Abstract
Within the United States, the majority of human plague cases are reported from New Mexico. We describe climatic factors involved in intra- and inter-annual plague dynamics using animal-based surveillance data from that state. Unlike the clear seasonal pattern observed at lower elevations, cases occur randomly throughout the year at higher elevations. Increasing elevation corresponded with delayed mean time in case presentation. Using local meteorological data (previous year mean annual precipitation, total degrees over 27°C 3 years before and maximum winter temperatures 4 years before) we built a time-series model predicting annual case load that explained 75% of the variance in pet cases between years. Moreover, we found a significant correlation with observed annual human cases and predicted pet cases. Because covariates were time-lagged by at least 1 year, intensity of case loads can be predicted in advance of a plague season. Understanding associations between environmental and meteorological factors can be useful for anticipating future disease trends.
Introduction
Plague, caused by Yersinia pestis, is a severe, primarily flea-borne zoonotic disease characterized by long periods of quiescence punctuated by rapidly spreading epizootics. Humans are at greatest risk of exposure to Y. pestis during epizootic periods when infected fleas that abandon rodent hosts, which succumb to plague infection, occasionally bite humans.1 In the United States, the majority of human infections are reported from the Southwest.2,3 Of the 456 cases reported to the Centers for Disease Control (CDC) from 1950 through 2008, 82.5% of cases occurred in either New Mexico (N = 245), Arizona (N = 63), Colorado (N = 55), or Utah (N = 13) (CDC, unpublished data). Although the frequency of disease is low, with an average of 8 (range: 1–40) human cases reported annually in the United States from 1950 through 2008, and an average of four cases per year (range: 0–26) in New Mexico during the same time period (CDC, unpublished data), case fatality rates are high if appropriate antibiotic therapy is delayed or inadequate.3,4 Early detection is paramount to a successful outcome.
In the southwestern United States, the majority of human infections are acquired in and around the home.2,5 The most common mode of exposure is through infectious flea bites, primarily those of ground squirrel fleas (Oropsylla montana), which are believed to serve as the primary bridging vector to humans.6,7 Less commonly, infection can occur by direct contact or by exposure to infectious respiratory droplets. Prevention recommendations typically include advising the public and health care providers of existing plague activity, reducing rodent food, and harborage in and around homes, avoiding handling sick or dead animals, not allowing pets to roam and hunt, and eliminating fleas from pets.3,8,9
Development of tools that identify when and where humans are at greatest risk of exposure to Y. pestis may aid in prevention and control efforts and ultimately reduce disease burden. Toward this goal, several decades of human surveillance data were used recently to construct spatial risk models for plague in the American Southwest.5,10 High-risk areas were predicted based on elevation, vegetation types (e.g., piñon-juniper and ponderosa pine) that were coincident with optimal elevation ranges and distance to water.
Other models have sought to define annual human case loads based on meteorological predictors. In a New Mexico study by Parmenter and others,11 human plague cases more frequently followed above average precipitation in winter–spring periods (October to May). Late winter precipitation and threshold temperatures were important in models of human plague in northern Arizona and New Mexico.12 Global climatic cycles such as the Pacific Decadal Oscillation, which influences precipitation in the Southwest and elsewhere in the western United States, were indicative of temporal variation in annual plague case occurrence in this country.13,14 Although these models were insightful in identifying climatic correlates of plague activity, they are of limited value as predictive tools either because they were fit to limited regions or include climatic data from the year of case onset.
In this study, we describe climatic factors involved in intra- and inter-annual plague dynamics in New Mexico based on 29 years of animal-based surveillance data. To identify climatic associations with intra-annual timing and abundance of pet case reports, we first exploited an elevation gradient across which meteorological factors also vary. Next, we identified time-lagged meteorological predictors of inter-annual variation in the number of cases reported. Finally, we compared between predicted pet and observed human plague cases, which indicated pet plague surveillance data are useful for refining predictions of when and where humans will be at highest risk of exposure to Y. pestis. Understanding associations between environmental and meteorological factors can be useful for anticipating future disease trends based on changing land-use and climate scenarios, provided the predictions achieve appropriate resolution.
Materials and Methods
Description of the study area.
Our study area, defined by where pet (cats and dogs) plague cases were acquired, included locations in the following New Mexico counties: Bernalillo, Cibola, Colfax, Doña Ana, Grant, Guadalupe, Lincoln, Los Alamos, Otero, Quay, Rio Arriba, San Juan, Sandoval, Santa Fe, Socorro, Taos, and Torrance (Figure 1). These counties constitute 76.4% of New Mexico's population or about 1.5 million residents. More than three-quarters (78%) of the pet cases reported in our study occurred within the previously defined high-risk plague areas of northern New Mexico (Figure 1).10 The average distance from the high-risk area to the remaining cases was 3.7 km (maximum distance 43.5 km). Plague risk areas in New Mexico are dominated by conifer woodland especially piñon-juniper or juniper and, like much of the Southwest, are relatively arid.
Figure 1.
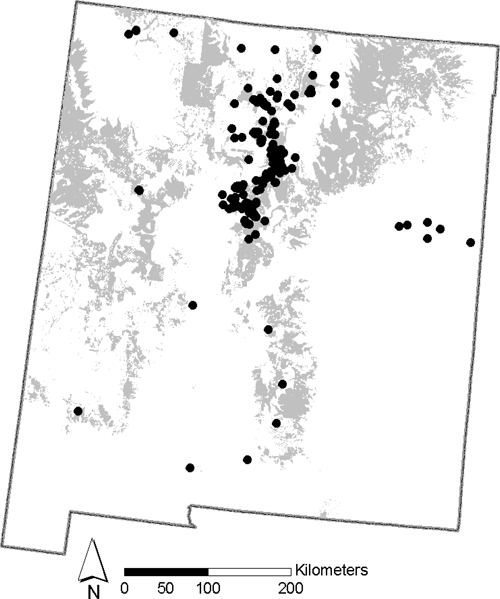
Pet cases reported to the New Mexico Department of Health. The shaded areas denote the risk area defined by Eisen and others.10
The Southwest hosts a unique climate in North America.13 Summer precipitation is focal and variable and is flanked by dry fore-summer and fall conditions.13,15 Lower elevations exhibit especially high temperatures and high rates of evapotranspiration and mean annual temperatures decrease with increasing elevation.13 It is one of the most diverse regions in the United States with respect to mammal species and climate may play a role in rodent diversity and plague activity in this region.16,17
Pet case data.
Because of the frequency of pet plague cases reported in New Mexico, the New Mexico Department of Health's Epidemiology and Response Division conducted thorough studies of all reported pet cases. All owner residences were visited, an environmental assessment was conducted, and samples were collected from domestic and wild animals on the premises. Residence locations were documented by a global positioning system (GPS) receiver to record geographic coordinates or addresses, which were subsequently geocoded (using Google Earth or http://www.batchgeocode.com/lookup/).
Because of the close association between pets and humans, there is increased risk to humans when a pet becomes infected or in situations where pets transport infected fleas into the home.18–20 Over the period of June 6, 1980 until June 18, 2008, 283 pet cases with geocoded exposure locations were identified in New Mexico (mean number of pet cases = 10 per year [range: 0–36]). An additional 77 cases were reported to the county but addresses were not recorded or could not be geocoded (for example, Post Office boxes). We do not believe the excluded data biases our results as there was no statistically significant difference in location when comparing the county from which cases were reported (Pearson χ2 [17] = 24.3, P = 0.11). The majority of these cases were feline (N = 221, 78%; canine = 62, 22%). Both probable (clinical symptoms, titer > 1:16) and confirmed (4-fold or greater increase in titer or positive culture) cases were included in these analyses.
Environmental variables.
Elevation data were extracted from 30 m resolution National Elevation Dataset digital elevation data. Cases were divided into quartiles based on elevation (a: 1,238–1,846 m; b: > 1,846–2,022 m; c: > 2,022–2,115 m; d: > 2,115–2,945 m).
Monthly average meteorological data were acquired from the Spatial Climate Analysis Service at Oregon State University (prism.oregonstate.edu/). These data included topographically weighted interpolated values for temperature and precipitation acquired with a spatial resolution of approximately 2 km at this latitude. For the intra-annual, seasonal analyses, 30 yr (1961–90) monthly average data were used. For the inter-annual analysis, monthly data were used covering the whole time series (June 1980–June 2008). Temperature and precipitation point values were extracted for each case and averaged for the analysis. Pet case data were aggregated by month for each elevation class for intra-annual analyses and by year for inter-annual analyses. Meteorological variables were summarized using seasonal definitions described for the Southwest by Comrie.21 In this scheme, winter includes January through April; May through July comprises the fore-summer; the monsoon season occurs in August and September, and the remaining months encompass the fall season. By aggregating point data we were able to generalize the meteorological data to the region of interest while maintaining its precision.
For ground cover analyses, Landsat 5 TM imagery cloud-free scenes from spring (fore-summer) [June 5, 2001] and fall (monsoon) [September 9, 2001] were downloaded from the USGS Earth Resources Observation and Science Center - Global Visualization Viewer (glovis.usgu.gov). These data were acquired at 30 m × 30 m resolution in the reflective wavelengths and 60 m × 60 m resolution for thermal emission wavelengths. The Tasseled Cap transformation was performed in ENVI v4.5 (ITT Visual Information Systems, Boulder, CO) to derive soil (brightness), soil moisture (wetness), and vegetation (greenness) indices.22
Statistical analyses.
Variance-to-mean ratios (VMRs) were calculated to characterize the temporal distribution (week of disease onset) of cases for each elevation class. A VMR ratio close to one indicates a random, Poisson-like distribution. Values greater than one indicate over-dispersal or “clumping” of cases during particular time periods. Values less than one infer an even distribution of cases.
We used analysis of variance (ANOVA) comparisons of seasonal meteorological variables, landcover (Tasseled Cap indices), and week of case onset to assess differences among elevation categories.
Time-series regression models were constructed to identify meteorological predictors of the number of pet plague cases per year. First, we tested cross correlations between square root transformed pet counts per year and meteorological variables for up to 5 lags. Lags up to 5 years were used as other researchers have shown associations with plague and 3- to 5-year cyclical weather indices, e.g., El Niño Southern Oscillation.23,24 Predictors at lags that were correlated (r > 0.25) with the square root transformed pet data were then used in univariate linear regression models (19 variables met the correlation threshold of > 0.25). A forward stepwise method was used to build the models and variables were selected for inclusion based on predictive accuracy (higher R2) and parsimony (lower Akaike information criterion [AIC]).25 A binary post-1990 surveillance variable was included because a concerted effort to increase surveillance and document pet plague cases by the NM Department of Health commenced in 1991. We used three tests of model fit: the link test to confirm the models were specified correctly, the Ramsey test for omitted variables, and the Breusch-Pagan/Cook-Weisberg test for heteroskedasticity. The predictors in the model were tested for multicollinearity by comparing variance inflation factors. Withholding a portion of the data for validation was not appropriate for this limited dataset. Model sensitivity was instead tested using the leave-one-out (L-O-O) method where the model was run dropping and then replacing each year sequentially allowing for an average R2 and 95% confidence intervals (CI) to be calculated across all models.26 All statistical analyses were performed in STATA version 10 (StataCorp LP, College Station, TX).
Results
Seasonal case occurrence patterns by elevation category.
Weekly pet case counts summarized over all years were plotted across the four elevation categories (a: 1,238–1,846 m; b: > 1,846–2,022 m; c: > 2,022–2,115 m; d: > 2,115–2,945 m) with monthly average minimum and maximum temperature and average precipitation (Figure 2). The VMR showed that the degree of over-dispersion was highest within the lowest elevation category (a: 1,238–1,846 m) indicating a clustered distribution (Table 1). The degree of over-dispersion decreased with increasing elevation. The highest elevation category (d: > 2,115–2,945 m) showed low numbers of cases occurring randomly across the year—a more stable pattern compared with visible peaks seen in the lower three elevation quartiles. The comparison across the epizootic curves by elevation quartiles also showed the mean week of onset occurred later in the year with increasing elevation. The mean for the lowest elevation was 5 weeks earlier compared with the highest elevation class (ANOVA F = 5.7, P = 0.021; Table 1).
Figure 2.
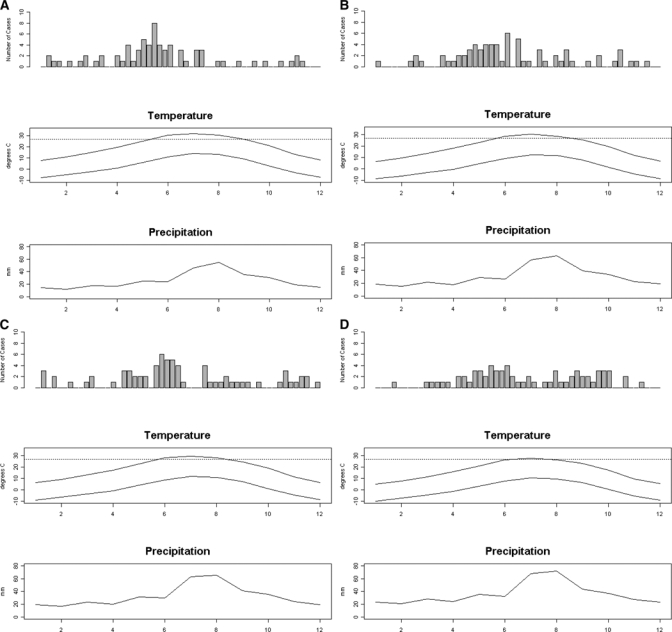
Total number of pet cases occurring by calendar week and 30 yr average monthly maximum/minimum temperature and precipitation data for the elevation quartiles (a: 1,238–1,846 m; b: > 1,846–2,022 m; c: > 2,022–2,115 m; d: > 2,115–2,945 m). The x axis is week of the year for the pet plague cases and month of the year for temperature and precipitation. The horizontal line in the temperature graph is drawn at 27°C.
Table 1.
Comparison of weekly pet cases by elevation of owner residence*
| Elevation (m) | Mean onset week | No. weeks with cases | Max count | Mean (µ) | Var (σ2) | VMR (σ/µ) |
|---|---|---|---|---|---|---|
| 1,238–1,846 | 23.1 (20.5–25.6) | 32 | 8 | 1.32 | 2.72 | 2.06 |
| > 1,846–2,022 | 25.8 (23.1–28.5) | 33 | 6 | 1.32 | 2.26 | 1.71 |
| > 2,022–2,115 | 27.4 (24.4–30.3) | 34 | 6 | 1.36 | 2.31 | 1.70 |
| > 2,115–2,945 | 28.5 (26.0–31.1) | 36 | 4 | 1.30 | 1.45 | 1.11 |
Data were summed by elevation quartile to week of year for all years for which data were available (1980–2008). Shown are the mean week of onset, number of weeks wherein at least one case was reported, and maximum number of cases ever occurring in a week. The VMRs were calculated to compare the distribution of cases where values near 1 show a Poisson-like distribution and values greater than 1 indicate over-dispersal or temporal clumping of case occurrence.
Distinctive differences between the highest elevation class and the similarity of the three others were presumed to be indicative of different disease transmission dynamics. Therefore, the data from the three lower elevation categories (i.e., 1,238–2,115 m) were combined for subsequent analyses.
Locations from which cases were reported within the higher elevation category (d: > 2,115–2,945 m) consistently had greater precipitation and lower maximum temperatures than case locations at lower elevations (Table 2). Based on the 30 yr average climate data, the highest elevations exceeded 27°C in July and August by only 1.6°C (1.4°C SD; category d) compared with 5.6°C (2.5°C SD; categories a–c) at the lower elevations. Furthermore, the points within the highest elevation category were significantly more vegetated (higher greenness, lower brightness) and wetter than lower elevation sites (Table 3). Examining difference values for greenness between September and June images revealed a more constant presence of green vegetation at high elevations compared with lower elevation (Table 3).
Table 2.
Seasonal comparison of climate variables based on 30 yr average values for highest elevation sites (> 2,115–2,945 m, N = 70) and all other sites (1,238–2,115 m, N = 213)*
| Winter | Fore-summer | Monsoon | Fall | |||||
|---|---|---|---|---|---|---|---|---|
| High | Other | High | Other | High | Other | High | Other | |
| Precipitation (mm) Mean (SD) | 97.2 (32.3) | 71.5 (15.1) | 134.4 (15.8) | 110.2 (22.6) | 115.4 (18.8) | 100 (14.5) | 87.6 (20.4) | 73.5 (13.2) |
| Maximum temperature (°C) | ||||||||
| Mean (SD) | 13.3 (2.1) | 16.4 (1.9) | 25.1 (1.8) | 27.8 (1.3) | 24.8 (1.6) | 27.4 (1.2) | 10.9 (1.4) | 13.1 (1.2) |
| July and August temp maximum > 27°C | ||||||||
| Mean (SD) | 1.6 (1.4) | 5.6 (2.5) | ||||||
Seasons are defined as winter (January–April), fore-summer (May–July), monsoon (August and September), fall (October–December). Precipitation comparisons for high vs. other analysis of variance (ANOVA) F > 44.39, P < 0.001. Temperature comparisons high vs. other ANOVA F > 141.6 P < 0.001 except for fore-summer (ANOVA F = 186.08, P = 0.0035).
Table 3.
Comparison of ground cover variables for highest (> 2,115–2,945 m, N = 68) and all other sites (1,238–2,115 m, N = 193 [N = 192 for September wetness]) elevation categories. Higher brightness values indicate more bare soil and higher greenness values indicate greater vegetation cover. Wetness is a measure of canopy and soil moisture and higher values indicate greater wetness
| Tassel Cap transformation–June | ||||||
|---|---|---|---|---|---|---|
| Brightness | Greenness | Wetness | ||||
| High | Other | High | Other | High | Other | |
| Mean (SD) | 204.2 (25.0) | 236.5 (26.2) | 5.5 (11.3) | 0.8 (7.9) | −88.4 (13.0) | −98.6 (12.2) |
| Tassel Cap transformation–September | ||||||
|---|---|---|---|---|---|---|
| Brightness | Greenness | Wetness | ||||
| High | Other | High | Other | High | Other | |
| Mean (SD) | 166.3 (22.7) | 195.9 (25.1) | 5.0 (8.7) | 2.8 (6.9) | −78.6 (12.6) | −88.2 (11.3) |
June Tassel Cap comparisons for high vs. other analysis of variance (ANOVA) F > 13.79, P < 0.001. September Tassle Cap comparisons high vs. other ANOVA F > 33.78 P < 0.001 except for greenness (ANOVA F = 4.42, P = 0.04).
Climatic predictors of inter-annual variation in case occurrence.
Predictive models of case occurrence for the three lower elevation sites combined (1,238–2,115 m) were constructed using linear regression. The number of cases per year was square root transformed to achieve normality (Shapiro–Wilk W = 0.96, P = 0.344). Competing models are shown in Table 4. Based on the selected model, the current year's total pet cases (square root transformed) was predicted based on positive associations with the previous year's average precipitation, total degrees over 27°C 3 years prior and winter temperature maximum 4 years prior (R2= 0.749, AIC = 63.33, P < 0.001). Model fit tests indicated an appropriate model (Table 4). The L-O-O validation indicated that the model was not overly sensitive to input from any one year (mean R2 = 0.747, 95% CI: 0.673–0.856) (Figure 3).
Table 4.
Forward stepwise models predicting annual pet cases (square root transformed)*
| Model | coef | P var | P model | R2 | AIC | df | Link | OV test | Het test |
|---|---|---|---|---|---|---|---|---|---|
| Model 1: Regress pet (square root transformed) on | |||||||||
| 0.0001 | 0.436 | 95.13 | 2 | – | – | 0.13 | |||
| Surveillance 1991 | 2.2 | 0.000 | |||||||
| Model 2: Regress pet (square root transformed) on | |||||||||
| < 0.0001 | 0.520 | 87.35 | 3 | 0.66 | 0.79 | 0.53 | |||
| Surveillance 1991 | 2.13 | 0.000 | |||||||
| 1 yr lag precip | 0.207 | 0.013 | |||||||
| Model 3: Regress pet (square root transformed) on | |||||||||
| < 0.0001 | 0.658 | 72.71 | 4 | 0.35 | 0.75 | 0.94 | |||
| Surveillance 1991 | 2.17 | 0.000 | |||||||
| 1 yr lag precip | 0.29 | 0.000 | |||||||
| 3 yr lag > 27°C | 0.11 | 0.002 | |||||||
| Model 4: Regress pet (square root transformed) on | |||||||||
| < 0.0001 | 0.749 | 63.33 | 5 | 0.49 | 0.32 | 0.14 | |||
| Surveillance 1991 | 1.89 | 0.000 | |||||||
| 1 yr lag precip | 0.36 | 0.000 | |||||||
| 3 yr lag > 27°C | 0.09 | 0.006 | |||||||
| 4 yr lag winter temperature maxium | 0.27 | 0.009 | |||||||
The final model included a dichotomized surveillance variable (0 = pre-1991), 1 year lagged annual average precipitation (precip), 3 year lagged total degrees over 27°C, and 4 year lagged winter temperature maximum. Models were compared by Akaike information criterion (AIC) and R2 and tested for validity using a link test, omitted variable test (OV test), and for heteroskedasticity (Het test). Winter spans the months of January through April.
Figure 3.
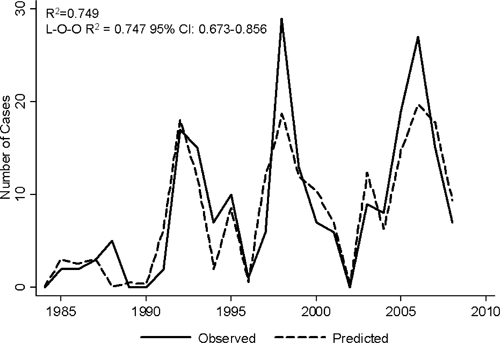
The final model explained 75% of the inter-annual variance in pet plague cases. The model was tested by singly dropping each year and running the model (Leave-One-Out). The mean R2 of this method was 0.747. The plotted observed vs. predicted cases show a good fit of pet case peak and trough, with limited discrepancy with respect to amplitude. Because of the reliance of the model on previous years' meteorological data, the predictions begin in 1984.
We tested whether the inclusion of the previous year's total pet cases (square root transformed) improved the model as has been shown in other models.14 When added to our best model, the lagged square root pet variable was not significant (P = 0.066) and, although there was slight improvement to the model (R2 = 0.780, AIC = 60.76, P < 0.001), the link test indicated the model was no longer correctly specified (P = 0.03). We also compared our model based on only the lower three elevation classes (1,238–2,115 m) with a model using all of the data (1,238–2945 m) and, while the R2 (0.787) and AIC (60.1) were comparable, it was incorrectly specified (link test P = 0.005) and included only precipitation above mean (lags, 1, 3, 4, and 5) and the current year's fall maximum temperature—reducing its predictive properties.
The model generated for pet cases was then compared with human plague cases reported from New Mexico. The human cases were not stratified by elevation and were restricted to those occurring after the increased pet surveillance began in New Mexico (1991 through 2007, N = 62). The correlation between human and the predicted number of pet cases was 0.520 (P = 0.032) (Figure 4).
Figure 4.
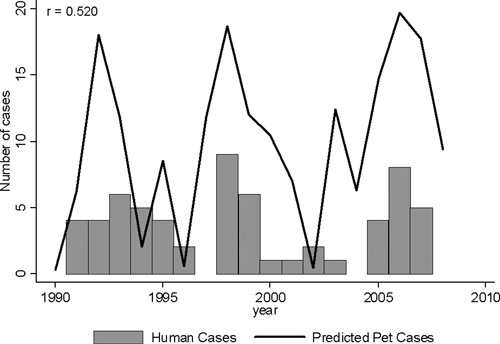
Predicted pet and the actual human plague cases since 1991. The bars represent the total number of human plague cases reported from New Mexico to the CDC (1991 through 2007). The line represents the model's predicted number of pet cases. We limited the data to post 1990 for this comparison to capture the increased surveillance that was significant in the model. The correlation between predicted pet cases and human cases is 0.520 (P = 0.032).
Discussion
Using 29 years of animal-based surveillance data, we identified distinctively different seasonal case occurrence patterns between high- and low-elevation sites. Specifically, more aggregated distributions and earlier onset dates were evident at lower elevation sites (a: 1,238–1,846 m; b: > 1,846–2,022 m; c: > 2,022–2,115 m) which, compared with higher elevation areas (d: > 2,115–2,945 m), are characterized by drier and hotter conditions with transient presence of limited vegetation. In addition, our time-series model accurately predicted the number of cases reported per year at lower elevations (1,238–2,115 m) based on warmer conditions 3–4 years prior, and increased precipitation in the previous year. Moreover, the predictions based on pet plague cases were significantly correlated with human plague cases in the same region.
Drivers of Y. pestis transmission dynamics are still poorly understood.1,27 It is not clear if the seasonal patterns detected here through animal-based surveillance represent different underlying transmission dynamics perhaps related to host and flea abundance or differences in host-seeking behavior of the fleas.7,28,29 The trophic cascade hypothesis suggests that suitable temperature and precipitation lead to increased primary vegetative production; the increased food supply increases the carrying capacity of rodent populations, which also increases the number of hosts for fleas and this in turn increases the likelihood of epizootic spread.7,11,12,23,30–32 Alternatively, flea survivorship and host-seeking behavior are closely associated with optimal temperature and moisture conditions.33–35 Thus, it is not surprising that epidemiological studies have revealed human plague cases are closely associated with temperature and precipitation patterns.11,12,29
Typically, plague cases are reported from areas with average temperatures in excess of 13°C, with most outbreaks reported when temperatures range from 24 to 27°C.29,36–39 Epidemic activity usually ceases when temperatures exceeded 27°C. Our analysis using 30 yr average precipitation and temperature data showed that, compared with lower elevation sites, highest elevation areas experience moister winter and fore-summer conditions and milder summer temperatures that rarely exceed 27°C. Optimal temperature and moisture conditions within our highest elevation class may result in persistent transmission for most of the year compared with lower elevations where most cases are reported from March to June and then decline as temperatures exceed 27°C (Figure 2).
A possible explanation for the sustained intra-annual transmission at higher elevation is that the combined cool and moist conditions from spring through the fall at high elevations may be optimal for flea survival and host seeking. Studies in California manipulating moisture levels in ground squirrel burrows showed that lack of moisture adversely effected Oropsylla montana breeding and abundance.34 Oropsylla montana is believed to be the primary bridging vector to humans and their pets because it is a highly efficient early-phase vector of Y. pestis, infests susceptible squirrels commonly associated with peridomestic settings (e.g., rock squirrels), and readily bites humans and domestic animals in the absence of its normal hosts.6,7,40
Consistent with previous studies, our inter-annual model identified a positive association between case occurrence and preceding moisture.11,12,14,23 The positive direction of the association with temperature maximums was somewhat unexpected, but the long delays provide a possible explanation. Milder winters 4 years prior may be indicative of reservoir host population increases as per the trophic cascade model. For example, milder winters could result in greater survival of rodents and fleas or longer growing periods because of earlier plant germination or hotter summers could be necessary for reproduction of specific types of plants. Rodents associated with plague in New Mexico (e.g., woodrats [Neotoma spp.], rock squirrels [Spermophilus variegatus], and the white-tailed antelope squirrel [Ammospermophilus leucurus]) typically produce one or two litters per year with limited numbers of offspring (2–4; 5–14 for antelope squirrels).41–44 In warmer areas, they are likely to have more litters per year. For rodents, as these with long reproductive periods, it may take a few years for their populations to build up, but warming conditions may facilitate population growth.31 Ecological studies of the interaction of rodents and fleas and the influence of climate on their behavior are necessary to explicitly define the processes that drive plague transmission dynamics.
Similar to other studies, we found that a spatial component exerted considerable influence on the predictive potential of meteorological-based models of plague case occurrence. Specifically, including the ecologically divergent high elevation cases in our inter-annual model yielded an inaccurate model. Elsewhere in the literature, meteorological-based models created for New Mexico did not fit those for Arizona and models specific to Montana were inadequate for Colorado.12,23 Weather indices that are not spatially explicit or are derived at an inappropriate spatial scale may not accurately capture local meteorological nuances.13,45 Exclusion of the ecologically divergent high elevation data from our inter-annual models may indicate a compelling move forward in understanding plague dynamics. An elevation gradient and its corresponding different ecological presentation indicate different biological drivers are active which, when aggregated, obfuscate delineation of plague cycles.
The exact nature of our findings with respect to the salient drivers of temporal plague dynamics—susceptibility differences among plague hosts at high versus low elevations, temperature limitations on flea questing behavior, population synchrony of reservoirs caused by climate and food resources, or other factors—remains unclear. Our intra-annual comparison supports the hypothesis that temperatures above 27°C limit the annual cycle of plague transmission possibly because of flea blockage, decreased questing behavior, or reductions in flea abundance. At the inter-annual scale, meteorological predictors with long time lags provide support for the trophic cascade hypothesis where rodent populations exhibit delayed response to more favorable conditions. Additional field studies are necessary to further refine the ecological mechanisms in both intra- and inter-annual plague dynamics. Nonetheless, though the biological mechanisms are still under investigation, we identified an epizootically distinct elevation difference and provided a strongly predictive model using local meteorological variables that is accurate and resilient to outliers.
Although the biological mechanisms behind the time lags included in our models remain speculative, the long lag times are advantageous from a practical perspective. Models that include meteorological estimates for the same year cases occur, even if they are based on an earlier season, are of limited value as predictive tools unless climate forecasting is significantly improved. The shortest time lag included in our model was 1 year. This makes it feasible to tabulate historic weather patterns in advance of the plague season, and thus may be useful for predictive purposes. Overall, our time-lagged inter-annual models of pet plague cases explained 75% of the variance between years. The majority of the unexplained variance seems to be related to the magnitude of cases in a year (with the model predicting somewhat fewer) rather than the timing of peak years. Although the model may underestimate the number of cases in a year, capturing the correct timing of inter-annual peaks and troughs is critical for forecasting and early warning purposes. Based on the significant association between predicted annual pet cases and observed human cases, our findings show the value of incorporating well-planned animal-based surveillance activities into plague prevention and control programs.
Acknkowledgments
We acknowledge Gabrielle Dietrich at the CDC—DVBID for her assistance in collecting the data from the New Mexico Department of Health. This research was supported in part by the appointment of Heidi E. Brown to the Research Participation Program at the CDC, NCZVED, DVBID administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the CDC.
Footnotes
Authors' addresses: Heidi E. Brown, Jennifer L. Holmes, Kenneth L. Gage, and Rebecca J. Eisen, Centers for Disease Control and Prevention (CDC), National Center for Zoonotic, Vector-Borne and Enteric Diseases, Division of Vector-Borne Infectious Diseases, Fort Collins, CO, E-mails: HEBrown@cdc.gov, JHolmes@cdc.gov, KGage@cdc.gov, and RJEisen@cdc.gov. Paul Ettestad, Ted Brown, and Elizabeth S. Hatton, Epidemiology and Response Division, New Mexico Department of Health, Santa Fe, NM, E-mails: Paul.Ettestad@state.nm.us, brownlizard2279@msn.com, and Elizabeth.Hatton@state.nm.us. Pamela J. Reynolds, Zoonoses Program, New Mexico Department of Health, Santa Fe, NM, E-mail: thunderpass@gmail.com. Gregory E. Glass, Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: gglass@jhsph.edu.
References
- 1.Gage KL, Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 2.Craven RB, Maupin GO, Beard ML, Quan TJ, Barnes AM. Reported cases of human plague infections in the United States, 1970–1991. J Med Entomol. 1993;30:758–761. doi: 10.1093/jmedent/30.4.758. [DOI] [PubMed] [Google Scholar]
- 3.MMWR Human plague—four states, 2006. MMWR. 2006;55:940–943. [PubMed] [Google Scholar]
- 4.Hull HF, Montes JM, Mann JM. Plague masquerading as gastrointestinal illness. West J Med. 1986;145:485–487. [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen RJ, Enscore RE, Biggerstaff BJ, Reynolds PJ, Ettestad P, Brown T, Pape J, Tanda D, Levy CE, Engelthaler DM, Cheek J, Bueno R, Targhetta J, Montenieri JA, Gage KL. Human plague in the southwestern United States, 1957–2004: spatial models of elevated risk of human exposure to Yersinia pestis. J Med Entomol. 2007;44:530–537. doi: 10.1603/0022-2585(2007)44[530:hpitsu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Barnes AM. Surveillance and control of bubonic plague in the United States. Symp Zoo Soc London. 1982;50:237–270. [Google Scholar]
- 7.Eisen RJ, Bearden SW, Wilder AP, Montenieri JA, Antolin MF, Gage KL. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proc Natl Acad Sci USA. 2006;103:15380–15385. doi: 10.1073/pnas.0606831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poland JD, Barnes AM. In: CRC Handbook Series in Zoonoses. Steele JH, editor. Boca Raton, FL: CRC Press, Inc; 1979. pp. 515–559. (Plague). [Google Scholar]
- 9.Centers for Disease Control and Prevention Prevention of plague: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2006;45:1–15. [PubMed] [Google Scholar]
- 10.Eisen RJ, Reynolds PJ, Ettestad P, Brown T, Enscore RE, Biggerstaff BJ, Cheek J, Bueno R, Targhetta J, Montenieri JA, Gage KL. Residence-linked human plague in New Mexico: a habitat-suitability model. Am J Trop Med Hyg. 2007;77:121–125. [PubMed] [Google Scholar]
- 11.Parmenter RR, Yadav EP, Parmenter CA, Ettestad P, Gage KL. Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am J Trop Med Hyg. 1999;61:814–821. doi: 10.4269/ajtmh.1999.61.814. [DOI] [PubMed] [Google Scholar]
- 12.Enscore RE, Biggerstaff BJ, Brown TL, Fulgham RF, Reynolds PJ, Engelthaler DM, Levy CE, Parmenter RR, Montenieri JA, Cheek JE, Grinnell RK, Ettestad PJ, Gage KL. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960–1997. Am J Trop Med Hyg. 2002;66:186–196. doi: 10.4269/ajtmh.2002.66.186. [DOI] [PubMed] [Google Scholar]
- 13.Sheppard PR, Comrie AC, Packin GD, Angersbach K, Hughes MK. The climate of the US Southwest. Clim Res. 2002;21:219–238. [Google Scholar]
- 14.Ben Ari T, Gershunov A, Gage KL, Snall T, Ettestad P, Kausrud KL, Stenseth NC. Human plague in the USA: the importance of regional and local climate. Biol Lett. 2008;4:737–740. doi: 10.1098/rsbl.2008.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernest SKM, Brown JH, Parmenter RR. Rodents, plants, and precipitation: spatial and temporal dynamics of consumers and resources. Oikos. 2000;88:470–482. [Google Scholar]
- 16.Parmenter RR, Brantley SL, Brown JH, Crawford CS, Lightfoot DC, Yates TL. In: Biodiversity of Rangelands: Natural Resources and Environmental Issues. West NE, editor. Logan, UT: Utah State University; 1995. pp. 50–71. (Diversity of animal communities on southwestern rangelands: species patterns, habitat relationship and land management). [Google Scholar]
- 17.Kolivras KN, Comrie AC. Climate and infectious disease in the southwestern United States. Prog Phys Geogr. 2004;28:387–398. [Google Scholar]
- 18.Kaufmann AF, Mann JM, Gardiner TM, Heaton F, Poland JD, Barnes AM, Maupin GO. Public health implications of plague in domestic cats. J Am Vet Med Assoc. 1981;179:875–878. [PubMed] [Google Scholar]
- 19.Gage KL, Dennis DT, Orloski KA, Ettestad P, Brown TL, Reynolds PJ, Pape WJ, Fritz CL, Carter LG, Stein JD. Cases of cat-associated human plague in the western US, 1977–1998. Clin Infect Dis. 2000;30:893–900. doi: 10.1086/313804. [DOI] [PubMed] [Google Scholar]
- 20.Gould LH, Pape J, Ettestad P, Griffith KS, Mead PS. Dog-associated risk factors for human plague. Zoonoses Public Health. 2008;55:448–454. doi: 10.1111/j.1863-2378.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 21.Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect. 2005;113:688–692. doi: 10.1289/ehp.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crist EP, Kauth RJ. The tasseled cap de-mystified. Photogramm Eng Remote Sensing. 1986;52:81–86. [Google Scholar]
- 23.Collinge SK, Johnson WC, Ray C, Matchett R, Grensten J, Cully JF, Gage KL, Kosoy MY, Loye JE, Martin AP. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landscape Ecol. 2005;20:941–955. [Google Scholar]
- 24.Stapp P, Antolin MF, Ball M. Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Nino events. Front Ecol Environ. 2004;2:235–240. [Google Scholar]
- 25.Akaike H. New look at statistical-model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 26.Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24:38–49. [Google Scholar]
- 27.Gage KL, Ostfeld RS, Olson JG. Nonviral vector-borne zoonoses associated with mammals in the United States. J Mammal. 1995;76:695–715. [Google Scholar]
- 28.Eisen RJ, Gage K. Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Vet Res. 2009;40:1–14. doi: 10.1051/vetres:2008039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis DHS. Plague in Africa from 1935 to 1949: a survey of wild rodents in African territories. Bull World Health Organ. 1953;9:655–700. [PMC free article] [PubMed] [Google Scholar]
- 30.Davis S, Begon M, De Bruyn L, Ageyev VS, Klassovskiy NL, Pole SB, Viljugrein H, Stenseth NC, Leirs H. Predictive thresholds for plague in Kazakhstan. Science. 2004;304:736–738. doi: 10.1126/science.1095854. [DOI] [PubMed] [Google Scholar]
- 31.Davis S, Calvet E, Leirs H. Fluctuating rodent populations and risk to humans from rodent-borne zoonoses. Vector-Borne Zoonotic Dis. 2005;5:305–314. doi: 10.1089/vbz.2005.5.305. [DOI] [PubMed] [Google Scholar]
- 32.Kausrud KL, Viljugrein H, Frigessi A, Begon M, Davis S, Leirs H, Dubyanskiy V, Stenseth NC. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proc Biol Sci. 2007;274:1963–1969. doi: 10.1098/rspb.2007.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacot AW, Martin CJ. The respective influence of temperture and moisture upon the survival of the rat flea (Xenopsylla cheopis) away from its host. J Hyg (Lond) 1924;23:98–105. doi: 10.1017/s0022172400008500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryckman RE. Plague vector studies: II: the role of climatic factors in determining seasonal fluctuations of flea species associated with the California ground squirrel. J Med Entomol. 1971;8:541–549. doi: 10.1093/jmedent/8.5.541. [DOI] [PubMed] [Google Scholar]
- 35.Stenseth NC, Samia NI, Viljugrein H, Kausrud KL, Begon M, Davis S, Leirs H, Dubyanskiy VM, Esper J, Ageyev VS, Klassovskiy NL, Pole SB, Chan KS. Plague dynamics are driven by climate variation. Proc Natl Acad Sci USA. 2006;103:13110–13115. doi: 10.1073/pnas.0602447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavanaugh DC, Williams JE. In: International Conference on Fleas. Traub R, Starcke H, editors. Ashton World, Peterborough, UK: A.A. Balkema; 1977. (Plague: some ecological interrelationships). [Google Scholar]
- 37.Cavanaugh DC, Marshall JD. The influence of climate on the seasonal prevalence of plague in the Republic of Vietnam. J Wildl Dis. 1972;8:85–94. doi: 10.7589/0090-3558-8.1.85. [DOI] [PubMed] [Google Scholar]
- 38.Brooks RJ. The influence of saturation deficiency and of temperature on the course of epidemic plague. J Hyg (Lond) 1917;5:881–899. [PMC free article] [PubMed] [Google Scholar]
- 39.Gage KL, Burkot TR, Eisen RJ, Hayes EB. Climate and vectorborne diseases. Am J Prev Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 40.Barnes AM, Quan TJ, Beard ML, Maupin GO. Plague in American Indians, 1956–1987. MMWR. 1988;37:11–16. [PubMed] [Google Scholar]
- 41.Cornely JE, Baker RJ. Neotoma mexicana. Mammalian Species. 1986;262:1–7. [Google Scholar]
- 42.Braun JK, Mares MA. Neotoma micropus. Mammalian Species. 1989;330:1–9. [Google Scholar]
- 43.Oaks EC, Young PJ, Kirkland GL, Schmidt DF. Spermophilus variegatus. Mammalian Species. 1987;272:1–8. [Google Scholar]
- 44.Belk MC, Smith HD. Ammospermophilus leucurus. Mammalian Species. 1991;368:1–8. [Google Scholar]
- 45.Hallett TB, Coulson T, Pilkington JG, Clutton-Brock TH, Pemberton JM, Grenfell BT. Why large-scale climate indices seem to predict ecological processes better than local weather. Nature. 2004;430:71–75. doi: 10.1038/nature02708. [DOI] [PubMed] [Google Scholar]


