living at Suratthani Province of southern Thailand in 1996.6 Several autochthonous cases with leishmaniasis were recently seen in northern, central, and southern Thailand.7–9 Interestingly, these patients were from provinces where a potential sandfly vector has never been reported.10–12 We describe the first report of visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient and review all previous reports of autochthonous cases of leishmaniasis in Thailand.
Case Report
A 37-year-old Thai fisherman with known HIV infection presented with progressive leg edema, ascites, and low-grade fever of 8 weeks duration. Seven weeks prior to admission (PTA), he was hospitalized at Chantaburi Provincial Hospital with a diagnosis of nephritonephrotic syndrome (hypertension, edema, heavy proteinuria, microscopic hematuria, azotemia, hypoalbuminemia, and hypercholesterolemia). He was treated with prednisolone 50 mg/day. Two weeks PTA, he had not improved and developed thrombocytopenia (platelet count of 85,000/µL) and anemia (hematocrit decreased from 29% to 23.4%). Bone marrow aspiration and biopsy were performed and revealed decreased cellularity and many “yeast-like” organisms 1–2 µM in size. Fungal cultures of both specimens did not grow any fungi. He was then transferred to King Chulalonkorn Memorial Hospital in Bangkok for further evaluation. The patient was born at Chantaburi, eastern Thailand. He had never been outside Thailand except for having worked as a fisherman in the Indian Ocean and northern Indonesian sea from 1999 to 2001. The HIV was diagnosed at 33 years of age presenting with active tuberculosis. He received a standard 6-month course of therapy comprised of isoniazid, rifampin, pyrazinamide, and ethambutol and also started on stavudine, lamivudine, nevirapine, and cotrimoxazole. His CD4 cell counts increased from 40 to 129 cells/µL and plasma HIV RNA levels became undetectable at 8 weeks PTA. He was also found to have chronic hepatitis C infection and a history of intravenous drug use. He smoked and drank alcohol daily. Physical examination showed a temperature of 38.5°C, moderate pallor, mild hepatomegaly, and moderate pedal edema. Blood complement levels of C3 and C4 were decreased. A kidney biopsy was performed and revealed membranoproliferative glomerulonephritis with focal segmental glomerulosclerosis. However, no organisms were demonstrated on Giemsa's staining in the renal biopsy slides. A review of the histopathology of the bone marrow revealed, amastigotes of Leishmania sp. within macrophages and bar-shaped kinetoplast were also seen (Figure 1). The blood and kidney biopsy specimens were sent to the Department of Parasitology, Chulalongkorn University, for identification of the species of Leishmania. The samples were then tested for Leishmania using the primers specific for 18S ribosomal RNA (rRNA) genes as described by Le Fichoux and others13 and for species differentiation using the primers specific for internal transcribed spacer1 (ITS1) regions of the rRNA as described by Uliana and others.14 Both specimens obtained from our patient were positive for Leishmania. Nucleotide sequences of the amplified PCR products for the 18S rRNA gene were identical to the sequence of Leishmania sp. previously reported in GenBank (GenBank accession no. AF303938). The species of Leishmania identified by nucleotide sequences of the amplified PCR products of the ITS1 region of the rRNA gene was identical to the new species of Leishmania (GenBank accession no. EF200011) (Figure 2), previously reported from a Thai patient with visceral leishmaniasis by Sukmee and others.8 Nucleotide sequences of the 18S rRNA gene and the ITS1 region of the rRNA gene of this report were submitted to GenBank under accession nos. GQ226033 and GQ226034. The patient gradually improved clinically along with his bone marrow and renal status after treatment with amphotericin B deoxycholate (2 mg/kg every other day) for 2 weeks. He was discharged on oral itraconazole (400 mg/day). He was doing well when last seen 3 months after diagnosis of leishmaniasis.
Figure 1.
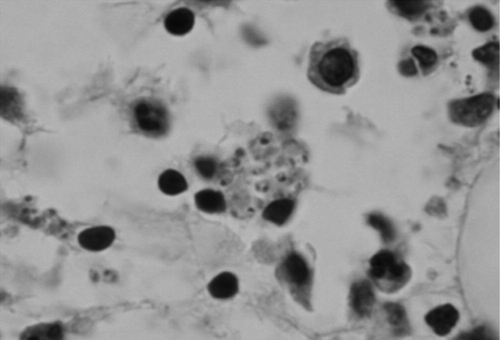
Leishmania sp. amastigotes are observed within a macrophage in a bone marrow aspirate. Both nucleus and kinetoplast can be seen within some amastigotes.
Figure 2.

The species of Leishmania of our patient (Leish_BC_R) identified by nucleotide sequences of the amplified polymerase chain reaction (PCR) products of the ITS1 region of the ribosomal RNA (rRNA) gene, was identical to the new species of Leishmania (GenBank accession no. EF200011). Nucleotide sequences of the 18S rRNA gene and the ITS1 region of the rRNA gene were then submitted to GenBank under accession nos. GQ226033 and GQ226034.
Conclusions
The clinical features of leishmaniasis can be categorized into visceral, cutaneous, and mucocutaneous forms depending on the species of Leishmania. Our patient had visceral leishmaniasis characterized by prolonged fever, anemia, thrombocytopenia, hepatomegaly, and nephritonephrotic syndrome. Glomerular involvement is rare in human visceral leishmaniasis.15–17 It can be associated with or without immune complex-mediated glomerulonephritis including proliferative glomerulonephritis17 and collapsing focal segmental glomerulosclerosis.16 Our patient presented with nephritonephrotic syndrome associated with immune-complex-mediated membranoproliferative glomerulonephritis and focal segmental glomerulosclerosis. He gradually improved after treatment with amphotericin B deoxycholate followed by itraconazole. Nephrotic syndrome complicating Leishmania/HIV coinfection has been reported in previously.15
We believe that our patient acquired the disease in Chantaburi province of Thailand even though he had been in northern Indonesia from 1999 to 2001. The incubation period of visceral leishmaniasis generally varies from 3 to 8 months, but it could be as short as 2 weeks or longer than 1 year.18 However, the infection may remain asymptomatic in some patients. However, there has never been a report of leishmaniasis from Indonesia. Therefore, ours is the first report of autochthonous visceral leishmaniasis in an HIV-infected patient from Thailand. To the best of our knowledge, there have seen five autochthonous cases of leishmaniasis (including our patient) in Thailand (Table 1).6–9 However, there are two additional non-reported autochthonous cases with visceral and cutaneous leishmaniasis from Nakorn Sri Thammarat and Chian Rai, respectively (Sukmee T, personal communication). All patients had visceral leishmaniasis, and lived in all parts of the country except for Northeast Thailand. All were males except one 2-year-old girl, and had no HIV infection. The species of Leishmania was identified in only three patients as Leishmania infantum (one patient from Bangkok) and a new species (one from Nan and one from Chantaburi [our patient]). The nucleotide sequence of the ITS1 region of the rRNA gene of Leishmania in our patient is identical to the suspected new species of Leishmania previously reported from a 40-year-old construction worker from Nan province in 2005.8 The phylogenetic tree of the ITS1 sequence of the rRNA gene from our patient is situated as a sister taxon of the clade of Leishmania brasiliensis and Leishmania guyanensis, which are the causative agents of New World visceral and cutaneous leishmaniasis.19
Table 1.
Summary of five case reports of autochthonous leishmaniasis in Thailand*
| Year, province, part of country | Age (years), sex | Occupation | Underlying disease | Clinical features; duration | Form of leishmaniasis, species of Leishmania | Investigations | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Sandfly vectors | Animal reservoirs (DAT) | ||||||||
| 1996, Suratthani, South6 | 2, Female | NA | No | Fever, hepatospleno- megaly, anemia, thrombocy topenia; 2 months | VL, no species identified | No study | No study | Pentamidine isethionate for 15 doses | Cured after 2 years of follow-up |
| 2005, Nan, North7 | 40, male | Construction worker in several provinces | Ampheta-mine and opium addiction | Fever, hepatospleno-megaly, pantypope- nia, mediastinal mass; 31 months | VL, no species identified | No potential vectors | Positive in 3 cows and 1 cat | 2 courses of ABd for 30 days | Remission |
| 2006, Phangnga, South8 | 55, male | Worker in rubber plantation | No | Fever, hepatospleno-megaly, pancytopenia; 3 years | VL, new species | No potential vectors | Positive in 9 cats | ABd (100 mg) mixed with 1 mg lipid for 14 days | Relapse 2 months after treatment |
| 2007, Bangkok, Center9 | 66, male | Lumber truck driver | Diabetes, hyper-tension | Fever, weight loss, hepatosplenomegaly pancytopenia; 6 months | VL, L. infantum | Inability to obtain vectors due to raining | Negative in 9 dogs, 1 cat, 3 rats | ABd every other day for 30 days | Remission |
| 2009, Chantaburi, East (present report) | 37, male | Fisherman, a history of travel to North Indonesia | AIDS, chronic HCV infection | Fever, nephritonephrotic syndrome, hepatomegaly, anemia, thrombocytopenia; 8 weeks | VL, new species | No potential vectors | Negative | ABd every other day for 14 days, and itraconazole (400 mg/day) | Remission |
NA = not applicable, ABd = amphotericin B deoxycholate, VL = visceral leishmaniasis, DAT = direct agglutination test for Leishmania antibody, AIDS = acquired immunodeficiency syndrome; HCV = hepatitis C virus.
There were no known potential vector sandfly species of Leishmania during surveys of sandflies collected in the village of our patients (Table 1), even though phlebotomine sandflies are widely distributed in Thailand.10–12 Previous studies in Thailand showed the presence of cow-biting sandflies, Phlebotomus major major and cow- and bat-biting cave-dwelling sandflies, Psilocybe argentipes, which have been shown to be vectors of Old World visceral leishmaniasis.10–12
A serologic study for Leishmania antibody using the direct agglutination test (DAT) was carried out to identify the animal reservoirs of the disease and published in four reports (Table 1). Positive DAT results in cats and cows were reported in two studies from Nan and Phangnga. The specificity and sensitivity of DAT to detect serum antibody for Leishmania have been shown to be very high.20 Domestic animals are one of the most important reservoirs of leishmaniasis.21 On the basis of the presence of potential sandfly vectors and animal reservoirs of leishmaniasis in Thailand, it is possible that the disease is transmitted from cows or cats with asymptomatic, subclinical, or viscerocutaneous infection to humans by these vectors.
In conclusion, to the best of our knowledge, this is the first report of autochthonous visceral leishmaniasis in an HIV-infected patient. The patient readily responded to conventional treatment. With an increasing number of patients with autochthonous leishmaniasis in association with the presence of potential vector, it remains to be determined whether this vector-borne disease will become an emerging infectious disease in Thailand.
Acknowledgments
We thank Theerayudh Sukmee, Department of Parasitology, Phramongkutklao College of Medicine, Bangkok; Bureau of Epidemiology, Department of Control Disease, Ministry of Public Health, Nonthburi; and Bureau of Vector-borne Disease Control, Department of Control Disease, Ministry of Public Health, Bangkok, for the information regarding the epidemiology and serology of some cases of leishmaniasis in Thailand.
Disclaimer: All authors have no conflicts of interest to declare and all have actively contributed to this study and review.
Footnotes
Authors' addresses: Chusana Suankratay and Gompol Suwanpimolkul, Division of Infectious Diseases, Department of Medicine, Chulalongkorn University Hospital, Bangkok, Thailand, E-mail: chusana.s@chula.ac.th. Henry Wilde, Department of Medicine and Division of Research Affairs, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Padet Siriyasatien, Department of Parasitology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 1996;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, Wasunna MK, Bryceson AD. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 3.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 4.Suttinont P, Thammanichanont C, Chantarakul N. Visceral leishmaniasis: a case report. Southeast Asian J Trop Med Public Health. 1987;18:103–106. [PubMed] [Google Scholar]
- 5.Viriyavejakul P, Viravan C, Riganti M, Punpoowong B. Imported cutaneous leishmaniasis in Thailand. Southeast Asian J Trop Med Public Health. 1997;28:558–562. [PubMed] [Google Scholar]
- 6.Thisyakorn U, Jongwutiwes S, Vanichsetakul P, Lertsapcharoen P. Visceral leishmaniasis: the first indigenous case report in Thailand. Trans R Soc Trop Med Hyg. 1999;93:23–24. doi: 10.1016/s0035-9203(99)90166-9. [DOI] [PubMed] [Google Scholar]
- 7.Kongkaew W, Siriarayaporn P, Leelayoova S, Supparatpinyo K, Areechokchai D, Duang-ngern P, Chanachai K, Sukmee T, Samung Y, Sridurongkathum P. Autochthonous visceral leishmaniasis: a report of a second case in Thailand. Southeast Asian J Trop Med Public Health. 2007;38:8–12. [PubMed] [Google Scholar]
- 8.Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, Samung Y, Kongkaew W, Bumrungsana K, Chanachai K, Apiwathanasorn C, Rujirojindakul P, Wattanasri S, Ungchusak K, Leelayoova S. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38:617–622. doi: 10.1016/j.ijpara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Maharom P, Siripattanapipong S, Mungthin M, Naaglor T, Sukkawee R, Pudkorn R, Wattana W, Wanachiwanawin D, Areechokchai D, Leelayoova S. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J Trop Med Public Health. 2008;39:988–990. [PubMed] [Google Scholar]
- 10.Apiwathnasorn C, Sucharit S, Rongsriyam Y, Leemingsawat S, Kerdpibule V, Deesin T, Surathin K, Vutikes S, Punavuthi N. A brief survey of phlebotomine sandflies in Thailand. Southeast Asian J Trop Med Public Health. 1989;20:429–432. [PubMed] [Google Scholar]
- 11.Apiwathnasorn C, Sucharit S, Surathin K, Deesin T. Anthropophilic and zoophilic phlebotomine sand flies (Diptera: Psychodidae) from Thailand. J Am Mosq Control Assoc. 1993;9:135–137. [PubMed] [Google Scholar]
- 12.Polseela R, Apiwathnasorn C, Samung Y. Seasonal variation of cave-dwelling phlebotomine sandflies (Diptera:Psychodidae) in Phra Phothisat Cave, Saraburi Province, Thailand. Southeast Asian J Trop Med Public Health. 2007;38:1011–1015. [PubMed] [Google Scholar]
- 13.Le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, Rousseau D, Kubar J. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol. 1999;37:1953–1957. doi: 10.1128/jcm.37.6.1953-1957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uliana SR, Nelson K, Beverley SM, Camargo EP, Floeter-Winter LM. Discrimination amongst Leishmania by polymerase chain reaction and hybridization with small subunit ribosomal DNA derived oligonucleotides. J Eukaryot Microbiol. 1994;41:324–330. doi: 10.1111/j.1550-7408.1994.tb06085.x. [DOI] [PubMed] [Google Scholar]
- 15.Alex S, Criado C, Fernández-Guerrero ML, de Górgolas M, Petkov V, Garcia Perez A, Egido J, Barat A, Manzarbeitia F, Caramelo C, Ortiz A. Nephrotic syndrome complicating chronic visceral leishmaniasis: re-emergence in patients with AIDS. Clin Nephrol. 2008;70:65–68. doi: 10.5414/cnp70065. [DOI] [PubMed] [Google Scholar]
- 16.Efstratiadis G, Boura E, Giamalis P, Mandala E, Leontsini M, Tsiaousis G, Memmos D. Renal involvement in a patient with visceral leishmaniasis. Nephrol Dial Transplant. 2006;21:235–236. doi: 10.1093/ndt/gfi157. [DOI] [PubMed] [Google Scholar]
- 17.Dutra M, Martinelli R, de Carvalho EM, Rodrigues LE, Brito E, Rocha H. Renal involvement in visceral leishmaniasis. Am J Kidney Dis. 1985;6:22–27. doi: 10.1016/s0272-6386(85)80034-2. [DOI] [PubMed] [Google Scholar]
- 18.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Bonfante C, Bonfante-Garrido R, Grimaldi G Jr, Momen H, Cupolillo E. Genotypically distinct Leishmania colombiensis isolates from Venezuela cause both cutaneous and visceral leishmaniasis in humans. Infect Genet Evol. 2003;3:119–124. doi: 10.1016/s1567-1348(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 20.Schallig HD, Schoone GJ, Kroon CC, Hailu A, Chappuis F, Veeken H. Development and application of ‘simple’ diagnostic tools for visceral leishmaniasis. Med Microbiol Immunol (Berl) 2001;190:69–71. doi: 10.1007/s004300100083. [DOI] [PubMed] [Google Scholar]
- 21.Abranches P, Campino L, Santos-Gomes GM. Canine leishmaniasis. New concepts of epidemiology and immunopathology: their impact in the control of human visceral leishmaniasis. Acta Med Port. 1998;11:871–875. [PubMed] [Google Scholar]


