Abstract
• Background and Aims Paris (Melanthiaceae) is a temperate genus of about 24 perennial herbaceous species distributed from Europe to eastern Asia. The delimitation of the genus and its subdivisions are unresolved questions in the taxonomy of Paris. The objective of this study is to test the generic and infrageneric circumscription of Paris with DNA sequence data.
• Methods Phylogenetic analysis of 21 species of Paris based on nuclear ITS and plastid psbA-trnH and trnL-trnF DNA sequence data, alone and in combination, was employed to assess previous classifications.
• Key Results Paris is monophyletic in all analyses. Neither of the two traditionally recognized subgenera (Paris and Daiswa) are monophyletic. Sections Axiparis, Kinugasa, Paris and Thibeticae are monophyletic in only some of the analyses. Species of sections Dunnianae, Fargesianae and Marmoratae are consistently intercalated among species of section Euthyra in all analyses. Strong discordance between nuclear and plastid lineages is detected.
• Conclusions The data support the classification of Paris as a single genus rather than as three genera (Daiswa, Kinugasa and Paris sensu stricto). They provide justification for the transfer of section Axiparis from subgenus Paris to subgenus Daiswa and for the combination of sections Dunnianae, Fargesianae and Marmoratae into section Euthyra. The nuclear-plastid discordance is interpreted as the result of interspecific hybridization among sympatric species.
Keywords: Classification, ITS, Melanthiaceae, nuclear-plastid incongruence, Parideae, Paris, phylogeny, psbA-trnH, trnL-trnF
INTRODUCTION
Paris (Melanthiaceae: Parideae) (APG II, 2003; Zomlefer et al., 2006) is a temperate genus of about 24 species of perennial herbs distributed from Europe to eastern Asia. Except for the European P. quadrifolia and the Caucasian P. incompleta, species are restricted to East Asia, chiefly in China (19 species), with the Yunnan-Guizhou Plateau as the centre of diversity (Li et al., 1988; Li, 1998). Paris spp. typically grow in montane evergreen forests, montane cloud forests, broadleaved forests, conifer forests, mixed conifer and broadleaved forests and bamboo and scrub thickets (Li, 1998; Liang and Soukup, 2000). Paris is notable in China for its medicinal value. The species with a thick rhizome are traditional medicinal herbs and the major source of raw material for some medicines, e.g. ‘Yunnan Baiyao’, well-known for its use as an analgesic and anti-coagulant (Long et al., 2002).
The classification of Paris, long in dispute, is still unresolved. Hara (1969), Li (1984, 1998) and Mitchell (1987, 1988) recognized it as a single genus (the broad concept of the genus is hereafter indicated by ‘Paris’) based on floral and leaf merosity. Paris is 4- to 15-merous, whereas Trillium, its sister group, is 3-merous. Based on fruit type, ovary shape, seed morphology and rhizome shape, Takhtajan (1983) divided Paris into three genera: Paris in the narrow sense (hereafter indicated by ‘Paris s.s.’), Kinugasa and Daiswa (Table 1). This treatment was adopted by Dahlgren et al. (1985), Tamura (1998) and Farmer and Schilling (2002). Hara (1969) divided the 14 species known at the time into three sections: Paris, Kinugasa and Euthyra, based on fruit and seed characters. In the most recent comprehensive taxonomic revision, Li (1998) recognized subgenus Paris (11 species) and subgenus Daiswa (13 species), delimited by axile or incompletely axile placentation versus parietal placentation, respectively. Subgenus Paris was divided into sections Kinugasa (one species), Paris (five species) and Axiparis (five species), whereas subgenus Daiswa was divided into sections Dunnianae (one species), Euthyra (eight species), Marmoratae (two species), Fargesianae (one species) and Thibeticae (one species).
Table 1.
Comparison of morphological features among Takhtajan's (1983) three segregate genera of Paris
| Daiswa | Kinugasa | Paris s.s. | |
|---|---|---|---|
| Rhizome | Thick | Thick | Long and slender |
| Fruit | Capsule | Berry | Berry |
| Ovary | Angular | Angular | Rounded |
| Seed | With sarcotesta | Without sarcotesta | Without sarcotesta |
Recent phylogenetic studies based on DNA sequence data have proved useful for estimating the phylogeny of the tribe Parideae. Analyses based on the plastid rbcL and matK genes and the nuclear ribosomal internal transcribed spacer (ITS) region supported the monophyly of Paris, with Trillium as its sister group (Kato et al., 1995a, b; Kazempour Osaloo and Kawano, 1999; Kazempour Osaloo et al., 1999). Other analyses combining sequence data (ITS and matK) and morphological data supported the division of Paris into three genera (Daiswa, Kinugasa and Paris s.s.; Farmer and Schilling, 2002). These studies have provided valuable insights for an initial molecular-based evaluation of Paris classification, but sampling within the genus (at most eight species) has been too low to address satisfactorily the issues of generic delimitation and infrageneric division.
Here the various classifications of Paris are tested with DNA sequence data by using a much larger taxon sample than has previously been available, including representatives from all described subgenera (or segregate genera) and sections. DNA sequence data are used from the ITS region and two regions of the plastid genome (trnL-trnF and psbA-trnH). These regions have proved to be of general utility for phylogenetic studies at the infrageneric level because of their relatively fast rate of sequence evolution and conserved flanking primer sites (Soltis and Soltis, 1998; Shaw et al., 2005).
MATERIALS AND METHODS
Taxon sampling, DNA extraction, amplification and sequencing
Twenty-one species and four varieties of Paris L. (Table 2) representing both subgenera and all eight sections sensu Li (1998) were sampled. Of the three species not included, one [P. tetraphylla, Japan (section Paris)] was unavailable, and the other two [P. undulata, China; P. birmanica, Burma (section Euthyra)] were not found in the areas in which they were known to be distributed and may be extinct. To assess the generic delimitation of Paris, nine Trillium spp. were included in the ingroup (Table 2), representing two of the subgenera of Trillium (Farmer and Schilling, 2002). Trillium rivale was used as outgroup in accordance with the results of Farmer and Schilling (2002).
Table 2.
Taxa represented in this study with voucher or source information, and GenBank accession numbers
| Taxa | Voucher or source | Accession no. |
||
|---|---|---|---|---|
| ITS | psbA-trnH | trnL-trnF | ||
| Paris axialis | Y. H. Ji 149 (KUN) | DQ404210 | DQ404244 | DQ404278 |
| P. bashanensis | Y. H. Ji 136 (KUN) | DQ404205 | DQ404239 | DQ404273 |
| P. cronquistii var. cronquistii | Y. H. Ji 133 (KUN) | DQ404214 | DQ404248 | DQ404281 |
| P. cronquistii var. xichouensis | X. Gong s. n. (KUN) | DQ404221 | DQ404255 | DQ404289 |
| P. daliensis | Q. Guo s. n. (KUN) | DQ404226 | DQ404260 | DQ404294 |
| P. delavayi var. delavayi | Y. H. Ji 135 (KUN) | DQ404215 | DQ404249 | DQ404283 |
| P. delavayi var. petiolata | S. T. Chen s. n. (KUN) | DQ404220 | DQ404254 | DQ404288 |
| P. dulongensis | GLGS Exp. 20534 (KUN) | DQ404207 | DQ404241 | DQ404275 |
| P. dunniana | Y. H. Ji 128 (KUN) | DQ404225 | DQ404259 | DQ404293 |
| P. fargesii | Y. H. Ji 129 (KUN) | DQ404217 | DQ404251 | DQ404285 |
| P. forrestii | Y. H. Ji 168 (KUN) | DQ404208 | DQ404242 | DQ404276 |
| P. incompleta | Cult. in Royal Botanic Garden, Edinburgh (19741458B) | DQ404203 | DQ404237 | DQ404271 |
| P. japonica | J. Maruta s. n. (KUN) | DQ404202 | DQ404236 | DQ404270 |
| P. luquanensis | Y. H. Ji 206 (KUN) | DQ404219 | DQ404253 | DQ404287 |
| P. mairei | W. Y. Xu s. n. (KUN) | DQ404213 | DQ404247 | DQ404282 |
| P. marmorata | Y. H. Ji 197 (KUN) | DQ404222 | DQ404256 | DQ404290 |
| P. polyphylla var. chinensis | Y. H. Ji 126 (KUN) | DQ404218 | DQ404252 | DQ404286 |
| P. polyphylla var. polyphylla | Y. H. Ji 174 (KUN) | DQ404224 | DQ404258 | DQ404292 |
| P. polyphylla var. yunnanensis | Y. H. Ji 131 (KUN) | DQ404223 | DQ404257 | DQ404291 |
| P. quadrifolia | P. Bruggeman s. n. (KUN) | DQ404204 | DQ404238 | DQ404272 |
| P. rugosa | GLGS Exp. 21597 (KUN) | DQ404211 | DQ404245 | DQ404279 |
| P. thibetica | GLGS Exp. 20193 (KUN) | DQ404216 | DQ404250 | DQ404284 |
| P. vaniotii | H. Li 8842 (KUN) | DQ404209 | DQ404243 | DQ404277 |
| P. vietnamensis | Y. H. Ji 139 (KUN) | DQ404212 | DQ404246 | DQ404280 |
| P. verticillata | L. X. Wang s. n. (KUN) | DQ404206 | DQ404240 | DQ404274 |
| Trillium albidum | Cult. in Royal Botanic Garden, Edinburgh (19623094B) | DQ404198 | DQ404232 | DQ404266 |
| T. cernuum | Cult. in Royal Botanic Garden, Edinburgh (19370524B) | DQ404193 | DQ404227 | DQ404261 |
| T. cuneatum | Cult. in Royal Botanic Garden, Edinburgh (19841344) | DQ404199 | DQ404233 | DQ404267 |
| T. erectum | Cult. in Royal Botanic Garden, Edinburgh (19653535A) | DQ404196 | DQ404230 | DQ404264 |
| T. grandiflorum | Cult. in Royal Botanic Garden, Edinburgh (19685241) | DQ404195 | DQ404229 | DQ404263 |
| T. camschatcense | Cult. in Royal Botanic Garden, Edinburgh (19741690) | DQ404197 | DQ404231 | DQ404265 |
| T. luteum | Cult. in Royal Botanic Garden, Edinburgh (19841338) | DQ404200 | DQ404234 | DQ404268 |
| T. ovatum | Cult. in Royal Botanic Garden, Edinburgh (19360567C) | DQ404194 | DQ404228 | DQ404262 |
| T. rivale | Kazempour Osaloo and Kawano (1999), Shaw et al. (2005) | AB018822 | AY727185 | AY727232 |
| T. tschonoskii | GLGS Exp. 20202 (KUN) | DQ404201 | DQ404235 | DQ404269 |
Genomic DNA was extracted from silica gel-dried or fresh leaves by using the method of Doyle and Doyle (1987). The ITS region was amplified with primers ITS4 and ITS5 (White et al., 1990). The PCR programme was as follows: 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s; and 72 °C for 7 min. The psbA-trnH region was amplified with primers psbA and trnH (Sang et al., 1997) in accordance with the protocol of Shaw et al. (2005). The trnL-trnF region was amplified with the primer pairs c–d and e–f (Taberlet et al., 1991) as follows: 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 56 °C (c–d) or 58 °C (e–f) for 30 s, 72 °C for 45 s; and 72 °C for 7 min.
Amplified fragments were purified by running PCR products on a 1·5 % low-melting temperature agarose gel followed by DNA recovery with a gel extraction kit (UNIQ-10, Sangon, Shanghai, China). Cycle sequence reactions of purified PCR products were performed by using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) in accordance with the manufacturer's instructions. Products were then run on a 3730XL genetic analyser (Applied Biosystems) at Sunbiotech Corp., Beijing, China. Forward and reverse strands of all ITS and psbA-trnH samples were sequenced. The trnL-trnF sequences of most samples were determined in one direction with primers c and f because the sequences were of sufficient quality to make sequence generation of the reverse strand unnecessary.
Data analysis
Sequences were compared and compiled with Sequencher 4.2 version (Gene Codes Corp., Ann Arbor, MI, USA). The alignment was performed manually in PAUP* version 4.0b10 (Swofford, 2002). The matrix is available upon request from the authors.
Phylogenetic analyses were conducted with maximum parsimony. Characters were equally weighted and unordered. Gaps were scored as missing data. Positions 510–641 in the aligned trnL-trnF data matrix encompassed an A/T-rich region of highly ambiguous alignment, and thus were excluded from phylogenetic analyses. The parsimony analyses were performed with the heuristic search option in PAUP*. Searches were conducted over 1000 random-taxon-addition replicates with tree bisection-reconnection branch-swapping and MulTrees enforced. All shortest trees were saved, and a strict consensus tree was computed. To estimate the support for individual clades, heuristic bootstrapping (1000 replicates) was performed. Partition homogeneity tests (Farris et al., 1994) were conducted with PAUP* to determine the degree of congruence between the two plastid data sets (trnL-trnF versus psbA-trnH) and plastid versus nuclear data sets (trnL-trnF + psbA-trnH versus ITS) by using 500 replicates and an heuristic tree search with 10 random-taxon-addition replicates. Only informative characters were included in analyses.
From the phylogenetic results the evolution of ovary placentation and seed morphology, two characters regarded as critical in the classification of Paris were examined (Takhtajan, 1983; Li, 1984, 1998). Character states for Paris and Trillium were obtained from the literature (Freeman, 1969; Takhtajan, 1983; Zomlefer, 1996; Li, 1998; Farmer, 2000; Liang and Soukup, 2000) and personal observation by the first author. Characters were traced onto the tree generated from the combined analysis of the ITS and plastid DNA data sets with MacClade 4.0 (Maddison and Maddison, 2000).
RESULTS
Plastid DNA analysis
The length of trnL-trnF ranged from 938 to 1058 base pairs (bp) among species of Paris and from 973 to 1018 bp among species of Trillium. Of the 1174 aligned positions, 106 were variable, of which 31 (29·2 % of the variable positions) were potentially phylogenetically informative. Parsimony analysis of trnL-trnF yielded 23 839 trees of 49 steps [consistency index (CI) = 0·67, retention index (RI) = 0·93]. In the strict consensus tree, Paris was monophyletic [bootstrap percentage (bt) = 96; Fig. 1]. The basal divergence within this Paris clade formed two major clades (‘A’ and ‘B’, bt = 68 and 90, respectively). Clade A comprised P. bashanensis, P. incompleta, P. japonica, P. quadrifolia and P. verticillata corresponding to Paris s.s. [= subgenus Paris sensu Li (1998) minus section Axiparis]. Clade B was resolved as two subclades: one (bt = 70) comprised section Axiparis of subgenus Paris and section Thibeticae of subgenus Daiswa, and the other (bt = 82) the rest of the species of subgenus Daiswa. The only other clades recovered were P. quadrifolia + P. verticillata (bt = 93) and P. forrestii + P. rugosa (bt = 58).
Fig. 1.
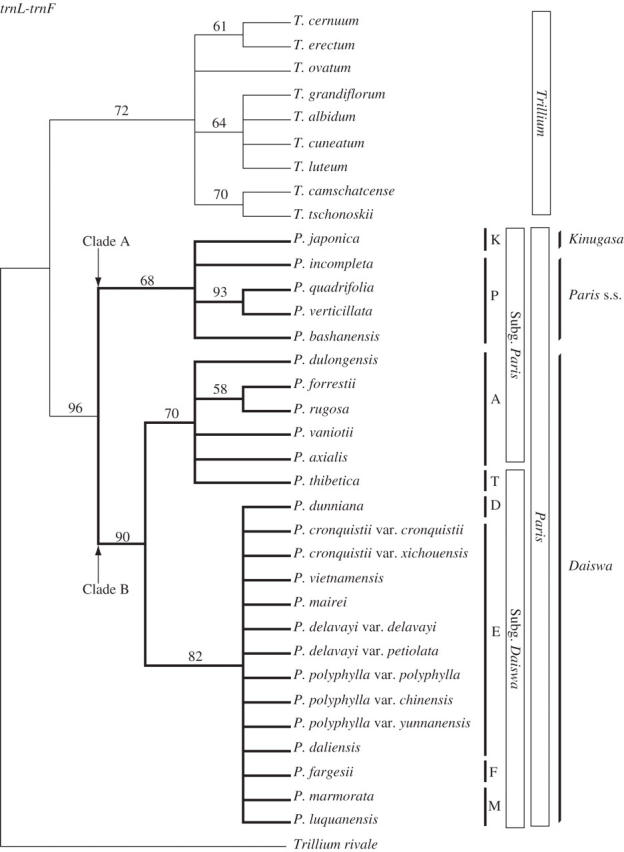
The strict consensus tree of 23 839 trees from parsimony analysis of trnL-trnF sequences of Paris and Trillium (length = 49, CI = 0·67, RI = 0·93). Bootstrap percentages >50 % are shown above branches. Two contrasting views of generic delimitation (see text) and the subgenera and sections of Li (1998) are indicated on the right. P = sect. Paris, A = sect. Axiparis, D = sect. Dunnianae, E = sect. Euthyra, F = sect. Fargesianae, K = sect. Kinugasa, M = sect. Marmoratae, T = sect. Thibeticae.
The length of psbA-trnH ranged from 1078 to 1103 bp among species of Paris and from 645 to 1120 bp among species of Trillium. Of the 1221 aligned positions, 102 were variable, of which 44 (43.1 % of the variable positions) were potentially phylogenetically informative. Parsimony analysis of psbA-trnH resulted in 6291 trees of 104 steps (CI = 0·61, RI = 0·85). The strict consensus tree showed a topology of Paris completely consistent with that from the analysis of trnL-trnF, but with somewhat higher clade resolution (Fig. 2). Paris was monophyletic (bt = 97) and the two first-diverging clades A and B were recovered (bt = 83 and 79, respectively). There were five other clades with bootstrap support >50 %: section Paris (bt = 88); section Thibeticae + section Axiparis (bt = 61); section Axiparis (bt = 53); a clade of subgenus Daiswa excluding section Thibeticae (bt = 79); and a clade of capsular species with 3- to 6-whorled stamens (bt = 69).
Fig. 2.
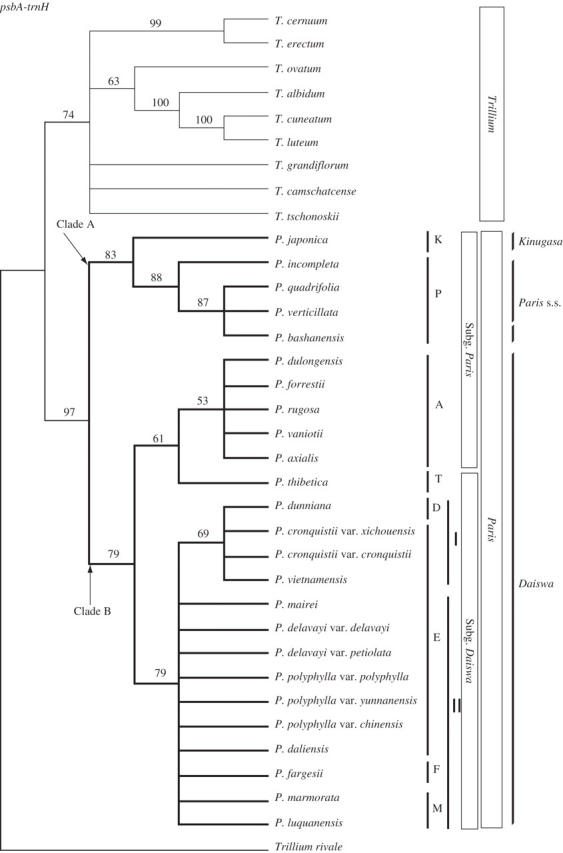
The strict consensus tree of 6291 trees from parsimony analysis of psbA-trnH sequences of Paris and Trillium (length = 104, CI = 0·61, RI = 0·85). Bootstrap percentages >50 % are shown above branches. Two contrasting views of generic delimitation (see text) and the subgenera and sections of Li (1998) are indicated on the right. Section abbreviations are as in Fig. 1. I = capsular species with 3- to 6-whorled stamens, II = capsular species with 2-whorled stamens.
The trnL-trnF and psbA-trnH data sets did not differ significantly in structure (P = 0·92); they were therefore combined into a single data set for phylogenetic analysis. Parsimony analysis of the combined plastid DNA data yielded 3158 trees of 270 steps (CI = 0·81, RI = 0·89). The topology of Paris in the combined strict consensus tree was congruent with that from the psbA-trnH analysis (Fig. 3). It differed from the psbA-trnH consensus only in the unresolved placement of P. incompleta. The ingroup nodes in the topology of the combined analysis received higher bootstrap support than those in the separate analyses of either plastid DNA region (cf. Figs 1–3).
Fig. 3.
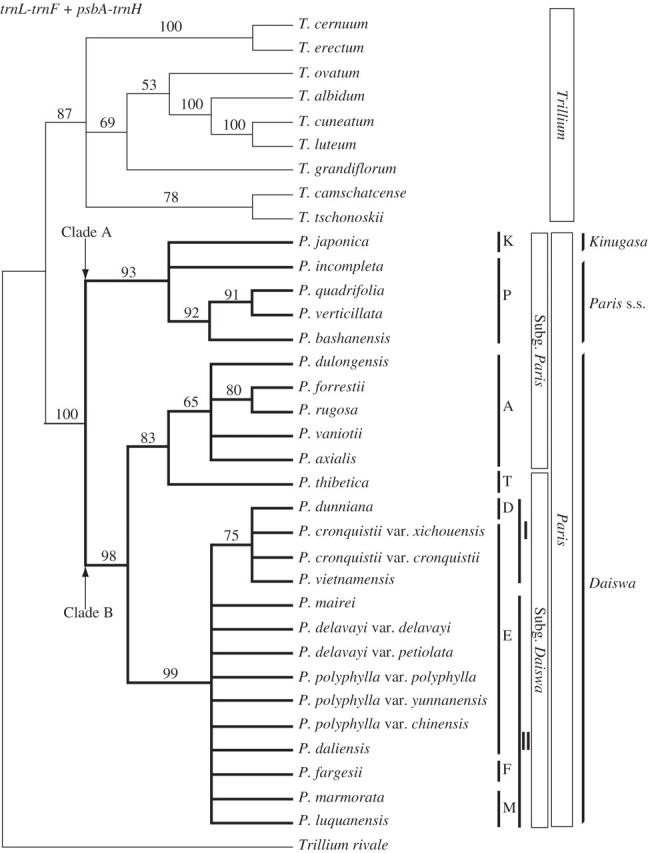
The strict consensus tree of 3158 trees from parsimony analysis of combined trnL-trnF and psbA-trnH sequences of Paris and Trillium (length = 270, CI = 0·81, RI = 0·89). Bootstrap percentages >50 % are shown above branches. Two contrasting views of generic delimitation (see text) and the subgenera and sections of Li (1998) are indicated on the right. Abbreviations are as in Fig. 2.
ITS analysis
The length of ITS ranged from 632 to 636 bp among species of Paris and 635 to 638 among species of Trillium. Of the 658 aligned positions, 208 were variable, of which 89 (42·8 % of the variable positions) were potentially phylogenetically informative. Parsimony analysis of ITS yielded 132 trees of 275 steps (CI = 0·62, RI = 0·81). In the strict consensus tree, Paris was monophyletic (bt = 100) and resolved into the same two major clades (A and B) recovered in the plastid DNA analyses (bt = 92 and 100, respectively; Fig. 4). The topology of clade A was consistent with those from plastid DNA; P. japonica was sister to a clade (bt = 59) comprising the remaining taxa (section Paris) of clade A. Clade B, however, differed substantially from that of the plastid DNA analyses. Paris dulongensis (section Axiparis), the first diverging lineage, was sister to a clade (bt = 94) comprising all other species of section Axiparis and subgenus Daiswa. There were two clades within the latter: a clade (bt = 93) comprising P. axialis and a clade in which P. forrestii and P. rugosa (section Axiparis) formed a group (bt = 99) sister to a clade comprising all species of subgenus Daiswa (bt = 80). Paris thibetica (subgenus Daiswa section Thibeticae) was sister to a clade comprising the remaining species of the subgenus (bt = 68). Three subclades formed a trichotomy within the latter clade: a clade (bt = 53) consisting of P. marmorata (section Marmoratae) and P. daliensis (section Euthyra); a clade (bt = 78) in which P. mairei (section Euthyra) was sister to a clade formed by P. luquanensis (section Marmoratae) and P. polyphylla var. yunnanensis (section Euthyra); and a clade (bt = 98) in which four species of section Euthyra formed a group (bt = 98) sister to a clade (bt = 80) comprising species of sections Dunnianae, Euthyra and Fargesianae. The species with 3- to 6-whorled stamens formed a monophyletic group (bt = 60), whereas the group of species with 2-whorled stamens was paraphyletic.
Fig. 4.
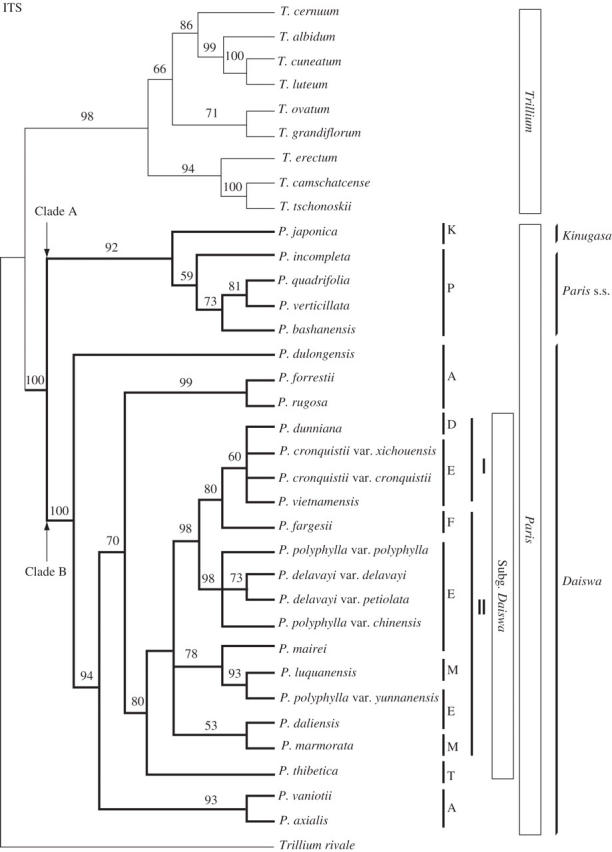
The strict consensus tree of 132 trees from parsimony analysis of ITS sequences of Paris and Trillium (length = 270, CI = 0·62, RI = 0·81). Bootstrap percentages >50 % are shown above branches. Two contrasting views of generic delimitation (see text) and the subgenera and sections of Li (1998) are indicated on the right. Abbreviations are as in Fig. 2.
Data incongruence and combined analysis
The plastid DNA and ITS data sets differed significantly in structure (P = 0·002). By visual comparison of the ITS and plastid DNA consensus topologies and experimentation with various taxon exclusion sets of minimal size, a non-significant P value (P = 0·36) was recovered when P. daliensis, P. dulongensis, P. luquanensis, P. mairei, P. marmorata, P. polyphylla var. polyphylla, P. polyphylla var. yunnanensis and P. thibetica were excluded from the test. To assess previous classifications of Paris the ITS and plastid DNA data were combined to form a single data set for phylogenetic analysis after removing these eight taxa.
Parsimony analysis of the combined plastid DNA and ITS data set yielded one tree of 347 steps (CI = 0·61, RI = 0·85). The topology of this tree was consistent with that of the combined plastid DNA consensus but exhibited higher resolution (Fig. 5). Within clade A (bt = 83), P. japonica (section Kinugasa) was sister to a clade comprising the species of section Paris (bt = 74). Within clade B (bt = 100), the species of section Axiparis formed a clade (bt = 73) sister to a clade comprising the rest of the species of subgenus Daiswa (bt = 100). The latter clade consisted of all species of section Euthyra included, with P. dunniana (section Dunnianae) and P. fargesii (section Fargesianae) nested within it. The species with 3- to 6-whorled stamens formed a monophyletic group (bt = 74), whereas the group of species with 2-whorled stamens was paraphyletic to the clade of species with 3- to 6-whorled stamens.
Fig. 5.
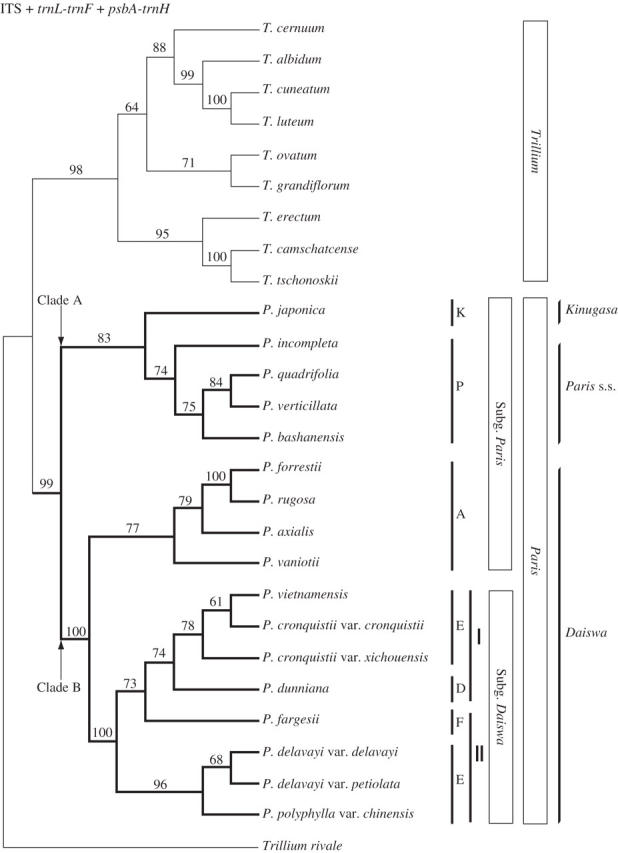
The single tree from parsimony analysis of combined plastid DNA and ITS analysis with nine taxa excluded (see text; length = 347, CI = 0·61, RI = 0·85). Bootstrap percentages >50 % are shown above branches. Two contrasting views of generic delimitation (see text) and the subgenera and sections of Li (1998) are indicated on the right. Abbreviations are as in Fig. 2.
Evolution of ovary placentation and seed morphology
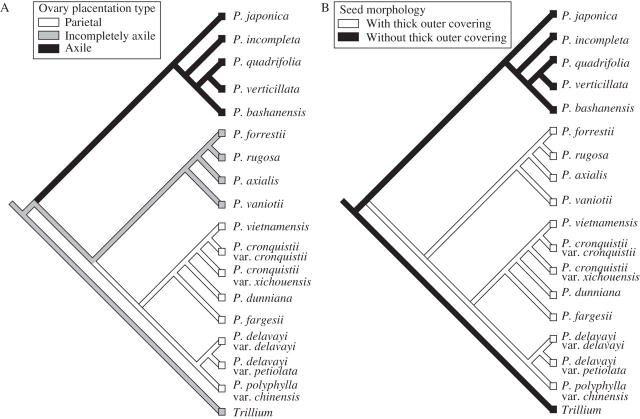
Optimization of ovary placentation onto the shortest tree for Paris from the combined ITS and plastid DNA analysis (see Fig. 5) recovered two equally optimal reconstructions of two steps (Fig. 6A). Incompletely axile placentation was inferred as the plesiomorphic state, whereas axile and parietal placentation were inferred as apomorphic states in the genus. Optimization of seed morphology recovered one optimal reconstruction of one step (Fig. 6B). The presence of a thick outer covering (imperfect aril or sarcotesta) was inferred as the apomorphic state.
Fig. 6.
The single shortest tree obtained for Paris from the combined ITS and plastid DNA analysis, onto which ovary placentation (A) and seed morphology (B) have been mapped.
DISCUSSION
Discordance between ITS and plastid DNA trees
Phylogenetic incongruence between nuclear and cytoplasmic genes has been reported in a number of previous studies (e.g. Soltis and Kuzoff, 1995; Wendel et al., 1995; Soltis et al., 1996; Hardig et al., 2000; Setoguchi and Watanabe, 2000; Yoo et al., 2002). The phenomenon has been attributed to lineage sorting (Neigel and Avise, 1986; Harrison, 1991; Hardig et al., 2000) or introgression of the cytoplasmic genome from one species onto the nuclear background of another (or vice versa) by interspecific hybridization (Soltis and Kuzoff, 1995; Soltis et al., 1996; Wendel and Doyle, 1998). Cytoplasmic gene flow with or without nuclear introgression is frequently observed (summarized by Rieseberg and Soltis, 1991; Rieseberg and Wendel, 1993). In contrast, nuclear introgression occurring without cytoplasmic gene flow appears to be rare, and only relatively few examples have been reported (e.g. Wagner et al., 1987; Arnold and Robinson, 1991; Setoguchi and Watanabe, 2000).
Although no natural interspecific hybridization has been reported in Paris, experimental outcrossing by manual pollination has been effected between most Chinese species with thick rhizomes (X. Gong, Kunming Institute of Botany, China, unpubl. res.). This indicates that natural hybridization between sympatric species is possible if other factors (e.g. pollination mechanisms) are compatible. The eight taxa removed prior to the recovery of a non-significant P value in the incongruence length difference tests between the nuclear and combined plastid data sets all share a distribution from the Hengduan Mountains to the Eastern Himalaya and are endemic to these areas, and some of them are sympatric. Some morphological intermediates have been reported, e.g. between P. marmorata and P. mairei, P. mairei and P. polyphylla var. yunnanensis, and P. polyphylla var. yunnanensis and P. polyphylla var. polyphylla (Li, 1998). Characters intermediate between those of P. luquanensis and P. polyphylla var. yunnanensis, P. mairei and P. thibetica, and P. mairei and P. daliensis have also been observed in the herbarium and in the field (Y. Ji, Kunming Institute of Botany, China, unpubl. res.). This suggests that natural hybridization occurs among at least some of these species.
Comparison of the morphology of the eight putative hybrids with nuclear and plastid topologies suggests that the ITS topology is consistent with the morphology of P. thibetica, whereas the plastid topology is consistent with that of the other taxa. Paris thibetica is unique in the genus in having characters that are intermediate between the species of section Axiparis and other capsular species (Table 3). Moreover, the species is sister to the clade of section Axiparis in plastid DNA topologies (Figs 2 and 3) but occupies the first diverging lineage within the clade consisting of capsular species in the ITS topology (Fig. 4). As such, P. thibetica is possibly a cross-bred derivative between an ancestor from section Axiparis and a capsular species, and ‘chloroplast capture’ (Soltis and Kuzoff, 1995) in an introgressant or maternal inheritance in the hybrid species could be the cause for the incongruence. Because the plastid topology best reflects phylogenetic predictions based on morphology for the other seven taxa, it is hypothesized that the discordance between nuclear and plastid lineages results from nuclear introgression without gene flow from the plastid genome. As Wagner et al. (1987) suggested, nuclear genes might be able to cross some species boundaries that plastid DNA is unable to cross. This hypothesis can be further tested in Paris through phylogenetic analysis of low-copy nuclear genes (e.g. waxy) and intensive sampling from putative hybrid populations.
Table 3.
Comparison of pollen ornamentation, ovary placentation and seed morphology among Paris thibetica, section Axiparis, and other capsular species
| Paris thibetica | Section Axiparis | Other capsular species | |
|---|---|---|---|
| Pollen ornamentation | Reticulate | Reticulate | Foveolate |
| Seeds | With imperfect aril | With imperfect aril | With sarcotesta |
| Ovary placentation | Parietal | Incompletely axile | Parietal |
Generic circumscription
The treatment of a single genus or three genera is an unresolved question in the taxonomy of Paris (Hara, 1969; Takhtajan, 1983; Li, 1984, 1998; Mitchell, 1987, 1988; Farmer and Schilling, 2002). According to the general principles of classification outlined by Backlund and Bremer (1998), a genus should be not only monophyletic with strong statistical support, but should also be recognizable from morphological characters. In the present study, Paris as circumscribed by Hara (1969), Li (1984, 1998) and Mitchell (1987, 1988) is consistently monophyletic in all separate and combined analyses of ITS, trnL-trnF and psbA-trnH data sets with strong bootstrap support. Furthermore, in all species of Paris, pollen is ellipsoidal (versus spherical in Trillium), and the pollen aperture is monosulcate (versus inaperturate in Trillium) (Takahashi, 1982, 1984; Wei, 1988, 1998; Wei and Wang, 2001). Likewise, the three segregated genera Kinugasa, Paris s.s., and Daiswa recognized by Takhtajan (1983) are each supported as monophyletic in all analyses except those of trnL-trnF and combined plastid DNA (in both of which relationships are partly unresolved). Paris (i.e. sensu lato) is supported by the probable morphological synapomorphy of 4- to 15-merous flowers and leaves (Hara, 1969; Li, 1984, 1998; Mitchell, 1987, 1988), as compared with the 3-merous condition of Trillium. Moreover, there are putative morphological synapomorphies for each of the segregate genera of Takhtajan (1983) (Table 1). On the basis of both monophyly and recognition by morphological characters, either classification is justifiable. The issue is nonetheless resolvable if one recognizes the strongly supported sister clade of Paris as a single genus, as have recent workers (as Trillium; Kato et al., 1995a, b; Kazempour Osaloo and Kawano, 1999; Kazempour Osaloo et al., 1999; Farmer and Schilling, 2002). If this clade is to be recognized at the genus level, then it follows logically that its sister clade (Paris s. l.) should be recognized at the level of genus.
Infrageneric division
Subgenera
The two subgenera recognized by Li (1984, 1998) do not accord with the topologies of Paris from all separate and combined analyses (Table 4). They become monophyletic only upon transfer of the species of section Axiparis subgenus Paris to subgenus Daiswa. In all other respects the present data support the subgeneric treatment of Li (1984, 1998). Thus, the previous use of both axile and incompletely axile placentation together to define subgenus Paris (Li, 1984, 1998) must be reassessed. Section Axiparis is unique in the genus Paris in its incompletely axile placentation (Li, 1984, 1998; Table 3). All 21 species of Trillium that have been examined for placentation type have incompletely axile placentation (Y. Ji, Kunming Institute of Botany, China, unpubl. res.). As concluded from Fig. 6A, incompletely axile placentation is most likely to be the plesiomorphic state in the genus Paris, and axile and parietal placentation thus define subgenus Paris and the rest of the species in subgenus Daiswa, respectively.
Table 4.
Bootstrap support for major clades in each of the analyses of Paris
| trnL-trnF | psbA-trnH | ITS | trnL-trnF + psbA-trnH | trnL-trnF + psbA-trnH + ITS | |
|---|---|---|---|---|---|
| Clade A [sect. Kinugasa (= Kinugasa) + sect. Paris (= Paris. s.s.)] | 68 % | 83 % | 92 % | 93 % | 83 % |
| Sect. Paris (= Paris s.s.) | Polytomy | 88 % | 59 % | Separate | 74 % |
| Clade B [(subg. Daiswa + sect. Axiparis) = Daiswa] | 98 % | 79 % | 100 % | 98 % | 100 % |
| Sect. Axiparis + P. thibetica | 70 % | 61 % | Separate | 83 % | P. thibetica excluded |
| Sect. Axiparis | Polytomy | 53 % | Separate | 65 % | 77 %1 |
| Sect. Dunnianae + sect. Fargesianae + sect. Marmoratae + sect. Euthyra | 82 % | 79 % | 68 % | 99 % | 100 %2 |
1with P. dulongensis excluded.
2with P. daliensis, P. dulongensis, P. luquanensis, P. mairei, P. marmorata, P. polyphylla var. polyphylla and P. polyphylla var. yunnanensis excluded.
The clade comprising the species of section Axiparis and those of subgenus Daiswa is supported by several characters. The plant height of the species of section Axiparis is >40 cm, similar to the species of subgenus Daiswa, whereas that of the species of subgenus Paris is <40 cm (Li, 1998; Liang and Soukup, 2000), as are all Trillium species. The seeds have an imperfect aril in section Axiparis and a sarcotesta in subgenus Daiswa. The presence of a thick outer covering in these two sections is probably a synapomorphy that unites these two groups (Fig. 6B). The basic karyotype of section Axiparis is k(2n) = 6m + 4t, which is similar to that of subgenus Daiswa. In contrast, other species of subgenus Paris are k(2n) = 6m + 2t + 2st (Li et al., 1988, 1998). Finally, both section Axiparis and subgenus Daiswa are distributed in tropical and subtropical areas, from northern Vietnam to southern, central and eastern China and the Hengduan Mountains and eastern Himalaya. The other species of subgenus Paris, in contrast, are concentrated in north-temperate regions of Asia and Europe (Li et al., 1988, 1998).
Sections
From the result of the combined plastid DNA and ITS analysis (Fig. 5), sections Kinugasa, Paris and Axiparis are monophyletic. Sections Kinugasa and Paris are also monophyletic in both the psbA-trnH and ITS analyses, and section Axiparis is also monophyletic in both the psbA-trnH and combined plastid DNA analyses (Table 4). The monophyly of these three sections and their distinctive characters (Table 5) justify their delimitation by Li (1998). The species of sections Dunnianae, Fargesianae, Marmoratae and Euthyra form a clade in all separate and combined analyses (Figs 1–5 and Table 4), which suggests that the previous delimitation of these sections (Li, 1998) must be reassessed. These sections share a capsular fruit, seeds with sarcotesta and pollen with foveolate ornamentation. Hence, section Euthyra should be expanded to accommodate all the species of the three sections. Paris thibetica is sister to the clade of Section Axiparis in the psbA-trnH and combined plastid DNA analyses, whereas it is sister to the clade of the other capsular species (the revised section Euthyra) in the ITS analysis. Because of its problematic morphology (Table 3) and the possible hybridization events involving this species as previously discussed, this species is retained here in its own section (Thibeticae).
Table 5.
Comparison of morphology among the five recircumscribed sections
| Subgenus Daiswa |
Subgenus Paris |
||||
|---|---|---|---|---|---|
| Sect. Euthyra | Sect. Thibeticae | Sect. Axiparis | Sect. Paris | Sect. Kinugasa | |
| Rhizome | Thick | Thick | Thick | Long and slender | Thick |
| Stamens | 2- to 6-whorled | 2-whorled | 2-whorled | 2-whorled | 2-whorled |
| Ovary shape | Angular | Angular | Angular | Rounded | Angular |
| Placentation type | Parietal | Parietal | Incompletely axile | Axile | Axile |
| Fruit | Capsule | Capsule | Berry | Berry | Berry |
| Seeds | With sarcotesta | With imperfect aril | With imperfect aril | Without sarcotesta or aril | Without sarcotesta or aril |
| Pollen ornamentation | Foveolate | Reticulate | Reticulate | Reticulate | Gemmate |
Based on the present study, the revised infrageneric system of Paris includes two subgenera: subgenus Paris and subgenus Daiswa. The former comprises sections Kinugasa and Paris, the latter sections Axiparis, Thibeticae and Euthyra. All five sections are distinctive on the basis of morphology (Table 5).
Acknowledgments
We are grateful to the Royal Botanic Garden, Edinburgh, P. Bruggeman, S. T. Chen, Z. L. Dao, X. Gong, Q. Guo, R. Li, J. Murata, J. McClements, L. X. Wang and W. Y. Xu for providing some of the samples used in this study, and to Drs W. B. Zomlefer, S. Kawano and M. F. Fay for their constructive comments in revising the manuscript. This work was financially supported by the United States National Science Foundation (DEB-0103795), the Ministry of Science and Technology of P. R. China (2004BA721A34) and a grant from the California Academy of Sciences to the first author funded by the Lakeside Foundation.
LITERATURE CITED
- APG II. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436. [Google Scholar]
- Arnold ML, Robinson JJ. 1991. Pollen mediated introgression and hybrid speciation in Louisiana irises. Proceedings of the National Academy of Sciences of the USA 88: 1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund A, Bremer K. 1998. To be or not to be—principles of classification and monophyletic plant families. Taxon 47: 391–400. [Google Scholar]
- Dahlgren RMT, Clifford HT, Yeo PF. 1985. The families of the monocotyledons: structure, evolution, and taxonomy. Berlin: Springer-Verlag.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin of the Botanical Society of America 19: 11–15. [Google Scholar]
- Farmer SB. 2000. Toward an understanding of the global phylogeny of Trilliaceae. MS Thesis, University of Tennessee, Knoxville, USA.
- Farmer SB, Schilling EE. 2002. Phylogenetic analyses of Trilliaceae based on morphological and molecular data. Systematic Botany 27: 674–692. [Google Scholar]
- Farris JS, Källersjö M, Kluge AC, Bult C. 1994. Testing significance of incongruence. Cladistics 10: 315–319. [Google Scholar]
- Freeman JD. 1969. A revisionary study of sessile-flowered Trillium L. (Liliaceae). PhD Dissertation, Vanderbilt University, Nashville, USA.
- Hara H. 1969. Variations in Paris polyphylla Smith with reference to other Asiatic species. Journal of the Faculty of Science, University of Tokyo, Sect. 3, Botany 10: 141–180. [Google Scholar]
- Hardig TM, Soltis PS, Soltis DE. 2000. Diversification of the North American shrub genus Ceanothus (Rhamnaceae): conflicting phylogenies from nuclear ribosomal DNA and chloroplast DNA. American Journal of Botany 87: 108–123. [PubMed] [Google Scholar]
- Harrison RG. 1991. Molecular changes at speciation. Annual Review of Ecology and Systematics 22: 281–308. [Google Scholar]
- Kato H, Kawano S, Terauchi R, Ohara M, Utech FH. 1995a. Evolutionary biology of Trillium and related genera (Trilliaceae). I. Restriction site mapping and variation of chloroplast DNA and its systematic implications. Plant Species Biology 10: 17–29. [Google Scholar]
- Kato H, Terauchi R, Utech FH, Kawano S. 1995b. Molecular systematics of the Trilliaceae sensu lato as inferred from rbcL sequence data. Molecular Phylogenetics and Evolution 4: 184–193. [DOI] [PubMed] [Google Scholar]
- Kazempour Osaloo S, Kawano S. 1999. Molecular systematics of the Trilliaceae. II. Phylogenetic analyses of Trillium and its allies using sequences of rbcL and matK genes of cpDNA and internal transcribed spacers of 18S-26S nrDNA. Plant Species Biology 14: 75–94. [Google Scholar]
- Kazempour Osaloo S, Utech FH, Ohara M, Kawano S. 1999. Molecular systematics of the Trilliaceae. I. Phylogenetic analysis of Trillium using matK gene sequences. Journal of Plant Research 112: 35–49. [Google Scholar]
- Li H. 1984. The phylogeny of the genus Paris L. Acta Botanica Yunnanica 6: 351–362. [Google Scholar]
- Li H. 1998. The phylogeny of the genus Paris L. In: Li H, ed. The genus Paris (Trilliaceae). Beijing: Science Press, 8–65.
- Li H, Gu ZJ, Na HY. 1988. Cytogeographic study of the genus Paris. Acta Phytotaxonomica Sinica 26: 1–10. [Google Scholar]
- Li H, Gu ZJ, Yang YP. 1998. Cytogeography of the genus Paris. In: Li H, ed. The genus Paris (Trilliaceae). Beijing: Science Press, 117–140.
- Liang SY, Soukup VG. 2000. Paris L. In: Wu ZY, Raven PH, eds. Flora of China. Vol. 24. Flagellariaceae through Marantaceae. Beijing: Science Press and St Louis, MO: Botanical Garden Press, 88–95.
- Long CL, Li H, Ouyang ZQ, Yang XY, Li Q, Trangmar B. 2002. Strategies for agrobiodiversity conservation and promotion: a case from Yunnan, China. Biodiversity and Conservation 11: 1146–1154. [Google Scholar]
- Maddison DR, Maddison WP. 2000. MacClade 4: analysis of phylogeny and character evolution. Sunderland, MA: Sinauer. [DOI] [PubMed]
- Mitchell B. 1987. Paris—Part I. Plantsman 9: 81–89. [Google Scholar]
- Mitchell B. 1988. Paris—Part II. Daiswa. Plantsman 10: 167–190. [Google Scholar]
- Neigel JE, Avise JC. 1986. Phylogenetic relationships of mitochondrial DNA under various demographic models of speciation. In: Karlin S, Nevo E, eds. Evolutionary processes and theory. New York, NY: Academic Press, 103–125.
- Rieseberg LH, Soltis DE. 1991. Phylogenetic consequences of cytoplasmic gene flow in plants. Evolutionary Trends in Plants 5: 65–84. [Google Scholar]
- Rieseberg LH, Wendel JF. 1993. Introgression and its consequences in plants. In: Harrison RG, ed. Hybrid zones and the evolutionary process. New York, NY: Oxford University Press, 70–114.
- Sang T, Crawford DJ, Stuessy TF. 1997. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). American Journal of Botany 84: 1120–1136. [PubMed] [Google Scholar]
- Setoguchi H, Watanabe I. 2000. Intersectional gene flow between insular endemics of Ilex (Aquifoliaceae) on the Bonin Islands and the Ryukyu Islands. American Journal of Botany 87: 793–810. [PubMed] [Google Scholar]
- Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J et al. 2005. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany 92: 142–166. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Kuzoff RK. 1995. Discordance between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae): evidence of chloroplast capture and paraphyly. Evolution 49: 727–742. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS. 1998. Choosing an approach and an appropriate gene for phylogenetic analysis. In: Soltis DE, Soltis PS, Doyle JJ, eds. Molecular systematics of plants. Vol. II. DNA sequencing. Boston: Kluwer Academic Press, 1–42.
- Soltis DE, Johnson LA, Looney C. 1996. Discordance between ITS and chloroplast topologies in the Boykinia group (Saxifragaceae). Systematic Botany 21: 169–176. [Google Scholar]
- Swofford DL. 2002. PAUP*: Phylogenetic analysis using parsimony (* and other methods), version 4.0 Beta10. Sunderland, MA: Sinauer.
- Taberlet P, Gielly L, Pautou G, Bouvet J. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- Takahashi M. 1982. Pollen morphology in North American species of Trillium. American Journal of Botany 69: 1185–1195. [Google Scholar]
- Takahashi M. 1984. Pollen morphology in Paris and its related genera. Botanical Magazine 97: 233–245. [Google Scholar]
- Takhtajan A. 1983. A revision of Daiswa (Trilliaceae). Brittonia 35: 255–270. [Google Scholar]
- Tamura MN. 1998. Trilliaceae. In: Kubitzki K, ed. The families and genera of vascular plants. Berlin: Springer-Verlag, 444–452.
- Wagner DB, Furnier GR, Saghai-Maroof MA, Williams SM, Dancik BP, Allard RW. 1987. Chloroplast DNA polymorphisms in lodgepole and jack pines and their hybrids. Proceedings of the National Academy of Sciences of the USA 84: 2097–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZX. 1988. Studies on the pollen morphology of Paris. Acta Botanica Yunnanica 10: 147–153. [Google Scholar]
- Wei ZX. 1998. Pollen morphology of Paris and the implication in systematics. In: Li H, ed. The genus Paris (Trilliaceae). Beijing: Science Press, 96–99.
- Wei ZX, Wang H. 2001. Studies of pollen morphology of four genera of Trilliaceae. Acta Botanica Yunnanica 23: 451–456. [Google Scholar]
- Wendel JF, Doyle JJ. 1998. Phylogenetic incongruence: window into genome history and speciation. In: Soltis PS, Soltis DE, Doyle JJ, eds. Molecular systematics of plants. New York, NY: Chapman and Hall, 265–296.
- Wendel JF, Schnabel A, Seelanan T. 1995. An unusual ribosomal DNA sequence from Gossypium gossypioides reveals ancient, cryptic, intergenomic introgression. Molecular Phylogenetics and Evolution 4: 298–313. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, eds. PCR protocols: a guide to methods and applications. San Diego, CA: Academic Press, 315–322.
- Yoo K, Lowry PP II, Wen J. 2002. Discordance of chloroplast and nuclear ribosomal DNA in Osmorhiza (Apiaceae). American Journal of Botany 89: 966–971. [DOI] [PubMed] [Google Scholar]
- Zomlefer WB. 1996. The Trilliaceae in the southeastern United States. Harvard Papers in Botany 1: 91–120. [Google Scholar]
- Zomlefer WB, Judd WS, Whitten WM, Williams NH. 2006. A synopsis of Melanthiaceae (Liliales), with focus on character evolution in tribe Melanthieae. In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG, eds. Comparative biology of the monocotyledons: proceedings of the third international conference. Claremont, CA: Rancho Santa Ana Botanic Garden, 564–576 (in press).