Abstract
• Background and Aims A phalanx growth form enables clonal plants to make better use of resource-rich patches, whereas a guerrilla growth form provides them with opportunities to escape from resource-poor sites. Leymus secalinus produces both spreading (guerrilla form) and clumping ramets (phalanx form). Here, the hypothesis that a trade-off between the two growth forms in L. secalinus exists under different resource levels is tested.
• Methods Ramets of L. secalinus were grown under three levels of nutrient supply.
• Key Results With increasing nutrient supply, the proportion of clumping ramets (in total number of ramets) increased, whereas that of spreading ramets decreased. With increasing nutrient supply, the number of buds increased, whereas biomass per bud decreased. A trade-off between bud number and size further supports the above hypothesis because larger buds were more likely to develop into spreading ramets, and smaller buds into clumping ramets. Mean spacer length between spreading ramets was significantly smaller under the high than under the medium nutrient supply.
• Conclusions The results suggest that a trade-off between the two growth forms in L. secalinus exists under different nutrient supplies. Such a trade-off together with plasticity in spacer morphology may enable L. secalinus to make better use of small-scale heterogeneity in resource supply.
Keywords: Architectural plasticity, bud size–number trade-off, clumping ramets, growth form trade-off, Leymus secalinus, morphological plasticity, spreading ramets
INTRODUCTION
Clonal plants are very common and diverse in the plant kingdom (Callaghan et al., 1992; Klimeš et al., 1997; Song et al., 2001). Based on the spatial arrangement of ramets, clonal plants can be classified into different clonal growth forms (Lovett-Doust, 1981; White, 1984). Clegg (1978; cited by Oborny and Cain, 1997) and Lovett-Doust (1981) distinguished two classes of clonal growth form: phalanx and guerrilla. In the phalanx growth form, connections between ramets (i.e. spacers sensu Bell, 1984) have few and/or short internodes, resulting in closely packed ramets termed ‘clumping ramets’ (Shaver and Billings, 1975; Lovett-Doust, 1981; Bernard, 1990; Navas and Garnier, 1990). In the guerrilla growth form, by contrast, connections between ramets have many and/or long internodes, resulting in widely spaced ramets termed ‘spreading ramets’ (Shaver and Billings, 1975; Lovett-Doust, 1981; Bernard, 1990; Navas and Garnier, 1990).
The ecological significance of these two clonal growth forms has long been recognized. The guerrilla growth form is very common in early successional stages, as well as in resource-heterogeneous and/or disturbed habitats, whereas the phalanx form is more favoured in late successional stages and in relatively homogeneous and/or less disturbed habitats (Schmid and Bazzaz, 1987; Navas and Garnier, 1990; Krystyna, 1995; Scott, 1995; Adachi et al., 1996). The guerrilla growth form enables clonal plants to spread quickly in horizontal space so that, in a spatially heterogeneous habitat, clonal plants can more readily escape from stressful microsites and find favourable ones (Lovett-Doust, 1981, 1987; Sutherland and Stillman, 1988; de Kroon and Hutchings, 1995; Humphrey and Pyke, 1998; Oborny et al., 2001). The phalanx growth form, by contrast, may enable clonal plants to tolerate more stressful conditions, make better use of locally abundant resources (monopolization strategy) and out-compete other species in a favourable microsite (Lovett-Doust, 1981, 1987; Schmid and Happer, 1985; Humphrey and Pyke, 1998).
The perennial grass Leymus secalinus, like many sedge species (Bernard, 1990), produces both spreading and clumping ramets, resulting in a combined growth form. In this growth form, spreading ramets are first produced at the end of long rhizomes and clumping ramets are then developed from the short rhizomes of the spreading ramets (Shaver and Billings, 1975; Bernard, 1990). A previous study has shown that severing rhizomes had little effect on the growth of the newly produced L. secalinus ramets in a spatially relatively homogeneous habitat (Dong, 1999), suggesting that the rhizomes of L. secalinus may be more important in foraging activities. Because spreading ramets have an advantage in exploiting open resources and clumping ramets may be more suited to monopolizing locally abundant resources, it is hypothesized here that a trade-off between the production of clumping and spreading ramets in L. secalinus exists under different resource supplies.
To test this hypothesis L. secalinus were grown under three levels of nutrient supply. With increasing nutrient supply it is predicted that the proportion of clumping ramets in L. secalinus will increase, whereas that of spreading ramets will decrease.
MATERIALS AND METHODS
The species
Leymus secalinus (Georgi) Tzvel. is a perennial grass approx. 45–90 cm high, distributed in the Autonomous Regions of Inner Mongolia and Tibet, and the provinces of Hebei, Shaanxi, Gansu, Shanxi and Qinhai of China, as well as in Mongolia, Japan and the Far East (Yang, 1994). It occurs in grasslands, sandy grasslands, mountain slopes, farmlands and roadsides (Dong, 1999).
Experimental design
In May, 2004, ten clones of L. secalinus were randomly collected in a sandy grassland near Ordos Sandland Ecological Station (OSES; 39 °02′N, 109 °51′E; Institute of Botany, the Chinese Academy of Sciences), located in the south-east of the Mu Us sandy grassland of China. Three similar-sized ramets of each clone were selected and planted into 30 × 20 × 20-cm plastic containers at OSES. On 15 June, 2004, after 2 weeks of recovery, the three ramets of each clone were randomly subjected to three nutrient treatments. For nutrient treatments, a commonly available granular lawn fertilizer was used (Osmocote 301, Scotts, Marysville, OH, USA) with 15N : 11P : 13K : 2 Mg (elemental ratio) as the basic fertilizer and the commercial nutrient solution (Peters1, Scotts) with 20N : 20P : 20K (elemental ratio) as the supplemental fertilizer. Under the high-nutrient treatment each container received 13·6 g Osmocote 301 once and 100 mL Peters1 nutrient solution (0·417 g L–1) once a week (Dong and Alaten, 1999). Both fertilizers (Osmocote 301 and Peters1) were reduced to 50 % in the medium-nutrient treatment and to 10 % in the low-nutrient treatment, respectively.
Measurement and analysis
The experiment was ended on 14 September, 2004. The number of clumping ramets, number of spreading ramets and number of buds (potential ramets) were counted. Spreading ramets are ramets produced by long rhizomes that eventually grow upright to form new ramets, whereas clumping ramets are ramets produced by tillering or very short rhizomes of spreading ramets. Each plant was then separated into roots, shoots, rhizomes and buds. Rhizome length and biomass of each plant part were measured after drying them at 80 °C for 48 h. Mean rhizome spacer length was calculated as the mean distance between two adjacent spreading ramets and specific spacer dry mass as the dry mass per unit spacer length. The data were analysed by using two-way ANOVA (without replication) followed by least-significant difference (LSD) tests to investigate the differences among the nutrient treatments.
RESULTS
Biomass
With increasing nutrient availability, total plant dry mass and root, shoot, bud and rhizome dry mass all increased considerably (Table 1, Fig. 1A). With increasing nutrient availability, biomass allocation to root, bud and rhizome decreased greatly, whereas that to shoot increased (Table 1, Fig. 1B).
Table 1.
Effects of nutrient availability and clone on Leymus secalinus
| Nutrient effect |
Clone effect |
|||
|---|---|---|---|---|
| Trait | F2,18 | P | F9,18 | P |
| (A) Biomass | ||||
| Plant dry mass | 47·129 | <0·001 | 2·640 | 0·038 |
| Root dry mass | 31·532 | <0·001 | 2·730 | 0·033 |
| Shoot dry mass | 54·713 | <0·001 | 2·420 | 0·053 |
| Bud dry mass | 23·583 | <0·001 | 2·317 | 0·062 |
| Rhizome dry mass | 25·729 | <0·001 | 0·792 | 0·628 |
| (B) Ramet number and size | ||||
| Number of clumping ramets | 34·875 | <0·001 | 1·919 | 0·114 |
| Number of spreading ramets | 20·555 | <0·001 | 0·422 | 0·906 |
| Percentage of clumping ramets | 17·513 | <0·001 | 1·603 | 0·188 |
| Percentage of spreading ramets | 17·513 | <0·001 | 1·603 | 0·188 |
| Dry mass per ramet | 0·371 | 0·695 | 2·768 | 0·032 |
| Number of leaves per ramet | 0·862 | 0·439 | 0·970 | 0·495 |
| (C) Bud number and size | ||||
| Number of buds | 35·717 | <0·001 | 1·221 | 0·342 |
| Dry mass per bud | 8·382 | 0·003 | 0·997 | 0·476 |
| (D) Spacer morphology | ||||
| Mean spacer length | 6·553 | 0·007 | 1·305 | 0·300 |
| Specific spacer dry mass | 0·003 | 0·997 | 1·784 | 0·142 |
Fig. 1.
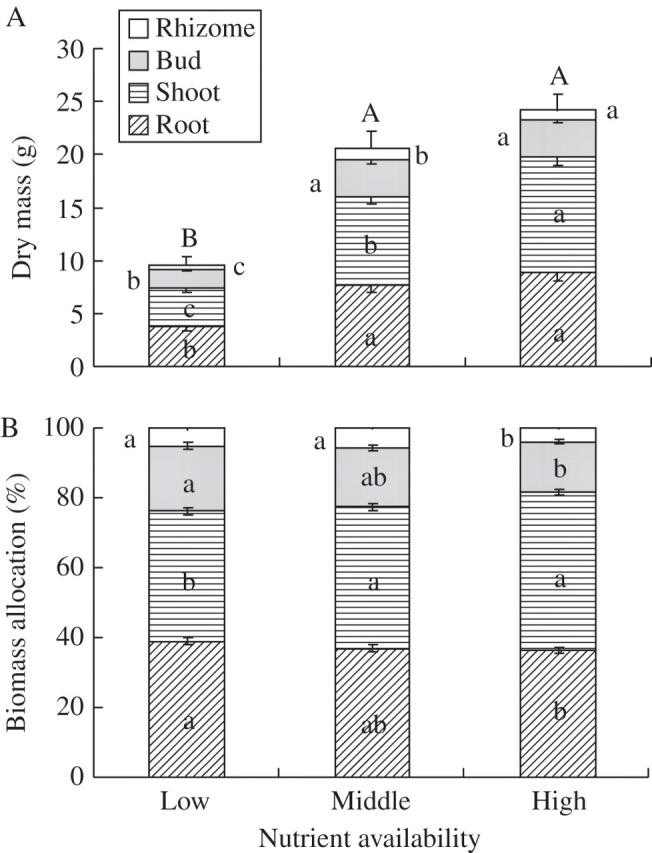
(A) Dry mass and (B) percentage biomass allocation in Leymus secalinus under the three nutrient treatments. Horizontal bars and vertical lines indicate mean and s.e. For each compartment, bars sharing the same letters are not significantly different at P = 0·05.
Ramet number and size
Both number of clumping ramets and number of spreading ramets were higher under higher nutrient supply (Table 1, Fig. 2A). The proportion of clumping ramets in total ramets increased significantly with increasing nutrient supply, whereas that of spreading ramets decreased (Table 1, Fig. 2B). Dry mass per ramet and number of leaves per ramet did not differ among the three nutrient treatments (Table 1).
Fig. 2.
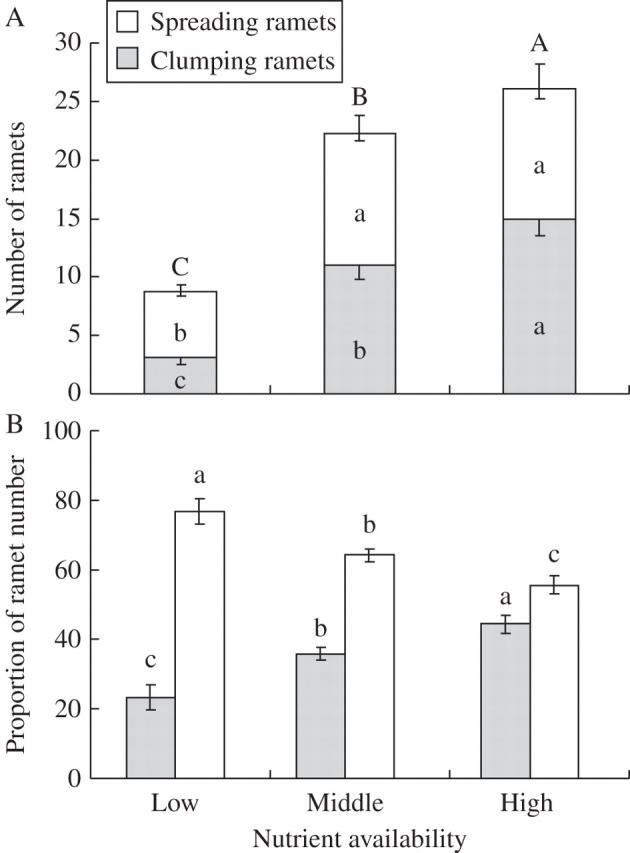
(A) Number of spreading and clumping ramets and (B) the proportion of ramet number (number of spreading and clumping ramets, respectively, divided by total number of ramets) in Leymus secalinus under the three nutrient treatments. Horizontal bars and vertical lines indicate mean and s.e. For each compartment, bars sharing the same letters are not significantly different at P = 0·05.
Bud number and size
With increasing nutrient supply, the number of buds increased considerably (Table 1, Fig. 3A), whereas dry mass per bud decreased (Table 1, Fig. 3B).
Fig. 3.
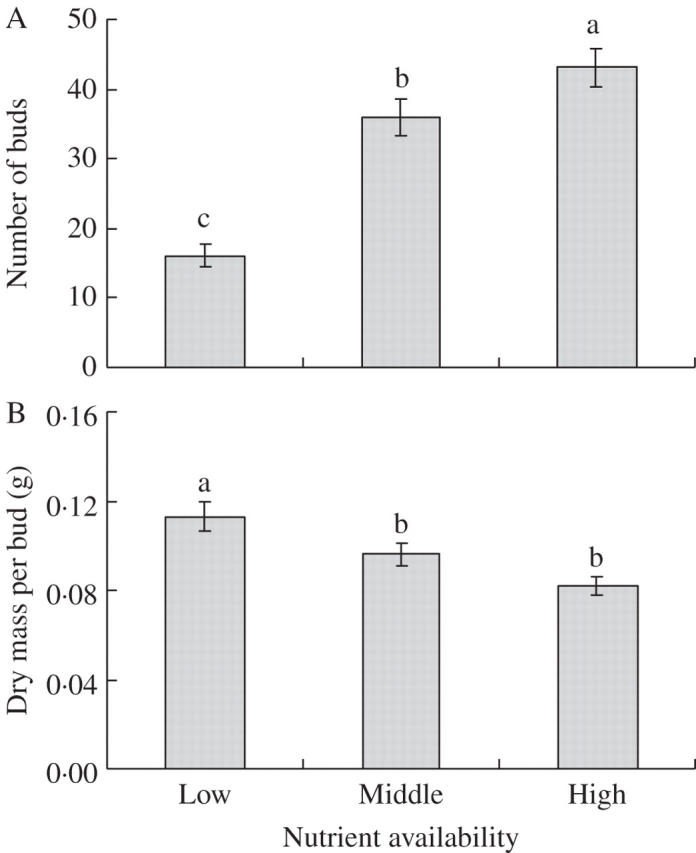
(A) Number of buds and (B) dry mass per bud in Leymus secalinus under the three nutrient treatments. Mean ± 1 s.e. is indicated. Bars sharing the same lower-case letters are not significantly different at P = 0·05.
Spacer morphology
Mean spacer length was the greatest under the medium level of nutrient supply and did not differ between the high- and low-nutrient treatments (Table 1, Fig. 4A). Specific spacer dry mass was statistically similar under the three nutrient treatments (Table 1, Fig. 4B).
Fig. 4.
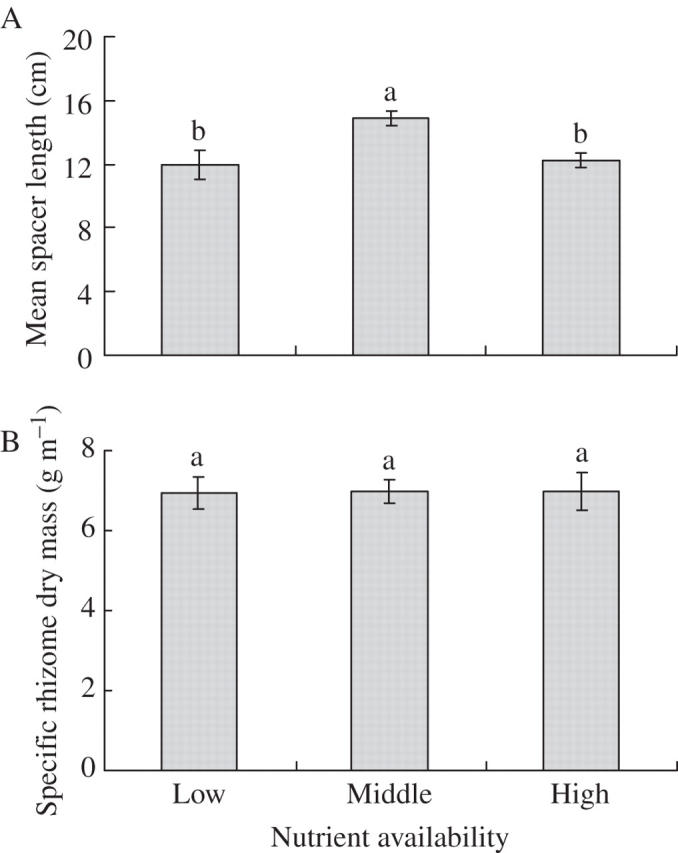
(A) Mean spacer length and (B) specific rhizome dry mass (dry mass per unit length of rhizome) in Leymus secalinus under the three nutrient treatments. Mean ± 1 s.e. is indicated. Bars sharing the same lower-case letters are not significantly different at P = 0·05.
DISCUSSION
Higher nutrient availability generally enhances biomass and module production, and decreases root to shoot ratio (Aung, 1974; Hunt and Nicholls, 1986; Sutherland and Stillman, 1988; Sakai, 1995; Stuefer et al., 1996; Verburg et al., 1996; Gardner and Mangel, 1999). This holds also for L. secalinus (Figs 1–3).
Trade-offs between growth forms
Within increasing nutrient supply, the proportion of clumping ramets in L. secalinus increased, whereas that of spreading ramets decreased (Fig. 2B). These results support our hypothesis that a trade-off between guerrilla and phalanx growth forms exists in the clonal grass L. secalinus, in response to resource availability.
Moreover, the number of buds of L. secalinus increased significantly with increasing nutrient supply (Fig. 3A), whereas dry mass per bud decreased (Fig. 3B), indicating a trade-off between bud number and size. The buds will develop into either spreading or clumping ramets. However, it is most likely that larger buds will develop into spreading ramets and smaller buds into clumping ramets, because developing into spreading ramets will involve more energy costs than developing into clumping ramets, and larger buds have a greater carbohydrate storage potential than smaller buds (McGinley et al., 1987; Dong et al., 1997). Producing more small buds under the high-nutrient supply enhanced production of clumping ramets. The number–size trade-off of L. secalinus buds therefore further supports the above hypothesis.
Mean spacer length between two adjacent spreading ramets was lower under the high- than under the medium-nutrient supply (Fig. 4A), whereas mean spacer thickness and potentially also mechanical strength, as indicated by specific spacer dry mass (Fig. 4B), were not affected by nutrient supply. Therefore, the architecture of L. secalinus was more aggregated under high- than under medium-nutrient supply, suggesting a plastic modification from a guerrilla form towards a phalanx form (de Kroon and Knops, 1990; Evans, 1992; Dong and Pierdominici, 1995; de Kroon and Hutchings, 1995). However, mean spacer length was lower under the low- than under the medium-nutrient supply and did not differ between the low- and the high-nutrient supply (Fig. 4A). It is possible that under the low-nutrient supply there were insufficient resources available to be allocated to rhizomes. Therefore, plasticity in spacer length of L. secalinus in response to nutrient supply only partly supports the hypothesis.
Ecological significance in heterogeneous environments
In a spatially heterogeneous habitat, shortening spacers under resource-rich patches and elongating spacers in resource-poor patches may potentially position more newly produced ramets (feeding sites sensu Bell, 1984) in resource-rich patches to acquire resources efficiently, and thereby to increase survival (de Kroon and Knops, 1990; Evans, 1992; Hutchings and de Kroon, 1994; Dong and Pierdominici, 1995; de Kroon and Hutchings, 1995; de Kroon et al., 2005). However, the extent of plasticity in spacer length is genetically determined and is also affected by the phylogeny of the species (de Kroon et al., 1994). Therefore, if the resource-rich patches are too small (e.g. patch size close to the canopy size of an individual ramet or to spacer length), even shortening spacers may not guarantee that newly produced ramets are positioned in the same resource-rich patches, and thus may not enable clonal species to forage for resources efficiently and, consequently, will fail to increase the fitness of the whole genet (Briske and Derner, 1998).
In resource-poor nutrient patches, L. secalinus will not only increase spacer length between spreading ramets, but also increase allocation to buds and biomass to spreading ramets. These combined responses may enable L. secalinus to search for resource-rich patches more efficiently. Once a spreading ramet is positioned in a resource-rich patch, a decrease in spacer length together with an increase in the proportion of clumping ramets could enable L. secalinus to monopolize these locally abundant resources and to compete efficiently with other species (de Kroon and Schieving, 1990; Derner, 1999). Even when the favourable patches are very small, clumping ramets may still be readily positioned in the resource-rich patches, because there are either no spacers between the clumping ramets of L. secalinus or the spacer is very short (Shaver and Billings, 1975; Bernard, 1990). A previous study has shown that clonal integration was of little importance in L. secalinus in relatively homogeneous habitats (Dong, 1999). Therefore, trade-offs between the two growth forms together with plasticity in spacer length may have been selected for in L. secalinus to make good use of spatial heterogeneity in resource supply, even where this occurs at very small scales.
Although decreasing nutrient availability greatly reduced biomass and ramet production in L. secalinus, overall ramet size was not affected by nutrient supply (Table 1). Similar results were found by Stuefer et al. (2002), in which low-light treatment reduced plant biomass and number of ramets, but did not affect average ramet weight. This may be interpreted as a strategy that enables L. secalinus to cope with spatial and temporal heterogeneity in resource supply, and to ensure its survival in different habitats.
CONCLUSIONS
It was predicted that there would be greater architectural plasticity in clonal species with a mixture of phalanx and guerrilla forms (e.g. L. secalinus) than in those with either a distinctly phalanx or a distinctly guerrilla growth form. A trade-off between the two growth forms together with plasticity in spacer length enables L. secalinus, and possibly other species with a similar architecture, to make better use of spatial heterogeneity in resource supply, even where this occurs at very small scales.
Acknowledgments
We thank Dr David R. Causton and two anonymous reviewers for their valuable comments on an earlier version of the manuscript. This research was supported by Key Project of the National Natural Science Foundation of China (30330130) and by the National Natural Science Foundation of China (30500070).
LITERATURE CITED
- Adachi N, Terashima I, Takahashi M. 1996. Central die-back of monoclonal stands of Reynoutria japonica in an early stage of primary succession on Mount Fuji. Annals of Botany 77: 477–486. [Google Scholar]
- Aung LG. 1974. Root–shoot relationship. In: Carson W, ed. The plant root and its environment. Charlottesville: University Press of Virginia, 29–61.
- Bell AD. 1984. Dynamic morphology: a contribution to plant population ecology. In: Dirzo R, Sarukhan J, eds. Perspectives on plant population ecology. Sunderland, MA: Sinauer, 48–65.
- Bernard JM. 1990. Life history and vegetative reproduction in Carex. Canadian Journal of Botany 68: 1441–1448. [Google Scholar]
- Briske DD, Derner JD. 1998. Clonal biology of caespitose grass. In: Cheplick GP, ed. Population biology of grasses. Cambridge: Cambridge University Press, 106–135.
- Callaghan TV, Carlsson BA, Jónsdóttir IS, Svensson BM, Jonasson S. 1992. Clonal plants and environmental change: introduction to the proceedings and summary. Oikos 63: 341–347. [Google Scholar]
- Derner JD. 1999. Intraclonal regulation in a perennial caespitose grass: a field evaluation of above-and below-ground resource availability. Journal of Ecology 87: 737–747. [Google Scholar]
- Dong M. 1999. Effects of severing rhizome on clonal growth in rhizomatous grass species Psammochloa villosa and Leymus secalinus. Acta Botanica Sinica 41: 194–198. [Google Scholar]
- Dong M, Alaten B. 1999. Clonal plasticity in response to rhizome severing and heterogeneous resource supply in the rhizomatous grass Psammochloa villosa in an Inner Mongolian dune, China. Plant Ecology 141: 53–58. [Google Scholar]
- Dong M, Pierdominici MG. 1995. Morphology and growth of stolons and rhizomes in three clonal grasses, as affected by different light supply. Vegetatio 116: 25–32. [Google Scholar]
- Dong M, During HJ, Werger MJA. 1997. Clonal plasticity in response to nutrient availability in the pseudoannual herb, Trientalis europaea L. Plant Ecology 131: 233–239. [Google Scholar]
- Evans JP. 1992. The effect of local resource availability and clonal integration on ramet functional morphology in Hydrocotyle bonariensis. Oecologia 89: 265–276. [DOI] [PubMed] [Google Scholar]
- Gardner SN, Mangel M. 1999. Modeling investments in seeds, clonal offspring, and translocation in a clonal plant. Ecology 80: 1202–1220. [Google Scholar]
- Humphrey LD, Pyke DA. 1998. Demographic and growth responses of a guerrilla and a phalanx perennial grass in competitive mixtures. Journal of Ecology 86: 854–865. [Google Scholar]
- Hunt R, Nicholls AO. 1986. Stress and the coarse control of growth and root–shoot partitioning in herbaceous plants. Oikos 47: 149–158. [Google Scholar]
- Hutchings MJ, de Kroon H. 1994. Foraging in plants: the role of morphological plasticity in resource acquisition. Advances in Ecological Research 25: 159–238. [Google Scholar]
- Klimeš L, Klimešová J, Hendriks R, Van Groenendael J. 1997. Clonal plant architecture: a comparative analysis of form and function. In: de Kroon H, van Groenendael J, eds. The ecology and evolution of clonal plants. Leiden: Backhuys, 1–29.
- de Kroon H, Hutchings MJ. 1995. Morphological plasticity in clonal plants: the foraging concept reconsidered. Journal of Ecology 83: 143–152. [Google Scholar]
- de Kroon H, Knops J. 1990. Habitat exploration through morphological plasticity in two chalk grassland perennials. Oikos 59: 39–49. [Google Scholar]
- de Kroon H, Schieving F. 1990. Resource partitioning in relation to clonal growth strategy. In: van Groenendael J, de Kroon H, eds. Clonal growth in plants: regulation and function. The Hague: SPB Academic Publishing, 113–130.
- de Kroon H, Stuefer JF, Dong M, During HJ. 1994. On plastic and non-plastic variation in clonal plant morphology and its ecological significance. Folia Geobotanica & Phytotaxonomica 29: 123–138. [Google Scholar]
- de Kroon H, Huber H, Stuefer JF, van Groenendael J. 2005. A modular concept of phenotypic plasticity in plants. New Phytologist 166: 73–82. [DOI] [PubMed] [Google Scholar]
- Krystyna F. 1995. Genet disintegration in Filipendula ulmaria: consequences for population dynamics and vegetation succession. Journal of Ecology 83: 9–21. [Google Scholar]
- Lovett-Doust L. 1981. Population dynamics and local specialization in a clonal perennial (Ranunculus repens). Journal of Ecology 69: 743–755. [Google Scholar]
- Lovett-Doust L. 1987. Population dynamics and clonal specialization in a clonal perennial (Ranunculus repens). Responses to light and nutrient supply. Journal of Ecology 75: 555–568. [Google Scholar]
- McGinley MA, Temme DH, Geber MA. 1987. Parental investment in offspring in variable environment: theoretical and empirical considerations. American Naturalist 130: 370–389. [Google Scholar]
- Navas ML, Garnier E. 1990. Demography and growth forms of the clonal perennial Rubia peregrine in Mediterranean vineyard and unmanaged habitats. Journal of Ecology 78: 691–712. [Google Scholar]
- Oborny B, Cain ML. 1997. Models of spatial spread and foraging in clonal plants. In: de Kroon H, van Groenendael J, eds. The ecology and evolution of clonal plants. Leiden: Backhuys, 155–183.
- Oborny B, Czárán T, Kun A. 2001. Exploration and exploitation of resource patches by clonal growth: a spatial model on the effect of transport between modules. Ecological Modelling 141: 151–169. [Google Scholar]
- Sakai S. 1995. Optimal resource allocation to vegetative and sexual reproduction of a plant growing in a spatially varying environment. Journal of Theoretical Biology 175: 271–282. [Google Scholar]
- Schmid B, Bazzaz FA. 1987. Clonal integration and population structure in perennials: effects of severing rhizome connection. Ecology 68: 2013–2022. [DOI] [PubMed] [Google Scholar]
- Schmid B, Happer JL. 1985. Clonal growth in grassland perennials. . Density and pattern dependent competition between plants with different growth forms. Journal of Ecology 73: 793–808. [Google Scholar]
- Scott WS. 1995. Physiological integration among clonal ramets during invasion of disturbance patches in a New England salt marsh. Annals of Botany 76: 225–233. [Google Scholar]
- Shaver GR, Billings WD. 1975. Root production and root turnover in a wet tundra ecosystem, Barrow, Alaska. Ecology 56: 401–409. [Google Scholar]
- Song MH, Dong M, Jiang GM, Li LH. 2001. Clonal plants along NECT and relation of their importance to environmental conditions. Acta Ecologica Sinica 21: 1096–1103. [Google Scholar]
- Stuefer JF, de Kroon H, During HJ. 1996. Exploitation of environmental heterogeneity by spatial division of labour in a clonal plant. Functional Ecology 10: 328–334. [Google Scholar]
- Stuefer JF, van Hulzen JB, During HJ. 2002. A genotypic trade-off between the number and size of clonal offspring in the stoloniferous herb Potentilla reptans. Journal of Evolutionary Biology 15: 880–884. [Google Scholar]
- Sutherland WJ, Stillman RA. 1988. The foraging tactics of plants. Oikos 52: 239–244. [Google Scholar]
- Verburg RW, Kwant R, Werger MJA. 1996. The effect on plant size on vegetative reproduction in a pseudo-annual. Vegetatio 125: 185–192. [Google Scholar]
- White EG. 1984. A multispecies simulation model of grassland producers and consumers: producers. Ecological Modelling 24: 241–262. [Google Scholar]
- Yang XL. 1994. Genus Leymus. In: Ma YQ, ed. Flora of Inner Mongolia, 5th vol., 2nd edn. Hohhut: Inner Mongolia People Press, 151–152 (in Chinese).


