Abstract
We conducted a randomized, placebo-controlled, triple-blinded trial to determine the health impact of daily use of sodium dichloroisocyanurate (NaDCC) tablets for household drinking water treatment in periurban Ghana. We randomized 240 households (3,240 individuals) to receive either NaDCC or placebo tablets. All households received a 20-liter safe water storage vvessel. Over 12 weeks, 446 diarrhea episodes (2.2%) occurred in intervention and 404 (2.0%) in control households (P = 0.38). Residual free chlorine levels indicated appropriate tablet use. Escherichia coli was found in stored water at baseline in 96% of intervention and 88% of control households and at final evaluation in 8% of intervention and 54% of control households (P = 0.002). NaDCC use did not prevent diarrhea but improved water quality. Diarrhea rates were low and water quality improved in both groups. Safe water storage vessels may have been protective. A follow-up health impact study of NaDCC tablets is warranted.
Introduction
Each year, diarrhea causes approximately 1.8 million deaths, mostly in children under 5 years old in developing countries.1 Consumption of contaminated drinking water is an important cause of diarrhea in developing countries, where safe water infrastructure is lacking. The definitive response to this problem would be the universal provision of piped, treated water, but because of insufficient resources, achievement of this goal remains remote. For this reason, a number of household water treatment technologies have been developed, tested, and disseminated to protect the health of populations lacking access to safe water.2–16
One of these household water treatment technologies is sodium dichloroisocyanurate (NaDCC) tablets, an alternative to sodium hypochlorite (NaOCl) solution, which is produced and distributed in many countries for water treatment.17 Both NaDCC tablets and NaOCl solution disinfect water by releasing free available chlorine in the form of hypochlorous acid, which is an effective microbicide against a wide range of bacteria, viruses, and parasites.18 Although NaOCl releases all its free available chlorine immediately, NaDCC releases half of its free available chlorine initially, leaving “reservoir chlorine” that is released once the original free available chlorine has been used up.18 NaDCC tablets' reservoir chlorine may be especially advantageous when water is subject to high organic loads, as is common in resource-poor and remote settings.18
NaDCC tablets, which have been used for emergency water treatment since the 1980s, were approved in 2004 for daily use as a drinking water disinfectant by the United States Environmental Protection Agency and World Health Organization.18–20 NaDCC tablets are lightweight, easily disseminated, and can be stored for over 5 years without losing efficacy.18,21 Results of field trials suggest that NaDCC tablets are acceptable and effective for water treatment;18–22 but no health impact data have been published. To assess the health impact of NaDCC tablets, we conducted a field trial in Tamale, Ghana between August and November 2006.
Materials and Methods
Study setting and participants.
We selected a periurban population in Tamale, Ghana. This population, which has experienced periodic cholera outbreaks,23 consisted of multi-family households that relied on community water sources and stored drinking water in the home. We enrolled households with at least one child under 5-years-old.
Study design and procedures.
We conducted a randomized, triple-blinded, placebo-controlled trial to assess the health impact of NaDCC tablets. The study included baseline data collection followed by twice weekly home visits over 12 weeks to verify tablet use and determine diarrhea rates. We analyzed stored drinking water samples for Escherichia coli at baseline, midpoint, and end of the study. We tested the hypothesis that daily use of NaDCC tablets would improve microbiological drinking water quality and decrease individual diarrhea rates among study households.
The baseline survey included demographic and socioeconomic characteristics, and knowledge, attitudes, and practices concerning water, hygiene, and sanitation. The questionnaire was translated into Dagbani, the local language, back-translated into English, and administered to the female head of household by bilingual field workers.
After the baseline survey, households were randomized into two groups designated as A and B using a random number table. Medentech, Ltd. (Wexford, Ireland) provided NaDCC and placebo tablets, which were packaged in sachets labeled only as A or B with identical instructions for use for the corresponding study groups. Only technical staff at Medentech knew which packets contained NaDCC or placebo tablets. Tablets for the intervention group, which were designed to disinfect 20 L of water, contained NaDCC with a pharmaceutical/food-grade effervescent base that allowed the tablets to dissolve rapidly in water; placebo tablets consisted only of the effervescent base. All study households were given a standard 20-liter plastic vessel with a plastic lid and metal spigot for drinking water storage to assure that participants treated the appropriate volume of water. Each household was provided a guinea worm cloth (commonly used in the community) by the Ghana Health Service for filtering turbid water. Alum was provided on request to clarify turbid water. Field officers provided oral, written, and pictorial instructions describing proper use of tablets; for turbid water, the recommended single-tablet dose was doubled. Field officers replenished tablets during home visits. No further information or instruction on water treatment or handling, sanitation, or hygiene was provided.
After distribution of the intervention, we initiated active diarrheal surveillance through twice weekly visits (every 3 to 4 days) to all households over a 12-week period; each household had two visits in each calendar week. Field workers recorded diarrhea episodes (defined as three or more loose or watery stools in 24 hours), symptoms, and treatment received since the preceding visit; provided oral rehydration salts free to persons with diarrhea; made referrals to health facilities as needed; and asked about use of the intervention. At the final visit, respondents were asked their opinions about the intervention and whether they believed their household was in the intervention or control group; if they responded “don't know,” they were asked to make their best guess.
A separate team of technicians visited each household twice weekly to measure free chlorine residuals in stored water using digital Colorimeters® (LaMotte, Chestertown, MD). To maintain blinding, technicians visited households at different times than field workers; did not share chlorine test results with study households, field officers, or investigators; and entered chlorine data into a separate database.
We selected a random sample of 20% of households from which we obtained stored water specimens at baseline, midpoint, and end of study; samples from principal water sources were tested at the end of study. Field officers collected water samples in sterile 125 mL Whirl-Pak® (Nasco, Fort Atkinson, WI) bags containing sodium thiosulfate to neutralize residual free chlorine; placed them in coolers with ice packs; and transported them to the Ghana Water Company laboratory for testing within 6 hours. For each water sample, we tested undiluted, 1:10, and 1:100 dilutions for E. coli using Colilert® (Idexx Co., Westbrook, ME) Quanti-Tray® test kits to obtain most probable number (MPN) estimates for each sample.24 A technician entered data into a separate database.
Outcomes.
The primary outcome was number of episodes and rates of diarrhea in individuals. Secondary outcomes were free residual chlorine levels in stored household drinking water and levels of E. coli contamination in randomly selected households at baseline, midpoint, and end of study.
Sample size.
To calculate household sample size, we assumed a 15% weekly diarrhea prevalence among children under 5-years-old based on Ghana's 2003 Demographic and Health Survey (DHS);25 reduction in diarrhea risk of 40% based on results of past field trials of household chlorination;7–16 type I error rate of 5%; and power of 80%. To accommodate a maximum design effect of three in a 12-week study with twice weekly visits and inevitable attrition of subjects, we aimed to enroll 120 households in each group, which would provide an estimated 3000 person-weeks of observation in children under 5-years-old in each group.
Statistical analysis.
Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC). After data collection was completed in November 2006, investigators maintained blinding by retaining unbroken treatment codes to differentiate study groups for data analysis. We compared diarrhea rates between intervention and control households using an intent-to-treat analysis. Diarrhea rates were determined by first aggregating the two visits per week into a weekly diarrhea event. Then an individual-level diarrhea rate was calculated by dividing the number of weeks with at least one episode of diarrhea by the person-weeks of observation. We estimated the difference in the mean individual-level diarrhea rates between the two study groups using a 2-sample t test. Taylor series linearization method was used to estimate the variance to account for household-level clustering. We used a stratified analysis to examine the size differences of demographic, socioeconomic, water handling, and sanitation co-variables between the study groups to assess the need for post-randomization adjustment.
After the treatment code was broken, the effectiveness of blinding was assessed using the Blinding Index proposed by James et al.26,27 The index, a variation of the kappa coefficient, is sensitive to the degree of disagreement between an individual's actual treatment status and what treatment an individual thinks he or she has received. A higher degree of disagreement indicates more effective blinding. James suggests that a Blinding Index ≥ 0.5 indicates adequate blinding.26,27
We used the Thomas equation28 to estimate E. coli MPN per 100 mL from results of undiluted, 1:10, and 1:100 dilutions of stored water samples. We used the signed rank test to compare median E. coli MPN within the intervention and control groups at baseline, midpoint, and end of study. Because of the skewed distribution of the water quality data, we used the Wilcoxon 2-sample test to compare the median estimated E. coli MPN between intervention and control groups.
Ethics.
We presented information about NaDCC tablets and the study to local health officials and community leaders, and obtained their consent to initiate the study. We obtained written informed consent from all participating households. Institutional review boards at the University of Development Studies in Tamale, Ghana, the Centers for Disease Control and Prevention [protocol 4730], and Emory University [802-2006] in Atlanta, Georgia reviewed and approved the protocol. The protocol is registered with Clinical Trials.gov, number NCT00252928.
Results
Participants.
We enrolled 240 households with 3,240 individuals (median 12 persons per household, range 2–42); 51% were female (Figure 1). Median age of household members was 18 years (range 1 month–95 years); 17% were children under 5-years-old. The median age of interviewed heads-of-household was 36 years (range 18–74); 99% were female. Of 240 interviewed heads-of-household, 32 (13%) had been to school; of these, 12 (38%) had not completed primary school whereas 20 (62%) had a complete primary or higher education level. Nine (4%) could read or write Dagbani and 19 (8%) could read or write English. There were no significant differences in demographic characteristics or household assets between intervention and control households (Table 1).
Figure 1.
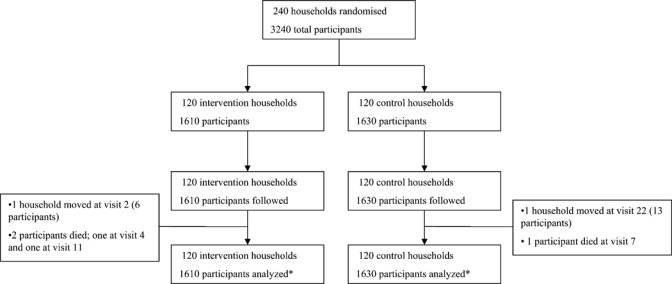
Given the intention-to-treat analysis, we analyzed all households and participants for the full time of surveillance (12.5 weeks) despite attrition in the study.
Table 1.
Demographic characteristics and household assets of household members and survey respondents among intervention and control households
| Household members | Total (N = 3,240) | Intervention (N = 1,610) | Control (N = 1,630) |
|---|---|---|---|
| Median age (range) | 18 years (1 month–95 years) | 18 years (1 month–95 years) | 19 years (1 month–90 years) |
| Female | 1,647 (51%) | 814 (50%) | 833 (52%) |
| Children ≤ 5 years* | 549 (17%) | 281 (18%) | 268 (16%) |
| Survey respondents | Total (N = 240) | Intervention (N = 120) | Control (N = 120) |
| Head of household | |||
| Median age (range) | 36 years (18–74) | 35.5 years (18–74) | 37 years (19–65) |
| Female | 238 (99%) | 119 (99%) | 119 (99%) |
| Any school | 32 (13%) | 20 (17%) | 12 (10%) |
| Method of earning a living | |||
| Small scale agriculture | 123 (51%) | 61 (51%) | 62 (52%) |
| Professional | 33 (14%) | 18 (15%) | 15 (12%) |
| Other | 84 (35%) | 41 (34%) | 43 (36%) |
| Household assets† | |||
| Coal pot | 222 (93%) | 107 (89%) | 115 (96%) |
| Bicycle | 213 (89%) | 108 (90%) | 105 (88%) |
| Bed | 202 (84%) | 101 (84%) | 101 (84%) |
| Sofa set | 123 (51%) | 62 (52%) | 61 (51%) |
| Electricity | 123 (51%) | 64 (53%) | 59 (49%) |
| Television | 95 (40%) | 49 (41%) | 46 (38%) |
| Mobile phone | 71 (30%) | 38 (32%) | 33 (28%) |
| Motorcycle | 69 (29%) | 37 (31%) | 32 (27%) |
| Refrigerator | 38 (16%) | 22 (18%) | 16 (13%) |
| Kerosene stove | 20 (8%) | 10 (8%) | 10 (8%) |
Age refers to the age of participants at the start of the study.
Participants were able to indicate more than one response; the total exceeds 100%.
Baseline characteristics.
At baseline, survey respondents reported that their water sources included water taps (95%), surface water (84%), wells (46%), rainwater (35%), and boreholes (25%); households typically used more than one source, depending on time of year (Table 2). Drinking water was mainly stored in clay pots (72%). Of 240 respondents, 186 (78%) reported treating their drinking water; most commonly reported methods were using a cloth sieve (29%) or alum (23%). For human waste disposal most relied on public latrines (84%) or open defecation (42%). There were no significant differences in water handling and sanitation practices between intervention and control groups (Table 2).
Table 2.
Baseline water handling and sanitation practices among intervention and control households
| Characteristic | Total | Intervention | Control |
|---|---|---|---|
| (N = 240) | (N = 120) | (N = 120) | |
| Water sources* | |||
| Water tap | 227 (95%) | 113 (94%) | 114 (95%) |
| Surface water | 202 (84%) | 100 (83%) | 102 (85%) |
| Well | 110 (46%) | 58 (48%) | 52 (43%) |
| Rainwater | 85 (35%) | 43 (36%) | 42 (35%) |
| Borehole | 61 (25%) | 34 (28%) | 27 (23%) |
| Water tanker | 12 (5%) | 6 (5%) | 6 (5%) |
| Drinking water storage container* | |||
| Clay pot | 173 (72%) | 94 (78%) | 79 (66%) |
| Jerry can | 37 (15%) | 22 (18%) | 15 (13%) |
| Barrel/water drum | 34 (14%) | 12 (10%) | 22 (18%) |
| Wide bucket | 12 (5%) | 1 (1%) | 11 (9%) |
| Treatment of water | 186 (78%) | 89 (74%) | 97 (81%) |
| Water treatment practices† | |||
| Sieve cloth | 70 (29%) | 30 (25%) | 40 (33%) |
| Alum | 54 (23%) | 23 (19%) | 31 (26%) |
| Nothing | 33 (14%) | 18 (15%) | 15 (13%) |
| Boil | 9 (4%) | 4 (3%) | 5 (4%) |
| Other | 15 (6%) | 6 (5%) | 9 (8%) |
| Type of sanitation access* | |||
| Public latrine | 201 (84%) | 102 (85%) | 99 (83%) |
| Bushes or ground | 100 (42%) | 51 (43%) | 49 (41%) |
| Latrine in compound | 4 (2%) | 2 (2%) | 2 (2%) |
| Hygiene observations | |||
| Soap in home | 226 (94%) | 113 (94%) | 113 (94%) |
| Presence of specific place to wash hands | 17 (7%) | 10 (8%) | 7 (6%) |
Participants were able to indicate more than one response; the total exceeds 100%.
Participants were able to indicate more than one response but due to missing values (approximately 25%), the total is less than 100%.
Active diarrheal surveillance.
Over the 12-week study period, diarrhea rates were low, with 446 episodes (2.2%) in the intervention and 404 episodes (2.0%) in the control group. Among children under 5-years-old, there was no significant difference in diarrhea rates between the two groups with 185 episodes (5.3%) in the intervention and 156 episodes (4.7%) in the control group (Table 3). Diarrhea rates decreased in both groups over time (Figure 2).
Table 3.
Diarrhea episodes, individuals, person-weeks of observation, and diarrheal rates in intervention and control households, by age group
| Age group (years) | Individuals in intervention households (N = 1,610) | Individuals in control households (N = 1,630) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of diarrheal episodes* | Number of individuals | PWO† | Diarrheal rate | Number of diarrheal episodes | Number of individuals | PWO† | Diarrheal rate | P‡ | |
| < 1 | 43 | 69 | 862 | 5.0 | 21 | 47 | 587 | 3.6 | 0.37 |
| 1–2 | 83 | 85 | 1,063 | 7.8 | 88 | 105 | 1,313 | 6.7 | 0.58 |
| 3–4 | 59 | 127 | 1,588 | 3.7 | 47 | 116 | 1,450 | 3.2 | 0.62 |
| ≥ 5 | 261 | 1,329 | 16,612 | 1.6 | 248 | 1,362 | 17,025 | 1.5 | 0.39 |
| Total | 446 | 1,610 | 20,125 | 2.2 | 404 | 1,630 | 20,375 | 2.0 | 0.38 |
An episode is a week with at least one reported episode of diarrhea.
PWO = person-week of observation was calculated by multiplying the number of individuals followed by the number of weeks of surveillance (12.5 weeks).
The mean individual-level diarrheal rates between intervention and control households were compared using the 2 sample t test, where Taylor series linearization method was used to estimate the variance to account for household-level clustering.
Figure 2.
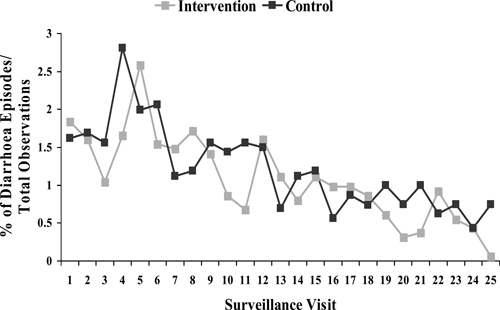
Percent of diarrhea episodes per total number of observations in intervention and control groups, by surveillance visit (N = 3240).
Chlorine results.
Over the study period, the percentage of households with free chlorine residuals ≥ 0.2 mg/L in stored drinking water ranged from 74–89% in the intervention group and 0–7% in the control group (Figure 3). For 93% of samples, the water source was tap water; ≤ 1% of household water samples were from surface water sources during the study period. Alum was used a total of 12 times by nine households (five control and four intervention households) during the study period.
Figure 3.
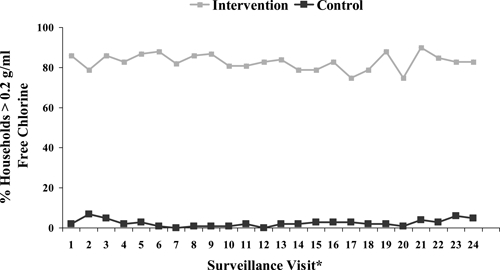
Percent of intervention and control households with ≥ 0.2 g/mL of free chlorine in stored water, by surveillance visit (N = 240). * Data was not obtained for surveillance visit number 25 due to constraints in the field.
Microbiological water quality.
At baseline, E. coli was isolated from stored drinking water samples from 96% of intervention (median E. coli MPN 93 per 100 mL) and 88% of control households (median E. coli MPN 219 per 100 mL); this difference was not statistically significant (Table 4). At the study midpoint, stored drinking water samples from 21% of intervention (median E. coli MPN 0 per 100 mL) and 92% of control households (median E. coli MPN 16 per 100 mL) yielded E. coli (P < 0.0001). At the end of the study, stored drinking water samples from 8% of intervention (median E. coli MPN 0 per 100 mL) and 54% of control households (median E. coli MPN 1 per 100 mL) yielded E. coli (P = 0.002) (Table 4). Heavily contaminated stored drinking water samples (E. coli MPN > 1000 per 100 mL) were found in 21% of intervention and 13% of control households at baseline, 4% of intervention and 8% of control households at midpoint, and no households at the end of the study.
Table 4.
Number and percent of stored drinking water samples contaminated with Escherichia coli, and median and range of Escherichia coli MPN/100 mL, among a random sample of intervention and control households at baseline, midterm, and endpoint of study
| Sampling round | Intervention households (N = 24) | Control households (N = 24) | |||
|---|---|---|---|---|---|
| Number (%) of samples with E. coli colonies | Median estimated E. coli MPN per 100 mL (min-max) | Number (%) of samples with E. coli colonies | Median estimated E. coli MPN per 100 mL (min-max) | P* | |
| Baseline | 23 (96%) | 93 (0–36,582) | 21 (88%) | 219 (0–6,824) | 0.22 |
| Midterm | 5 (21%) | 0 (0–1,885) | 22 (92%) | 16 (0–1,221) | < 0.0001 |
| Endpoint | 2 (8%) | 0 (0–292) | 13 (54%) | 1 (0–58) | 0.002 |
Median estimated Escherichia coli counts between the intervention and control households were compared using the Wilcoxon 2-sample test.
The median E. coli MPN for stored water samples in intervention households at the midpoint and the end of the study was significantly lower than at baseline (P < 0.0001); there was no significant difference from the midpoint to the end of the study (P = 0.13). Among control households, the median E. coli MPN for stored water samples at the end of the study was significantly lower than at baseline (P < 0.0001) or midpoint (P = 0.0005); there was no significant difference from baseline to midpoint (P = 0.37). Tap water samples yielded no E. coli. The median MPN of E. coli of surface water was 178 per 100 mL.
Final perceptions and blinding index.
The final survey included 238 respondents. Respondents believed that the tablets' effect included the following: improved health (63%), prevented disease (45%), made water safer (43%), were easy to use (21%), and made their water taste better (16%); 2% complained of bad smell and 1% bad taste. Respondents believed that the water vessel made their water safer (69%), improved health (46%), prevented disease (29%), was easy to use (28%), and made water taste better (12%).
Of 238 respondents, 127 (53%) believed they received NaDCC tablets whereas only 4 (2%) thought they received placebo tablets; 107 (45%) did not know. When those who did not know were asked to make a guess, 37 (35%) believed they received NaDCC tablets; the rest were unsure but none believed they received placebo tablets. There were no differences between intervention and control groups in beliefs about which group they were in. The Blinding Index was 0.65.
Discussion
Results of this study suggest that the use of NaDCC tablets, despite contributing to marked improvement in stored water quality, had no impact on diarrheal diseases in the study population. This finding occurred in spite of very high adherence to recommended water treatment practices and a statistically significant improvement in stored water quality in the intervention group. There was no apparent bias as intervention and control groups were similar in age, gender, and socioeconomic characteristics, and had comparable water handling and sanitation practices. The lack of health impact in the context of successful treatment of stored drinking water was not consistent with results of previous studies of other chlorine-based, point-of-use water treatment interventions.7,9,12–16
There are several possible explanations for the observed lack of health impact. First, it is possible that disinfected stored water does not prevent diarrhea. This possibility is unlikely based on over a century's experience with chlorination of water supplies in developed countries, where waterborne enteric disease morbidity and mortality have decreased dramatically.29 Furthermore, a recent Cochrane review of point-of-use household water treatment intervention trials, many of which assessed chlorination as the treatment technology, demonstrated a pooled protective effect of nearly 40%.2 Second, although the study took place during the rainy season (when waterborne diarrhea outbreaks, including cholera, have occurred in the past),23 diarrhea rates were much lower than anticipated, particularly in children under 5-years-old. This finding suggests that enteric pathogens were not circulating widely at that time in the population. Third, source water at the time of the study had very low levels of contamination, which suggests that waterborne transmission of diarrheal pathogens may have been low. Previous research has suggested that diarrhea risk increases with the intensity of E. coli contamination in source water, with a significantly increased risk occurring above a threshold of 1,000 colonies/100 mL;30 in this study, the percentage of stored water samples that exceeded this threshold was low and decreased over time. However, point-of-use water treatment can reduce diarrhea risk even in settings with low levels of E. coli contamination in both source and stored water samples, as demonstrated in an intervention trial that evaluated the health impact of household chlorination and safe storage in a Zambian population.9 Finally, the improved water storage vessels, by protecting stored water from the introduction of potential contaminants, may have served as a water quality intervention independent of tablet use and contributed to decreased diarrhea rates in both the intervention and control groups. This possibility is supported by the finding that stored water quality in the control group at the end of the study was significantly better than at baseline and midpoint. Previous research has shown that safe water storage can decrease diarrhea risk.8,31,32
To our knowledge, only one other blinded study examining the health impact of point-of-use chlorination has been published in a peer-reviewed journal. That study evaluated sodium hypochlorite solution in a Brazilian periurban slum and found no significant difference in diarrheal prevalence between intervention and control households despite a significant improvement of stored water quality in intervention households.33 The study authors suggested that, because of the taste of chlorine, true blinding may not have occurred, and acknowledged that the trial was limited by small size, drop-out of 4 of 20 study households, uncertainty about whether water was adequately disinfected, and the likelihood that study participants drank contaminated water outside the home. The difficulties encountered by the authors highlighted the challenges in conducting placebo-controlled, blinded trials of sodium hypochlorite solution because of the smell, taste, and texture of bleach.
Aside from the Brazilian study, all other field trials of chlorine-based point-of-use water treatment interventions, none of which were blinded, have shown efficacy in reducing diarrhea risk.2–5,7,9,12–16 A recent meta-epidemiological study assessed the evidence for bias in controlled trials due to lack of blinding34 and, although the review did not specifically assess point-of-use water treatment intervention trials, it did estimate that trials with subjective outcomes exaggerated the actual effect of those outcomes by approximately 25%. Thus, even if the pooled estimate of the effect of household water treatment were adjusted by this factor of 25%, the protective effect would still be greater than 30%. It is unknown whether the lack of other published studies documenting no health impact of point-of-use chlorination is a result of publication bias or the robustness of the interventions in the settings where they were tested.2–5
Two double-blinded, placebo-controlled trials of non-chlorine based point-of-use water treatment interventions have been published in peer-reviewed literature, and neither found an impact on health.27,35 Both took place in the United States and evaluated active and sham water filtration devices in households relying on water that met federal and state drinking water standards and requirements.27,35 These studies were not comparable to most trials of point-of-use water treatment because of the setting, in which waterborne disease risk in the group with sham filters was exceedingly low.
Our study had several important limitations. First, the principal outcome was self-reported diarrhea, which is subject to imperfect recall.36,37 To improve recall, we visited homes twice a week. Second, frequent home visits by the field officers may have influenced water handling and household hygienic practices in both the intervention and control groups. To mitigate the influence of our field workers on hygienic habits, they limited the advice they gave study participants to tablet utilization. Third, the short duration of the study limited the data collection to one season, which reduced the power of the study to detect an impact of the intervention. Fourth, water treated with NaDCC may have a detectable odor or taste, which would make it difficult to effectively blind study participants. This possibility seems unlikely because the Blinding Index was 0.65. Finally, because of low diarrhea prevalence in the study population, the sample size was insufficient to measure an effect. Our sample size calculation was based on 2003 DHS data from the Northern region of Ghana, which was the only relevant available data source from which diarrhea estimates could be made. However, DHS data are based on cross-sectional surveys using self-reported 2-week recall which is subject to over-reporting.37 In addition, diarrhea rates vary by season, by climatic conditions, by population, and by year. During this study, heavy rains maintained ground water supplies which are delivered through piped water networks; the expected seasonal use of surface water, which usually coincides with an increase in diarrheal disease rates, did not occur. This characteristic variability of diarrhea prevalence makes it difficult, regardless of the accuracy of diarrhea estimates, to generalize from the results of geographically circumscribed intervention trials.
Findings of this study indicate that NaDCC tablets are a promising method of water disinfection for routine daily household water treatment. In this and other field trials,18,22 high-risk populations in low educational and socioeconomic categories were able to effectively disinfect water with NaDCC tablets. In addition, high adherence to water treatment recommendations and positive attitudes to the tablets expressed by the study population implied that the tablets were acceptable. The evidence base already established for other point-of-use water chlorination technologies suggests that comparable findings would be expected for NaDCC tablets under the right study conditions and that a follow-up health impact study is warranted. Because of the ease of blinding investigators and research subjects to this intervention, a double-blinded, placebo-controlled trial of sufficient duration to fully examine the efficacy of NaDCC tablets would be an important contribution to the evidence base for point-of-use water treatment.
Acknowledgments
The authors, first and foremost, express their appreciation to the study participants, who warmly and graciously tolerated their frequent interruptions of their daily activities. They are indebted to Ayuba Abukari, Jennifer Apiung, Issah Baba, Chief M.S. Caesar, Luqman Mahama, Issahaku Mohammed, Alhassan Tahiru Seini, and Imoro Z. Tuu-naa for their work as field officers; Musin Salifu, Musah Abdul-Wahab, Sayibu Imoro Wunpini, and Bukari Mohammed Yakubu as water quality testers; Ibrahim Mohammed Ali and Abdallah Mashud for data entry. The authors thank Emmanuel Agyemang Ansaaku and Benjamin K. Moses for their laboratory work at the Ghana Water Company. The authors thank all the staff at New Energy for their logistical support with special thanks to Thomas Sayibu Imoro. They are grateful to Rochelle Rainey and Sharon Murray from the United States Agency for International Development and the West Africa Water Initiative for their assistance in finding local partners. They appreciate the technical expertise and support provided by Daniele Lantagne, PE. The authors thank James M. Hughes, M.D. for his insight and guidance.
Footnotes
Financial support: The United States Agency for International Development, the Bureau of Oceans and Environmental Science of the United States Department of State, Medentech Ltd, and the Chlorine Chemistry Council funded the study. Medentech, Ltd provided NaDCC and placebo tablets.
Authors' addresses: Seema Jain, Elizabeth Blanton, Ann Schmitz, Kathleen A. Wannemuehler, Robert M. Hoekstra, and Robert E. Quick, Enteric Diseases Epidemiology Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: bwc8@cdc.gov. Osman K. Sahanoon, NewEnergy, Tamale, Ghana.
References
- 1.World Health Organization . World Health Report 2005. Genev: World Health Organization; 2005. [Google Scholar]
- 2.Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhea: systematic review and meta-analysis. BMJ. 2007;334:782–791. doi: 10.1136/bmj.39118.489931.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold BF, Colford JM. Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg. 2007;76:354–364. [PubMed] [Google Scholar]
- 4.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM. Water, sanitation, and hygiene interventions to reduce diarrhea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 5.Sobsey MD. Accelerated Health Gains from Improved Water Supply. Geneva: World Health Organization; 2002. (Managing Water in the Home). [Google Scholar]
- 6.Mintz ED, Reiff FM, Tauxe RVM. Safe water treatment and storage in the home: a practical new strategy to prevent waterborne disease. JAMA. 1995;273:948–953. [PubMed] [Google Scholar]
- 7.Quick R, Venczel L, Mintz ED, Soleto L, Aparicio J, Gironaz M, Hutwagner L, Greene K, Bopp C, Maloney K, Chavez D, Sobsey MD, Tauxe RV. Diarrhea prevention in Bolivia through point-of-use water treatment and safe storage: a promising new strategy. Epidemiol Infect. 1999;122:83–90. doi: 10.1017/s0950268898001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deb BC, Sircar BK, Sengupta PG, De SP, Mondal SK, Gupta DN, Saha NC, Ghosh S, Mitra U, Pal SC. Studies on interventions to prevent eltor cholera transmission in urban slums. Bull World Health Organ. 1986;64:127–131. [PMC free article] [PubMed] [Google Scholar]
- 9.Quick RE, Kimura A, Thevos A, Tembo M, Shamputa I, Hutwagner L, Mintz E. Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am J Trop Med Hyg. 2002;66:584–589. doi: 10.4269/ajtmh.2002.66.584. [DOI] [PubMed] [Google Scholar]
- 10.Macy JT, Quick RE. Evaluation of a novel drinking water treatment and storage intervention in Nicaragua: letter to the editor. Rev Panam Salud Publica/Pan. Am J Public Health. 1998;3:135–136. doi: 10.1590/s1020-49891998000200017. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Safe Water System (SWS) 2005. Available at: http://www.cdc.gov/safewater/index.htm. Accessed July 1, 2006.
- 12.Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer W, Keswick B, Hoekstra RM. Combining drinking water treatment and hand washing for diarrhea prevention, a cluster randomised controlled trial. Trop Med Int Health. 2006;11:479–489. doi: 10.1111/j.1365-3156.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 13.Crump JA, Otieno PO, Slutsker L, Keswick BH, Rosen DH, Hoekstra RM, Vulule JM, Luby SP. Household based treatment of drinking water with flocculant-disinfectant for preventing diarrhea in areas with turbid source water in rural western Kenya: cluster randomised controlled trial. BMJ. 2005;331:478–483. doi: 10.1136/bmj.38512.618681.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luby SP, Agboatwalla M, Hoekstra RM, Rahbar MH, Billhimer W, Keswick BH. Delayed effectiveness of home-based interventions in reducing childhood diarrhea, Karachi, Pakistan. Am J Trop Med Hyg. 2004;71:420–427. [PubMed] [Google Scholar]
- 15.Semenza JC, Roberts L, Henderson A, Bogan J, Rubin CH. Water distribution system and diarrheal disease transmission: a case study in Uzbekistan. Am J Trop Med Hyg. 1998;59:941–946. doi: 10.4269/ajtmh.1998.59.941. [DOI] [PubMed] [Google Scholar]
- 16.Reller ME, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Olson CA, Baier KG, Keswick BH, Luby SP. A randomized controlled trial of household-based flocculant-disinfectant drinking water treatment for diarrhea prevention in rural Guatemala. Am J Trop Med Hyg. 2003;69:411–419. [PubMed] [Google Scholar]
- 17.Population Services International Water/Child Survival. 2007. Available at: http://www.psi.org/child-survival/safe-water.html. Accessed July 1, 2007.
- 18.Clasen T, Edmondson P. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at the household level. Int J Hyg Environ Health. 2006;209:173–181. doi: 10.1016/j.ijheh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.United States Environmental Protection Agency . Product Label for Occidental Chemical Corporation Sodium Dichloroisocyanurate. Washington, DC: United States Environmental Protection Agency; 2005. [Google Scholar]
- 20.World Health Organization . Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives: WHO Technical Report Series. Vol. 922. Geneva: World Health Organization; 2004. [Google Scholar]
- 21.Solsona F, Méndez J. Water Disinfection. Lima, Peru: Pan American Center for Sanitary Engineering and Environmental Sciences (Pan American Health Organization); 2003. [Google Scholar]
- 22.Clasen T, Saeed TF, Boisson S, Edmondson P, Shipin O. Household water treatment using sodium dichloroisocyanurate (NaDCC) tablets: a randomized, controlled trial to assess microbiological effectiveness in Bangladesh. Am J Trop Med Hyg. 2007;76:187–192. [PubMed] [Google Scholar]
- 23.deMagny GC, Cazelles B, Guégan JF. Cholera threat to humans in Ghana is influenced by both global and regional climatic variability. EcoHealth. 2006;3:223–231. [Google Scholar]
- 24.Idexx Co Colilert®. 2007. Available at: http://www.idexx.com/water/colilert/. Accessed July 1, 2007.
- 25.Ghana Statistical Service (GSS) Noguchi Memorial Institute for Medical Research (NMIMR), and ORC Macro; Calverton, MD: 2004. pp. 159–160. Ghana Demographic and Health Survey 2003. GSS, NMIMR, and ORC Macro. [Google Scholar]
- 26.James KE, Block DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi-centre clinical trial: disulfiram for alcohol cessation—a VA cooperative study. Stat Med. 1996;15:1421–1434. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1421::AID-SIM266>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Colford JM, Rees JR, Wade TJ, Khalakdina A, Hilton JF, Ergas IJ, Burns S, Benker A, Ma C, Bowen C, Mills DC, Vugia DJ, Juranek DD, Levy DA. Participant blinding and gastrointestinal illness in a randomized, controlled trial of an in-home drinking water intervention. Emerg Infect Dis. 2002;8:29–36. doi: 10.3201/eid0801.001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration Bacteriological Analytical Manual Appendix 2: Most Probable Number from Serial Dilutions. 2006. Available at: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm109656.htm. Accessed March 11, 2007.
- 29.Galal-Gorchev H. Chlorine in water disinfection. Pure Appl Chem. 1996;68::1731–1735. [Google Scholar]
- 30.Moe CL, Sobsey MD, Samsa GP, Mesolo V. Bacterial indicators of risk of diarrheal disease from drinking-water in the Philippines. Bull World Health Organ. 1991;69:305–317. [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts L, Chartier Y, Chartier O, Malenga G, Toole M, Rodka H. Keeping clean water clean in a Malawi refugee camp: a randomized intervention trial. Bull World Health Organ. 2001;79:280–287. [PMC free article] [PubMed] [Google Scholar]
- 32.Pinfold JV. Faecal contamination of water and fingertip-rinses as a method for evaluating the effect of low-cost water supply and sanitation activities on faeco-oral disease transmission. II: a hygiene intervention study in north-east Thailand. Epidemiol Infect. 1990;105:377–389. doi: 10.1017/s0950268800047968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchhoff LV, McClelland KE, Do Carmo Pinho M, Araujo JG, De Sousa MA, Guerrant RL. Feasibility and efficacy of in-home water chlorination in rural north-eastern Brazil. J Hyg (Lond) 1985;94:173–180. doi: 10.1017/s0022172400061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colford JM, Wade TJ, Sandhu SK, Wright CC, Lee S, Shaw S, Fox K, Burns S, Benker A, Brookhart MA, van der Laan M, Levy DA. A randomized, controlled trial of in-home drinking water intervention to reduce gastrointestinal illness. Am J Epidemiol. 2005;161:472–482. doi: 10.1093/aje/kwi067. [DOI] [PubMed] [Google Scholar]
- 36.Alam N, Henry FJ, Rahaman MM. Reporting errors in one-week diarrhoea recall surveys: experience from a prospective study in rural Bangladesh. Int J Epidemiol. 1989;18:697–700. doi: 10.1093/ije/18.3.697. [DOI] [PubMed] [Google Scholar]
- 37.Boerma JT, Black RE, Sommerfelt AE, Rutstein SO, Bicego GT. Accuracy and completeness of mothers' recall of diarrhoea occurrence in pre-school children in demographic and health surveys. Int J Epidemiol. 1991;20:1073–1080. doi: 10.1093/ije/20.4.1073. [DOI] [PubMed] [Google Scholar]


