Abstract
The United States Department of Defense Global Emerging Infections Surveillance and Response System (DoD-GEIS) conducted a review in 2008 of projects funded by DoD-GEIS at five partner overseas laboratories from 1999 through 2007. During this period, the annual overseas programming budget grew from US$1.038 million to US$21 million. The review describes the distribution of project priorities and geographic locations over the years, the types of outcomes the projects generated, and the frequency with which they involved collaboration with other public health agencies and organizations, including CDC and WHO. Areas for further program strengthening are identified.
Introduction
Since 1946, the United States Department of Defense (DoD) has co-operated 20 laboratories with host-country agencies to study infectious diseases of mutual interest.1 Five persist, based in Peru, Indonesia, Egypt, Thailand, and Kenya (Table 1). Since 1998, they have emphasized their role as regional public health laboratories through project support from the DoD Global Emerging Infections Surveillance and Response System (DoD-GEIS). DoD-GEIS was created in 1997 by the DoD as part of the US Government's response to a 1996 Presidential Decision Directive calling for increased effort to reduce the threat posed by emerging infectious diseases (EIDs) through “surveillance, training, research, and response.”2
Table 1.
DoD-GEIS Partner Overseas Laboratories
| Abbreviation | Title | Location |
|---|---|---|
| NMRCD | Naval Medical Research Center Detachment | Lima, Peru |
| NAMRU-2 | Naval Medical Research Unit–2 | Jakarta, Indonesia |
| NAMRU-3 | Naval Medical Research Unit–3 | Cairo, Egypt |
| USAMRU-K | U.S. Army Medical Research Unit–Kenya | Nairobi, Kenya |
| AFRIMS | Armed Forces Research Institute of the Medical Sciences | Bangkok, Thailand |
DoD-GEIS has made these overseas laboratories the backbone of its globally focused efforts. Projects supported at the laboratories have been collaborative efforts with host-country public health agencies and have focused on the surveillance of EIDs in local populations, the improvement of local EID surveillance infrastructure, and the professional development of host-country scientists and public health professionals.3
DoD-GEIS has undergone five external reviews: two by the Armed Forces Epidemiology Board (AFEB), two by the US Institute of Medicine (IOM), and one by the US Government Accountability Office (GAO).4–7 Reviews by the AFEB considered the entirety of domestic and overseas DoD-GEIS programs, rather than focusing on the five overseas laboratories as this review does. The most recent reviews—GAO 2007 and IOM 2007—focused on limited components of DoD-GEIS overseas programs: capacity-building and influenza surveillance, respectively.
This review includes DoD-GEIS-funded projects at five DoD overseas laboratories for the period 1999–2007. Projects were selected by DoD-GEIS for funding on the basis of their scientific merit, their consistency with DoD-GEIS goals and disease/agent focus areas, and their military relevance or interest. The review describes the growth of the DoD-GEIS program at the overseas laboratories, analyzes the focus of the projects during this period with reference to the DoD-GEIS goals of surveillance, response, capacity-building, and cooperation, and discusses the importance of impact evaluation.8,9
Materials and Methods
The annual reports of projects funded by DoD-GEIS and executed at the five overseas laboratories between 1999 and 2007 were reviewed. All projects were funded for one year. Projects were categorized by primary objective and, if applicable, their disease or agent surveillance focus. DoD-GEIS has emphasized the surveillance of respiratory illness, gastrointestinal illness, febrile and vector-borne illness, antimicrobial resistance, and sexually transmitted infections. Project locations were recorded at the country level. Projects were categorized by reported outcomes: enhancement of physical host-country laboratory or surveillance infrastructure, training of host-country partners, response to local or regional disease outbreaks, direct epidemiologic support of deployed US troops, and publications in peer-reviewed journals. Last, projects were categorized by their reported collaborations with organizations identified as priorities for collaborations in DoD-GEIS strategic plans.8,9
Results
Between 1999 and 2007, DoD-GEIS supported 466 1-year projects with the annual number of projects trebling compared to 1999 (Figure 1). When broken down by primary objective, 343 (74%) were categorized as surveillance projects (Table 2). An additional 101 projects (22%) were almost evenly split between infrastructure enhancement (32, 7%), training (33, 7%), and outbreak response (36, 8%). Table 2 additionally shows average figures for 1999–2000 and for 2006–2007 in an attempt to compare the program at the commencement and conclusion of the period in question.
Figure 1.
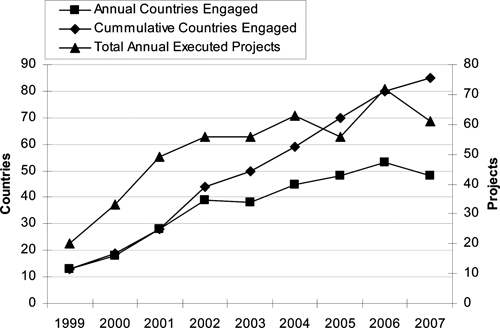
Annual and cumulative countries engaged and annual total projects, 1999–2007.
Table 2.
Results, 1999–2007: projects by primary objective, disease or agent, outcome, organization collaboration, as well as publication in peer-reviewed journals
| Average 1999–2000 | Average 2006–2007 | Total | Percent of all | |
|---|---|---|---|---|
| Projects by primary objective (N = 466) | ||||
| Surveillance | 24 | 48.5 | 343 | 74% |
| Surveillance projects by disease/agent (N = 343)* | ||||
| Respiratory illness | 7.5 | 22 | 115 | 34% |
| Gastrointestinal illness | 9 | 19.5 | 105 | 31% |
| Febrile illness | 14 | 24 | 217 | 63% |
| Antimicrobial resistance | 11 | 15.5 | 122 | 36% |
| Sexually transmitted infections | 1 | 1.5 | 17 | 5% |
| Surveillance projects by select outcomes (N = 343)* | ||||
| Training of foreign collaborators | 13 | 28 | 193 | 56% |
| Physical infrastructure enhancement | 10.5 | 19 | 150 | 44% |
| Capacity building† | 14 | 30.5 | 214 | 62% |
| Response to ≥ 1 outbreak | 2 | 7 | 58 | 17% |
| Direct support of deployed US troops | 1.5 | 2 | 20 | 6% |
| Outbreak response | 1 | 5.5 | 36 | 8% |
| Training | 0 | 2.5 | 33 | 7% |
| Infrastructure enhancement | 0 | 6 | 32 | 7% |
| Other | 0 | 4 | 22 | 5% |
| Publications in peer-reviewed journals | 7.5 | 51.5 | 207 | n/a |
| All projects by organizational collaboration (N = 466)* | ||||
| WHO | 3 | 12 | 120 | 26% |
| DoD and military health system organizations‡ | 9.5 | 9.5 | 116 | 25% |
| US CDC | 6.5 | 8.5 | 95 | 20% |
| USAID | 2.5 | 0 | 25 | 5% |
| Private and educational institutions§ | 5.5 | 5 | 59 | 13% |
Not mutually exclusive categories.
Either physical infrastructure enhancement, training of foreign collaborators, or both.
Other DoD-GEIS military partner organizations or other DoD organizations, not including DoD-GEIS Central Hub.
Except private and educational partners within the host country.
When surveillance projects (N = 343) were characterized by their disease/agent focus or foci, 217 (63%) reported addressing febrile illnesses. Antimicrobial resistance was addressed by 122 (36%) projects, respiratory illnesses by 115 (34%) projects, gastrointestinal illnesses by 105 (31%) projects, and sexually transmitted infections by 17 (5%) projects. Cumulatively, from 1999 to 2007, DoD-GEIS-supported projects conducted work in 85 countries (Figure 1). The annual number of countries engaged grew from 13 in 1999 to > 45 between 2004 and 2007, with a peak of 53 in 2006.
Of surveillance projects (N = 343), 193 (56%) reported conducting training and 150 (44%) reported enhancing physical infrastructure (Table 2). A composite value titled “capacity-building,” which requires a project to have reported at least one of the preceding outcomes included 214 (62%) surveillance projects. Additionally, 50 (17%) projects reported responding to at least one disease outbreak and 20 (6%) reported providing direct epidemiologic or diagnostic support for deployed US troops. Partner laboratories reported publishing 207 articles relating to GEIS supported work in peer-reviewed journals during the years under review. A large number of projects included organizational collaboration with the WHO (120, 26%) other DoD organizations (116, 25%), and the US CDC (95, 20%). Projects also reported collaboration with private and educational institutions 59 times (13%) and with USAID 25 times (5%).
Discussion
Between 1999 and 2007, DoD-GEIS overseas surveillance expanded significantly, measured by annual projects, countries engaged, funds awarded, and peer-reviewed publications. The majority of surveillance projects addressed one or more DoD-GEIS priority diseases or agents. Additionally, the majority of surveillance projects made investments toward local sustainability by training host-country scientists and investing in laboratory and public health infrastructure. These investments were considered critical to maintaining collaborative relationships with host-country scientists and officials. Furthermore, DoD-GEIS funded 65 projects with infrastructure development or training as their primary objective. These contributions were independently appraised by a GAO report that compared DoD (GEIS) overseas surveillance capacity-building efforts to those of CDC and USAID.4
We found a favorable level of collaboration with non-DoD agencies, both within the US Government and outside of it. These collaborations suggest that GEIS funds have been leveraged with funding from other governmental organizations, creating a stronger whole. DoD has assigned liaisons to, hosted scientists from, and engaged in routine coordination with CDC and WHO, whereas collaborations with USAID and private and educational institutions have taken place on a more limited basis.
This review additionally identified a few areas that could be strengthened. First, only 5% of projects reported surveillance for sexually transmitted infections. This fifth disease or agent category was added as a DoD-GEIS priority after a 2004 Armed Forces Epidemiology Board Review without additional resources. Second, project reports document a small number of projects (25, 5%) involving collaborations with USAID. DoD-GEIS is intent on increasing collaboration, where appropriate. Additionally, this figure does not include coordination—not measured directly in this review—which has taken place frequently either in-country or through DoD-GEIS headquarters. Third, as a military organization, DoD-GEIS might be expected by some to involve > 6% of its projects in direct support of US troops. This number, however, comes only from DoD-GEIS overseas host-country collaborative surveillance efforts. DoD-GEIS surveillance efforts within the military health system—not considered here—contribute routinely and substantially to troop health. In addition, direct-support projects often took the form of providing epidemiologic support to major, multi-country military training exercises in Asia and North Africa.10–14
The presidential directive adding EID surveillance and response to DoD's responsibilities named the overseas laboratories because of their strategic positioning and considerable expertise. During the 1980s and 1990s, the Military Infectious Diseases Research Program prioritized funding toward the development of specific drugs and vaccines at the overseas laboratories. Through DoD-GEIS, National and DoD leaders sought to broaden the mission of the overseas laboratories, using stable, flexible funding to develop public health laboratory capacity for detecting and responding to EIDs.15
This emphasis on laboratory capacity as a first step toward disease detection followed from 1992 IOM recommendations that urged broad-based laboratory capacity to detect novel pathogens.16,17 It also followed the logic of what former Secretary of Defense William Perry termed preventive diplomacy, the practice of addressing threats—emerging diseases in this instance—before they arrive in the United States.18 Subsequently, in 2005, the DoD issued a directive regarding Support for Stability, Security, Transition, and Reconstruction (SSTR) Operations that elevated stability operations—including their humanitarian and public health components—to the same level of strategic importance as combat.19,20 Both the surveillance of diseases and the trust engendered by capacity building can be viewed as furthering these goals.
The projects included in this review represent collaborative efforts with many public health professionals around the globe, through many ministries of health and defense, private institutions, the US CDC, and the WHO. The development and completion of these projects reflect successful partnerships. However, our consideration of the impact of these projects was limited to the peer-reviewed publications they generated, an important but narrow measure. Evaluating the lasting impact of the surveillance and capacity-building efforts on disease prevention and control was beyond the scope of this review.
Nevertheless, impact evaluation is critical to the maintenance and relevance of any surveillance system. In an age of budgetary scrutiny and many competing, worthy priorities, we are challenged to show the value of surveillance to a broader audience. Surveillance systems are typically measured by their execution of stated goals, rather than by their impact—primarily because such measurement is difficult and imprecise. Bearing in mind the ultimate goal of surveillance information leading to public health action, designing surveillance systems from the onset in a manner most conducive to impact measurement is advisable. A current priority of DoD-GEIS is to explore the most effective means of incorporating such measurement into our routine project reviews.
We have begun this process and it is worth mentioning, in the context of this narrow review, some of the system-wide accomplishments that have been described in the peer-reviewed literature. DoD-GEIS has consolidated and expanded DoD global influenza surveillance efforts—the program coordinates > 300 sites in 72 countries and contributes annual, geographically unique viral isolates to the WHO surveillance network that have been selected as seed viruses for vaccines.21 DoD-GEIS scientists in Asia, East Africa, and South America have documented the continued spread of markers suggesting the potential for malaria drug resistance emergence—notably recent artesunate-class drug resistance—while working to standardize laboratory methodology and microscopy.22–30 A collaboration between the National Aeronautics and Space Administration, USAMRU-K, and DoD-GEIS headquarters has developed an effective, satellite-based early warning tool for rift valley fever in East Africa and is now working to expand its applicability to other climate-dependent epidemic diseases.31–36 The network has also overseen the development and refinement of a series of systems designed to bring real-time disease surveillance to remote or low-resource settings, including Cambodia, Laos, Thailand, the Philippines, and Andean Ridge countries of South America.37–41 GEIS partners at NAMRU-3 and WRAIR have additionally conducted revealing studies on the burden of diarrheal diseases among US and coalition troops in the Eurasian theater, recommending more aggressive empiric treatment.11–14,42–51 Last, the DoD-GEIS network has supported the training of thousands of host-country scientists, epidemiologists, physicians, and laboratorians while simultaneously investing in physical laboratory capacity to extend the return on the local training.52,53
Looking forward, DoD-GEIS expects the five DoD overseas laboratories discussed herein to continue in their current roles, conducting infectious disease surveillance of value to their various stakeholders and serving as health ambassadors around the globe.
Acknowledgments
Jay Mansfield provided critical assistance with the data input and analysis, and Jennifer Bondarenko, Dr. Victor MacIntosh, and Dr. Ralph Loren Erickson also provided key assistance and support.
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the Department of the Army or Department of Defense.
Footnotes
Authors' addresses: J. Jeremy Sueker, Joel C. Gaydos, and Kevin L. Russell, Division of GEIS Operations, Armed Forces Health Surveillance Center, 2900 Linden Lane, Silver Spring, MD 20910. Jean-Paul Chretien, Division of Preventive Medicine, Walter Reed Army Institute of Research, 503 Robert Grant Ave., Silver Spring, MD 20910; and Division of Health Sciences Informatics, Johns Hopkins University School of Medicine, 2024 East Monument St, Suite 1–200, Baltimore, MD 21205.
References
- 1.Gambel JM, Richard G, Hibbs J. U.S. Military Overseas Medical Research Laboratories. Mil Med. 1996;161:638–645. [PubMed] [Google Scholar]
- 2.National Science and Technology Council, Executive Office of the President . Presidential Decision Directive NSTC-7: Emerging Infections. Washington, DC: Government Printing Office; 1996. [Google Scholar]
- 3.Chretien JP, Gaydos JC, Malone JL, Blazes DL. Global network could avert pandemics. Nature. 2006;440:25–26. doi: 10.1038/440025a. [DOI] [PubMed] [Google Scholar]
- 4.Government Accountability Office . U.S. Agencies Support Programs to Build Overseas Capacity for Infectious Disease Surveillance. Washington, DC: Government Printing Office; 2007. [Google Scholar]
- 5.Institute of Medicine . Perspectives on the Department of Defense Global Emerging Infections Surveillance and Response System: A Program Review. Washington, DC: Institute of Medicine; 2001. [PubMed] [Google Scholar]
- 6.Institute of Medicine . Review of the DoD-GEIS Influenza Programs: Strengthening Global Surveillance and Response. Washington, DC: Institute of Medicine; 2007. [Google Scholar]
- 7.Armed Forces Epidemiology Board . Memorandum: Review of Department of Defense Global Emerging Infections Surveillance and Response System (DoD-GEIS) AFEB 2004-5. 2005. [Google Scholar]
- 8.DoD-GEIS . Addressing Emerging Infectious Disease Threats: A Strategic Plan for the Department of Defense. Washington, DC: DoD-GEIS; 1998. [Google Scholar]
- 9.DoD-GEIS . DoD-GEIS Emerging Infections Surveillance and Response System Strategic Plan. Washington, DC: DoD-GEIS; 2005. [Google Scholar]
- 10.Fuller J, Hanley K, Schultz R, Lewis M, Freed NE, Ellis M, Ngauy V, Stoebner R, Ryan M, Russell K. Surveillance for febrile respiratory infections during Cobra Gold 2003. Mil Med. 2006;171:357–359. doi: 10.7205/MILMED.171.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Mohamady H, Francis W, Shaheen HI, Rozmajzl P, Rockabrand DM, Karim AK, Hall ER, Sanders JW. Detection of fecal and serum antibodies against enterotoxigenic Escherichia coli toxins and colonization factors in deployed U.S. military personnel during Operation Bright Star 2001–Egypt. Egypt J Immunol. 2006;13:189–198. [PubMed] [Google Scholar]
- 12.Riddle MS, Halvorson HA, Shiau D, Althoff J, Monteville MR, Shaheen H, Horvath EP, Armstrong AW. Acute gastrointestinal infection, respiratory illness, and noncombat injury among US military personnel during Operation Bright Star 2005, in Northern Egypt. J Travel Med. 2007;14:392–401. doi: 10.1111/j.1708-8305.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- 13.Rockabrand DM, Shaheen HI, Khalil SB, Peruski LF, Jr, Rozmajzl PJ, Savarino SJ, Monteville MR, Frenck RW, Svennerholm AM, Putnam SD, Sanders JW. Enterotoxigenic Escherichia coli colonization factor types collected from 1997 to 2001 in US military personnel during operation Bright Star in northern Egypt. Diagn Microbiol Infect Dis. 2006;55:9–12. doi: 10.1016/j.diagmicrobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Sanders JW, Putnam SD, Gould P, Kolisnyk J, Merced N, Barthel V, Rozmajzl PJ, Shaheen H, Fouad S, Frenck RW. Diarrheal illness among deployed U.S. military personnel during Operation Bright Star 2001–Egypt. Diagn Microbiol Infect Dis. 2005;52:85–90. doi: 10.1016/j.diagmicrobio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Chretien JP, Blazes DL, Gaydos JC, Bedno SA, Coldren RL, Culpepper RC, Fyrauff DJ, Earhart KC, Mansour MM, Glass JS, Lewis MD, Smoak BL, Malone JL. Experience of a global laboratory network in responding to infectious disease epidemics. Lancet Infect Dis. 2006;6:538–540. doi: 10.1016/S1473-3099(06)70556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emerging Infections: Microbial Threats to Health in the United States. Washington, DC: Institute of Medicine; 1992. [PubMed] [Google Scholar]
- 17.Microbial Threats to Health: Emergence, Detection and Response. Washington, DC: Institute of Medicine; 2003. [PubMed] [Google Scholar]
- 18.Perry WJ. Defense in an age of hope. Foreign Aff. 1996;75:64–79. [Google Scholar]
- 19.US Joint Forces Command . Military Support to Stabilization, Security, Transition, and Reconstruction Operations Joint Operating Concept, version 2.0. Washington, DC: US Department of Defense; 2006. [Google Scholar]
- 20.US Undersecretary of Defense for Policy . DoDD 3000.05: Military Support for Stability, Security, Transition, and Reconstruction (SSTR) Operations. Washington, DC: US Department of Defense; 2005. [Google Scholar]
- 21.Owens AB, Canas LC, Russell KL, Neville J, Pavlin JA, MacIntosh VH, Gray GC, Gaydos JC. Department of defense global laboratory-based influenza surveillance: 1998–2005. Am J Prev Med. 2009;37:235–241. doi: 10.1016/j.amepre.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Miller RS, Wongsrichanalai C, Buathong N, McDaniel P, Walsh DS, Knirsch C, Ohrt C. Effective treatment of uncomplicated Plasmodium falciparum malaria with azithromycin-quinine combinations: a randomized, dose-ranging study. Am J Trop Med Hyg. 2006;74:401–406. [PubMed] [Google Scholar]
- 23.Noedl H, Krudsood S, Leowattana W, Tangpukdee N, Thanachartwet W, Looareesuwan S, Miller RS, Fukuda M, Jongsakul K, Yingyuen K, Sriwichai S, Ohrt C, Knirsch C. In vitro antimalarial activity of azithromycin, artesunate, and quinine in combination and correlation with clinical outcome. Antimicrob Agents Chemother. 2007;51:651–656. doi: 10.1128/AAC.01023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohrt C, Obare P, Nanakorn A, Adhiambo C, Awuondo K, O'Meara WP, Remich S, Martin K, Cook E, Chretien JP, Lucas C, Osoga J, McEvoy P, Owaga ML, Odera JS, Ogutu B. Establishing a malaria diagnostics centre of excellence in Kisumu, Kenya. Malar J. 2007;6:79. doi: 10.1186/1475-2875-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J. 2009;8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor WR, Richie TL, Fryauff DJ, Ohrt C, Picarima H, Tang D, Murphy GS, Widjaja H, Braitman D, Tjitra E, Ganjar A, Jones TR, Basri H, Berman J. Tolerability of azithromycin as malaria prophylaxis in adults in northeast papua, indonesia. Antimicrob Agents Chemother. 2003;47:2199–2203. doi: 10.1128/AAC.47.7.2199-2203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wongsrichanalai C, Meshnick SR. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg Infect Dis. 2008;14:716–719. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 29.Wongsrichanalai C, Sirichaisinthop J, Karwacki JJ, Congpuong K, Miller RS, Pang L, Thimasarn K. Drug resistant malaria on the Thai-Myanmar and Thai-Cambodian borders. Southeast Asian J Trop Med Public Health. 2001;32:41–49. [PubMed] [Google Scholar]
- 30.Wongsrichanalai C, Wimonwattrawatee T, Sookto P, Laoboonchai A, Heppner DG, Kyle DE, Wernsdorfer WH. In vitro sensitivity of Plasmodium falciparum to artesunate in Thailand. Bull World Health Organ. 1999;77:392–398. [PMC free article] [PubMed] [Google Scholar]
- 31.Anyamba A, Chretien JP, Small J, Tucker CJ, Formenty PB, Richardson JH, Britch SC, Schnabel DC, Erickson RL, Linthicum KJ. Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci USA. 2009;106:955–959. doi: 10.1073/pnas.0806490106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anyamba A, Chretien JP, Small J, Tucker CJ, Linthicum KJ. Developing global climate anomalies suggest potential disease risks for 2006–2007. Int J Health Geogr. 2006;5:60. doi: 10.1186/1476-072X-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Britch SC, Linthicum KJ, Anyamba A, Tucker CJ, Pak EW, Maloney FA, Jr, Cobb K, Stanwix E, Humphries J, Spring A, Pagac B, Miller M. Satellite vegetation index data as a tool to forecast population dynamics of medically important mosquitoes at military installations in the continental United States. Mil Med. 2008;173:677–683. doi: 10.7205/milmed.173.7.677. [DOI] [PubMed] [Google Scholar]
- 34.Epstein PR. Climate and health. Science. 1999;285:347–348. doi: 10.1126/science.285.5426.347. [DOI] [PubMed] [Google Scholar]
- 35.Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 36.Martin V, De Simone L, Lubroth J, Ceccato P, Chevalier V. Perspectives on using remotely-sensed imagery in predictive veterinary epidemiology and global early warning systems. Geospatial Health. 2007;2:3–14. doi: 10.4081/gh.2007.250. [DOI] [PubMed] [Google Scholar]
- 37.Huaman MA, Araujo-Castillo RV, Soto G, Neyra JM, Quispe JA, Fernandez MF, Mundaca CC, Blazes DL. Impact of two interventions on timeliness and data quality of an electronic disease surveillance system in a resource limited setting (Peru): a prospective evaluation. BMC Med Inform Decis Mak. 2009;9:16. doi: 10.1186/1472-6947-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soto G, Araujo-Castillo RV, Neyra J, Fernandez M, Leturia C, Mundaca CC, Blazes DL. Challenges in the implementation of an electronic surveillance system in a resource-limited setting: Alerta, in Peru. BMC Proc. 2008;2((Suppl 3)):S4. doi: 10.1186/1753-6561-2-s3-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres-Slimming PA, Mundaca CC, Moran M, Quispe J, Colina O, Bacon DJ, Lescano AG, Gilman RH, Blazes DL. Outbreak of cyclosporiasis at a naval base in Lima, Peru. Am J Trop Med Hyg. 2006;75:546–548. [PubMed] [Google Scholar]
- 40.Siswoyo H, Permana M, Larasati RP, Farid J, Suryadi A, Sedyaningsih ER. EWORS: using a syndromic-based surveillance tool for disease outbreak detection in Indonesia. BMC Proc. 2008;2((Suppl 3)):S3. doi: 10.1186/1753-6561-2-s3-s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chretien JP, Burkom HS, Sedyaningsih ER, Larasati RP, Lescano AG, Mundaca CC, Blazes DL, Munayco CV, Coberly JS, Ashar RJ, Lewis SH. Syndromic surveillance: adapting innovations to developing settings. PLoS Med. 2008;5:e72. doi: 10.1371/journal.pmed.0050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteville MR, Riddle MS, Baht U, Putnam SD, Frenck RW, Brooks K, Moustafa M, Bland J, Sanders JW. Incidence, etiology, and impact of diarrhea among deployed US military personnel in support of Operation Iraqi Freedom and Operation Enduring Freedom. Am J Trop Med Hyg. 2006;75:762–767. [PubMed] [Google Scholar]
- 43.Porter CK, El Mohammady H, Baqar S, Rockabrand DM, Putnam SD, Tribble DR, Riddle MS, Frenck RW, Rozmajzl P, Kilbane E, Fox A, Ruck R, Lim M, Johnston YJ, Murphy E, Sanders JW. Case series study of traveler's diarrhea in U.S. military personnel at Incirlik Air Base, Turkey. Clin Vaccine Immunol. 2008;15:1884–1887. doi: 10.1128/CVI.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Putnam SD, Sanders JW, Frenck RW, Monteville M, Riddle MS, Rockabrand DM, Sharp TW, Frankart C, Tribble DR. Self-reported description of diarrhea among military populations in operations Iraqi Freedom and Enduring Freedom. J Travel Med. 2006;13:92–99. doi: 10.1111/j.1708-8305.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 45.Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891–900. [PubMed] [Google Scholar]
- 46.Riddle MS, Tribble DR, Jobanputra NK, Jones JJ, Putnam SD, Frenck RW, Sanders JW. Knowledge, attitudes, and practices regarding epidemiology and management of travelers' diarrhea: a survey of front-line providers in Iraq and Afghanistan. Mil Med. 2005;170:492–495. doi: 10.7205/milmed.170.6.492. [DOI] [PubMed] [Google Scholar]
- 47.Riddle MS, Tribble DR, Putnam SD, Mostafa M, Brown TR, Letizia A, Armstrong AW, Sanders JW. Past trends and current status of self-reported incidence and impact of disease and nonbattle injury in military operations in Southwest Asia and the Middle East. Am J Public Health. 2008;98:2199–2206. doi: 10.2105/AJPH.2007.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders JW, Frenck RW, Putnam SD, Riddle MS, Johnston JR, Ulukan S, Rockabrand DM, Monteville MR, Tribble DR. Azithromycin and loperamide are comparable to levofloxacin and loperamide for the treatment of traveler's diarrhea in United States military personnel in Turkey. Clin Infect Dis. 2007;45:294–301. doi: 10.1086/519264. [DOI] [PubMed] [Google Scholar]
- 49.Sanders JW, Putnam SD, Frankart C, Frenck RW, Monteville MR, Riddle MS, Rockabrand DM, Sharp TW, Tribble DR. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan) Am J Trop Med Hyg. 2005;73:713–719. [PubMed] [Google Scholar]
- 50.Sanders JW, Putnam SD, Riddle MS, Tribble DR. Military importance of diarrhea: lessons from the Middle East. Curr Opin Gastroenterol. 2005;21:9–14. [PubMed] [Google Scholar]
- 51.Sanders JW, Putnam SD, Riddle MS, Tribble DR, Jobanputra NK, Jones JJ, Scott DA, Frenck RW. The epidemiology of self-reported diarrhea in operations Iraqi freedom and enduring freedom. Diagn Microbiol Infect Dis. 2004;50:89–93. doi: 10.1016/j.diagmicrobio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Lescano AG, Salmon-Mulanovich G, Pedroni E, Blazes DL. Epidemiology. Outbreak investigation and response training. Science. 2007;318:574–575. doi: 10.1126/science.1146837. [DOI] [PubMed] [Google Scholar]
- 53.Lescano AR, Blazes DL, Montano SM, Moran Z, Naquira C, Ramirez E, Lie R, Martin GJ, Lescano AG, Zunt JR. Research ethics training in Peru: a case study. PLoS One. 2008;3:e3274. doi: 10.1371/journal.pone.0003274. [DOI] [PMC free article] [PubMed] [Google Scholar]


