Abstract
A cross-sectional study was carried out in Água Comprida, MG, Brazil, a region previously endemic to Chagas disease whose vectorial transmission was interrupted around 20 year ago. A total of 998 individuals were examined for anti-Trypanosoma cruzi antibodies. Seropositivity was observed in 255 subjects (25.5%), and 743 subjects were negative. Forty-one families with 5–80 individuals with similar environmental conditions were selected for familial analysis. In 15 families, seropositivity to T. cruzi was observed in > 50% of individuals. The segregation analysis confirmed family aggregation for the seropositivity to the T. cruzi. Heart commitment was the major clinical form observed, and in six families, > 50% of the individuals display cardiopathy that may be attributed to T. cruzi infection. Our results support the hypothesis that there is a family aggregation for the seropositivity but without the effect of one major gene.
Introduction
Genetic studies on susceptibility to infectious diseases and their pathological consequences in human populations are relatively recent.1–3 Several methods can be used to identify the genes that influence the host's susceptibility to pathogenic agents. Epidemiological studies suggest a familial aggregation with features associated with resistance or susceptibility that, with modern techniques of analysis, made clear the presence of genetic control by the main gene.4
Recent advances in statistics and molecular genetics allowed for in-depth investigation of both genetic and environmental factors that influence susceptibility to several infectious diseases.2,5,6
In the worldwide context, parasitic diseases represent an enormous onus, and nowadays, many studies are being carried out for the identification of genetic factors that may imply susceptibility for a variety of diseases.
Chagas disease, caused by the hemoflagellate protozoan Trypanosoma cruzi, is prevalent in Latin American populations with about 9–13 million individuals chronically infected. Forty million people still live in areas where risks of infection transmission exist.7–9 Research carried out with experimental models and humans has shown a variation in the susceptibility to infection by T. cruzi, and possibly, genetic factors may play an important role in the susceptibility to the infection and in the development of lesions in the chronic phase.10–17
This study aims to verify the current prevalence of seropositivity, clinical forms, and familial aggregation of these characteristics in a population living in an endemic area where the vectorial transmission was interrupted.
Materials and Methods
Study area.
The study was performed in the Municipality of Água Comprida, (20°03′23″ S, 48°06′31″ W at 540 m above sea level), situated in the Vale do Rio Grande, South of Triângulo Mineiro, Minas Gerais State, Brazil. The city was an endemic area for Chagas disease and was included in the first National Campaign against T. cruzi that began in 1975. Epidemiological and entomological data showed evidence of the interruption of the vectorial transmission of the parasite to humans in 1999, and an international commission certified Brazil as free of transmission in 2005.18,19
Studied population.
All individuals > 25 year old living in the rural and urban zones of the municipality were invited to participate in this study.
For the familial analysis the families were recorded as well as relationship, name, date of birth, gender, and time of exposure to the domiciliary triatomines of all the individuals to ensure correct identification. This information was double checked with another member of the family to avoid inconsistencies.
Ethical considerations.
The protocol of this study was approved by the Ethical Committee on Research of the Federal University of the Triângulo Mineiro and local health authorities. Informed consent was obtained from all who were involved in the study.
Serological test.
The detection of anti-T. cruzi antibodies was performed through passive haemaglutination techniques (Salck Laboratory, São Paulo, Brazil), enzyme-linked immunosorbent assay (ABBOTT, Brazil), and indirect immunofluorescence using FITC (Fluorescein isothiocyanate) conjugated to rabbit anti-human IgG (Sigma). Assays were done in accordance with orientations of each manufacturer, and the results were qualitatively expressed.
The individual who presented at least two positive reactions was considered positive.
Characterization of clinical forms.
Clinical examination, radiography of the thorax contrasted with that of the esophagus and colon, and conventional 12-branch electrocardiogram were performed in all individuals. The individual was considered an indeterminate form carrier when presenting only positive serological reaction along with an absence of signs and symptoms and normal conventional electrocardiogram and radiography of the thorax, esophagus, and colon.20 Cardiopathy was characterized by at least one of the following electrocardiographic alterations: atrioventricular blocks, intraventricular blocks (right bundle branch block and block of the anterosuperior division of the left branch), sinus bradycardia (with pre-mature ventricular contractions or primary and diffuse alterations of ventricular repolarisation), and pre-mature ventricular contractions (five or more per minute).21 We considered the individual who presented with alteration of the esophagus and/or colon on barium-contrast radiological examination as digestive clinical form.22,23 When both cardiac and digestive alterations existed, the clinical form was considered mixed.21
The characterization of clinical forms was realized in 224 subjects from the 41 families selected.
Statistical analysis.
The Mann-Whitney non-parametric test for comparing clinical form, age, and gender of all the individuals was used. The χ2 test was also used for comparing gender, serology, age, and clinical form of the positive individuals; P < 0.05 was considered significant.
Segregation analysis.
Segregation analysis was performed using the computer program REGRESS in a model developed by Bonney.24 All tests were performed using a type I error of 0.05. The major gene is not the only gene involved in the expression of the phenotype. A binary clinical phenotype (affected/unaffected by disease) specifies a regression relationship between each individual phenotype and explanatory variables. The dependence on phenotypes of preceding relatives is parameterized in terms of familial correlations: in the present study, father-offspring (FO) and sibling-sibling (SS) correlations. The effect of a major gene is defined by q (the frequency of allele A that is assumed to pre-dispose to infection), αAA, αAa, and αaa (the genotypic means of individuals with genotypes AA, Aa, and aa, respectively); the regression coefficient is associated with the time of exposure.
Results
The study encompassed 998 individuals distributed in 114 families. Of these, 468 (46.9%) were male, and the mean age was 41.6 year (SD ± 42.4 year). The mean age was 38.5 year (SD ± 20.2 year) for females. No significant difference was observed on mean age and gender (P = 0.1344). The research for anti-T. cruzi antibodies in these individuals showed that 255 (25.6%) were serologically positive, 114 of which were males (44.7%); 743 (74.5%) individuals presented with negative serology, 354 of which were males (47.6%). No significant difference was observed with seropositivity and gender. Forty-one families with at least five individuals were selected for familiar analysis, which totaled 526 individuals: 224 (42.6%) with positive serology results, 235 (44.7%) with negative serology results, and 67 (12.7%) without serology results. In analyzing the familial serological positivity, it was observed that, in 15 families, > 50% of the individuals presented positive serology for anti-T. cruzi antibodies (Table 1), which showed aggregation in some families. Among the 224 seropositive individuals of the selected families, 151 were submitted to clinical and laboratorial examination and than grouped on clinical forms of chronic Chagas disease: 58 (38.4%) presented the indeterminate form, 72 (47.7%) presented the cardiac form, 10 (6.6%) presented the digestive form, and 11 (7.3%) presented the mixed form. The subjects with cardiac clinical form were older than those individuals with indeterminate clinical form (62.7 year [SD ± 11.7 year] and 54.9 year [SD ± 13.2 year], respectively; P = 0.002). It was not possible to examine for diagnosis of clinical form in 73 individuals. Of the 41 families analyzed, 12 did not present any individual with the cardiac form (Figure 1). However, in six families, the cardiac form was observed in > 50% of individuals (Table 2;Figure 2), and in two families, 100% of the individuals presented the indeterminate form.
Table 1.
Distribution of the families according to the percentage of individuals serologically positive for Chagas disease in Agua Comprida City, Minas Gerais State, Brazil
| Positivity | Number of families | Percentage |
|---|---|---|
| 1–25% | 5 | 11.1 |
| 26–50% | 21 | 46.7 |
| 51–75% | 11 | 24.4 |
| ≥ 76% | 4 | 8.9 |
| Total | 41 | 100.0 |
Figure 1.
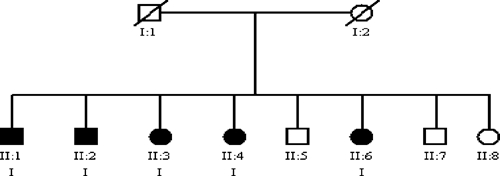
Pedigree showing a family with 100% of the individuals with the indeterminate form of Chagas disease. (Circles, females; squares, males; filled symbols, individuals with positive serology to Chagas disease; symbols with trace, dead individuals; I, indeterminate form.)
Table 2.
Distribution of the families according to the percentage of individuals with chagasic cardiopathy in Agua Comprida City, Minas Gerais State, Brazil
| Cardiac form | Number of families | Number of individuals | Percentage of total families |
|---|---|---|---|
| Absent | 12 | 40 | 29.3 |
| 1–25% | 6 | 41 | 14.6 |
| 26–50% | 17 | 116 | 41.5 |
| 51–75% | 2 | 11 | 4.9 |
| ≥ 75% | 4 | 16 | 9.7 |
| Total | 41 | 224 | 100.0 |
Figure 2.
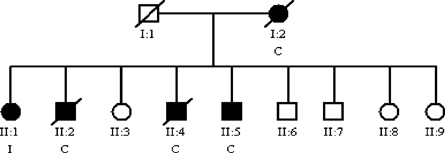
Pedigree showing a family with > 50% of the individuals with the cardiac form of Chagas disease. (Circles, females; squares, males; filled symbols, individuals with positive serology to Chagas disease; symbols with trace, dead individuals; I, indeterminate form.)
The evidence of a possible family aggregation in relation to the seropositivity was confirmed by segregation analysis. The results in Table 3 show strong evidence for familial aggregation of serological positivity to T. cruzi (χ2 = 22.8; gl = 4; P < 0.001), because the sporadic model without familial correlation was rejected. Concerning clinical forms, there was a tendency for aggregation in certain families in cardiac and indeterminate forms. The small number of cases of digestive and mixed forms did not show the evidence of a possible familial aggregation.
Table 3.
Segregation analysis in 41 families with individual serological positivity
| Model and hypothesis | Q* | αaa† | αAa | αAA | γPO1‡ | γSS1 | γPO2 | γSS2 | βdur§ | τAAA¶ | τAAa | τAaa | −2l nL + C** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I.Sporadic | [0] | −6.22 | [=αaa] | [=αaa] | [0] | [0] | [0] | [0] | 1.77 | – | – | – | 23.1 |
| II. Familial dependence | |||||||||||||
| a. Father-offspring and sib-sib | [0] | −4.49 | [=αaa] | [=αaa] | −0.95 | −0.34 | −0.03 | 0.19 | 1.34 | – | – | – | 0.3 |
| III. Mendelian major gene | |||||||||||||
| a. Codominant with residual familiar dependence | 0.62 | −6.78 | −11.0 | −11.0 | −1.55 | 0.0 | −0.49 | 0.0 | 2.79 | 1 | 0.5 | 0.0 | 0.0 |
χ2 = 22.8; gl = 4; P < 0.001.
Frequency of allele A pre-disposing to T. cruzi infection.
Baseline risks of being T. cruzi positive on logit scale corresponding to three genotypes: AA (αAA), Aa (αAa), and aa (αaa).
Regression coefficients associated with the following familial dependences: father-offspring (γPO1, γPO2) and sib-sib (γSS1, γSS2).
Regression coefficient associated with the time of exposure to the domiciliary triatomines (βdur).
Probabilities of transmitting A for individuals AA (τAAA), Aa (τAaa), and aa (τAaa).
C represents twice the log likelihood (21 nL) of the general model.
Discussion
In this study, the research for anti-T. cruzi antibodies was performed in individuals > 25 year old, and 25.56% were found to have positive serology. Despite the high positivity, a decrease over the years as a consequence of the interruption of vectorial transmission was verified. In Brazil, the prevalence of T. cruzi has reduced in incidence by 98% from 1980 to 1999.25
In the distribution of clinical forms in the 151 individuals analyzed, 47.7% presented the cardiac form, 38.4% presented the indeterminate form, 6.6% presented the digestive form, and 7.3% presented the mixed form. These data are similar to those found by Pereira and Coura26 in Virgem da Lapa, Minas Gerais. The manifestation of clinical forms has regional characteristics, and the State of Minas Gerais is one of those that presents a predominance of cardiac form. Chapadeiro and others27 showed 41.8% cardiac impairment among the individuals seropositive to Chagas disease found in the region of Triângulo Mineiro. Other authors pointed out that the percentage of individuals with chagasic cardiopathy in endemic areas varied from 8% to 50%.28–34
The frequency of the digestive form of this casuistry (6.3%) is included in that signalized in other endemic areas of Brazil, and it oscillates between 3% and 10% of the population living in these areas.26,35
The differences in clinical form presentations have raised questions about the potential for evolving forms. Patients are able to remain in an indeterminate form, or they can progress to the cardiac, digestive (megas), or mixed forms.36 In endemic areas where there still is a vector transmission for Chagas disease, it is important to highlight the role of re-exposition. In the case of the endemic area of Água Comprida, vectorial transmission of Chagas disease has been interrupted since 1985 and continues that way through the present.37,38
At the present, there are no solid explanations that justify this difference of behavior, although some hypotheses try to associate it to different factors connected to the infective sampling of T. cruzi, the socioeconomic status of the patient, or the immune response in the interaction with this parasite.39
The clinical diagnosis of the 73 individuals was not possible, because since 1980, several clinical, epidemic, immunological, and therapeutic studies were performed in this area. For this reason, those patients, in the moment of the investigation, did not agree to participate in the evaluation.
The influence of the genetic characteristics of the host on susceptibility to other human parasitic infections was not easy proven, probably because environmental factors, such as vectors and reservoirs, can play an important role. It is also thought that alteration in parasite characteristics contributes to the heterogeneity of the manifestations observed in individuals in endemic areas.40 The idea of susceptibility genes of the host has developed recently. However, solid evidence points out that the intrinsic capacity for human resistance to parasitic infections varies widely among individuals and may be determined by genetic polymorphism of the host.41 A few genetic studies on the susceptibility to infectious diseases in human populations have come to light, and recent advancement in genetics has allowed for better exploration of these factors in a large number of infectious diseases. Much controversy exists about the factors that could influence the evolvement of Chagas disease, because some patients develop severe forms, whereas other ones remain asymptomatic for their whole lives.
In this study performed in 41 families, it was possible to observe the familial aggregation of serological positivity to T. cruzi. Similar data regarding the familial aggregation of seropositivity to T. cruzi were found by Williams-Blangero and others15 in a region of Goiás State, Brazil. However, the mean number of individuals per studied family does not allow comparison between the studies. The familial aggregation of seropositivity to T. cruzi is normally ascribed to the bigger or smaller presence of triatomines in the homes as well as to habitation conditions. In this study, distinct familial seropositivity was observed in families homogeneously exposed to domiciliary vectors. However no evidence of a major gene control was observed, it does not exclude the participation of genetic factors. Moreover, these results may indicate a multigenetic and complex host–parasite relationship that can affect the susceptibility to infection and the potential for the development of clinical forms of Chagas disease.
Acknowledgments
The authors gratefully acknowledge the cooperation of the population of the municipality of Água Comprida and Laurent Abel for his help on segregation analysis.
Footnotes
Authors' addresses: Roseane L. Silva-Grecco and Marly A. S. Balarin, Disciplina de Genética, Federal University of the Triângulo Mineiro, Uberaba, Minas Gerais, Brazil, E-mails: rlope@terra.com.br and balarin@mednet.com.br. Dalmo Correia and Aluízio Prata, Disciplina de Doenças Infecciosas e Parasitárias, Federal University of the Triângulo Mineiro, Uberaba, Minas Gerais, Brazil, E-mail: dalmo@mednet.com.br. Virmondes R. Rodrigues Jr., Disciplina de Imunologia, Federal University of the Triângulo Mineiro, Uberaba, Minas Gerais, Brazil, E-mail: vrodrigues@mednet.com.br.
References
- 1.Dessein A, Marquet S, Hillaire D, Rodrigues V, Abel L. Susceptibilité génétique aux infections parasitaires humaines: étude de la bilharziose. Annales de L'Institut Pasteur. 1996;7:59–62. [Google Scholar]
- 2.Williams-Blangero S, Subedi J, Upadhayay RP, Manral DB, Rai DR, Jha B, Robinson ES, Blangero J. Genetic analysis of susceptibility to infection with Ascaris lumbricoides. Am J Trop Med Hyg. 1999;60:921–926. doi: 10.4269/ajtmh.1999.60.921. [DOI] [PubMed] [Google Scholar]
- 3.Segal S, Hill AVS. Genetic susceptibility to infectious disease. Trends Microbiol. 2003;11:445–448. doi: 10.1016/s0966-842x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 4.Abel L, Dessein A. Predisposição genética a altos níveis de infecção em área endêmica de esquistossomose mansônica. Rev Soc Bras Med Trop. 1991;24:1–3. doi: 10.1590/s0037-86821991000100001. [DOI] [PubMed] [Google Scholar]
- 5.Abel L, Demenais F, Prata A, Souza AE, Dessein A. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet. 1991;48:959–970. [PMC free article] [PubMed] [Google Scholar]
- 6.Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbash J, Dessein AJ. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31q33. Nat Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 7.Schmunis GA. Iniciativa del Cono Sur. Proceedings of the II International Workshop on Population Genetics and Control of Triatominae; Tegucigalpa, Honduras: 1999. pp. 26–31. [Google Scholar]
- 8.Moncayo A. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz. 2003;98:577–591. doi: 10.1590/s0074-02762003000500001. [DOI] [PubMed] [Google Scholar]
- 9.Morel CM, Lazdins J. Focus: Chagas disease. Nature Reviews Microbiology. 2003;1:14–15. doi: 10.1038/nrmicro735. [DOI] [PubMed] [Google Scholar]
- 10.Trischmann TM, Bloom BR. Genetics of murine resistance to Trypanosoma cruzi. Infect Immun. 1982;35:546–551. doi: 10.1128/iai.35.2.546-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva JS, Vespa GNR, Cardillo F, Cardoso MAG, Martins GA, Rodrigues V.1993O papel de citocinas no determinismo de resistência ou susceptibilidade à infecção pelo Trypanosoma cruzi. Rev Soc Bras Med Trop 26(Suppl 2)17–18.7855355 [Google Scholar]
- 12.Reed SG, Brownell CE, Russo DM, Silva JS, Grabstein KH, Morrissey PJ. IL-10 mediates susceptibility to Trypanosoma cruzi infection. Journal of Immunology. 1994;153:3135–3140. [PubMed] [Google Scholar]
- 13.Chandrasekar B, Melby PC, Troyer DA, Freeman GL. Induction of proinflammatory cytokyne expression in experimental acute chagasic cardiomyopathy. Biochem Biophys Res Commun. 1996;223:365–371. doi: 10.1006/bbrc.1996.0900. [DOI] [PubMed] [Google Scholar]
- 14.Andrade ALS, Zicker F, Silva IG, Souza JMP, Martelli CMT. Risk factors for Trypanosoma cruzi infection among children in central Brazil: a case-control study in vector control settings. Am J Trop Med Hyg. 1995;52:183–187. doi: 10.4269/ajtmh.1995.52.183. [DOI] [PubMed] [Google Scholar]
- 15.Williams-Blangero S, Vandeberg JL, Blangero J, Teixeira ARL. Genetic epidemiology of seropositivity for Trypanosoma cruzi infection in rural Goiás, Brazil. Am J Trop Med Hyg. 1997;57:538–543. doi: 10.4269/ajtmh.1997.57.538. [DOI] [PubMed] [Google Scholar]
- 16.Bahia-Oliveira LMG, Gomes JAS, Rocha MOC, Moreira MCV, Lemos EM, Luz ZMP, Pereira MES, Coffman RL, Dias JCP, Cançado JR, Gazzinelli G, Côrrea-Oliveira R. IFN-γ in human Chagas' disease: protection or pathology? Braz J Med Biol Res. 1998;31:127–131. doi: 10.1590/s0100-879x1998000100017. [DOI] [PubMed] [Google Scholar]
- 17.Corrêa-Oliveira R, Gomes JAS, Lemos EM, Cardoso GM, Reis DD, Adad S, Crema E, Martins-Filho AO, Costa MOR, Gazzinelli G, Bahia-Oliveira LMG. The role of immune response on the development of severe clinical forms of human Chagas disease. Mem Inst Oswaldo Cruz. 1999;94((Suppl 1)):253–255. doi: 10.1590/S0074-02761999000700042. [DOI] [PubMed] [Google Scholar]
- 18.Vinhaes MC, Dias JCP. Doença de Chagas no Brasil. Cad Saude Publica. 2000;16((Suppl 2)):7–12. [PubMed] [Google Scholar]
- 19.Villela MM, Souza JMB, Melo VP, Dias JCP. Avaliação do Programa de Controle da Doença de Chagas em relação à presença de Panstrongylus megistus na região centro-oeste do Estado de Minas Gerais, Brasil. Cad Saude Publica. 2009;25:907–917. doi: 10.1590/s0102-311x2009000400022. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous Validade do conceito de forma indeterminada de doença de Chagas. Rev Soc Bras Med Trop. 1985;18:46. [Google Scholar]
- 21.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 22.Rezende JM, Lauar KM, Oliveira AR. Aspectos clínicos e radiológicos da aperistalse do esôfago. Revista Brasileira de Gastroenterologia. 1960;12:247–262. [PubMed] [Google Scholar]
- 23.Rezende JM, Moreira H. In: Clínica das Doenças Intestinais. Porto JAF, editor. Rio de Janeiro; Atheneu: 1976. pp. 451–474. (Megacolo chagásico). [Google Scholar]
- 24.Bonney GE. On the statistical determination of major gene mechanisms in continuous human traits. Am J Med Genet. 1984;18:731–749. doi: 10.1002/ajmg.1320180420. [DOI] [PubMed] [Google Scholar]
- 25.Moncayo A, Yanine MIO. An update on Chagas disease (human American trypanosomiasis) Ann Trop Med Parasitol. 2006;100:1–15. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 26.Pereira JB, Coura JR. Morbidade da doença de Chagas. Estudo seccional em uma área endêmica, Virgem da Lapa, Minas Gerais. Rev Soc Bras Med Trop. 1986;19:139–148. doi: 10.1590/s0037-86821986000300003. [DOI] [PubMed] [Google Scholar]
- 27.Chapadeiro E, Lopes ER, Mesquita PM, Pereira FEL. Incidência de “megas” associados à cardiopatia chagásica. Rev Inst Med Trop Sao Paulo. 1964;6:287–291. [PubMed] [Google Scholar]
- 28.Coura JR, Abreu LL, Dubois LEG, Lima FC, Arruda Júnior E, Willcox HPF, Anunziato N, Petana W. Morbidade da doença de Chagas. II—Estudo seccionais em quatro áreas de campo no Brasil. Mem Inst Oswaldo Cruz. 1984;79:101–124. doi: 10.1590/s0074-02761984000100012. [DOI] [PubMed] [Google Scholar]
- 29.Maguire JH, Hoff R, Sherlock I, Guimarães AC, Sleigh AC, Ramos NB, Mott KE, Weller TH. Cardiac morbidity and mortality due to Chagas' disease: prospective electrocardiographic study of a Brazilian community. Circulation. 1987;75:1140–1145. doi: 10.1161/01.cir.75.6.1140. [DOI] [PubMed] [Google Scholar]
- 30.Laranja FS, Dias E, Duarte E, Pellegrino J. Observações clínicas e epidemiológicas sobre a moléstia de Chagas no oeste de Minas Gerais. Hospital Med. 1951;40:945–988. [PubMed] [Google Scholar]
- 31.Dias E, Laranja FS, Nóbrega G. Doença de Chagas. Mem Inst Oswaldo Cruz. 1945;42:495–582. [Google Scholar]
- 32.Lucena DT, Costa EG, Cordeiro E. Alterações eletrocardiográficas na doença de Chagas, no nordeste do Brasil. Revista Brasileira de Malariologia e Doenças Tropicais. 1963;15:369–390. [PubMed] [Google Scholar]
- 33.Baruffa G, Aquino Neto JO, Alcântara Filho A, Bettini VN, Bertinetti ES. Dados preliminares de inquérito sorológico e eletrocardiográfico para doença de Chagas em populações rurais não selecionadas da zona sul do Rio Grande do Sul. Rev Soc Bras Med Trop. 1972;6:362–363. [Google Scholar]
- 34.Macêdo V, Prata A, Silva GR, Castilho E. Prevalência de alterações eletrocardiográficas em chagásicos. (Informações preliminares sobre o inquérito eletrocardiográfico nacional) Arq Bras Cardiol. 1982;34:261–264. [PubMed] [Google Scholar]
- 35.Castro C, Macêdo V, Rezende JM, Prata A. Estudo radiológico longitudinal do esôfago, em área endêmica de doença de Chagas, em um período de 13 anos. Rev Soc Bras Med Trop. 1994;27:227–233. doi: 10.1590/s0037-86821994000400005. [DOI] [PubMed] [Google Scholar]
- 36.Pereira JB, Cunha RV, Willcox HPF, Coura JR. Evolução da cardiopatia chagásica crônica humana no sertão do estado da Paraíba, Brasil, no período de 4,5 anos. Rev Soc Bras Med Trop. 1990;23:141–147. doi: 10.1590/s0037-86821990000300002. [DOI] [PubMed] [Google Scholar]
- 37.Correia D, Junqueira LF, Molina RJ, Prata A. Cardiac autonomic modulation evaluated by heart interval variability is unaltered but subtly widespread in the indeterminate Chagas' disease. Pacing Clin Electrophysiol. 2007;30:772–780. doi: 10.1111/j.1540-8159.2007.00749.x. [DOI] [PubMed] [Google Scholar]
- 38.Resende LAPR, Molina RJ, Ferreira BDC, Carneiro AC, Ferreira LAA, Silva VJD, Prata A, Correia D. Cardiac autonomic function in chagasic elderly patients in an endemic área: a time and frequency domain analysis approach. Auton Neurosci. 2007;131:94–101. doi: 10.1016/j.autneu.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Pereira JB, Willcox HPF, Coura JR. Evolução da cardiopatia chagásica crônica. I—Influência da parasitemia. Rev Soc Bras Med Trop. 1992;25:101–108. doi: 10.1590/s0037-86821992000200003. [DOI] [PubMed] [Google Scholar]
- 40.Souza W. In: Trypanosoma cruzi e doença de Chagas. Brenner Z, Andrade ZA, Barral-Neto M, editors. Rio de Janeiro: Guanabara Koogan; 2000. pp. 88–126. (O parasita e sua interação com os hospedeiros). [Google Scholar]
- 41.Abel L, Dessein AJ. The impact of host genetics on susceptibility to human infectious diseases. Curr Opin Immunol. 1997;9:509–516. doi: 10.1016/s0952-7915(97)80103-3. [DOI] [PubMed] [Google Scholar]


