Abstract
We have reported the epidemic patterns of dengue disease in the Region of the Americas from 1980 through 2007. Dengue cases reported to the Pan American Health Organization were analyzed from three periods: 1980–1989 (80s), 1990–1999 (90s), and 2000–2007 (2000–7). Age distribution data were examined from Brazil, Venezuela, Honduras, and Mexico. Cases increased over time: 1,033,417 (80s) to 2,725,405 (90s) to 4,759,007 (2000–7). The highest concentrations were reported in the Hispanic Caribbean (39.1%) in the 80s shifting to the Southern Cone in the 90s (55%) and 2000–7 (62.9%). From 1980 through 1987, 242 deaths were reported compared with 1,391 during 2000–7. The most frequently isolated serotypes were DENV-1 and DENV-2 (90s) and DENV-2 and DENV-3 (2000–7). The highest incidence was observed among adolescents and young adults; dengue hemorrhagic fever incidence was highest among infants in Venezuela. Increasing dengue morbidity/mortality was observed in the Americas in recent decades.
Introduction
Dengue is a mosquito-borne disease, caused by serologically related but antigenically distinct single-strand positive-sense RNA viruses; the viruses have been grouped into four serotypes (DENV-1 through DENV-4) belonging to the genus Flavivirus (family Flaviviridae).1 Aedes aegypti is the primary mosquito vector; however, other species from the genus Aedes, such as Aedes albopictus, can also be vectors of dengue virus transmission.
The clinical spectrum of disease includes asymptomatic infection, mild dengue fever (DF), dengue hemorrhagic fever (DHF), or dengue shock syndrome, which is frequently fatal because of abnormal capillary permeability and plasma leakage.2 Unusual manifestations such as miocardiopathy, hepatic failure, and neurological disorders have also been reported.3 There is no specific treatment of dengue illness as yet, and vector control is the only preventive strategy.
Dengue incidence and prevalence are rising in endemic areas of the tropical and subtropical regions. On the basis of mathematical model estimates, approximately 50 million infections occur each year.4 Dengue infection occurs in more than 100 countries in the Asia-Pacific, the Americas, the Middle East, and Africa, and cases continue to rise worldwide.5,6 In the past, the burden of the disease has fallen on countries in South-East Asia and the Western Pacific regions of the World Health Organization (WHO). However, a dramatic increase of cases has been reported in the Americas during the last decade.7 Recent dengue virus transmission has been reported in almost every country in this region, and Uruguay and continental Chile are the only countries without indigenous transmission in Latin America.
Although there is a perceived increase in disease burden, dengue epidemiology of the Americas region has not been well documented. Here, we characterize dengue disease dynamics during the last three decades and track the spread of dengue across the Americas.
Methods
To examine the disease patterns, we obtained the dengue infection records of WHO and of the Pan American Health Organization (PAHO), the regional body of WHO responsible for the 35 nations and 9 territories of the Americas.7 Because age and monthly distribution of cases are not routinely reported to PAHO, we obtained data from the Ministries of Health of selected countries to assess age distribution and seasonality patterns.
Annual PAHO reports of DF and DHF cases, reported according to the PAHO dengue case definitions,8 were analyzed by subregions and by country. Seven PAHO subregions were considered: North America (excluding Mexico), Central America/Mexico, Andean (i.e., Bolivia, Colombia, Ecuador, Peru, and Venezuela), Southern Cone (i.e., Argentina, Brazil, Chile, Paraguay, and Uruguay), Hispanic Caribbean, and Non-Hispanic Caribbean. Three time periods were considered: 1980 through 1989 (80s), 1990 through 1999 (90s), and 2000 through 2007 (2000–7). Population data from the U.S. Census Bureau Population Division, Department of Commerce for 1980 through 2006 and from PAHO for the year 2007 only were used to calculate country incidence rates for each study time period.7,9 Fatality records from this source were unavailable from 1990 to 1994.
Because serotype identity had been systematically determined since 1995, serotype circulation could be assessed from PAHO records beginning in 1995. The identity and proportions of serotypes circulating in each country were compared.
To examine case age distribution, we analyzed available data from Brazil, Venezuela, Honduras, and Mexico, because these countries reported the highest number of DF and DHF cases during the last two decades. Incidence rates by age-adjusted population (per 100,000 inhabitants) were calculated. The dengue case age distribution for Brazil was obtained from that country's Ministry of Health (MoH) website and Sistema de Informação de Agravos de Notificação (SINAN) for the period 2001–2006; population data were obtained from the same sources.10,11 Venezuelan age incidence data were obtained from the MoH website for 2005 through 2007.12 We also reviewed data from the Secretariat of Health of Honduras (Dirección General de Vigilancia en Salud, [DGVS]) for 2004 through 2007. Mexican case, incidence and population data were obtained from the MoH, General Directorate of Epidemiology (GDE) website for 2002 through 2006.13
To examine seasonality, we obtained data from the Brazilian MoH and SINAN, and Mexican MoH, GDE, and the Honduran DGVS websites. We examined available gender distribution data from Mexico (2003 through 2007) and Brazil (2001 through 2005) at their respective MoH websites.10,11,13
Results
Dengue disease patterns.
The total dengue cases reported in the Region were 1,033,417 (16.4/100,000) during the 80s, 2,725,405 (35.9/100,000) during the 90s, and 4,759,007 (71.5/100,000) during 2000–7. Similarly, the number of DHF cases increased over time from 13,398 (0.2/100,000) during the 80s, to 58,419 (0.8/100,000) during the 90s, to 111,724 (1.7/100,000) during 2000–7. The DHF cases as a percentage of total dengue cases also increased from 1.3% to 2.1% to 2.4% (Table 1). Epidemic cycles were observed every 3–5 years with an increased frequency of DHF cases and fatalities caused by dengue during epidemic years (Figure 1). From 1980 through 1987, 242 deaths were reported (1.8% of all DHF cases) compared with 1,391 deaths during 2000–7 (1.2% of all DHF cases).
Table 1.
Dengue fever (DF) dengue hemorrhagic fever (DHF) cases, incidence/100,000, and fatalities by subregion and decade region of the Americas 1980–2007*
| Time period | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1980–9 | 1990–9 | 2000–7 | Total | |||||
| No. of cases | Average incidence/100,000 | No. of cases | Average incidence/100,000 | No. of cases | Average incidence/100,000 | No. of cases | Average incidence/100,000 | |
| Subregion | ||||||||
| North America | ||||||||
| Dengue total cases | 1,213 | 0.05 | 350 | 0.01 | 796 | 0.03 | 2,359 | 0.03 |
| DF cases | 1,213 | 0.05 | 350 | 0.01 | 796 | 0.03 | 2,359 | 0.03 |
| DHF cases | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Fatalities | 0 | 0 | 0 | 0 | ||||
| Central America and Mexico | ||||||||
| Dengue total cases | 280,584 | 28.67 | 519,777 | 41.42 | 604,507 | 53.65 | 1,404,868 | 41.24 |
| DF cases | 280,412 | 28.65 | 512,665 | 40.86 | 567,301 | 50.35 | 1,360,378 | 39.95 |
| DHF cases | 172 | 0.02 | 7,112 | 0.56 | 37,206 | 3.29 | 44,490 | 1.29 |
| Fatalities | 11 | 172 | 258 | 441 | ||||
| Andean subregion | ||||||||
| Dengue total cases | 120,875 | 14.85 | 564,640 | 55.94 | 891,838 | 96.44 | 1,577,353 | 55.74 |
| DF cases | 118,208 | 14.55 | 515,926 | 51.15 | 826,489 | 89.39 | 1,460,623 | 51.7 |
| DHF cases | 2,667 | 0.30 | 48,714 | 4.79 | 65,349 | 7.05 | 116,730 | 4.05 |
| Fatalities | 73 | 279 | 331 | 683 | ||||
| Southern Cone | ||||||||
| Dengue total cases | 196,497 | 10.15 | 1,499,598 | 66.30 | 3,056,054 | 155.22 | 4,752,149 | 77.22 |
| DF cases | 196,493 | 10.15 | 1,498,721 | 66.26 | 3,049,262 | 154.87 | 4,744,476 | 77.09 |
| DHF cases | 4 | 0.00 | 877 | 0.04 | 6,792 | 0.34 | 7,673 | 0.13 |
| Fatalities | 0 | 21 | 500 | 521 | ||||
| Hispanic Caribbean | ||||||||
| Dengue total cases | 404,514 | 210.53 | 111,634 | 50.39 | 89,525 | 46.95 | 605,673 | 102.62 |
| DF cases | 394,128 | 205.01 | 110,448 | 49.86 | 88,206 | 46.26 | 592,782 | 100.38 |
| DHF cases | 10,386 | 5.52 | 1,186 | 0.53 | 1,319 | 0.69 | 12,891 | 2.25 |
| Fatalities | 158 | 78 | 256 | 492 | ||||
| Non-Hispanic Caribbean | ||||||||
| Dengue total cases | 29,734 | 24.02 | 29,406 | 20.60 | 89,874 | 70.97 | 149,014 | 38.52 |
| DF cases | 29,565 | 23.88 | 28,876 | 20.23 | 88,816 | 70.12 | 147,257 | 38.08 |
| DHF cases | 169 | 0.14 | 530 | 0.37 | 1,058 | 0.84 | 1,757 | 0.45 |
| Fatalities | 0 | 27 | 46 | 73 | ||||
| Total region | ||||||||
| Dengue total cases | 1,033,417 | 16.42 | 2,725,405 | 35.88 | 4,732,594 | 71.1 | 8,491,416 | 41.13 |
| DF cases | 1,020,019 | 16.21 | 2,666,986 | 35.10 | 4,620,870 | 69.43 | 8,307,875 | 40.25 |
| DHF cases | 13,398 | 0.21 | 58,419 | 0.77 | 111,724 | 1.67 | 183,541 | 0.89 |
| Fatalities | 242 | 577 | 1,391 | 2,210 | ||||
A total of 1,159 cases were not included because the country was not specified.
Deaths from 1990–4 are not reported.
Figure 1.
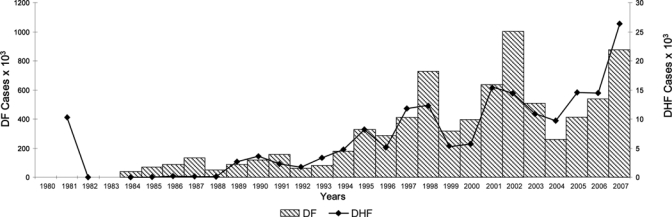
Number of dengue fever (DF) and dengue hemorrhagic fever (DHF) cases, Region of the Americas, 1980–2007.
The Southern Cone and Andean subregions reported the highest proportion of cases during the 90s: 55% (66.3/100,000) for Southern Cone and 20.7% (55.9/100,000) for Andean subregion. In 2000–7, the proportion of cases remained approximately the same but the incidence increased substantially: 64.2% (155.3/100,000) for Southern Cone and 19% (97.7/100,000) for Andean. Interestingly, two different regions reported the highest percentages of cases during the 80s: Hispanic Caribbean 39.1% (210.5/100,000), and Central America/Mexico 27.2% (28.7/100,000). In general, reported cases increased over time in most subregions. In contrast, the number of reported cases was highest in the 80s in the Hispanic Caribbean and declined over time (Table 1). Similarly, the majority of DHF cases and the highest incidence of disease occurred in the Hispanic Caribbean in the 80s: 77.5% (5.5/100,000); the Andean subregion reported the most DHF cases during the 90s (83.4% [4.8/100,000]) and 2000–7 (58.5% [7.1/100,000]).
The number of countries with an average incidence greater than 100/100,000 increased from 5 during the 80s, to 7 during the 90s to 15 during 2000–7 (Figure 2). Brazil reported the majority of dengue cases (54.5%) during the 27-year study period but ranked sixth in total DHF cases (Figure 3 ). Venezuela reported the highest number of DHF cases in the (35.1%) during the same period. During the 80s by contrast, Cuba experienced the highest number of dengue and DHF cases. By the end of 2007, only 2 countries (Uruguay and Chile) reported an absence of indigenous transmission. Endemic dengue transmission was reported in the Easter Island, a Chilean territory at the Pacific Ocean.
Figure 2.
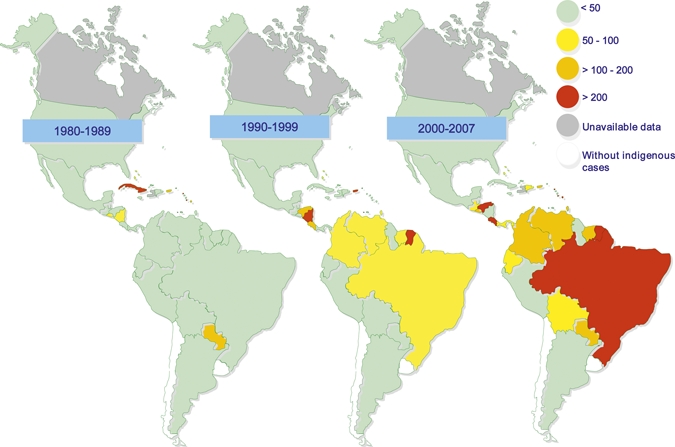
Average dengue incidence per 100,000 by country, Region of the Americas, 1980–2007.
Figure 3.
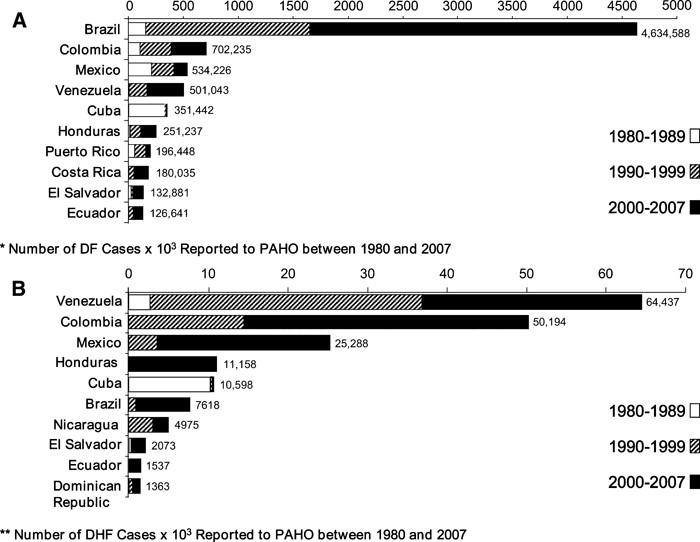
A, Countries with the highest number of dengue fever (DF) cases, Region of the Americas, 1980 and 2007. B, Countries with the highest number of dengue hemorrhagic fever (DHF) cases, Region of the Americas, 1980 and 2007.
Dengue serotypes.
During 1995 through 1999, all four serotypes were reported in Central America and the Caribbean subregions. Serotypes DENV-1, DENV-2, and DENV-4 were detected in the Andean subregion, but only DENV-1 and DENV-2 were circulating in the Southern Cone. By 2000–7, all four serotypes were circulating throughout the Americas, except for the Southern cone, where only DENV-4 was not detected during the period. The number of countries circulating ≥ 3 serotypes in any given year was 5 during 1995 through 1999 and rising to 15 during 2000–7.
The circulating serotypes reported most frequently during the 90s were DENV-1 and DENV-2. This pattern changed during 2000–7 when DENV-2 and DENV-3 were the most frequently reported serotypes (Figure 4). DENV-3 circulation increased throughout the Americas after its introduction in the Andean and Southern Cone subregions in 2000.
Figure 4.
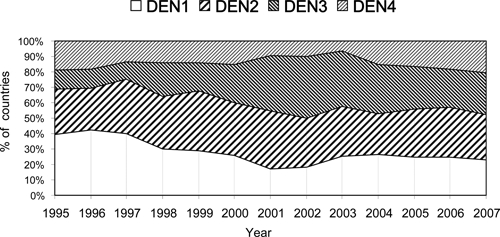
Percentage of countries reporting serotypes 1–4 to the Pan American Health Organization by year, Region of the Americas, 1995–2007.
Age distribution of cases.
In Brazil, the highest incidence of DF during 2000–7 was among young adults. In Venezuela, incidence was highest among 10- to 14-year-old children and adolescents, but peaked among 5 to 9 year olds in 2007 (570/100,000). In Honduras, the incidence across age groups was similar. In Mexico, children and adolescents 10 through 14 years of age had the highest dengue incidence rates followed by adolescents 16 through 18 years of age (Figure 5).
Figure 5.
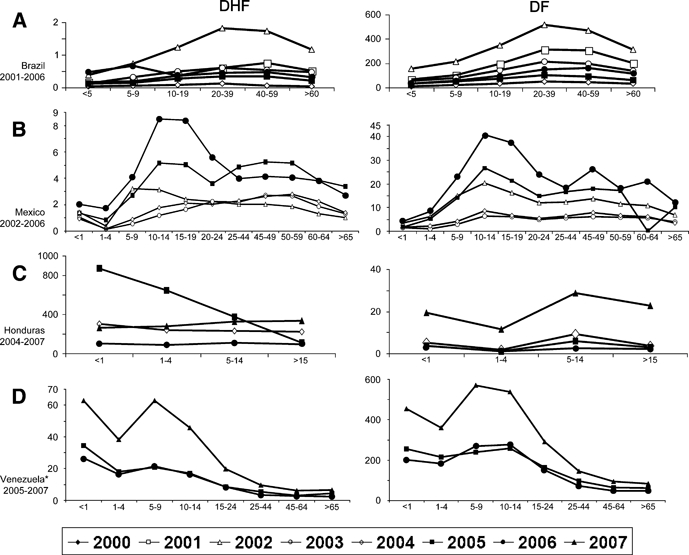
Incidence rates per 100,000 of dengue fever (DF) and dengue hemorrhagic fever (DHF) cases by age: (A) Brazil, (B) Mexico, (C) Honduras, and (D) Venezuela.
Figure 6.
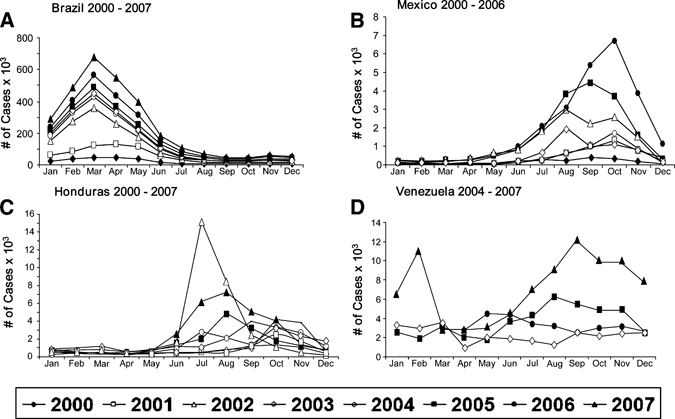
Monthly distribution of dengue cases: (A) Brazil, (B) Mexico, (C) Honduras, and (D) Venezuela.
In general, DHF incidences by age group were similar to those observed for DF. However, in Venezuela the highest incidence rates were observed among infants starting in 2005 and peaking in 2007 (62.9/100,000). In Brazil, the highest DHF incidence rates were among young adults during 2001 through 2005 and were coincident with the highest incidence of DF. However, in 2006 the incidence rate among < 5 year olds increased dramatically (0.47/100,000) and was higher than that observed among 10 through 19 year olds (0.36/100,000) and 20 through 39 year olds (0.46/100,000).
Other epidemiological features.
A seasonal pattern was observed in the countries with available monthly case distribution data. In Brazil, most cases occur in the first half of the year, peaking in March–April. However, in Honduras and Mexico the cases began to increase in June–July, and most cases were concentrated in the second half of the year. In Mexico, cases peaked between August and October throughout 2000–7 (Figure 6).
The case reports indicate that women were more frequently infected with dengue than men. The male: female ratio ranged from 1:1.22 to 1:1.34 in Brazil during 2001–5 and from 1:1.27 to 1:1.41 in Mexico during 2003 through 2007.
Discussion
The data reported herein suggest that the Americas region overall is trending toward the higher dengue incidence rate levels. The Region of the Americas reports the most WHO-reported cases of dengue worldwide (68%, 2000 through 2006).14 Although the greatest proportion of dengue-related deaths are reported in the South-East Asia and Western Pacific regions and some of these countries may underreport dengue-related disease by recording only DHF cases, the disease burden in the Americas is a significant public health concern. Over the last three decades, a 4.6-fold increase in reported cases was observed in the Americas (~1 million cases during the 80s to 4.7 million during 2000–7).
The endemo-epidemic nature of dengue in the Americas features recurring peaks of reported cases at 3- to 5-year intervals. Over time, the peaks have become progressively higher. More worrisome is the increasing trend of observed DHF cases. During the study period, DHF cases increased 8.3-fold, (~13,400 in the 80s to ~111,700 DHF in 2000–7).
Since 1980, the disease has broadly expanded throughout the region. The Southern Cone reported the most cases since 1990, with Brazil alone accounting for 63% of reported cases since 2000. In contrast, the Latin Caribbean and Central America/Mexico reported the highest number of DF and DHF cases after the emergence of dengue in Cuba in 1981.15 In the following two decades, the Andean subregion reported the majority of DHF cases, after a large epidemic in Venezuela in 1989.16
The epidemiological patterns of dengue in the Americas Region may be related to outbreaks of disease caused initially by a single dengue serotype followed by infections by a different serotype. DENV-1 was reported for the first time in the region in 1977 causing an epidemic, which began in Jamaica expanded to Cuba, Puerto Rico, and Venezuela, and eventually to the rest of the Caribbean countries, Mexico, Central America, and the northern countries of South America. DENV-2 caused the first DHF epidemic in the region when reported in Cuba in 1981.17 During the same year, DENV-4 was introduced into the eastern Caribbean islands. This serotype then expanded to the rest of the Caribbean and Central, South America and Mexico.18 Despite the increasing number of dengue cases during 1981 through 1988, the incidence of DHF in the region remained low until 1989 when a second DHF epidemic was reported in Venezuela. DENV-1, -2, and -3 serotypes were isolated from clinical samples with DENV-2 predominant.16 During the 80s, dengue started to circulate in Brazil, Bolivia, Paraguay, Ecuador, and other South American countries that had not experienced the disease before.19 In Brazil, the first laboratory-confirmed dengue outbreak was reported in 1981–1982 in Roraima state.20 This outbreak was contained after local vector control measures were implemented. No further dengue activity was reported until 1986 with the introduction of DENV-1 in Rio de Janeiro state.21
In 1990, DENV-2 serotype was introduced in Rio de Janeiro during a period of DENV-1 serotype circulation.22 DENV-2 serotype in Brazil spread to other parts of the country with more severe clinical presentations, and the first fatal cases caused by secondary dengue infections.23 In 1994, DENV-3 virus was reintroduced in the Americas after an absence of 16 years. Colombia and Puerto Rico reported this serotype in 1977 and 1978, but it was not observed again until 1994 in Nicaragua and Panama.24 DENV-3 serotype initially expanded to Central American countries and Mexico, and later to Puerto Rico, other Caribbean islands, and South America.25
In 2000, DENV-3 serotype was introduced in Rio de Janeiro causing a widespread, 3-year outbreak.25–27 Although DENV-3 was the predominant serotype and was the only serotype associated to fatalities, DENV-1 and -2 serotypes were also circulating.26 DENV-3 virus later spread more broadly in Brazil.26–28 DENV-2 was reintroduced to Rio de Janeiro in 2007 and 2008 causing the majority of fatal cases during that time.29,30 These data suggest that a prior dengue infection may set the stage for a more deadly infection by a different serotype in a few years time. Several seroepidemiological studies support this hypothesis.31,32
Although the populations in Southeast Asia and the Americas are similar and that all dengue serotypes are endemic in both regions, DHF rates in the Americas are lower compared with those reported in Southeast Asia since World War II.33 One potential explanation may be the failure of clinicians in the Americas to collect data to fulfill the requirements of the PAHO case definition. Another explanation is the association of severe disease to a specific dengue genotype. The introduction of the DEN-2 Asian genotype in the Americas region has been previously associated with DHF epidemics.34 Clade changes have also been associated with dengue severity.35–37 However, there are no viral or immunological mechanisms that can be associated to the absence of major DHF epidemics in Brazil. Infection with DENV-1, followed by DENV-2 viruses, known to have circulated in Brazil, exactly reproduced the conditions that led to DHF in Cuba. Lower DHF rates in Brazil have been related to a high prevalence of dengue resistance genes.33
In contrast with the DHF age distribution in Asian countries, in which DHF infected primarily young children6,33 dengue and DHF cases in the Americas most commonly affect older age groups, as shown in the selected countries of our study. These differences are not fully understood, but may be related to host, epidemiologic, and virologic factors. A trend toward infections in younger age groups was found in countries such as Venezuela, and Honduras with high circulation levels of several serotypes over several years. A similar trend has been reported in Nicaragua and Honduras.38,39 In Brazil, the incidence rates have been typically higher among adults.40 However, during 2007–2008 a new and severe epidemic was reported in Rio de Janeiro initiated by DENV-3 and later DENV-2 serotypes.29,30 DENV-2 was responsible for most fatal cases primarily in children.29,30,41,42
The majority of cases in countries in the southern hemisphere were observed in the first half of the year, but in the northern hemisphere countries examined in most cases occurred in the second half, which may be related with the rainy seasons in the countries. A slightly higher percentage of cases and higher incidence rates in females compared with males were observed in Mexico and Brazil. A higher proportion of dengue cases among females were also observed in Pernambuco and in Belem (at Pará state) in Brazil and in Cuba in 1981.28,43,44
Several factors may be involved in the increasing incidence of dengue in the Americas. The deterioration of the Aedes aegypti eradication program implemented by PAHO in the 1940s has resulted in increasing density of the vector since the 1970s.45 Although the introduction of Aedes albopictus, a secondary vector reported for the first time in the continent in 1985, could play a role in the maintenance of the virus cycle, it has not been associated with dengue transmission until recently. In addition, population growth, unplanned urbanization with poor sanitary conditions, deterioration of the public health infrastructure, and a decreased access to health care has also contributed to the increase of disease burden. Globalization of the economy, international travel, and climatic changes might also explain the disease expansion. In Mexico, increases in the amount of rainfall, higher sea-surface temperature, and increases in weekly minimum temperature may be related to increases of reported cases of dengue.46Although the association between climatic changes and the explosion of dengue in the Americas remains controversial; in April 2007, the Intergovernmental Panel on Climate Change concluded that global warming and climate change would cause an upsurge in dengue and other tropical diseases.47
Our study has some limitations. First, our data rely on a passive surveillance system, which typically underestimates the true number of cases as documented in a recent Puerto Rican epidemiological study.48 In a passive system, dengue reporting can vary over time, by country, or even by different regions within a country. Nevertheless, all countries reported dengue cases to PAHO and the number of reporting countries has remained unchanged throughout the study period. Although most reported cases were not laboratory confirmed, no major changes in the case definitions or reporting practices were observed during the study period. Second, the age distribution of cases is not collected systematically, and age range boundaries differ by country. Therefore, a trend in age distribution can be inferred but direct comparisons cannot be made. In large countries such as Brazil, seasonality may vary widely in different regions. In fact, dengue outbreaks may occur during both halves of the year as reported in the state of Rio de Janeiro during 1990–1991.22
In conclusion, the Americas Region evolved from a low dengue-endemic state to hyperendemic state with indigenous transmission now observed in almost all countries. The increasing trend in DHF cases and the occurrence of more severe cases in children is alarming. The only available control measures relies on vector control programs, which are costly and difficult to maintain. The development of effective vaccines to protect against the four dengue serotypes, in conjunction with the broad implementation of a PAHO initiative, the Integrated Management Strategy for Dengue Prevention, seem to be the most promising approaches for disease control.49
Received June 21, 2009. Accepted for publication September 17, 2009.
Acknowledgments
We thank Pilar Alvarez for her kind assistance compiling some epidemiological data and for the production of graphs. Editorial guidance and medical writing support was provided by Robert Lersch of Sanofi Pasteur.
Footnotes
Financial support: The manuscript was a collaboration between the authors. No funding was involved in the development of the manuscript.
Disclosure: Some of the authors are employed by Sanofi Pasteur, Inc. This statement is made in the interest of full disclosure and not because the authors consider this to be a conflict of interest. Information of this manuscript was previously presented at the IDSA/ICAAC 2008.
Authors' addresses: José Luis San Martín, Dengue Regional Program, Pan American Health Organization, Panamá, Republic of Panama, E-mail: sanmarjl@pan.ops-oms.org. Olivia Brathwaite, Dengue Regional Program, Pan American Health Organization (PAHO), Panama, Republic of Panama, E-mail: brathwao@pan.ops-oms.org. Betzana Zambrano, Clinical Department, Sanofi Pasteur, Montevideo, Uruguay, E-mail: betzana.zambrano@sanofipasteur.com. José Orlando Solórzano, General Directorate of Health Surveillance, Secretariat of Health, Tegucigalpa, Honduras, E-mail: josghn@yahoo.com. Alain Bouckenooghe, Clinical Department, Sanofi Pasteur, Singapore, E-mail: alain.bouckenooghe@sanofipasteur.com. Gustavo H. Dayan, Clinical Department, Sanofi Pasteur, Swiftwater, PA, E-mail: gustavo.dayan@sanofipasteur.com. María G. Guzmán, Virology Department, PAHO/World Health Organization Collaborating Center for the Study of Dengue and its Vector, Institute of Tropical Medicine ‘Pedro Kouri’, Havana, Cuba, E-mail: lupe@ipk.isld.cu.
References
- 1.Chang G-J. In: Dengue and Dengue Hemorrhagic Fever. Gubler DJ, Kuno G, editors. London: CAB International; 1997. pp. 175–98. (Molecular biology of dengue viruses). [Google Scholar]
- 2.Nimmannitya S. In: Dengue and Dengue Hemorrhagic Fever. Gubler DJ, Kuno G, editors. London: CAB International; 1997M. pp. 133–45. (Dengue hemorrhagic fever: diagnosis and management). [Google Scholar]
- 3.World Health Organization Dengue and dengue hemorrhagic fever: diagnosis, treatment, prevention and control. 1997. pp. 1–58.http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/ Available at. Accessed April 1, 2009.
- 4.World Health Organization . Strengthening Implementation of the Global Strategy for Dengue Fever and Dengue Hemorrhagic Fever, Prevention and Control. Geneva: WHO HQ; 1999. http://www.who.int/csr/resources/publications/dengue/whocdsdenic20001.pdf Report of the informal consultation, October 18–20, 1999. Available at. Accessed April 1, 2009. [Google Scholar]
- 5.Guzmán MG, Kouri G. Dengue and dengue hemorrhagic fever: research priorities. Rev Panam Salud Publica. 2006;19:204–215. doi: 10.1590/s1020-49892006000300015. [DOI] [PubMed] [Google Scholar]
- 6.Periago MR, Guzmán MG. Dengue and hemorrhagic dengue in the Americas. Rev Panam Salud Publica. 2007;21:187–191. doi: 10.1590/s1020-49892007000300001. [DOI] [PubMed] [Google Scholar]
- 7.Pan American Health Organization (PAHO) Number of reported cases of dengue and dengue hemorrhagic fever (DHF), Region of the Americas (by country and subregion) from 1995 through 2007. 2007. http://www.paho.org/english/ad/dpc/cd/dengue.htm Available at. Accessed April 1, 2008.
- 8.Pan American Health Organization . Dengue and Dengue Hemorrhagic Fever in the Americas: Guidelines for Prevention and Control. Washington, DC: Pan American Health Scientific Organization; 1994. Publication no. 548 ISBN 92 75 11548 6. [Google Scholar]
- 9.US Census Bureau Department of Commerce, International Database. http://www.census.gov/ipc/www/idb/summaries.html Available at. Accessed April 1, 2009.
- 10.Ministério da Saúde Dengue: Notificações registradas no Sistema de Informação de Agravos de Notificação – SINAN, Brazil. http://dtr2004.saude.gov.br/sinanweb/tabnet/dh?sinan/dengue/bases/denguebr.def Available at. Accessed April 1, 2009.
- 11.Ministério da Saúde . SINAN. D. Indicadores de Morbidade e fatores de risco, D.2.3 Taxa de incidência da dengue. Brazil: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?idb2006/d0203.def Available at. Accessed, April 1, 2009. [Google Scholar]
- 12.Ministerio del Poder Popular para la Salud Venezuela. Epidemiología, Alertas Epidemiológicos. http://www.mpps.gob.ve/ms/modules.php?name=Downloads&cid=4 Available at. Accessed April 1, 2009.
- 13.Secretaría de Salud de México Dirección General Adjunta de Epidemiología. http://www.dgepi.salud.gob.mx/index.htm Available at. Accessed April 1, 2009.
- 14.World Health Organization Dengue Net. http://www.who.int/csr/disease/dengue/denguenet/en/index.html Available at. [registration required]. Accessed April 1, 2009.
- 15.Kourí G, Guzmán MG, Bravo J. Hemorrhagic dengue in Cuba: history of an epidemic. Bull Pan Am Health Organ. 1986;20:24–30. [PubMed] [Google Scholar]
- 16.Pan American Health Organization Dengue haemorrhagic fever in Venezuela. Epidemiol Bull. 1990;11:7–9. [PubMed] [Google Scholar]
- 17.Kourí GP, Guzmán MG, Bravo JR, Triana C. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull World Health Organ. 1989;67:375–380. [PMC free article] [PubMed] [Google Scholar]
- 18.Pinheiro FP, Corber SJ. Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q. 1997;50:161–169. [PubMed] [Google Scholar]
- 19.Pan American Health Organization Dengue in the Americas: an update. Epidemiol Bull. 1993;14:1–3. [PubMed] [Google Scholar]
- 20.Osanai CH, Travassos da Rosa AP, Tang AT, do Amaral AS, Passos AD, Tauil PL. Outbreak of dengue in Boa Vista, Roraima. Preliminary report. Rev Inst Med Trop Sao Paulo. 1983;25:53–54. [PubMed] [Google Scholar]
- 21.Schatzmayr HG, Nogueira RM, Travassos da Rosa AP. An outbreak of dengue virus at Rio de Janeiro. Mem Inst Oswaldo Cruz. 1986;81:245–246. doi: 10.1590/s0074-02761986000200019. [DOI] [PubMed] [Google Scholar]
- 22.Nogueira RM, Miagostovich MP, Lampe E, Souza RW, Zagne SMO, Schatzmayr HG. Dengue epidemic in the state of Rio de Janeiro, Brazil, 1990–1: co-circulation of dengue 1 and dengue 2 serotypes. Epidemiol Infect. 1993;11:163–170. doi: 10.1017/s0950268800056788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Costa Vasconcelos PF, de Menezes DB, Melo LP, Pessoa P, Rodrigues SG, Travassos da Rosa E, Timbó MJ, Coelho CB, Montenegro F, Travassos da Rosa J, Andrade F, Travassos da Rosa A. A large epidemic of dengue fever with dengue hemorrhagic cases in Ceará State, Brazil, 1994. Rev Inst Med Trop Sao Paulo. 1995;37:253–255. doi: 10.1590/s0036-46651995000300012. [DOI] [PubMed] [Google Scholar]
- 24.Guzmán MG, Vázquez S, Martínez E, Alvarez M, Rodriguez R, Kouri G, de los Reyes J, Acevedo F. Dengue in Nicaragua, 1994: reintroduction of serotype 3 in the Americas. Pan Am J Publ Health. 1997;1:193–199. [PubMed] [Google Scholar]
- 25.Figueroa R, Ramos C. Dengue virus serotype 3 circulation in endemic countries and its reappearance in America. Arch Med Res. 2000;31:429>–430. doi: 10.1016/s0188-4409(00)00082-5. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira RM, Shcatzmayr HG, de Filippis AM, dos Santos FB, da Cunha RV, Coelho JO, de Souza LJ, Guimaraes FR, de Araújo ES, De Simone TS, Baran M, Teixeira G, Jr, Miagostovich MP. Dengue type 3, Brazil, 2002. Emerg Infect Dis. 2005;11:1376–1381. doi: 10.3201/eid1109.041043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melo PR, Reis EA, Ciuffo IA, Góes M, Blanton RE, Reis MG. The dynamics of dengue virus serotype 3 introduction and dispersion in the state of Bahia, Brazil. Mem Inst Oswaldo Cruz. 2007;102:905–912. doi: 10.1590/s0074-02762007000800003. [DOI] [PubMed] [Google Scholar]
- 28.Cordeiro MT, Schatzmayr HG, Nogueira RM, Oliveira VF, Melo WT, Carvalho EF. Dengue and dengue hemorrhagic fever in the State of Pernambuco, 1995–2006. Rev Soc Bras Med Trop. 2007;40:605–611. doi: 10.1590/s0037-86822007000600001. [DOI] [PubMed] [Google Scholar]
- 29.Ministério de Saúde, Secretaría de Vigilância em Saúde Informe Epidemiológico da Dengue. Janeiro a Dezembro 2007. http://portal.saude.gov.br/portal/arquivos/pdf/boletim_dengue_010208.pdf Available at. Accessed April 1, 2009.
- 30.Ministério de Saúde, Secretaría de Vigilância em Saúde Informe Epidemiológico da Dengue. Janeiro a Novembro 2008. http://portal.saude.gov.br/portal/arquivos/pdf/boletim_dengue_janeiro_novembro.pdf Available at. Accessed April 1, 2009.
- 31.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 32.Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma'roef C, Sutaryo E, Porter KR, Halstead SB. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I, studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 33.Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, da Rosa AT. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 34.Halstead SB. Dengue in the Americas and Southeast Asia: do they differ? Rev Panam Salud Publica. 2006;6:407–415. doi: 10.1590/s1020-49892006001100007. [DOI] [PubMed] [Google Scholar]
- 35.Salda LT, Parquet MD, Matias PR, Natividad FF, Kobayashi N, Morita K. Molecular epidemiology of dengue 2 viruses in the Philippines: genotype shift and local evolution. Am J Trop Med Hyg. 2005;73:796–802. [PubMed] [Google Scholar]
- 36.Balmaseda A, Gomez T, Henn M, Lennon N, Kuan G, Rocha C, Silva S, Gordon A, Birren B, Harris E. Increased dengue disease severity in Nicaragua is associated with a clade replacement in dengue virus 2. Am J Trop Med Hyg. 2008;79:15. [Google Scholar]
- 37.Aquino JD, Tang W-F, Ishii R, Ono T, Eshita Y, Aono H, Makino Y. Molecular epidemiology of dengue virus serotypes 2 and 3 in Paraguay during 2001–2006: the association viral clade introductions with shifting serotype dominance. Virus Res. 2008;137:266–270. doi: 10.1016/j.virusres.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Pérez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, González A, Harris E. Differences in dengue severity in infants, children and adults in a 3-year hospital-based study in Nicaragua. Am Trop Med Hyg. 2005;73:1063, 1071. [PubMed] [Google Scholar]
- 39.de Rivera IL, Parham L, Murillo W, Moncada W, Vázquez S. Humoral response of dengue hemorrhagic fever cases in children from Tegucigalpa, Honduras. Am Trop Med Hyg. 2008;79:262–266. [PubMed] [Google Scholar]
- 40.Nunes-Araújo FR, Ferreira MS, Nishioka Sde A. Dengue fever in Brazilian adults and children: assessment of clinical findings and their validity for diagnosis. Ann Trop Med Parasitol. 2003;97:415–419. doi: 10.1179/000349803235002263. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira MG, Costa NM, Coelho G, Barreto ML. Recent shift in age pattern of dengue hemorrhagic fever, Brazil. Emerg Infect Dis. 2008;14:1663. doi: 10.3201/eid1410.071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira MG, Costa NM, Barreto F, Barreto ML. Dengue: twenty-five years since reemergence in Brazil. Cad Saude Publica. 2009;25((Supp 1)):S7–S18. doi: 10.1590/s0102-311x2009001300002. [DOI] [PubMed] [Google Scholar]
- 43.Travassos da Rosa AP, Vasconcelos PF, Travassos da Rosa ES, Rodríguez SG, Mondet B, Cruz AC, Sousa MR, Travassos da Rosa JF. Dengue epidemic in Belém, Pará, Brazil, 1996–97. Emerg Infect Dis. 2000;6:298–301. doi: 10.3201/eid0603.000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kouri GP, Guzmán MG, Bravo JR. Why dengue haemorrhagic fever in Cuba? An integral analysis. Trans R Soc Trop Med Hyg. 1987;81:821–823. doi: 10.1016/0035-9203(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 45.Guzmán MG, Kourí G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 46.Hurtado-Díaz M, Riojas-Rodríguez H, Rothenberg SJ, Gomez-Dantés H, Cifuentes E. Impact of climate variability on the incidence of dengue in Mexico. Trop Med Int Health. 2007;12:1327–1337. doi: 10.1111/j.1365-3156.2007.01930.x. [DOI] [PubMed] [Google Scholar]
- 47.Barclay E. Is climate change affecting dengue in the Americas? Lancet. 2008;371:973–974. doi: 10.1016/s0140-6736(08)60435-3. [DOI] [PubMed] [Google Scholar]
- 48.Ramos MM, Argüello DF, Luxemburger C, Quiñones L, Muñoz JL, Beatty M, Lang J, Tomashek KM. Epidemiological and clinical observations on patients with dengue in Puerto Rico: results from the first year of enhanced surveillance, June 2005–May 2006. Am J Trop Med Hyg. 2008;79:123–127. [PubMed] [Google Scholar]
- 49.San Martin JL, Brathwaite-Dick O. La Estrategia de Gestión Integrada para la prevención y el control del dengue en la Región de las Américas. Rev Panam Salud Publica. 2007;21:55–63. doi: 10.1590/s1020-49892007000100011. [DOI] [PubMed] [Google Scholar]


