Abstract
• Background and Aims Once human skin contacts stinging hairs of Urtica spp. (stinging nettles), the irritant is released and produces pain, wheals or a stinging sensation which may last for >12 h. However, the existence of pain-inducing toxins in the stinging hairs of Urtica thunbergiana has never been systematically demonstrated. Experiments were therefore conducted to identify the persistent pain-inducing agents in the stinging hairs of U. thunbergiana.
• Methods The stinging hairs of U. thunbergiana were removed and immersed in deionized water. After centrifugation, the clear supernatants were then subjected to high-performance liquid chromatography (HPLC), enzymatic analysis and/or behavioural bioassays.
• Key Results The HPLC results showed that the major constituents in the stinging hairs of U. thunbergiana were histamine, oxalic acid and tartaric acid. However, the well-recognized pain-inducing agents, serotonin and formic acid, existed at a low concentration as estimated by HPLC and/or enzymatic analyses. The behavioural tests showed that 2 % oxalic acid and 10 % tartaric acid dramatically elicited persistent pain sensations in rats. In contrast, 10 % formic acid and 2 % serotonin only elicited moderate pain sensation in the first 10 min. Moreover, no significant pain-related behavioural response was observed after injecting 10 % acetylcholine and histamine in rats.
• Conclusions Oxalic acid and tartaric acid were identified, for the first time, as major long-lasting pain-inducing toxins in the stinging hairs of U. thunbergiana. The general view that formic acid, histamine and serotonin are the pain-inducing agents in the stinging hairs of U. dioica may require updating, since their concentrations in U. thunbergiana were too low to induce significant pain sensation in behavioural bioassays.
Keywords: Formalin test, high-performance liquid chromatography (HPLC), oxalic acid, stinging hairs, tartaric acid, Urtica thunbergiana
INTRODUCTION
In Urtica spp. (stinging nettles), stinging hairs are known to induce persistent pain when their tips touch human skin. After contacting human skin, the irritant is released and produces pain, wheals or a stinging sensation which may last for >12 h (Oliver et al., 1991). Many potential pain-inducing toxins have been proposed as the causative agents during the study of these hairs (Thurston and Lersten, 1969), including histamine, acetylcholine and serotonin (Emmelin and Feldberg, 1947; Collier and Chesher, 1956; Oliver et al., 1991; Taskila et al., 2000). However, the existence of these pain-inducing toxins in the stinging hairs of Urtica thunbergiana has never been systematically demonstrated. Pain can be triggered by various natural stimuli, such as mechanical, thermal, chemical and electrical means, and many methods have been developed for assessing pain in animals (Jourdan et al., 2001). Previous studies on the identification of pain-inducing toxins were based on contraction reactions of guinea pig jejunum and intestine (Emmelin and Feldberg, 1947) or rat uterus and colon (Collier and Chesher, 1956) exposed to pain-producing chemicals. Human reactions by pricking the skin with histamine or acetylcholine were detected only in the first 2 min (Emmelin and Feldberg, 1947), which does not fully explain why pain sensations may persist for 1–2 h, followed by tingling paraesthesia which can persist for up to 12 h after contact with Urtica spp. Although part of the immediate reaction can be mediated by histamine (Oliver et al., 1991), a microdialysis analysis performed by Taskila indicated only a weak release of histamine and serotonin (Taskila et al., 2000). These results might not fully support the hypothesis that histamine is the persistent pain-inducing toxin in Urtica.
The stinging hairs of Urtica spp. were first reported by Hooke (1665). In 1849, Gorup-Besanez proposed that formic acid was the major toxin involved (Gorup-Besanez, 1849). Although this assumption was challenged by Haberlandt's studies in 1886 (Emmelin and Feldberg, 1947; Thurston and Lersten, 1969), formic acid is still considered to be the major pain-inducing agent in the stinging hair of Urtica spp. in some areas (Lin et al., 1991; Edom, 2002). Since little work has been done on the morphology and/or toxicology of plant stinging hairs for the past century, much of our knowledge is based on unsound references and is superficial or inaccurate (Thurston and Lersten, 1969; Edom, 2002). These uncertainties indicate that systematic investigation is required to clarify the toxic elements in the stinging hairs of Urtica.
The toxic effects of extracts from stinging hairs of Urtica spp. have not been well characterized due to the difficulty in extracting toxins effectively from the hairs. Whereas some earlier preparations employed individual hairs removed with fine forceps to investigate the toxins in stinging hairs (Emmelin and Feldberg, 1947; Collier and Chesher, 1956), most studies identified the pain-inducing agents in the stinging hairs using whole leaves (Saxena et al., 1965; Antonopoulou et al., 1996). However, extracts from individual hairs or whole leaves contain a mixture of compounds, not only the toxins (Emmelin and Feldberg, 1948).
In this study, we attempted to clarify the pain-producing toxins in U. thunbergiana, the most common species of nettle in Taiwan, using high-performance liquid chromatography (HPLC), enzymatic analysis and an animal behavioural assay. The stinging hairs consist of a minute capillary tube, silicified at its upper end and calcified at its lower end. The latter breaks off on contact with skin, exposing a fine needle-like point which penetrates the skin, with pressure leading to expression of the fluid contents into the dermis (Kulze and Greaves, 1988). A preparation of dipping the fluids exuded from the tip of stinging hairs into water was adopted here. This preparative extract contained only compounds injected into human skin when it contacts the stinging hairs of U. thunbergiana.
MATERIALS AND METHODS
Sample preparation and chemicals
Standard solutions of acetylcholine chloride, histamine dihydrochloride and serotonin hydrochloride were obtained from Sigma-Aldrich. Formic acid was bought from Chem Service, oxalic acid from Yakuri Chemicals and l-tartaric acid from J. T. Baker.
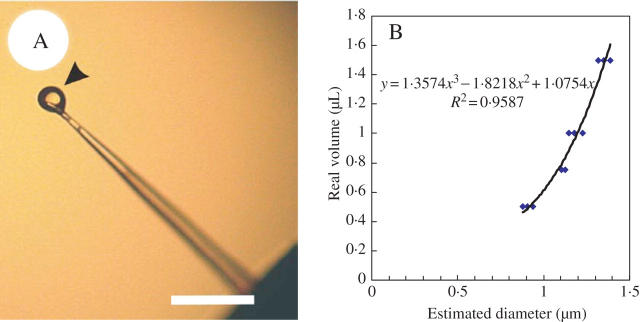
Urtica thunbergiana was collected in Dungshih, Taichung County and Hsitou, Nantou County in west-central Taiwan in June 2004 and grown in a greenhouse at the Department of Life Science, National Taiwan University. To collect fluids of stinging hairs, the hairs on the surfaces of leafs and stalks were carefully cut from the base, and the tips of the stinging hairs were broken using forceps. Afterwards, the base parts of the hairs were compressed with forceps to force out any fluids (Fig. 1A) into deionized water. The fluids collected were stored at −20 °C if not used immediately.
Fig. 1.
Fluid extract from a stinging hair. (A) Microphotograph of fluid exuded by pressing the base of a stinging hair of Urtica thunbergiana. The fluids (arrowhead) were dipped into water for further identification. Scale bar = 300 μm. (B) Calibration curve of the estimated fluid diameter by image analysis software vs. fluid volume by micropipette. The volumes of fluids in the stinging hairs were converted from the estimated diameter by the formula of this curve.
Estimation of fluid volumes in a single stinging hair
To estimate the volume of fluids in the stinging hairs, fluids exuded on the tips were photographed under a microscope (Fig. 1A); volumes of 0·5, 0·75, 1·0 and 1·5 μL of water on the tip of the micropipette were used as a regression reference. Images were analysed by Image-Plus Pro (version 4.5) to measure the diameter of fluids exuded from stinging hairs and to convert then to volumes using the water reference.
HPLC-UV detection system
Prior to HPLC analysis, the optimized UV spectra of acetylcholine, histamine, serotonin and formic acid were determined by wavelength scanning between 200 and 320 nm using a standard UV system. The HPLC system used to analyse the potential chemicals in the stinging hairs of U. thunbergiana was an Agilent 1100 series with a UV detector. Separation was performed on a 250 × 4·6 mm (5 μm particles) Hypersil GOLD C-18 RP column from Thermo Incorporated using 25 mm phosphate buffer (pH 2·5):acetonitrile at 95 : 5 (v/v) at a flow rate of 1 mL min−1. The peaks were identified by comparison with the retention times of the standard solutions and confirmed by the standard addition of the target compounds.
The calibration curves were based on three replicates using the standards and used to estimate the concentrations of the sample solutions, which were converted to concentrations of the respective compounds in a single stinging hair. Five different concentrations of each standard were added, respectively, to the sample solutions. Serotonin was used as an internal standard for inclusion in the sample solutions, since it was undetectable in the stinging hair fluids.
Formic acid assay
The presence of formic acid in the stinging hairs of U. thunbergiana was also analysed using a formic acid test kit according to a procedure recommended by the manufacturer (R-Biopharm). In brief, 1·0 mL of phosphate buffer solution (pH 7·5) containing NAD and Li salt was mixed with 0·1 mL of sample, and water was added to bring the total solution to 3·0 mL. After incubation with formate dehydrogenase solution (0·05 mL) for 20 min, the absorbance at 340 nm was recorded and the concentration of formic acid was calculated according to the standard curve. To ensure that no formic acid degradation occurred during sample preparation, stinging hairs were extracted both in water and in standard solutions, and a pure standard and blank were also analysed at the same time for comparison.
Determination of oxalic acid
The content of oxalic acid was deduced from the oxalate concentration measured by a diagnostic kit (Sigma-Aldrich, catalogue no. 591-D) according to the manufacturer's instructions with minor modifications. In brief, 100 μL of reagent A [3-methyl-2-benzothiazolinone hydrazone (MBTH, 0·22 mm) and 3-(dimethylamino) benzonic acid (DMAB, 3·2 mm), pH = 3·1] was mixed with 50 μL of the sample solutions, followed by the addition of 20 μL of reagent B [oxalate oxidase (3000 U L−1) and peroxidase (100 000 U L−1)]. After a 5 min reaction, the absorbance at 590 nm was recorded. The concentration of oxalic acid in the sample solutions was estimated according to the calibration curve of the standard solutions.
The presence of oxalic acid in the stinging hairs was also confirmed by a histochemical technique according to Yasue (1969). Fresh stinging hairs were picked and immersed in a solution containing 5 % (v/v) glacial acetic acid for 30 min. At the end of the incubation, the hairs were washed with distilled water, placed in 5 % (w/v) silver nitrate for 15 min and thoroughly washed with distilled water again. To enhance the staining, samples were further immersed in saturated dithio-oxamide (in 70 % ethanol) containing two drops per 100 mL of a 28 % ammonia solution for 1 min, and sequentially washed with 50 % ethanol and water. Soluble oxalic acid and insoluble calcium oxalate stained black.
In order to distinguish calcium oxalate from other forms of calcium precipitates, such as calcium carbonate, 1 % (w/v) alizarin red S was utilized to identify the calcium. The existence of calcium oxalate showed a positive calcium reaction to alizarin red S after 5 % (v/v) glacial acetic acid treatment for 30 min, but a negative reaction after 2 % (v/v) hydrochloric acid for 30 min. Negative reactions after both acetic acid and hydrochloric acid treatment indicated another calcium precipitate besides calcium oxalate.
Evaluation of pain in the rat
We adopted the formalin method to identify the pain-inducing agents in stinging hairs of U. thunbergiana (Coderre et al., 1993). Experiments were carried out on 30 adult female Wistar rats weighing 180–230 g. Animals were housed three or four to a cage on a 12 h light–dark cycle and supplied with food and water ad libitum. Each animal received a maximum of two rounds of toxin injections with at least a 7 d interval to avoid the development of tolerance, and to ensure that the previous drugs had been eliminated from the injection site (Smith et al., 1988). In addition, the second injection was applied to the alternate hind paw in the same rat.
Before testing, rats were allowed to acclimatize to the experimental arena, a plastic chamber (30 × 30 × 50 cm), for approximately 15−20 min. Afterward, the hind paw of a rat was injected with 20 μL of toxic agents using a 1 mL syringe with a 27-gauge needle, or challenged by repeated stroking with U. thunbergiana leaves five times. The rat was then put into the plastic chamber for behavioural observation for 60 min. The responses were analysed based on the weighted-scores method of Coderre (Coderre et al., 1993). The time spent in each of four behavioural categories was measured and weighted as follows: 0, the injected paw was not favoured; 1, little or no weight was placed on the injected paw; 2, the injected paw was elevated and was not in contact with any other surface; and 3, the injected paw was licked, bitten or shaken.
The scores were recorded in minutes by summing the time spent in each of the four behavioural categories multiplied by the weighting scores. The scores were averaged per 5 min for further statistical analyses, which used two-way analysis of variance (ANOVA) followed by post hoc multiple comparisons (all pairwise and vs. the control) using the Holm–Sidak method. All of these analyses were performed using SigmaStat (version. 3.1) on a Windows PC.
RESULTS
Fluid volumes in a single stinging hair
Figure 1B shows the calibration curve of fluids exuded from a single stinging hair. The regression analysis of the water reference showed the linear equation to be y = 1·3574x3 − 1·8218x2 + 1·0754x (R2 = 0·9587), where y is the theoretical volume calculated from the diameter measured by the image processor, and x is the diameter measured under microscopy. The estimated volume of fluid per stinging hair was 3·97 nL.
HPLC
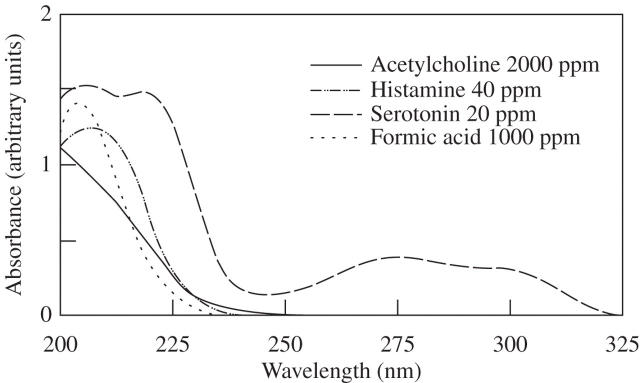
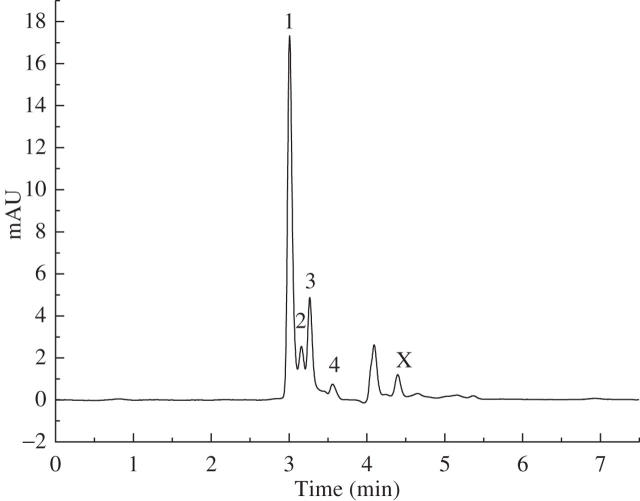
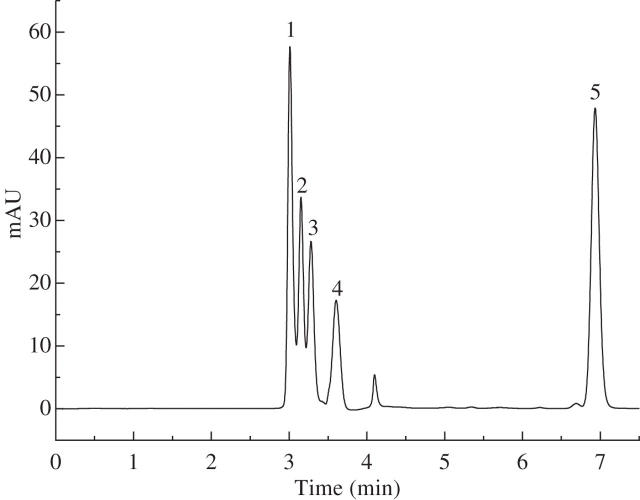
The optimal UV spectra for histamine, serotonin and formic acid detection were calibrated at 210 nm (Fig. 2). Acetylcholine could not be detected in the current HPLC system because of its low UV absorption. Figure 3 shows the HPLC chromatogram of sample solutions from stinging hairs. By comparison with internal standards, the chemicals in peaks 1–4 of Fig. 3 were shown to be histamine, oxalic acid, tartaric acid and formic acid, respectively, as compared with the standards in HPLC (Fig. 4). Serotonin was not found in the chromatograms of the sample solutions in the current study. The peak at 4 min in both the plant extract and the standard solution results from the HPLC system, and the chemical responsible for peak X in Fig. 3 is still unknown.
Fig. 2.
UV spectra of four proposed pain-inducing toxins. Standard solutions of acetylcholine, histamine, serotonin and formic acid were prepared in deionized water at the concentrations indicated and subjected to UV spectrophotometric scanning.
Fig. 3.
HPLC chromatogram of a sample solution from stinging hairs. The sample solution was prepared by dipping fluid from 100 stinging hairs into 1 mL of deionized water, and 20 μL of sample solution was injected for chromatographic separation. The retention times for peaks 1–4 were 3·008, 3·159, 3·267 and 3·562 min, respectively. Peak X did not occur in the HPLC chromatogram of the standards (Fig. 4), and the chemical structure of the peak is unknown. The ordinate scale is arbitrary units (AU) of UV absorbance at 210 nm.
Fig. 4.
HPLC chromatogram of the proposed pain-inducing agents. The column and the conditions of the chromatography were the same as those described in Fig. 3. Standards were prepared by dissolving pure compounds in deionized water. Peak 1, histamine [5 ppm (w/v)] at 3·01 min; peak 2, oxalic acid [10 ppm (w/v)] at 3·153 min; peak 3, tartaric acid [50 ppm (w/v)] at 3·281 min; peak 4, formic acid [100 ppm (v/v)] at 3·605 min; peak 5, serotonin [4 ppm (w/v)] at 6·934 min. The ordinate scale is arbitrary units (AU) of UV absorbance at 210 nm.
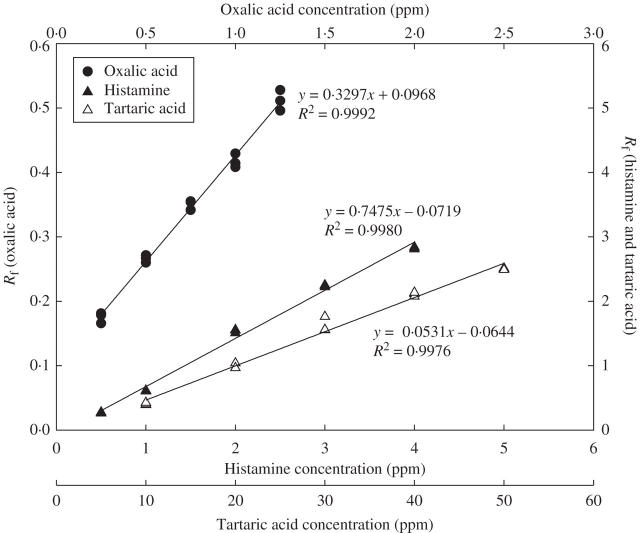
The calibration curves of histamine, oxalic acid and tartaric acid standards (with an internal standard of serotonin) are shown in Fig. 5. These calibration curves were used to deduce the concentrations of histamine, oxalic acid and tartaric acid shown in Fig. 3. The results given in Table 1 are expressed as the weight or concentration per stinging hair based on the volume per stinging hair.
Fig. 5.
HPLC calibration curves of histamine, oxalic acid and tartaric acid. Five concentrations of each chemical were prepared in deionized water, and 20 μL of each sample was injected into a 250 × 4·6 mm C-18 column for chromatographic separation. Serotonin in deionized water was used as an internal standard, and each sample was triple-duplicated. The Rf values were estimated by the peak area of the standard divided into that of serotonin. Regression analysis from all above curves showed that coefficients of x are statistically significant (t-test, P < 0·001).
Table 1.
Estimated concentration of three pain-inducing toxins in the stinging hairs of U. thunbergiana
| Histamine | Oxalic acid | Tartaric acid | |
|---|---|---|---|
| Sample solution (mg L−1) | 2·12 | 0·48 | 5·72 |
| Weight per stinging hair (ng)* | 21·2 | 4·8 | 57·2 |
| Concentration per stinging hair (%)† | 0·53 | 0·12 | 1·44 |
*Weight per stinging hair (ng) = [sample solution (mg L−1/100 hairs) × sample volume (0·001 L)]/100 hairs × 106 ng mg−1.
†The estimated average volume of fluids per stinging hair was 3·97 nL. Concentration per stinging hair (% = 10 ng nL−1) = weight per stinging hair (ng)/volume of fluids per stinging hair (3·97 nL)/10.
Formic acid in the stinging hairs of U. thunbergiana
During the chromatographic separation, the formic acid peak was ambiguous, as shown in Fig. 3. To confirm the existence of formic acid, the fluids exuded from the stinging hairs were further subjected to enzymatic analysis. The results in Table 2 indicate that the concentration of formic acid was approx. 9·4 ppm in the fluid exuded from the hairs. No decrease in the concentration of the standard solutions in the fluids of the stinging hairs indicated that no formic acid-degrading catalyser exists in the stinging hairs.
Table 2.
Enzymatic assay of formic acid in the stinging hairs of U. thunbergiana
| Absorbance (340 nm) | Concentration (ppm) | |
|---|---|---|
| Sample in water | −0·001 | −0·2 |
| Sample in standard solution | 0·452 | 101 |
| Standard solution only | 0·411 | 91·6 |
Samples were derived from 150 stinging hairs in 0·2 mL of deionized water or a standard solution. The standard solution was 100 ppm (w/v) formic acid. All three solutions were kept in the same conditions from the time of extraction of the stinging hairs.
Oxalic acid in the stinging hairs of U. thunbergiana
Oxalic acid has never been reported to induce pain sensations, and these studies show that it occurs only as a minor component in stinging hairs. To estimate the concentration of oxalate ion in the stinging hairs more accurately, the fluid exuded from 100 stinging hairs in 0·1 mL of water was subjected to enzymatic analysis. The results showed that obvious colour changes were detected in sample solutions, and the estimated concentration of oxalate ions was 40·8 μm in solution, equal to 0·13 % (w/v) in a single stinging hair, according to the calibration curve. Histochemical results showed that the oxalate ions were mainly located at the top of the stinging hair (Fig. 6A), and some appeared as bubbles moving towards the tip (Fig. 6B). Direct staining with silver nitrate after cutting the tip of the stinging hair showed a positive reaction on both sides of the cut edge (Fig. 6C), especially the broken stub (Fig. 6C, inset). Calcium identification by Alizarin red S showed positive staining only in a neutral condition (water) and negative staining after the stinging hairs were treated with 5 % acetic acid or 2 % hydrochloric acid. These results indicated that soluble oxalic acid was the dominant form of oxalate in the stinging hairs of U. thunbergiana, and that calcium oxalate was not present.
Fig. 6.
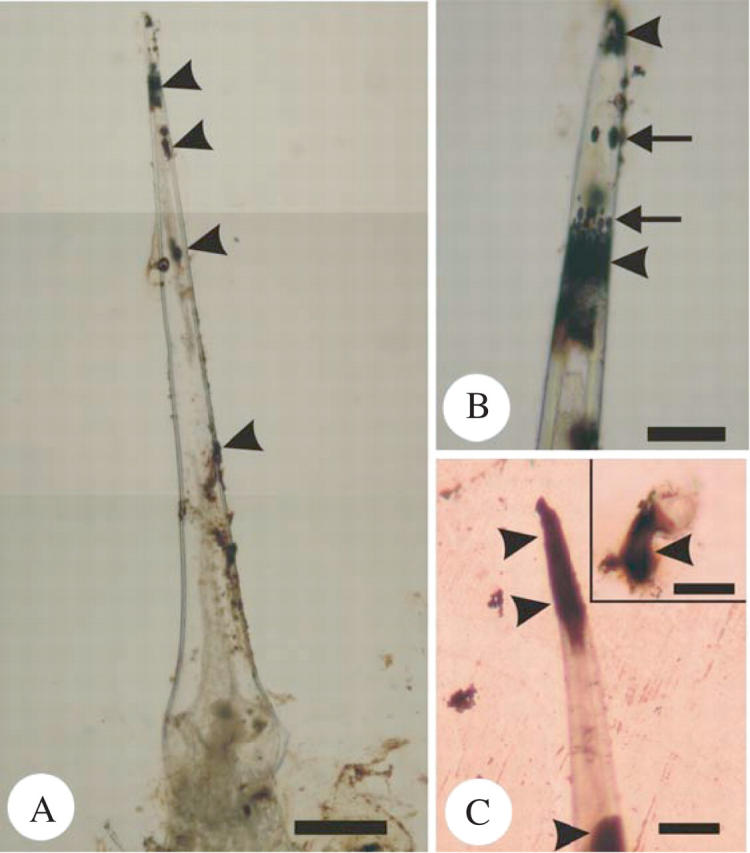
Histochemical localization of oxalic acid in the stinging hairs of Urtica thunbergiana. The oxalic acid in the stinging hairs was histochemically stained with silver nitrate (arrowheads in A and B). Note that the uppermost tip of the stinging hair was removed. Scale bar = 200 μm. (B) Enlargement of (A) showing oxalic acid at the tip of the stinging hair (arrowheads) and some oxalic acid showing as bubbles (arrows) moving toward the tip. Scale bar = 50 μm. In (C), the stinging hairs were immersed in a silver nitrate solution, and the tip was cut with a knife. The stinging hairs showed positive staining (arrowheads), especially at the cut edge and the uppermost stub (inlet). Scale bar = 50 μm; inset bar = 25 μm.
Rat behavioural test of pain-inducing agents in the stinging hairs of U. thunbergiana
Preliminary studies showed that 5 % (w/v) oxalic acid was more than sufficient to induce distinct pain-related behaviour in the first 10 min. Therefore, 2 % (w/v) oxalic acid was used in the following behavioural test. However, to achieve distinct pain-related behaviour, concentrations of 10 % (w/v) acetylcholine, histamine, formic acid and tartaric acid, respectively, were employed, all of which were twice the concentrations used in the primary study. In the stinging hair group, approximately half of a mature leaf (6–10 cm2 in area) was rubbed back and forth on the hind paw of the rat five times to achieve an obvious intense sensation of pain.
Figure 7 shows the time course effect of toxin-induced pain measured by the weighted pain score. The stinging hair group showed significantly higher weight scores than the control (saline injection) group at 0–5 min, followed by stationary weighted scores at 5–20 min (Fig. 7A). Afterward, the pain scores steadily decreased with time. The weighted scores in the 2 % oxalic acid- and 10 % tartaric acid-injected groups significantly increased at 0–10 min (peaking at 5–10 min), and then steadily decreased in a manner similar to that of the stinging hair group (Fig. 7A). Despite the decrease in the weighted scores, Holm–Sidak analysis (vs. the control) showed significant differences between the control group (saline) and the treated groups of oxalic acid and tartaric acid at 10 and 20 min, respectively, after the injection. Serotonin (2 %) in rats produced a similar response to oxalic acid and tartaric acid (Fig. 7B); however, it was not detected in the stinging hairs by HPLC (Figs 3 and 4). A formic acid (10 %) injection induced an obvious pain response in two of six rats only in the first 10 min. The treatment with histamine and acetylcholine (both highly recognized pain-inducing agents) induced no significant pain response compared with that of the control (Fig. 7).
Fig. 7.
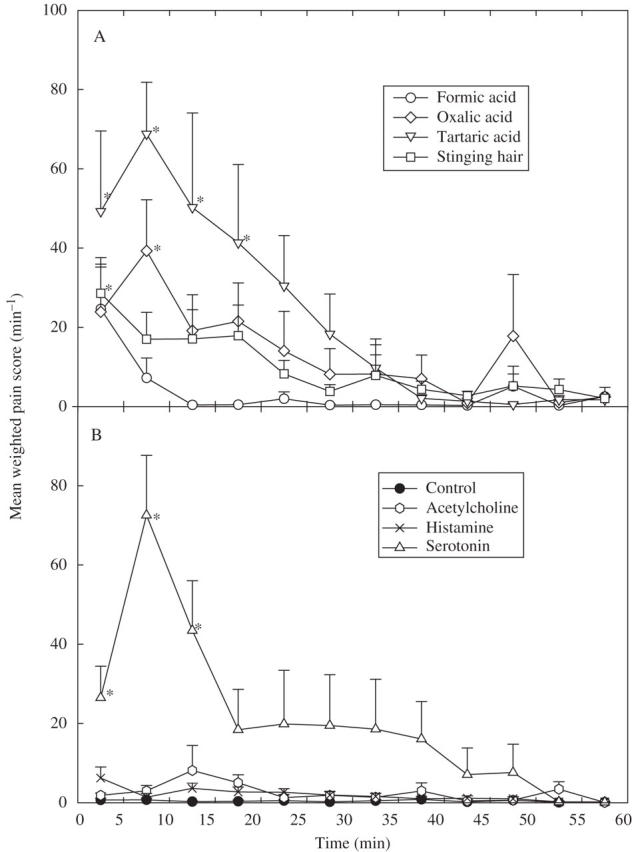
Time course effect of rat pain behaviour induced by pain-inducing agents. The hind paws of rat were subjected to repeated stroking with the leaves of Urtica thunbergiana five times or were respectively injected with 20 μL of various toxic agents [10 % (w/v) formic acid and tartaric acid, 2 % (w/v) oxalic acid in (A); and 10 % (w/v) acetylcholine, histamine, 2 % (w/v) serotonin in saline and pure saline as the control group in (B)]. Each rat was then placed in a plastic chamber for behavioural observation (average per 5 min ± s.e., n = 6) for 60 min. Asterisks indicate significant differences vs. the control (P < 0·05, ANOVA followed by the Holm–Sidak test).
DISCUSSION
The objective of this study was to identify the possible long-lasting pain-inducing agents in the stinging hairs of U. thunbergiana. The formalin test was adopted as a method for long-lasting pain measurement elicited by stinging hairs or the known toxins, since the formalin test is an important animal model in the study of acute long-lasting pain by the hypodermal injection of noxious substances (Capone and Aloisi, 2004). The current studies identified, for the first time, oxalic acid and tartaric acid as major long-lasting pain-inducing toxins in the stinging hairs of the nettle, U. thunbergiana.
The current study of U. thunbergiana supports the observations of Haberlandt showing that formic acid is not the long-lasting pain-inducing toxin in the stinging hairs (Haberlandt, 1886). Our studies demonstrate that the level of formic acid was low as estimated by HPLC (Fig. 3, peak 4) and enzymatic assay of the stinging hairs. The low concentration of formic acid in the extract of stinging hairs could not be attributed to instability or degradation of formic acid during preparation, since formic acid alone and in the surrogate samples showed no degradation. Moreover, a high concentration of formic acid (10 %) only induced a temporary pain sensation in the animal behavioural assay (Fig. 7). This result provides further support for formic acid not being the long-lasting pain-inducing agent in U. thunbergiana. Kulze's review in 1988 was also highly sceptical that formic acid is involved in the pathophysiology of the prolonged tingling sensation which may persist for 12 h or more, long after visible changes have faded (Kulze and Greaves, 1988).
The discovery of histamine, acetylcholine and serotonin as toxins in stinging hairs of Urtica occurred over half a century ago (Emmelin and Feldberg, 1947; Collier and Chesher, 1956). These authors identified serotonin as one of the major toxins of U. thunbergiana using stems with leaves rather than fluid from the stinging hairs as in the present study. In addition, Kulze suggested that histamine, acetylcholine and 5-hydroxytryptamine collectively might account for the vascular changes and immediate discomfort due to nettle stings, but the sensation of stricking and persistent paraesthesia might be induced by unidentified neurotoxic factors (Kulze and Greaves, 1988). In the current study, the estimated amounts of histamine in a single hair were 21·2 ng, almost double that of previous studies (Oliver et al., 1991). In contrast, we did not detect serotonin using HPLC, which disagrees with a past study of U. thunbergiana (Regula and Devide, 1980). The presence of acetylcholine and serotonin was also investigated by histochemical techniques as described by Corsi (1992), but the results were negative (data not shown).
The previous identification of histamine, acetylcholine and serotonin as pain-inducing toxins in the stinging hairs of Urtica used bioassays (Thurston and Lersten 1969): a contraction reaction of either guinea pig jejunum and intestine (Emmelin and Feldberg, 1947) or rat uterus and colon (Collier and Chesher, 1956) as indicators. Although acetylcholine is a common neurotransmitter in neuromuscular junctions in humans (Nicoll and Malenka, 1998) and is involved in cholinergic mechanisms for the descending inhibition of nociception (Millan, 2002), it has also been shown to induce pain sensations directly in a microdialysis test (Schmelz et al., 2003). However, the current study showed that a high concentration of acetylcholine (10 %) did not elicit significant pain sensations in the rat behavioural test. Histamine and serotonin are recognized as inflammatory signals released by mast cells and platelets (Oliver et al., 1991). Earlier studies showed that histamine can elicit itching (Millan, 1999) and, at high concentrations (10 and 20 %), may induce pain (Carstens, 1997). However, microdialysis analysis indicated only weak release of histamine, which may not be sufficient to cause inflammatory reactions such as flares and wheals (Taskila et al., 2000). In addition, it is uncertain whether histamine is released from mast cells or by diffusion from stinging hairs (Oliver et al., 1991; Taskila et al., 2000). Pricking of human skin with 0·05 % histamine plus 1·67 % acetylcholine induced a sensation like that induced by stinging hairs, but the feeling disappeared after 2 min (Emmelin and Feldberg, 1947) and did not resemble the persistent sensation of a nettle sting. Our behavioural tests on rats indicated that even 10 % acetylcholine or histamine induced neither immediate nor long-lasting pain responses, indicating that neither acetylcholine nor histamine was the long-lasting pain-inducing agent. Although the current study showed that 2 % serotonin treatment induced obvious enduring pain-related behaviours lasting for nearly 1 h, it also revealed that serotonin was undetectable by HPLC in the fluid from stinging hairs. This result also suggests that serotonin was not a long-lasting pain-inducing agent in the stinging hairs of U. thunbergiana. Nevertheless, it has been shown that degradation of serotonin in stinging hair suspensions occurs within 30 min (Collier and Chesher, 1956). Additional studies are needed to estimate the half-life of serotonin in the stinging hair fluid.
The physiological level of acetylcholine and histamine in human blood is approx. 1264 pg mL−1 (equivalent to 8·65 nm) (Fujii et al., 1995) and 35–240 nm, respectively (Taskila et al., 2000). However, higher concentrations are usually employed in in vitro studies to obtain a significant response. For instance, concentrations of 150–500 and 1500–5000 nm histamine, respectively, were used to induce a visible flare and wheal response (Peterson et al., 1997; Taskila et al., 2000). Moreover, concentrations of 0·1 % histamine (9000 nm) and 0·01 % (568 nm) serotonin were also used to increase the permeability of blood vessels by an intravenous injection (Majno and Palade, 1961). In the current study, dosages much higher than the physiological concentrations [10 % histamine dihydrochloride (543 mm), acetylcholine chloride (550 mm) and 2 % serotonin hydrochloride (94 mm)] were employed for the rat behavioural test to reach a local high concentration in hypodermal tissues; however, no obvious results in the acetylcholine or histamine group were observed. These results further indicate that neither acetylcholine nor histamine is the pain-inducing agent in the stinging hairs of U. thunbergiana.
The pain reaction related to nettle stings had only been studied using a skin prick test with acetylcholine or histamine for 2 min (Emmelin and Feldberg, 1947), and questions regarding persistent pain parenthesis caused by stinging hairs in Urtica have never been addressed. One hypothesis is that the persistent pain may be caused by other neurotoxins mediated by the stinging hairs (Kulze and Greaves, 1988; Oliver et al., 1991), but no responsible chemicals were identified to support this idea. By using the modified formalin test, we detected that the chronological patterns of oxalic acid-, tartaric acid- and serotonin-induced pain sensations were most similar to that of the stinging hairs, and the pain sensation could last for at least 30 min after treatment. Since serotonin was not detectable in the stinging hairs of U. thunbergiana in the current study, we propose that oxalic acid and tartaric acid are the major persistent pain sensation-inducing agents in the stinging hairs of this species. The chemical structures of the two newly identified pain-inducing agents, oxalic acid and tartaric acid, are most similar to that of formic acid, but are unrelated to any of the well-recognized pain-inducing agents, such as acetylcholine, histamine, serotonin, etc. The mechanisms of oxalic acid- and/or tartaric acid-induced pain sensations are currently unknown, and deserve further investigation.
In summary, the current study identified oxalic acid and tartaric acid as the possible persistent pain-inducing agents in the stinging hairs of U. thunbergiana. Earlier speculation that formic acid is the pain-inducing toxin requires an update, since it exists only at a low concentration and induced no significant pain sensation as estimated by HPLC, enzymatic analyses and rat behavioural tests. Moreover, the contributions of acetylcholine, histamine and serotonin alone to the persistent pain sensation after human skin touches the stinging hairs of Urtica may be negligible, since they either exist at a low concentration or could not induce pain sensations at high concentrations. However, pain is a variable, subjective experience involving a class of sensations which can be triggered by a combination of various natural stimuli. The synergistic effects of acetylcholine, histamine and serotonin from U. thunbergiana on pain sensation could not be the exclusive cause, as their concentrations are low. Stinging hairs, although studied for a long time, are still mysterious, particularly concerning the mechanism of the skin reaction after being stung. In the current studies, the newly identified pain-inducing agents, oxalic acid and tartaric acid, are two ubiquitous components of terrestrial plant tissues. Tartaric acid has been suggested as a component of Urtica (Thurston and Lersten, 1969), but oxalate was excluded as a component of U. ferox (Pilgrim, 1959). Their role in plants and the mechanism underlying the induced pain are issues which remain to be fully addressed.
Acknowledgments
We thank the University System of Taiwan, Joint Research Program—Tsou's Foundation—VGHUST95-P4-10 and National Science Council of Taiwan, ROC for financial support of this project (NSC93-2815-C-008-029-B and NSC93-2313-B-002-091) and the two referees for their valuable comments on the manuscript.
LITERATURE CITED
- Antonopoulou S, Demopoulos CA, Andrikopoulos NK. 1996. Lipid separation from Urtica dioica: existence of platelet-activating factor. Journal of Agricultural and Food Chemistry 44: 3052–3056. [Google Scholar]
- Capone F, Aloisi AM. 2004. Refinement of pain evaluation techniques. The formalin test. Annali dell'Istituto Superiore di Sanita 40: 223–229. [PubMed] [Google Scholar]
- Carstens E. 1997. Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. Journal of Neurophysiology 77: 2499–2514. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R. 1993. The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain 54: 43–50. [DOI] [PubMed] [Google Scholar]
- Collier HOJ, Chesher GB. 1956. Identification of 5-hydroxytryptamine in the sting of the nettle (Urtica dioica). British Journal of Pharmacology 11: 186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi G. 1992. The stinging hair of Urtica membranacea poiret (Urticaceae). II. Histochemistry. Phyton-Annales Rei Botanicae 32: 247–253. [Google Scholar]
- Edom G. 2002. The uncertainty of the toxic effect of stings from the Urtica nettle on hunting dogs. Veterinary and Human Toxicology 44: 42–44. [PubMed] [Google Scholar]
- Emmelin N, Feldberg W. 1947. The mechanism of the sting of the common nettle (Urtica urens). Journal of Physiology 106: 440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelin N, Feldberg W. 1948. Distribution of acetylcholine and histamine in nettle plants. New Phytologist 48: 143–148. [Google Scholar]
- Fujii T, Yamada S, Yamaguchi N, Fujimoto K, Kawashima K. 1995. Species differences in the concentration of acetylcholine, a neurotransmitter, in whole blood and plasma. Neuroscience Letters 20: 207–210. [DOI] [PubMed] [Google Scholar]
- Haberlandt G. 1886. Zur Anatomie und Physiologie der pflanzlichen Brennhaare. Sitzungberichte Akademie Wien 93: 122–145. [Google Scholar]
- Hooke R. 1665. Micrographia: or some physiological descriptions of minute bodies made by magnifying glasses with observations and inquiries thereupon. New York: Dover facsimile of the first edition, Dover Publishing Co.
- Jourdan D, Ardid D, Eschalier A. 2001. Automated behavioural analysis in animal pain studies. Pharmacological Research 43: 103–110. [DOI] [PubMed] [Google Scholar]
- Kulze A, Greaves M. 1988. Contact urticaria caused by stinging nettles. British Journal of Pharmacology 119: 269–270. [DOI] [PubMed] [Google Scholar]
- Lin CT, Yao YN, Chung NJ. 1991. Morphology and habitats of devil's-leaf (Urtica thunbergiana) in Chi-tou Forest Recreation Area. Quarterly Journal of the Experimental Forest, National Taiwan University 5: 167EP–183JA. [Google Scholar]
- Majno G, Palade GE. 1961. Studies on inflammation I. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. Journal of Biophysical and Biochemical Cytology 11: 571–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. 1999. The induction of pain: an integrative review. Progress in Neurobiology 57: 1–164. [DOI] [PubMed] [Google Scholar]
- Millan MJ. 2002. Descending control of pain. Progress in Neurobiology 66: 355–474. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. 1998. A tale of two transmitters. Science 281: 360–361. [DOI] [PubMed] [Google Scholar]
- Oliver F, Amon EU, Breathnach A, Francis DM, Sarathchandra P, Kobza Black A, Greaves MW. 1991. Contact urticaria due to the common stinging nettle (Urtica dioica)—histological, ultrastructural and pharmacological studies. Clinical and Experimental Dermatology 16: 1–7. [DOI] [PubMed] [Google Scholar]
- Peterson LJ, Church MK, Scov PS. 1997. Histamine is released in the wheal but not the flare following challenge of human skin in vivo: a microdialysis study. Clinical and Experimental Allergy 27: 284–295. [DOI] [PubMed] [Google Scholar]
- Pilgrim RLC. 1959. Some properties of the sting of the New Zealand nettle, Urtica ferox. Proceedings of the Royal Society of London: B 151: 48–56.
- Regula I, Devide Z. 1980. The presence of serotonin in some species of genus Urtica. Acta Botanica Croatica 39: 47–50. [Google Scholar]
- Saxena PR, Pant MC, Kishor K, Bhargava KP. 1965. Identification of pharmacologically active substances in the Indian stinging nettle, Urtica parviflora (Roxb.). Journal of Physiology and Pharmacology 4: 869–876. [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. 2003. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. Journal of Neurophysiology 89: 2441–2448. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Perrotti JM, Crisp T, Cabral ME, Long JT, Scalzitti JM. 1988. The μ opiate receptor is responsible for descending pain inhibition originating in the periaqueductal gray region of the rat brain. European Journal of Pharmacology 156: 47–54. [DOI] [PubMed] [Google Scholar]
- Taskila K, Saarinan JV, Harvima IT, Harvima RJ. 2000. Histamine and LTC4 in stinging nettle-induced urticaria. Allergy 55: 680–681. [DOI] [PubMed] [Google Scholar]
- Thurston EL, Lersten NR. 1969. The morphology and toxicology of plant stinging hairs. Botanical Review 63: 393–412. [Google Scholar]
- Yasue T. 1969. Histochemical identification of calcium oxalate. Acta Histochemica et Cytochemica 2: 83–95. [Google Scholar]