Abstract
Chagas' disease, or American trypanosomiasis, is caused by the protozoan parasite Trypanasoma cruzi. It is estimated that 15,000 new cases of congenital T. cruzi transmission occur in the Americas each year. The aim of this study was to estimate the rate of congenital T. cruzi infection in infants born to infected women living in Ushuaia, Argentina, as well to assess a serologic test using Shed Acute Phase Antigen (SAPA) for a timely diagnosis of congenital infection. The rate of congenital infection among children in the study was 4.4% (3/68). Our results show that for infants younger than 30 days of age, matched blood samples from mother and infant were capable of identifying congenital transmission of infection using an enzyme-linked immunosorbent assay with SAPA. For infants older than 3 months, congenital infection could be ruled out using the same procedure.
Chagas' disease, or American trypanosomiasis, is caused by the protozoan parasite Trypanasoma cruzi. It is a major cause of morbidity and mortality in Latin America.1 It is estimated that 15,000 new cases of congenital T. cruzi transmission occur in Latin America each year2 and 2,000 in North America.3 Currently, only direct parasite detection tests are able to confirm infection at birth. However, after the age of 9 months, with the disappearance of maternal antibodies, conventional immune-globulin G (IgG) serology may allow the diagnosis of congenital infection.4,5 The long-term follow-up on most infants to confirm whether they are infected often causes decreases of the adherence, with a consequent lack of opportunity for almost 70% of newborns to infected mothers, to be diagnosed and treated timely.4,6,7
The aim of this study was to estimate the rate of congenital T. cruzi infection in infants born to women infected and living in Ushuaia, Tierra del Fuego, Argentina, as well to assess a serologic test using Shed Acute Phase Antigen (SAPA) for a timely diagnosis of congenital infection. The study populations were mothers infected with T. cruzi and their children up to 2 years old, recruited from February 2001 to December 2002. We conducted a cohort study from birth to 9 months of age. Blood samples of mothers and children were collected at birth. From age 3 months and older, only the children's blood samples were analyzed during the study. The methods for diagnosis of congenital infection were (1) the direct examination of blood parasites using the microhematocrit8 at the time of birth and/or (2) serologic tests: indirect hemagglutination (IHA; Polychaco, Buenos Aires, Argentina) and enzyme-linked immunosorbent assay (EIA; Conventional EIA; WIENER Laboratory, Rosario, Argentina or ORGANON, Buenos Aires, Argentina). These tests were performed according to the manufacturer's instructions. During this study, the IgG antibody test was also performed to compare the results to the SAPA of T. cruzi (EIA-SAPA).9–12 This technique was standardized by the Departamento de Biología Molecular, Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción, Paraguay (IICS-UNA).7,13 The antigen used was a recombinant SAPA of plasmid pGex (the insert used was released by Dr. Carlos Frasch [Instituto de Investigaciones Biotecnológicas, Universidad Nacional de San Martín, Argentina] in 1998 to the Department Biología Molecular, IICS, Paraguay) obtained by genetic engineering techniques at IICS-UNA. Serologic tests (IHA, conventional EIA, and EIA-SAPA) were performed on matched blood samples of mother and child from birth to 30 days of age and on blood samples taken from the child during follow-up. All infants had samples taken at least at 9 months of age or older. Serologic tests were considered reactive when the titer of IHA was ≥ 16, index of positivity (IP) of conventional EIA test result was ≥ 1, where IP is the optical density/cut-off, and the EIA-SAPA results were OD ≥ 0.300.7
Detection of antibodies using any serologic test is an indirect way of measuring infection. In addition, in the early months after birth, there is a great limitation because of the presence of antibodies transferred from the mother to offspring. Congenital infection was defined when a child born to a mother infected had a positive parasitologic test or IHA and conventional EIA reactive after 9 months of age. This procedure was used as the gold standard to estimate the rate of congenital T. cruzi transmission and to assess EIA-SAPA.
The data were analyzed to determine the prevalence of infection, with a 95% confidence interval or as means and SD for continuous data. Comparisons of proportions were done with χ2 or Fisher tests. Means were compared by analysis of variance (ANOVA) and the Mann-Whitney test. Results of IHA were analyzed by conversion log2 of 2-fold dilutions of serum samples. To analyze results of the serologic test at birth, an index was built called a “differential” by subtracting the results of the mother (Log2 of dilution to IHA, IP to conventional EIA, and OD to EIA-SAPA) from the results of the child (result of the child – results of the mother). Data were analyzed using Epi Info version 3.4 (CDC).
The study was approved by the Institutional Board of the Hospital Regional Ushuaia and the Ministry of Health of the Province. Oral informed consent to take samples was obtained from all women for themselves and their children.
The study population consisted of 61 infected mothers and 68 children. The mean age of the mothers was 29.1 years old, and the mean length of residence in Tierra del Fuego was 7.1 years. Fifty percent of mothers were EIA-SAPA reactive. The rate of EIA SAPA reactivity in women chronically infected was consistent with other studies.7,14,15 Among the 68 children in the study, 52.9% were girls and 60.3% were younger than 3 months of age. No children received transfusions.
The rate of congenital infection among children being studied was 4.4% (3/68). All of them were diagnosed by a parasitologic test (microhematocrit) at birth (Table 1). All infants with congenital T. cruzi infection were treated by the health providers according to official guidelines. The serologic tests were non-reactive when non-infected children reached 9 months of age or older.
Table 1.
Tests used to diagnose congenital T. cruzi infection at the time of birth or within 30 days of age, Hospital Regional Ushuaia, Ushuaia, 2001–2002
| Technique | Case 1 (age: 10 days) | Mother 1 | Case 2 (age: 10 hours) | Mother 2 | Case 3 (age: 2 hours) | Mother 3 |
|---|---|---|---|---|---|---|
| Direct parasitemia (microhematocrit) | 5 parasites | NA | 2 parasites | NA | 4 parasites | NA |
| IHA reactive ≥ 1/16 | 1/512 (reactive) | 1/256 (reactive) | 1/512 (reactive) | 1/256 (reactive) | 1/128 (reactive) | 1/64 (reactive) |
| EIA (IP) reactive ≥ 1 | 5.3 (reactive) | 6.9 (reactive) | 6.6 (reactive) | 5.9 (reactive) | 4.7 (reactive) | 5.0 (reactive) |
| EIA-SAPA cut-off = 0.300 | OD = 1.885 (reactive) | OD = 0.201 (not reactive) | OD = 1.118 (reactive) | OD = 0.102 (not reactive) | OD = 2.607 (reactive) | OD = 0.179 (not reactive) |
NA = not applicable; IP = index of positivity (OD/cut-off); IHA = indirect hemagglutination; EIA = enzyme-linked immunosorbent assay.
Among 34 samples from mothers and their children who were 30 days of age, the median differential EIA-SAPA was −0.05 (range, −0.5 to 0.4) among children not infected and 1.7 (range, 1.02–2.43) among three children with congenital infection (P < 0.05). In contrast, the median differential of IHA was −1.0 (range, −0.5 to 1.0) among 36 children without congenital infection and 1.0 (range, 1.0–1.0) among three children with congenital infection (P > 0.05). For the conventional EIA for detecting IgG, the median differential was −0.3 (range, −4.5 to 1.8) and −0.3 (range, −1.6 to 0.7) among non-infected and infected children, respectively (P > 0.05). In the three cases with congenital infection, the mothers had an OD of EIA-SAPA < 0.300 (Table 1). The differential of EIA-SAPA could distinguish the three children with congenital infection. The differential of IHA and conventional EIA of children without congenital infection overlapped with those who had congenital infection; hence, these tests could not distinguish congenital infection within 30 days of birth (Figure 1).
Figure 1.
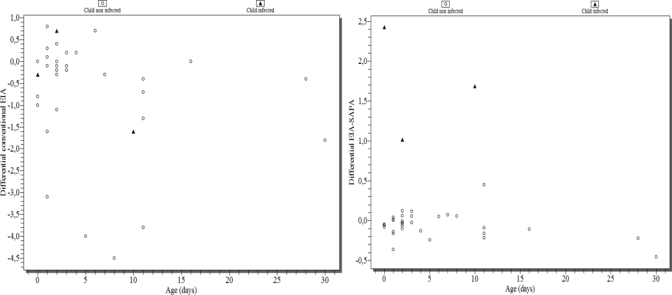
Differential of results of conventional EIA and EIA-SAPA among children born to mothers infected with T. cruzi regarding age in days. Hospital Regional Ushuaia, Ushuaia, 2001–2002.
All children without congenital infection who tested EIA-SAPA reactive at birth or within 30 days of birth and their mothers who were EIA-SAPA reactive also had a differential index < 1 at birth and were EIA-SAPA non-reactive after 3 months of age (Figure 2).
Figure 2.
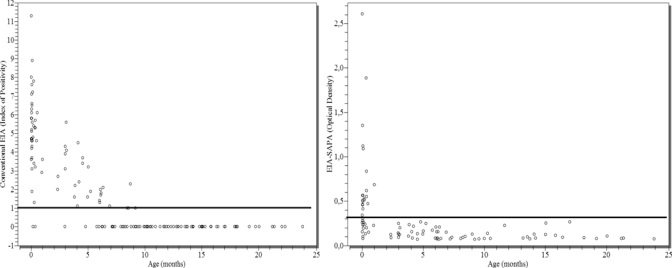
Detection of transferred maternal antibodies using conventional EIA and EIA-SAPA among children without congenital T. cruzi infection. Hospital Regional Ushuaia, Ushuaia, 2001–2002.
We used the subtraction to highlight the reactivity produced by the infant's own antibody production, deducing the reactivity produced for antibodies transferred from the mother. Some SAPA-EIA–reactive mothers had infants with low reactivity (because of transference of maternal antibodies or the lack of antibodies detected by SAPA-EIA), resulting in a value < 1. All infants with values < 1 were non-infected. In contrast, the differential index of conventional EIA had results < 1 or negative values among infants with congenital infection (Figure 1).
During the study, maternal antibodies were detected in children without congenital infection at 6 months with HIA and 8 months with conventional EIA (Figure 2). All of them were non-reactive at 9 months of age or older. No non-infected children were EIA-SAPA reactive against maternal antibodies after 3 months of age. Reactivity of transferred maternal antibodies against SAPA in non-infected infants disappeared earlier than reactive transferred maternal antibodies against epimastigote antigens used in conventional-EIA. This phenomenon is caused by antibodies against recombinant SAPA detecting an antigen more defined than those of epimastigotes. Although SAPA has multiple epitopes, they are repetitive and have high affinity. Meanwhile, the reactivity of transferred antibodies against epimastigotes is more heterogeneous, and more time may be needed to show the decrease in reactivity (as least 9 months using conventional EIA).
Table 2 shows the response to the etiologic treatment of newborns diagnosed with congenital T. cruzi infection. The treatments started between 2 and 11 days after diagnosis was obtained. All infants became non-reactive according to their serologic tests during the follow-up.
Table 2.
Treatment and follow-up of newborns with congenital T. cruzi infection diagnosed at the time of birth or within 30 days of age, Hospital Regional Ushuaia, Ushuaia, 2001–2002
| Variable | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age of diagnosis | 10 days | 2 days | 10 hours |
| IHA before treatment | 1/512 | 1/512 | 1/128 |
| EIA (IP) before treatment | 5.3 | 6.6 | 4.7 |
| EIA-SAPA cut-off = 0.300 | DO = 1.885 reactive | DO = 1.118 reactive | DO = 2.607 reactive |
| Clinical manifestation | No | No | No |
| Age when treatment started | 21 days | 9 days | 2 days |
| Tolerance of treatment | Good (no adverse events) | Good (no adverse events) | Good (no adverse events) |
| Age at first control post-treatment | 9 months | 10 months | 8 months |
| IHA at first control post-treatment | Not reactive | Not reactive | Not reactive |
| EIA (IP) at first control post-tratment | 1.0 | 1.0 | Not reactive |
| EIA-SAPA at first control post-treatment | DO = 0.319 (low reactive) | DO = 0.188 (not reactive) | DO = 0.132 (not reactive) |
| Age at second control post-treatment | 4 years old | 4 years old | 17 months |
| IHA at second control post-treatment | Not reactive | Not reactive | Not reactive |
| EIA (IP) at second control post-treatment | Not reactive | Not reactive | Not reactive |
| EIA-SAPA at second control post-treatment | DO = 0.164 (not reactive) | DO = 0.132 (not reactive) | DO = 0.106 (not reactive) |
IP = index of positivity (OD/cut-off); IHA = indirect hemagglutination; EIA = enzyme-linked immunosorbent assay.
Congenital T. cruzi infections occur in Ushuaia, and the migration of population can explain16 why the rate observed was above the national average (3.2%) and similar to the rate observed in the endemic area.17 The microhematocrit proved to be an excellent instrument, but it requires a long, laborious procedure and is dependent on the operator. This operator dependence explains the variations that occur in this test's performance and why other authors have reported a detection rate ranging from 97.4% to 2.9%.4,7,18–21
Indirect hemagglutination and conventional EIA could not distinguish children with congenital infection when the tests were made at birth.5 In contrast, within 30 days of birth, EIA-SAPA results from samples taken from mother and child were capable of detecting children with congenital infection. These results also indicated an ability to identify the absence of congenital infection for children > 3 months of age. The use of SAPA for diagnosis of congenital infection in children in endemic areas was described previously.11,22,23 Our results show that, using corresponding samples of mother and child at birth, the test was successful in detecting congenital transmission in newborns up to 30 days, and it could rule out the possibility of congenital infection after 90 days of age.
Based on the results of these EIA-SAPA tests, it is possible to identify, within 30 days of birth, that an offspring born to an infected mother has congenital T. cruzi infection when (1) the mother is EIA-SAPA non-reactive and the child is EIA-SAPA reactive and (2) the differential of EIA-SAPA using matched samples of mother and child is ≥ 1. In contrast, the offspring does not have congenital T. cruzi infection within 30 days after birth when (3) the child is SAPA non-reactive, (4) both samples of the mother and child are reactive but the differential is < 1; or (5) the child is EIA-SAPA non-reactive after 90 days.
A high level of absorption of EIA-SAPA at birth is not a sufficient indicator of infection when no maternal sample is available because of the inability to distinguish between the offspring's own antibodies and those of the mother. On the other hand, it is necessary to assess whether samples of newborn with EIA-SAPA ≥ 0.300 at birth, and a differential with a value 1 or more can diagnose congenital T. cruzi infection.
Neonates treated between 10 hours and 10 days of birth exhibited excellent tolerance to the treatment. All serologic tests, IHA, EIA, and EIA-SAPA, showed through serological test non-reactive that the treatment was effective. We could not test this series if EIA-SAPA showed an early negativization in comparison with conventional tests.
The main limitation of this study is the small sample size and the paucity of cases with congenital infection. Long-term follow-up of a large sample size of infants born to infected mothers is currently being analyzed (G. Russomando, personal communication). Other studies involving larger numbers of people in other regions will be necessary to validate this diagnostic method.
The prevalence of T. cruzi infection found in pregnant women in Ushuaia was 5.9%,16 similar to a region of endemic vector transmission in Argentina.17 This rate showed a real risk of congenital transmission, indicating that controls must be implemented, and the follow-up of children born to infected mothers should be strengthened.16
Prevention of congenital transmission of T. cruzi is not possible at present, but it can be detected early, and treatment of infected newborns is highly effective.24–27 Congenital T. cruzi infection is recognized as a major cause of transmission in endemic countries where relatively successful vector control and blood screening programs exist.2 The disease is also observed along the major migration routes leading from endemic into non-endemic countries. After eliminating transmission of Chagas' disease by vectors and blood transfusions, a critical final step toward eliminating the disease will involve finding new tools to diagnose and provide timely treatment to infants with congenital T. cruzi infection at the primary health care level.
Acknowledgments
The authors thank our colleagues of the laboratory of Hospital Regional de Ushuaia, Direction of Epidemiology and Biostatistics of Tierra del Fuego, the Primary Care Health Agents, and María Torres (data management). We Christina Vella for her assistance in the editing of this manuscript.
Footnotes
Financial support: This work was supported by the Minister of Health, Tierra del Fuego, Argentina; Instituto de Investigaciones de Ciencias de la Salud, Universidad Nacional de Asunción, Paraguay; and Administración Nacional de Laboratorios e Institutos de Salud, Dr. Carlos G. Malbrán, Minister of Health, Argentina.
Authors' addresses: María Cristina Mallimaci, Carina Sijvarger, Isabel Marcela Alvarez, Lola Barrionuevo, and Carlos Lopez, Laboratorio Central, Hospital Regional Ushuaia, 12 de octubre y Maipú, Ushuaia, Tierra del Fuego, Argentina, Tel: 54-2901-44-1066/1070, Fax: 54-2901-421173, E-mails: crismallimaci@yahoo.com.ar, carina.sijvarger@gmail.com, chabelaush@hotmail.com, loladvb@hotmail.com, and rufianmelanco41@hotmail.com. Sergio Sosa-Estani, Centro Nacional de Diagnóstico e Investigación de Endemoepidemias (CeNDIE) ANLIS Dr. Carlos G. Malbrán, Av. Paseo Colón 568, 1063 Buenos Aires, Argentina, Tel: 54-11-4331-4010, Fax: 54-11-4331-2536 and Instituto de Efectividad Clínica y Sanitaria, Viamonte 2146 - 3er piso, 1056 Buenos Aires, Argentina, Tel/Fax: 54-11-4953-4058, E-mail: ssosa@msal.gov.ar. Graciela Russomando and Zunilda Sanchez, Departamento de Biología Molecular y Genética, Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción, Rio de la Plata y Lagerenza, Asunción, Paraguay, Tel: 595-21-424 520, Fax: 595-21-480 185, E-mails: grusso@rieder.net.py and zunysanchez@hotmail.com. Elsa Leonor Segura, Instituto Nacional de Parasitología Dr. Mario Fatala Chaben/ANLIS, Av. Paseo Colón 568, 1063 Buenos Aires, Argentina, Tel: 54-11-4331-4010, Fax: 54-11-4331-2536, E-mail: elsasegura@fibertel.com.ar.
References
- 1.World Health Organization . Report of the Expert Committee on the Control of Chagas' Disease. Geneva: World Health Organization; 2002. [Google Scholar]
- 2.Pan-American Health Organization Report of the technical consultation on information, education and communication (IEC) on congenital Chagas' disease. 2007. http://www.paho.org/English/AD/DPC/CD/dch-congenita-iec-07.doc Available at. Accessed December 3, 2007.
- 3.Buekens P, Almendares O, Carlier Y, Dumonteil E, Eberhard M, Gamboa-Leon R, James M, Padilla N, Wesson D, Xiong X. Mother-to-child transmission of Chagas' disease in North America: why don't we do more? Matern Child Health J. 2008;12:283–286. doi: 10.1007/s10995-007-0246-8. [DOI] [PubMed] [Google Scholar]
- 4.Blanco SB, Segura EL, Cura EN, Chuit R, Tulian L, Flores I, Garbarino G, Villalonga JF, Gurtler RE. Congenital transmission of Trypanosoma cruzi: an operational outline for detecting and treating infected infants in northwestern Argentina. Trop Med Int Health. 2000;5:293–301. doi: 10.1046/j.1365-3156.2000.00548.x. [DOI] [PubMed] [Google Scholar]
- 5.Carlier Y, Torrico F.2003Congenital infection with Trypanosoma cruzi: from mechanisms of transmission to strategies for diagnosis and control. Conclusions of round tables and synopsis of an international colloquium. Cochabamba, Bolivia, November 6–8 2002. Rev Soc Bras Med Trop 36767–771. [DOI] [PubMed] [Google Scholar]
- 6.Gurtler RE, Segura EL, Cohen JE. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Infect Dis. 2003;9:29–32. doi: 10.3201/eid0901.020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russomando G, Almiron M, Candia N, Franco L, Sanchez Z, de Guillen I. Implementación y evaluación de un sistema localmente sustentable de diagnóstico prenatal que permite detectar casos de transmisión congénita de la enfermedad de Chagas en zonas endémicas del Paraguay. Rev Soc Bras Med Trop. 2005;38:49–54. [PubMed] [Google Scholar]
- 8.Freilij H, Muller L, Gonzales Cappa SM. Direct micro-method for diagnosis of acute and congenital Chagas'desease. J Clin Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Affranchino JL, Ibanez CF, Luquetti AO, Rassi A, Reyes MB, Macina RA, Aslund L, Pettersson U, Frasch AC. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol. 1989;34:221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 10.Frasch AC, Reyes MB. Diagnosis of Chagas' disease using recombinant DNA technology. Parasitol Today. 1990;6:137–139. doi: 10.1016/0169-4758(90)90234-u. [DOI] [PubMed] [Google Scholar]
- 11.Reyes MB, Lorca M, Munoz P, Frasch AC. Fetal IgG specificities against Trypanosoma cruzi antigens in infected newborns. Proc Natl Acad Sci USA. 1990;87:2846–2850. doi: 10.1073/pnas.87.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazzulo JJ, Frasch AC. SAPA/trans-sialidase and cruzipain: two antigens from Trypanosoma cruzi contain immunodominant but enzymatically inactive domains. FASEB J. 1992;6:3259–3264. [PubMed] [Google Scholar]
- 13.Russomando G. Assessment of a locally sustainable system for Chagas' disease diagnosis in two endemic regions of Paraguay. TDR News 16. 1999. http://www.who.int/tdrold/research/finalreps/pdf/fr16.pdf Available at. Accessed June 29, 2009.
- 14.Breniere SF, Bosseno MF, Noireau F, Yacsik N, Liegeard P, Aznar C, Hontebeyrie M. Integrated study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas' disease. Mem Inst Oswaldo Cruz. 2002;97:289–295. doi: 10.1590/s0074-02762002000300002. [DOI] [PubMed] [Google Scholar]
- 15.Vergara U, Veloso C, Gonzalez A, Lorca M. Evaluation of an enzyme-linked immunosorbent assay for the diagnosis of Chagas' disease using synthetic peptides. Am J Trop Med Hyg. 1992;46:39–43. doi: 10.4269/ajtmh.1992.46.39. [DOI] [PubMed] [Google Scholar]
- 16.Mallimaci MC, Sijvarger C, Dates A, Alvarez M, Sosa-Estani S. Seroprevalence of Chagas' disease in Ushuaia, Argentina, an area without triatominae. Rev Panam Salud Publica. 2001;9:169–171. doi: 10.1590/s1020-49892001000300006. [DOI] [PubMed] [Google Scholar]
- 17.Ministerio de Salud de Argentina, Programa Federal de Chagas' Situación de la enfermedad de Chagas y su control en Argentina. 2007. http://www.msal.gov.ar/chagas/home.htm Available at. Accessed April 20, 2009.
- 18.Brutus L, Schneider D, Postigo J, Delgado W, Mollinedo S, Chippaux JP. Evidence of congenital transmission of Trypanosoma cruzi in a vector-free area of Bolivia. Trans R Soc Trop Med Hyg. 2007;101:1159–1160. doi: 10.1016/j.trstmh.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Mora MC, Sanchez Negrette O, Marco D, Barrio A, Ciaccio M, Segura MA, Basombrio MA. Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. J Parasitol. 2005;91:1468–1473. doi: 10.1645/GE-549R.1. [DOI] [PubMed] [Google Scholar]
- 20.Diez CN, Manattini S, Zanuttini JC, Bottasso O, Marcipar I. The value of molecular studies for the diagnosis of congenital Chagas' disease in northeastern Argentina. Am J Trop Med Hyg. 2008;78:624–627. [PubMed] [Google Scholar]
- 21.Zaidenberg M, Segovia A. Congenital Chagas' disease in the city of Salta, Argentina. Rev Inst Med Trop Sao Paulo. 1993;35:35–43. [PubMed] [Google Scholar]
- 22.Lorca M, Veloso C, Munoz P, Bahamonde MI, Garcia A. Diagnostic value of detecting specific IgA and IgM with recombinant Trypanosoma cruzi antigens in congenital Chagas' disease. Am J Trop Med Hyg. 1995;52:512–515. doi: 10.4269/ajtmh.1995.52.512. [DOI] [PubMed] [Google Scholar]
- 23.Breniere SF, Yaksic N, Telleria J, Bosseno MF, Noireau F, Wincker P, Sanchez D. Immune response to Trypanosoma cruzi shed acute phase antigen in children from an endemic area for Chagas' disease in Bolivia. Mem Inst Oswaldo Cruz. 1997;92:503–507. doi: 10.1590/s0074-02761997000400011. [DOI] [PubMed] [Google Scholar]
- 24.Moya PR, Paolasso RD, Blanco S, Lapasset M, Sanmartino C, Basso B, Moretti E, Cura D. Treatment of Chagas' disease with nifurtimox during the first months of life. Medicina (B Aires) 1985;45:553–558. [PubMed] [Google Scholar]
- 25.Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, Freilij H. Aetiological treatment of congenital Chagas' disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother. 2003;52:441–449. doi: 10.1093/jac/dkg338. [DOI] [PubMed] [Google Scholar]
- 26.Pan American Health Organization 1999Tratamiento etiológico de la enfermedad de Chagas. Conclusiones de una consulta técnicaOPS/HCP/HCT/140/99. Available athttp://www.paho.org/Spanish/AD/DPC/CD/chagas.pdfAccessed April 20, 2009
- 27.Sosa-Estani S. Congenital transmission of Trypanosoma cruzi infection in Argentina. Rev Soc Bras Med Trop. 2005;38((Suppl 2)):29–32. [PubMed] [Google Scholar]


