Abstract
• Background and Aims Caffeoylquinic acids are cinnamate conjugates derived from the phenylpropanoid pathway. They are generally involved in plant responses to biotic and abiotic stress and one of them, chlorogenic acid (5-O-caffeoylquinic acid, 5-CQA), is an intermediate in the lignin biosynthesis pathway. Caffeoylquinic acids, and particularly 5-CQA, are accumulated in coffee beans, where they can form vacuolar complexes with caffeine. Coffea canephora beans are known to have high caffeoylquinic acid content, but little is known about the content and diversity of these compounds in other plant parts. To gain new insights into the caffeoylquinic acid metabolism of C. canephora, caffeoylquinic acid content and in situ localization were assessed in leaves at different growth stages.
• Methods HPLC analyses of caffeoylquinic acid content of leaves was conducted in conjunction with detailed histochemical and microspectrofluorometrical analysis.
• Key Results and Conclusions HPLC analyses revealed that caffeoylquinic acid content was 10-fold lower in adult than in juvenile leaves. The most abundant cinnamate conjugate was 5-CQA, but dicaffeoylquinic acids (particularly in juvenile leaves) and feruloylquinic acids were also present. Using specific reagents, histochemical and microspectrofluorometrical analysis showed that caffeoylquinic acids (mono- and di-esters) were closely associated with chloroplasts in very young leaves. During leaf ageing, they were found to first accumulate intensively in specific chlorenchymatous bundle sheath cells and then in phloem sclerenchyma cells. The association with chloroplasts suggests that caffeoylquinic acids have a protective role against light damage. In older tissues, their presence in the leaf vascular system indicates that they are transported via phloem and confirms their involvement in lignification processes. In accordance with the hypothesis of a complex formation with caffeine, similar tissue distribution was observed for alkaloids and this is further discussed.
Keywords: Coffea canephora, developing leaves, caffeoylquinic acids, alkaloids, histolocalization, microspectrofluorometry, high performance liquid chromatography
INTRODUCTION
Esters formed between hydroxycinnamates and quinic acid represent a major family of plant phenolics. Chlorogenic acid (5-CQA) is the most widespread of all monoesters formed between caffeic and quinic acids (CQA) (Molgaard and Ravn, 1988). It is commonly considered to be a storage form of cinnamic acid derivatives and appears to be an intermediate in the lignin pathway (Schoch et al., 2001). Some plant families, including Solanaceae, Asteraceae and Rubiaceae, also produce diesters, principally dicaffeoylquinic acids.
Due to their antioxidant and antibiotic properties, hydroxycinnamoylquinic acids are involved in numerous biological plant functions such as pest and disease resistance (Takahama, 1998; Matsuda et al., 2003). Mono- (Szalma et al., 2005) and di-caffeoylquinic acids (Cole, 1984) are involved in insect resistance in different cultivated species. Using transgenic tobacco plants that overexpress phenylpropanoid pathway genes, it has been shown that reduced levels of chlorogenic acid increase plant susceptibility to virulent pathogens such as Cercospora nicotianae (Maher et al., 1994). Chlorogenic acid also appears to be involved in the response to different abiotic stresses. An increase in its content was noted in Mahonia repens in response to drought (Grace et al., 1998), and in Betula pendula seedlings subjected to far red irradiation (Tegelberg et al., 2004). Caffeoylquinic acids are potent antioxidants that are synthesized in response to oxidative stresses, and act particularly against lipid peroxidation (Rice-Evans et al., 1997; Tamagnone et al., 1998). This antioxidant activity may also be beneficial for human health, e.g. limiting atherosclerosis and carcinogenesis (Niggeweg et al., 2004; Jin et al., 2005), or inhibiting HIV-1 replication (Zhu et al., 1999).
Hydroxycinnamoylquinic acids are involved in a broad range of stress responses, but mechanisms underlying their biosynthesis and protective action in vivo are still unclear. Like other phenolics, they are accumulated inside vacuoles or in the apoplast during leaf ageing (Takahama, 1998), and their biosynthesis apparently occurs within chloroplasts since the last enzyme that catalyses their biosynthesis is described as chloroplastic (Alibert et al., 1977).
In coffee trees, hydroxycinnamoylquinic acids accumulate in beans. This is particularly marked in Coffea canephora where their content can exceed 10 % of dry bean weight (Ky et al., 2001). In Coffea, as in other plants, 5-CQA is the most abundant soluble ester. However, in the Coffea genus, the noteworthy feature is that there is wide interspecific hydroxycinnamoylquinic acid diversity in beans, and this concerns both the nature (Clifford, 2000) and the content of these esters. Depending on the coffee species, feruloylquinic acids (3-, 4- and 5-FQA) and caffeoylquinic acids (CGAss), i.e. the isomers of the monoester CQA (3-, 4- and 5-CQA) and the diesters (3,4-, 3,5- and 4,5-DiCQA), also occur (Anthony et al., 1993). Coffea canephora green beans contain all of the above compounds (Anthony et al., 1993). Hydroxycinnamoylquinic acid content is well documented in coffee beans as these compounds are involved in the bitterness of the coffee beverage due to their degradation into phenolics during roasting (Leloup et al., 1995). They are also thought to be involved in the vacuolar sequestration of caffeine in seeds due to their ability to form complexes with this alkaloid (Spencer et al., 1988; Mösli Waldhauser and Baumann, 1996).
Nevertheless, little is known about their biosynthesis in coffee trees or their presence in other parts of the plant. Aerts and Baumann (1994) showed that 5-CQA accumulation in C. arabica cotyledons preceded phenolic polymer synthesis, and they suggested that lignification may occur via the utilization of stored 5-CQA. Studies based on cell suspension culture and protoplast isolation from C. arabica leaves revealed that 5-CQA may be vacuolar or cell-wall associated (Mösli Waldhauser and Baumann, 1996).
The present study investigated the biochemical composition and histochemical localization of CGAss during C. canephora leaf development. The results are discussed on the basis of the multipurpose functions these compounds may have in plants.
MATERIALS AND METHODS
Plant material
Leaves were collected during spring, 3 h after sunrise, from newly formed shoots of two Coffea canephora Pierre trees maintained in tropical greenhouses (natural daylight, 25 °C night, 28 °C day, 80 % humidity) at the IRD research centre in Montpellier (France). Three five-node axes were selected on two 15-year-old trees. Nodes were ranked from node 1, for the youngest (juvenile leaves), to node 5, for the oldest (mature leaves). Expanding buds were not used. Node 6 corresponded to the opposite leaves situated at the base of a new shoot on the lignified part of the branch. For each tree, the two leaves of the same node from the three axes were pooled (12 samples of six leaves each) before being weighed and measured (length). Five of the six leaves collected were pooled for biochemical evaluation, immediately frozen in liquid nitrogen and then stored at –80 °C until lyophilization. The remaining leaf was stored in wet conditions until histochemical analysis.
Biochemical evaluation of hydroxycinnamoylquinic acids
After grinding the leaves into a fine powder, hydroxycinnamoylquinic acids were extracted three times from the 12 samples using the method previously described by Ky et al. (2001). Compounds were identified on the basis of their retention time and UV spectra compared with 5-CQA (Sigma-Aldrich) using a previously described HPLC analysis procedure (Bertrand et al., 2003). Coumaroylshikimate and caffeoylshikimate (a generous gift from D. Werck-Reichhart at the IBMP, Strasbourg, France) were also used as controls. Quantification was performed by comparison to a 5-CQA standard, and hydroxycinnamoylquinic acid content was expressed as mean percentage of dry weight (% d. wt) from three different evaluations. Peak identity was confirmed by evaluating the molecular weight by LC-MS (LCMS Alliance, Waters).
Statistical analysis
All the data were analysed using the Statistica software package (version 5.1, 1997, for Microsoft Windows). Between-stage differences were tested using a one-way ANOVA (Tukey, HSD). For each node, the individual value expressed as percentage of dry matter (% d. wt) was the average of six mean values resulting from three different extractions of five leaves pooled from each of the two trees.
Histochemical analysis
Small pieces of freshly collected Coffea canephora leaves were embedded in 3 % agarose (type II EEO, Panreac) before cutting for histochemical examination. Transverse sections (40 μm) were obtained using a Leica VT 1000S vibrating blade microtome (frequency 7, speed 2). Neu's reagent (Neu, 1957), a standard reagent for phenolic compounds, was used. Transverse sections were immersed (30 s) in Neu's reagent [1 % 2-amino-ethyldiphenylborinate (Fluka) in absolute methanol] and then mounted in glycerol–water (10 : 90, v/v) solution. Specimens were viewed under a light microscope (Nikon Optiphot) with UV light (filter UV-1A: 365 nm excitation filter, 400 nm barrier filter). In these conditions, CGAss were detected by a greenish-white fluorescence (Mondolot-Cosson et al., 1997) while feruloylquinic derivatives were bright blue. This specific CGAss fluorescence has been assessed by thin layer chromatography using standard solutions of these compounds (Wagner and Bladt, 2001). For alkaloids, transverse sections were immersed (1 min) in Dragendorff's reagent modified by Schute (Merck, 1968) and then mounted in glycerine–water (10 : 90, v/v) solution. Specimens were examined under visible light. Alkaloids and associated compounds such as caffeine, theobromine and trigonelline were reddish-brown. Photographs were taken with a Nikon F301 camera or a digital Nikon coolpix 4500 camera.
Microspectrofluorometry
A microspectrofluorometer (Jobin-Yvon) equipped with an Olympus BX 60 microscope was used to obtain emission fluorescence spectra from fresh leaf transverse sections previously immersed in Neu's reagent for 30 s (an area of 5 μm in diameter was selected). Each leaf was analysed in triplicate. Using a xenon lamp and monochromators, 361·5–368·5 nm wavelengths were produced to excite compounds in the sample. The subsequent fluorescence was detected with a CCD camera, and the fluorescence emission spectra were produced by the SpectraMax software package. Standards (Sigma-Aldrich, St Louis) of CGAss (5-caffeoylquinic acid, 3,5-dicaffeoylquinic acid) and alkaloids (trigonelline, theobromine and caffeine) were tested at 0·2 % (w/v) concentration in Neu's reagent.
RESULTS
Hydroxycinnamoylquinic acid content
Leaves were collected on growing shoots of C. canephora trees according to their node origin. When the leaf lengths of these five node-shoots (node 6 was located at the base of the newly formed shoot) were compared, leaf growth appeared to be complete at node 3 (Table 1). HPLC analyses of the hydroxycinnamoylquinic acid (CGA) content were performed on leaves from each node. As already observed in fruits, two main classes of CGA were found to have accumulated, i.e. caffeoylquinic (CGAss) and feruloylquinic (FQA) acids. In contrast to FQA, where only 5-FQA was detectable, four compounds were present at all stages for CGAss: a monocaffeoylquinic acid (5-CQA) and the dicaffeoylquinic acids (3,4-, 3,5- and 4,5-DiCQA). Younger leaves (from node 1) showed the highest CGA content and were characterized by a high DiCQA content, almost equivalent to that of 5-CQA. All three DiCQA isomers were accumulated, but 3,5-DiCQA was the most abundant (Fig. 1). Compared to juvenile leaves from node 1 (under 10 cm long), mature leaves (node 5) showed a 10-fold lower concentration of CGAss, which is probably due to the lower DiCQA content. The mature leaf content of these compounds was 18-fold lower than in juvenile leaves and represented 25·8 % and 46·9 % of the total CGA, respectively. Interestingly, leaves from node 6 (from 1-year-old shoots) had a higher CGAss content than mature leaves (node 5) from the new axis (0·82 % of the dry weight instead of 0·57 %) and 5-CQA appeared to be the major CGA, representing about 90 % of the total CGA content.
Table 1.
Hydroxycinnamoylquinic acid content of Coffea canephora leaves harvested at different growth stages, from the youngest (1) to the oldest node (6)
| Hydroxycinnamoylquinic acid content (% d. wt) |
||||||
|---|---|---|---|---|---|---|
| Node no. | Minimal– maximal leaf length (cm) | 5-CQA | DiCQA | 5-FQA | CGAss | CQA/CGA (%) |
| 1 | 2·5–10·0 | 2·94 a | 2·75 a | 0·09 a | 5·77 a | 50·8 |
| 2 | 12·5–21·0 | 0·96 b | 0·55 b | 0·02 b | 1·35 b | 62·6 |
| 3 | 11·0–29·0 | 0·89 b | 0·31 c | 0·02 b | 1·19 bc | 73·4 |
| 4 | 13·5–29·0 | 0·59 c | 0·18 cd | 0·01 b | 0·78 d | 75·3 |
| 5 | 11·0–27·0 | 0·43 c | 0·15 cd | 0·01 b | 0·57 d | 73·4 |
| 6 | 17·0–19·0 | 0·74 bc | 0·08 d | 0·01 b | 0·82 cd | 89·3 |
For each node, the individual value, expressed in percentage of dry matter (% d. wt), was the average of six values issued from three different extractions of a mixture of five leaves from each of two trees.
5-CQA, 5-caffeoylquinic acid; DiCQA, dicaffeoylquinic acids (3,4-, 3,5- and 4,5-dicaffeoylquinic acid); 5-FQA, 5-feruloylquinic acid; CGAss, caffeoylquinic acids (CQA + DiCQA); CGA, hydroxycinnamoylquinic acids (CQA + DiCQA + FQA).
For the same node, values followed by the same letter indicate no significant difference between stages at P ≤ 0·05 according to one-way ANOVA.
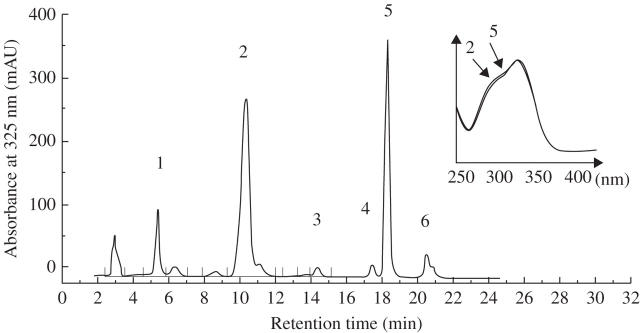
Fig. 1.
HPLC chromatogram of Coffea canephora juvenile leaf (node 1) extracts using UV detection at 325 nm. Peak 1, 3-CQA, 3-caffeoylquinic acid; peak 2, 5-CQA, 5-caffeoylquinic acid; peak 3, 5-FQA, 5-feruloylquinic acid; peak 4, 3,4-DiCQA, 3,4- dicaffeoylquinic acid; peak 5, 3,5-DiCQA, 3,5- dicaffeoylquinic acid; peak 6, 4,5-DiCQA, 4,5- dicaffeoylquinic acid. The two major peaks (2 and 5) have the same UV spectrum (shown in the insert) characteristic of caffeoylquinate esters.
When the CGA content was expressed in mg leaf−1 (instead of dry matter percentage), the 5-CQA and 5-FQA contents did not change significantly with ageing, with the values remaining steady around 4·0 and 0·10 mg leaf−1 from nodes 1–6, respectively (Table 2). A regular decrease was only observed in DiCQA content. The youngest growing leaves (node 1) showed a 3-fold higher DiCQA content than the mature leaves (nodes 3, 4 and 5) which had reached maximal length (Table 1). The lowest DiCQA content was observed in 1-year-old leaves (node 6).
Table 2.
Hydroxycinnamoylquinic acid level in Coffea canephora leaves collected at different growth stages, from the youngest (1) to the oldest node (6)
| Hydroxycinnamoylquinic acid level (mg leaf−1) |
||||
|---|---|---|---|---|
| Node no. | 5-CQA | DiCQA | 5-FQA | CGAss |
| 1 | 4·04 a | 3·78 a | 0·13 a | 7·82 a |
| 2 | 4·25 a | 2·44 b | 0·10 a | 6·68 ab |
| 3 | 4·43 a | 1·53 c | 0·08 a | 5·95 b |
| 4 | 4·49 a | 1·37 c | 0·10 a | 5·86 bc |
| 5 | 3·66 a | 1·24 c | 0·09 a | 4·90 cd |
| 6 | 3·65 a | 0·37 d | 0·07 a | 4·02 d |
For each node, the individual value, expressed in percentage of dry matter (% d. wt) was the average of six values resulting from three different extractions of a mixture of five leaves from each of two trees.
5-CQA, 5-caffeoylquinic acid; DiCQA, dicaffeoylquinic acids (3,4-, 3,5- and 4,5-dicaffeoylquinic acid); 5-FQA, 5-feruloylquinic acid; CGAss, caffeoylquinic acids (CQA + DiCQA).
For the same node, values followed by the same letter indicate no significant difference between stages at P ≤ 0·05 according to one-way ANOVA.
Histolocalization of caffeoylquinic acids
Neu's reagent is a standard histochemical reagent for phenols. This borate salt forms complexes with phenolics which then emit a specific fluorescence with some phenolic groups. During immersion of transverse sections, this methanol-containing reagent also dissolves pigments such as chlorophyll, thus enabling easy in-situ localization of CGAss by the specific greenish-white fluorescence they emit under UV light. However, it is not possible to distinguish monocaffeoylquinic acids from dicaffeoylquinic acids.
In juvenile leaf blades (node 1), an intense greenish-white fluorescence was observed in all chlorenchyma cells, including the palisade and spongy mesophyll cells, indicating a high concentration of CGAss (Fig. 2A1). In mature leaves, from node 3, cortical parenchyma emitted a more dispersed specific fluorescence. Interestingly, the highest intensity was observed in chlorenchymatous bundle sheath cells as well as in phloem cells. A greenish-white fluorescence was also observed in xylem vessel walls (Fig. 2A2) which differed from the usual blue fluorescence emitted by lignins as can be seen in old xylem vessels (Fig. 2A3) or in sclerenchyma (Fig. 2D2). In 1-year-old leaves (node 6), this specific fluorescence was particularly intense in the cell walls of newly formed xylem vessels and in the walls of sclerenchyma cells resulting from chlorenchymatous bundle sheath cells (Fig. 2A3). A very pale and diffuse fluorescence was detected in parenchyma cells. Magnification of the vascular tissues in a transverse section of leaves from node 2 (Fig. 2B1) and node 5 (Fig. 2C1) highlighted modifications in the localization of the fluorescence during leaf growth. In maturing leaves from node 2, an intense, specific fluorescence was observed in the bundle sheath cells surrounding the vascular system, in the primary phloem cells, as well as in the secondary phloem parenchyma and in the xylem vessel walls. In older leaves (node 5), fluorescence decreased in bundle sheath cells and was not detected in phloem cells. On the contrary, the walls of secondary xylem vessels were highly fluorescent. In similar transverse sections, Dragendorff's reagent was used to localize alkaloids (Fig. 2B2 and C2). A brownish colour appeared in the same leaf tissues as the fluorescence, except in the xylem vessels.
Fig. 2.
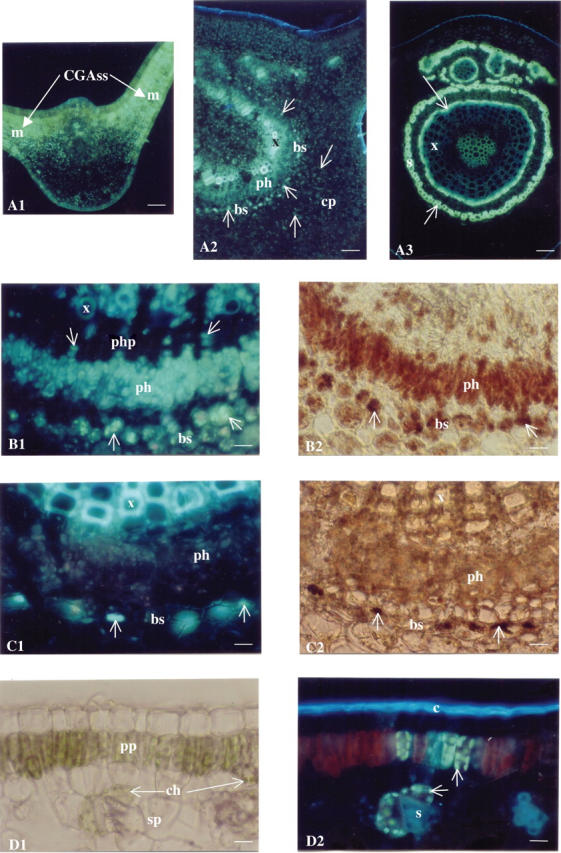
Histochemical localization of caffeoylquinic acids and alkaloids in C. canephora leaves. (A) Transverse section of C. canephora leaves (midrib), Neu's reagent, UV light, ×100. A1, juvenile leaf (node 1): the specific greenish-white fluorescence of caffeoylquinic acids (CGAss), (arrows) is highly concentrated in mesophylls (m). A2, mature leaves (node 3): a decrease in specific fluorescence (arrows) is observed. The compounds are still dispersed in the cortical parenchyma (cp) but concentrated in specific chlorenchymatous bundle sheath (bs) cells, while also present in phloem (ph) cells and impregnated in xylem (x) cell walls. A3, 1-year-old leaf (node 6): fluorescence (arrows) remains bound to the cell walls of the newly formed xylem (x) and is located in sclerenchyma (s) cells. Scale bars = 74 μm. (B) Transverse section of a C. canephora growing leaf (node 2), ×400. B1, Neu's reagent, UV light. The greenish-white fluorescence (arrows) is particularly intense in specific chlorenchymatous bundle sheath (bs) cells. It is also present in primary phloem cells (ph), in phloem parenchyma (php) of secondary phloem and in xylem (x) cell walls. B2, Dragendorff's reagent, visible light: note the distribution of the reddish-brown colour (arrows), located in the same area as the greenish-white fluorescence (bs and ph). Scale bar = 18 μm. (C) Transverse section of a C. canephora mature leaf (node 5), ×400. C1, Neu's reagent, UV light. The greenish-white fluorescence (arrows) is still present in some chlorenchymatous bundle sheath (bs) cells but is almost completely absent from phloem cells (ph). This fluorescence, however, is very strongly present in xylem (x) cell walls. C2, the distribution of the reddish-brown colour is identical to that of the greenish-white fluorescence, i.e. present in some chlorenchymatous bundle sheaths (bs) and almost absent from phoem cells (ph). However, no brown colour was observed in xylem cell walls (x). Scale bars = 18 μm. (D) Transverse section of a C. canephora leaf blade (node 2), Neu's reagent, ×400. D1, visible light. Chloroplasts (ch) are visible in palisade parenchyma (pp) and spongy parenchyma (sp) cells. D2, UV light. Some chloroplasts (those where chlorophyll has been dissolved) display specific greenish-white fluorescence (arrows). The bright blue fluorescence of the cuticle (c) is due to ferulic derivatives and the blue fluorescence of the sclerenchyma (s) is attributable to hydroxycinnamic compounds bound to cell walls. Scale bars = 18 μm.
Observations were also undertaken to localize CGAss in chlorenchymatous cells of newly formed leaves. With a short immersion time of a transverse section of a node 2 leaf in Neu's reagent, chlorophyll was not dissolved in all cells. Chloroplasts were easily localized under visible light (Fig. 2D1). Under UV light, the greenish-white fluorescence was observed in some cells without interference with red chlorophyll fluorescence (Fig. 2D2). This specific fluorescence appeared in the area where chlorophyll was almost completely dissolved, highlighting the close association between CGAss and chloroplasts in chlorenchyma cells.
Microspectrofluorometry
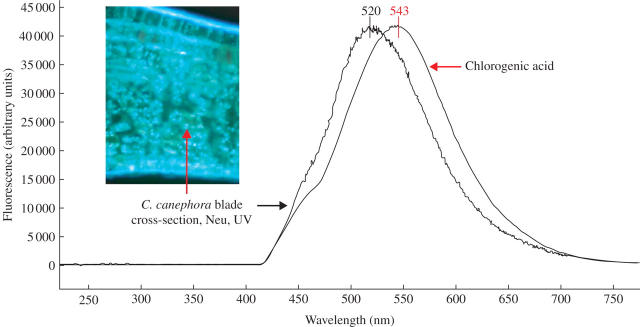
Microspectrofluorometrical analysis of transverse sections of young C. canephora leaf blades reinforced the histochemical observations (Fig. 3). The fluorescence emission spectrum of chloroplasts showing a specific greenish-white fluorescence for CGAss in Neu's reagent had a maximal peak at 520 nm, which is very close to the value obtained for the standard 5-CQA spectrum (peak at 543 nm). CGAss may have formed complexes with other compounds, thus modifying the spectrum profile, which could explain the slight differences noted between these spectra.
Fig. 3.
Comparison of the fluorescence emission spectra of Coffea canephora chloroplasts with chlorogenic acid (5-CQA) standard. Samples were treated with Neu's reagent and their spectra were obtained by microspectrofluorometry, under excitation at 365 nm.
DISCUSSION
Analysis of the hydroxycinnamoylquinic acid (CGA) content of C. canephora leaves harvested on newly formed shoots showed that caffeoylquinic acids (mono- and di-caffeoylquinic acids: CGAss) were the most abundant. This trend was also observed when the CGA content of C. canephora green beans was evaluated (Ky et al., 2001). When the origin of the leaf node and the growth stage were taken into account, it was found that the concentration, nature and localization of CGAss varied throughout leaf development. Juvenile leaves (from node 1) were clearly shown to be highly accumulating organs; this is in agreement with previous analyses of C. arabica and C. pseudozanguebariae leaves (Aerts and Baumann, 1994; Bertrand et al., 2003). Interestingly, and for the first time, this study demonstrated that chlorogenic acid (5-CQA), the most widespread CGA, and also 3,5-DiCQA, a diester of quinic acid, were both present at high concentration in very young leaves. DiCQA content, expressed in percentage of dry matter or in quantity per leaf, then drastically decreased in mature leaves to reach a value as low as 10 % of the CGA content in 1-year-old leaves. A decrease in 5-CQA content was also observed during ageing, but only when values were expressed in percentage of dry matter. Expressed per leaf, there was no statistical difference in 5-CQA content between growth stages. This indicates that CQAs and DiCQAs undergo different metabolic processes during leaf ageing, the former being biosynthesized in the early stage of organ development and stored, the latter being degraded or exported as the organ matures.
In immature leaf blades, CGAss were specifically localized in chlorenchyma cells, where they appeared to be associated with chloroplasts. This localization has already been observed in leaves of another very CGAss-rich plant, Helianthus sp. (L. Mondolot, unpubl. res.). Most phenolics, including CGAss, are usually reported to be accumulated in vacuoles or apoplasts (Mösli Waldhauser and Baumann, 1994), where they could help peroxidases in hydrogen peroxide scavenging (Takahama and Oniki, 1997). This close link between CGAss and chloroplasts in C. canephora young leaves suggests that, in the newly formed organs whose tissue architecture is not complete, CGAss could play a special role by protecting chloroplasts against light damage. Hydroxycinnamate esters were shown to constitute a UV screen in tobacco and Arabidopsis leaves (Cerovic et al., 2002) demonstrating an active protective role against UV-B-induced injury (Landry et al., 1995). Higher 5-CQA concentrations were observed in silver birch (Betula pendula) leaves exposed to UV-B irradiation or to a relative increase in far-red light (Tegelberg et al., 2004). Through their potent antioxidant activity due to their ability to directly interact with reactive oxygen species (Zang et al., 2003), CGAss may be present in young coffee leaf tissues to protect them against oxidative stress, plant-pathogen infections or UV irradiation. As diesters display higher antioxidant activity than monoesters, the presence of a high concentration of DiCQAs in newly formed organs would confirm this protective role in C. canephora leaves. As suggested by Alibert et al. (1977), another explanation for the chloroplastic localization of CGAss may be that CGAss synthesis takes place in chloroplasts and the compounds are then accumulated in vacuoles. Complementary biochemical and enzymatic analyses on isolated chloroplasts are needed to clarify this point.
Another interesting finding in this study was the presence of CGAss in vascular bundles. Their localization in phloem cells of developing leaves indicates that they are transported throughout the plant. In this case, they would be synthesized in the upper part of the plant and transported by the phloem sap to other plant organs where they would be required in the lignification process. Their impregnation in xylem cell walls of mature leaves argues in favour of the assumption that CGAss contribute to cell wall building (Aerts and Baumann, 1994; Schoch et al., 2001). Their accumulation in bundle sheath cells of growing leaves and in phloem sclerenchyma of 1-year-old leaves also confirms that they are involved in the development of sclerified cell walls. Carvalho Carelli et al. (2003) showed that, as in other C3 plants, the coffee tree bundle sheath is simply formed by a distinctive layer of chloroplast-rich cells. One of the physiological functions of the C3 plant bundle sheath is to participate in phloem loading and unloading (van Bel, 1993). The present data identified 5-CQA as the main accumulated CGAs in fully expanded leaves. Its high concentration in vascular bundles, and more specifically in the primary phloem, leads to the hypothesis that 5-CQA is one of the lignin precursors transported from younger to older C. canephora leaves.
The present study also revealed that CGAss and alkaloids accumulate in the same area. This is fully in line with the hypothesis of a complex formation between chlorogenic acid and caffeine, a purine alkaloid (Spencer et al., 1988; Mösli Waldhauser and Baumann, 1996). In C. arabica, purine alkaloid biosynthesis appeared to occur in very young leaf tissues (Zheng and Ashihara, 2004). High indole alkaloid synthesis was also described in young developing Catharanthus roseus leaves, particularly in zones of active cell division (St-Pierre et al., 1999). As proposed by Frischknecht et al. (1986), alkaloids may accumulate in young leaves of Coffea and Catharanthus as protection against predators. It is possible that DiCQA and alkaloid synthesis take place in young tissues of coffee leaves where they act as protective agents. Their transport to mature organs could occur via the phloem vessels, especially when they are required for response to biotic or abiotic stress.
Coffea canephora leaves are excellent material for studying caffeoylquinic acid biosynthesis since they have high contents of these phenolic compounds and high diversity, not only in the accumulated forms of esters but also in their tissue and cell localization during maturation. The results obtained in this study suggest that CGAss (and particularly DiCQA) biosynthesis occurs in the apical zone of C. canephora plants. Now it would be of interest to gain further insights into the biosynthetic pathway leading to caffeoylquinic acid biosynthesis in Coffea species through molecular characterization of the genes and enzymes involved.
Acknowledgments
We thank Professor Claude Andary for all the helpful discussions.
LITERATURE CITED
- Aerts RJ, Baumann TW. 1994. Distribution and utilization of chlorogenic acid in Coffea seedlings. Journal of Experimental Botany 45: 497–503. [Google Scholar]
- Alibert G, Ranjeva R, Boudet AM. 1977. Organisation subcellulaire des voies de synthèse des composés phénoliques. Physiologie Végétale 15: 279–301. [Google Scholar]
- Anthony F, Clifford MN, Noirot M. 1993. Biochemical diversity in the genus Coffea L.: chlorogenic acids, caffeine and mozambioside contents. Genetic Resources and Crop Evolution 40: 61–70. [Google Scholar]
- van Bel AJB. 1993. Strategies of phloem loading. Annual Review of Plant Physiology and Plant Molecular Biology 44: 253–281. [Google Scholar]
- Bertrand C, Noirot M, Doulbeau S, de Kochko A, Hamon S, Campa C. 2003. Chlorogenic acid content swap during fruit maturation in Coffea pseudozanguebaria: qualitative comparison with leaves. PlantScience 165: 1355–1361. [Google Scholar]
- Carvalho Carelli ML, Benetti Queiroz-Voltan R, Irineu Fahl J, Ocheuze Trivelin PC. 2003. Leaf anatomy and carbon isotope composition in Coffea species related to photosynthetic pathway. Brazilian Journal of Plant Physiology 15: 19–24. [Google Scholar]
- Cerovic ZG, Ounis A, Cartelat A, Latouche G, Goulas Y, Meyer S, Moya I. 2002. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant, Cell and Environment 25: 1663–1676. [Google Scholar]
- Clifford MN. 2000. Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. Journal of the Science of Food and Agriculture 80: 1033–1043. [Google Scholar]
- Cole RA, 1984. Phenolic acids associated with the resistance of lettuce cultivars to the lettuce root aphid. Annals of Applied Biology 105: 129–145. [Google Scholar]
- Frischknecht PM, Ulmer-Dufek J, Baumann TW. 1986. Purine alkaloid formation in buds and developing leaflets of Coffea arabica: expression of an optimal defense strategy? Phytochemistry 25: 613–616. [Google Scholar]
- Grace SC, Logan BA, Adams III WW. 1998. Seasonal differences in foliar content of chlorogenic acid, a phenylpropanoid antioxidant, in Mahonia repens. Plant, Cell and Environment 21: 513–521. [Google Scholar]
- Jin U-H, Lee J-Y, Kang S-K, Kim J-K, Park W-H, Kim J-G, et al. 2005. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sciences 77: 2760–2769. [DOI] [PubMed] [Google Scholar]
- Ky L, Louarn J, Dussert S, Guyot B, Hamon S, Noirot M. 2001. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chemistry 75: 223–230. [Google Scholar]
- Landry LG, Chapple CCS, Last RL. 1995. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiology 109: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup V, Louvrier A, Liardon R. 1995. Degradation mechanisms of chlorogenic acids during roasting. Proceedings of the International Congress ASIC 16: 192–198. [Google Scholar]
- Maher EA, Bate NJ, Ni W, Elkind Y, Dixon RA, Lamb CJ. 1994. Increased disease susceptibility of transgenic tobacco plants with suppressed levels of performed phenylpropanoid products. Proceedings of the National Academy of Sciences of the USA 91: 7802–7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F, Morino K, Miyashita M, Miyagawa H. 2003. Metabolic flux analysis of the phenylpropanoid pathway in wound-healing potato tuber tissue using stable isotope-labeled tracer and LC-MS spectroscopy. Plant Cell Physiology 44: 510–517. [DOI] [PubMed] [Google Scholar]
- Merck E. 1968. Révélateurs pour la chromatographie sur couche mince et sur papier. Darmstadt, Allemagne.
- Molgaard P, Ravn A. 1988. Evolutionary aspects of caffeoyl ester distribution in dicotyledonous. Phytochemistry 27: 2411–2421. [Google Scholar]
- Mondolot-Cosson L, Andary C, Guang-Hui D, Roussel J-L. 1997. Histolocalisation de substances phénoliques intervenant lors d'interactions plante-pathogène chez le tournesol et la vigne. Acta Botanica Gallica 144: 353–362. [Google Scholar]
- Mösli Waldhauser SS, Baumann TW. 1996. Compartmentation of caffeine and related purine alkaloids depends exclusively on the physical chemistry of their vacuolar complex formation with chlorogenic acids. Phytochemistry 42: 985–996. [Google Scholar]
- Neu R. 1957. A new reagent for differentiating and determining flavones on paper chromatograms. Naturwissenschaften 43: 82. [Google Scholar]
- Niggeweg R, Michael AJ, Martin C. 2004. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nature Biotechnology 22, 746–754. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. 1997. Antioxidants effect of phenolic compounds. Trends in Plant Science 2: 152–159. [Google Scholar]
- Schoch G, Goepfert S, Morant M, Hehn A, Meyer D, Ullmann P, et al. 2001. CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. Journal of Biological Chemistry 276: 36566–36574. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Cai Y, Martin R, Gaffney SH, Goulding PN, Magnolato D, et al. 1988. Polyphenol complexation: some thoughts and observations. Phytochemistry 27: 2397–2409. [Google Scholar]
- St-Pierre B, Vazquez-Flota FA, de Luca V. 1999. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intracellular translocation of a pathway intermediate. The Plant Cell 11: 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalma, SJ, Buckler ES, Snook ME, McMullen MD. 2005. Association analysis of candidate genes for maysin and chlorogenic acid accumulation in maize silks. Theoretical and Applied Genetics 110: 1324–1333. [DOI] [PubMed] [Google Scholar]
- Takahama U. 1998. Ascorbic acid-dependant regulation of redox levels of chlorogenic acid and its isomers in the apoplast of leaves of Nicotiana tabacum L. Plant Cell Physiology 39: 681–689. [Google Scholar]
- Takahama U, Oniki T. 1997. A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiologia Plantarum 101: 845–852. [Google Scholar]
- Tamagnone L, Merida A, Stacey N, Plaskitt K, Parr A, Chang C-F, et al. 1998. Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. The Plant Cell 10: 1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelberg R, Julkunen-Tiitto R, Aphalo PJ. 2004. Red : far-red light ratio and UV-B radiation: their effects on leaf phenolics and growth of silver birch seedlings. Plant, Cell and Environment 27: 1005–1013. [Google Scholar]
- Wagner H, Bladt S. 2001. Plant drug analysis: a thin layer chromatography atlas, 2nd edn. Berlin/Heidelberg: Springer-Verlag
- Zang L-Y, Cosma G, Gardner H, Castranova V, Vallyathan V. 2003. Effect of chlorogenic acid on hydroxyl radical. Molecular and Cellular Biochemistry 247: 205–210. [DOI] [PubMed] [Google Scholar]
- Zheng X-q, Ashihara H. 2004. Distribution, biosynthesis and function of purine and pyridine alkaloids in Coffea arabica seedlings. Plant Science 166: 807–813. [Google Scholar]
- Zhu K, Cordeiro ML, Atienza J, Robinson Jr WE, Chow SA. 1999. Irreversible inhibition of human immunodeficiency Virus type 1 integrase by dicaffeoylquinic acids. Journal of Virology 73: 3309–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]