Abstract
• Background and Aims Moss food-conducting cells (leptoids and specialized parenchyma cells) have a highly distinctive cytology characterized by a polarized cytoplasmic organization and longitudinal alignment of plastids, mitochondria, endoplasmic reticulum and vesicles along endoplasmic microtubules. Previous studies on the desiccation biology of mosses have focused almost exclusively on photosynthetic tissues; the effects of desiccation on food-conducting cells are unknown. Reported here is a cytological study of the effects of de- and rehydration on food-conducting cells in the desiccation-tolerant moss Polytrichum formosum aimed at exploring whether the remarkable subcellular organization of these cells is related to the ability of mosses to survive desiccation.
• Methods Shoots of Polytrichum formosum were dehydrated under natural conditions and prepared for transmission and scanning electron microscopy using both standard and anhydrous chemical fixation protocols. Replicate samples were then fixed at intervals over a 24-h period following rehydration in either water or in a 10 µm solution of the microtubule-disrupting drug oryzalin.
• Key Results Desiccation causes dramatic changes; the endoplasmic microtubules disappear; the nucleus, mitochondria and plastids become rounded and the longitudinal alignment of the organelles is lost, though cytoplasmic polarity is in part retained. Prominent stacks of endoplasmic reticulum, typical of the hydrated condition, are replaced with membranous tubules arranged at right angles to the main cellular axis. The internal cytoplasm becomes filled with small vacuoles and the plasmalemma forms labyrinthine tubular extensions outlining newly deposited ingrowths of cell wall material. Whereas plasmodesmata in meristematic cells at the shoot apex and in stem parenchyma cells appear to be unaffected by dehydration, those in leptoids become plugged with electron-opaque material. Starch deposits in parenchyma cells adjoining leptoids are depleted in desiccated plants. Rehydration sees complete reestablishment over a 12- to 24-h period of the cytology seen in the control plants. Oryzalin effectively prevents leptoid recovery.
• Conclusions The results point to a key role of the microtubular cytoskeleton in the rapid re-establishment of the elaborate cytoplasmic architecture of leptoids during rehydration. The reassembly of the endoplasmic microtubule system appears to dictate the time frame for the recovery process. The failure of leptoids to recover normal cytology in the presence of oryzalin further underlines the key role of the microtubules in the control of leptoid cytological organization.
Keywords: Bryophytes, desiccation-tolerance, microtubules, oryzalin, plasmodesmata, Polytrichum formosum, rehydration, vascular tissue
INTRODUCTION
Since the 18th century it has been known that representatives of many groups of organisms including algae, lichens, animals (‘infusoria’, rotifers, nematodes and tardigrades) and land plants, are able to survive dehydration and recover upon rewetting (Alpert, 2000). The ability of cells to revive from the air-dry state is referred to as desiccation tolerance (Bewley, 1979) or anhydrobiosis (Crowe et al., 1992). Virtually all plants produce desiccation-tolerant spores (or pollen grains) and most seeds are desiccation-tolerant, but vegetative desiccation-tolerance is much less common. Many, perhaps most, bryophytes are desiccation-tolerant, but in vascular plants this condition is rare, occurring sporadically in widely scattered taxonomic groups (Proctor and Tuba, 2002).
Desiccation-tolerant plants can be divided broadly into those where tolerance appears to be constitutive (most bryophytes, some small pteridophytes) and those that are desiccation-tolerant only if this is induced by a period of stress. In both categories many vascular resurrection plants and most bryophytes are able to survive the loss of most of their protoplasmic water. However, induced and constitutive desiccation-tolerance are not sharply separated, and there is evidence that some desiccation-tolerance may be induced to a greater or less degree in most species (Werner et al., 1991; Oliver et al., 1998; Beckett, 2001).
Apart from a handful of investigations on liverworts (Höfler, 1946; Clausen, 1952; Hinshiri and Proctor, 1971; Hearnshaw and Proctor, 1982; Marschall and Proctor, 1999; Proctor, 2003), studies of desiccation-tolerance in bryophytes have mostly been confined to mosses. These have focused either on the ability of mosses to recover rapidly their synthetic metabolism during rehydration (Tuba et al., 1998; Proctor and Smirnoff, 2000; Proctor and Tuba, 2002) or on the effects of desiccation and rehydration on membrane integrity (Platt et al., 1994). Studies of recovery in Tortula ruralis showed that this moss was able to achieve a positive carbon balance within 20 min of rehydration (Tuba et al., 1996; Proctor and Smirnoff, 2000), while Platt et al. (1994) demonstrated that the plasmalemma and other cellular membranes in photosynthetic tissues of Tortula ruralis and the pteridophyte Selaginella lepidophylla retained structural integrity in the dry state. Changes in the metabolism of carbohydrates and proteins have been increasingly implicated in desiccation-tolerance and seem to play a major protective role during the drying and recovery processes (Oliver et al., 1998, 2000a, b).
In contrast to the substantive studies on relationships between metabolism and de- and rehydration, very little research has been conducted on the effects of desiccation on plant transport systems. Preliminary studies include the report of a lipid layer lining the cell walls in xylem vessels in the resurrection angiosperm Myrothamnus flabellifolia. It has been proposed that in the dry state the lipid lining provides waterproofing, thus restricting water loss of the tissues within tolerable levels (Canny, 2000; Wagner et al., 2000). Coupled with the ability of vessels to refill rapidly after cavitation, this might account for the rapid restoration of the hydraulic system (Wagner et al., 2000; Schneider et al., 2003). On the other hand, experimental studies of highly specialized water-conducting cells in some mosses (hydroids) have shown that these are able to avoid cavitation by collapsing in the desiccated condition and that they resume normal structure and functioning on rehydration very rapidly (Schmid, 1998).
In addition to hydroids, bryoid mosses possess highly specialized cells, scattered in a ring surrounding the central strand of hydroids. These are generally considered to be analogous to the sieve elements of vascular plants and accordingly are reported as ‘food-conducting cells’ or also, mainly in polytrichaceous mosses, as leptoids (Hébant, 1976, 1977; Ligrone et al., 2000). These are elongate cells with a distinctive cytology characterized by cytoplasmic polarization along the source-to-sink axis. Plastids, mitochondria, membranous tubules and vesicles are longitudinally aligned along endoplasmic microtubules extending from the poles of the spindle-shaped nucleus towards the ends of the cells (Ligrone and Duckett, 1994).
The leptoids have oblique end walls displaying a high concentration of plasmodesmata with constricted ends and a conspicuous enlargement of the desmotubule in the middle. Plasmodesmata are also seen occasionally along the common longitudinal walls between leptoids and adjoining parenchyma cells. As in leptoids, the parenchyma cells also have oblique end walls with a high frequency of plasmodesmata and an elongate nucleus but lack the distinctive system of endoplasmic microtubules and the cytoplasmic polarization typical of leptoids. Moreover, while the plastids in leptoids have only a rudimentary inner thylakoid system and contain little or no starch, those in parenchyma cells have a well-developed thylakoid system and in fully hydrated plants contain abundant starch. Cell types with characteristics intermediate between leptoids and parenchyma cells are also regularly observed in P. formosum.
Leptoids in mosses are particularly amenable to experimental manipulation. Experiments using cytoskeleton-disrupting drugs (oryzalin, cytochalasins, carbamate herbicides) as well as mechanical interruption of the source-to-sink gradient indicate a causal relationship between the highly characteristic cytoplasmic architecture and the endoplasmic microtubule system (Ligrone and Duckett, 1996).
To date no study has been conducted on the cytological consequences of drying and rewetting on leptoids in mosses. Because of their unique cytology, built upon their endoplasmic microtubular system, these cells are a particularly intriguing system to investigate the effects of dehydration and rehydration on the microtubular cytoskeleton and cytoplasmic organization.
Although numerous studies of the cellular responses of plants to cold and freezing injuries have pointed to the microtubule cytoskeleton as a primary target (Carter and Wick, 1984; Wang and Nick, 2001; Abdrakhamanova et al., 2003; Schwarzerova et al., 2003), the de- and rehydration biology of microtubules has been largely overlooked. As far as is known, the only suggestions of possible links between desiccation and microtubules in the literature relate to studies of desiccation-sensitivity in recalcitrant seeds (Berjak and Pammenter, 2000; Mycock et al., 2000; Rocha Faria et al., 2004) and descriptions of the disassembly of microtubules during maturation of moss spores (Brown and Lemmon, 1980, 1982, 1987). A characteristic feature of both seeds and dormant spores appears to be the absence of microtubules, the activation of the microtubule cytoskeleton being one of the earliest visible signs of germination.
This paper reports on an ultrastructural study of food-conducting cells (referred to herein as leptoids) in the moss Polytrichum formosum in the dried condition and during rehydration.
MATERIALS AND METHODS
Plant material
The moss Polytrichum formosum Hedw. (Polytrichales) was chosen because its leafy shoots have highly specialized leptoids and an optimal fixation protocol for experimental ultrastructural studies, including dehydration and the use of drugs that affect the cytoskeleton, had already been worked out (Ligrone and Duckett, 1994, 1996).
Wild plants were collected from mixed Quercus/Fagus woodland, Box Hill, Surrey, UK (OSGB grid reference TQ 182513; 51°15′N, 0°18′W). The de- and rehydration experiment was repeated three times over a 3-year period with identical results on each occasion.
Light and electron microscopy
The controls were freshly collected, fully hydrated shoots. The desiccated samples were obtained from moss tufts collected in the field and allowed to dry slowly outdoors, under cover and away from direct sunlight, for at least 18 d. The naturally desiccated shoots were rehydrated by immersion in distilled water or in 10 µm oryzalin solution (the lowest concentration previously found to affect leptoid microtubules; Ligrone and Duckett, 1996). The total water contents of control and dehydrated shoots examined at intervals during rehydration are reported in Table 1. Excess water was carefully removed with tissues from the rehydrated shoots before weighing. The water content of shoots was evaluated by oven drying at 80 °C for 12 h. Fully hydrated and dehydrated shoots, and shoots rehydrated for 0·5, 1, 2, 4, 12 and 24 h, were fixed in 3 % (v/v) glutaraldehyde, 1 % (v/v) formaldehyde (freshly prepared from paraformaldehyde) and 0·5 % (w/v) tannic acid in 0·05m Na-phosphate buffer, pH 7·0. The apical part of the shoots, about 3 cm long, was dissected and, after removing the leaves, was left in the fixative for 1 h at room temperature under low vacuum. Afterwards, the stem portion between 1 and 2 cm below the apex was cut into 1-mm segments and left in the fixative for further 2 h. The shoot apices, about 1 mm long, were also isolated and fixed.
Table 1.
Water content of Polytrichum formosum leafy shoots (means of three groups of 20 shoots ± s.d.)
| Sample | Water content (% of total weight) |
|---|---|
| Freshly collected shoots | 56·2 ± 2·0 |
| Naturally desiccated shoots | 7·5 ± 0·4 |
| 0·5-h rehydrated shoots | 45·1 ± 1·2 |
| 1-h rehydrated shoots | 51·2 ± 1·7 |
| 2-h rehydrated shoot | 54·3 ± 2·2 |
| 12-h rehydrated shoots | 59·6 ± 0·8 |
| 24-h rehydrated shoots | 59·5 ± 0·6 |
Desiccated shoots were also fixed anhydrously in a solution of heptane/glutaraldehyde prepared as follows: 6 % (w/v) glutaraldehyde in 0·05 m PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 7·4 was added to an equal volume of heptane, shaken thoroughly for at least 1 h at room temperature and left to stand until complete separation into two phases. Aliquots of the heptane/glutaraldehyde solution so obtained were used to fix the material for 2 h at room temperature.
After rinsing in 0·1 m Na-phosphate buffer, both sets of samples were post-fixed with 1 % (w/v) osmium tetroxide in 0·1 m Na-phosphate buffer, pH 6·8, overnight at 4 °C, dehydrated through an ethanol series and embedded in Spurr's resin via propylene oxide as described previously (Ligrone and Duckett, 1994). Thin sections, cut with a diamond knife, were sequentially stained with 5 % (v/v) methanolic uranyl acetate for 15 min and lead citrate for 10 min and examined with a Jeol 1200 EX2 electron microscope.
Sections, 0·5 µm thick, stained with 1 % toluidine-blue, were photographed with a Leica DM RXA2 microscope equipped with differential interference contrast optics.
For scanning electron microscopy, 2- to 3-mm lengths of fresh shoots from the control and each of the treatments detailed above were dehydrated over 24 h in a 1 : 1 mixture of acetone and ethanol to remove the cytoplasmic contents, transferred to anhydrous ethanol, critical point dried and observed with a Hitachi S570 scanning electron microscope operating at 20 kV.
RESULTS
Polytrichum formosum forms dark green tufts usually on acidic soil in woodlands, heaths and moorlands (Hill et al., 1992). The plants are regularly exposed to dehydration and can survive in a desiccated state for several weeks. Dry shoots have a very low water content but can absorb water and resume a normal appearance extremely rapidly (Table 1 and Fig. 1). The leptoids in the fully hydrated control plants showed the distinctive cytology described previously by Ligrone and Duckett (1994, 1996) and Ligrone et al. (2000) (Fig. 2E) and summarized in the Introduction.
Fig. 1.

Changes in the appearance of Polytrichum formosum in the hydrated and desiccated condition. Fully hydrated (A) and desiccated shoots (B) in the wild. Isolated shoots in fully hydrated (C), desiccated condition (D) and after 5-min rehydration (E).
Fig. 2.
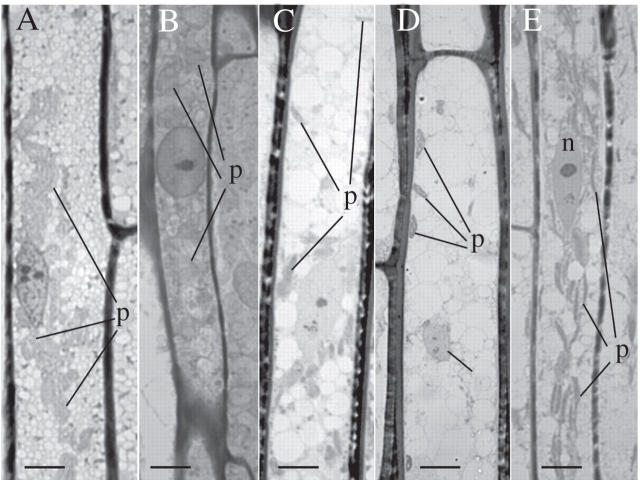
Light micrographs of leptoids in the leafy shoot of Polytrichum formosum. (A and B) Details of desiccated leptoids: (A) aqueous chemical fixation; (B) anhydrous chemical fixation. In both cases note the vesiculated cytoplasm, the ovoid, centrally located nuclei and ovoid to spherical plastids. Cells rehydrated for 4 h (C) and 12 h (D) in 10 µm oryzalin. The nuclei and plastids are ovoid; the vacuoles are like those in the controls. (E) Fully hydrated leptoids. Aqueous chemical fixation. Note the longitudinal alignment of the highly elongate plastids and the spindle-shaped nucleus. n, Nucleus; p, plastids. Scale bars = 10 µm.
Cytology of leptoids in the desiccated condition
Dramatic differences between desiccated and hydrated leptoids are clearly visible at the light microscope level (Fig. 2). Whether standard or anhydrous fixation is used, the cytoplasm in desiccated leptoids appears packed with small vesicles, plastids are rounded and the nucleus is spherical to ovoid (Fig. 2A, B). Although the cytoplasm appears highly vesiculate, cytoplasmic polarity is still visible after desiccation; the nucleus and most organelles remain clustered in the portion of the cells proximal to the shoot apex (Fig. 3A). The nuclei are ovoid or irregularly shaped and contain masses of condensed chromatin, not present in the hydrated samples; the nucleolus is inconspicuous (Figs 3B and 5A). Rounded plastids and mitochondria are clumped together in a central mass alongside the nucleus. Endoplasmic microtubules are absent (Fig. 3B, C; cf. Figs 7E and 8A, B; see also Fig. 6C). The large stacks of endoplasmic reticulum (ER), typically visible in the hydrated condition at the cellular ends towards the shoot apex (Ligrone and Duckett, 1994, 1996) also are absent and, as one of the most conspicuous features of dehydrated cells, numerous membranous tubules appear in the cytoplasm; unlike the microtubules, these are arranged at right angle to the longitudinal cellular axis and have diameters ranging between 15 and 47 nm (Fig. 4D, E). Also scattered in the cells are cytoplasmic areas containing aggregates of free ribosomes, dictyosomes, coated vesicles and partially coated reticulum (Fig. 4A).
Fig. 3.
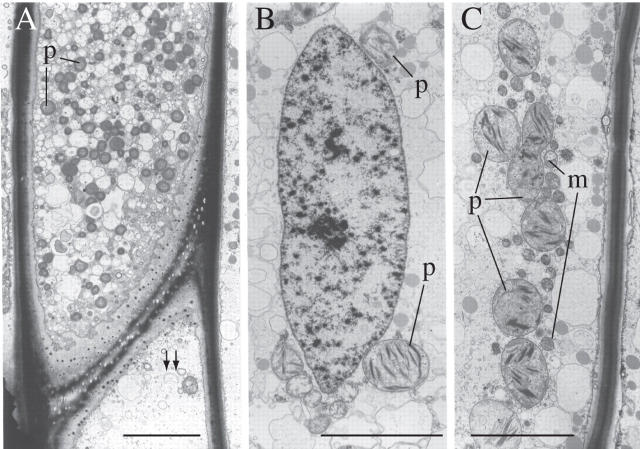
Transmission electron micrographs (TEMs) of P. formosum desiccated leptoids. (A) Polarity is maintained but cytoplasmic organization is severely disrupted. Note the highly vesiculate cytoplasm and rounded plastids. Numerous plasmodesmata are visible in the end walls. The double arrow points to the direction of the stem apex. (B) Ovoid nucleus with partially condensed chromatin and spherical plastids. (C) A row of spherical plastids and associated mitochondria in the central cytoplasm. m, Mitochondria; p, plastids. Scale bars = 5 µm.
Fig. 4.
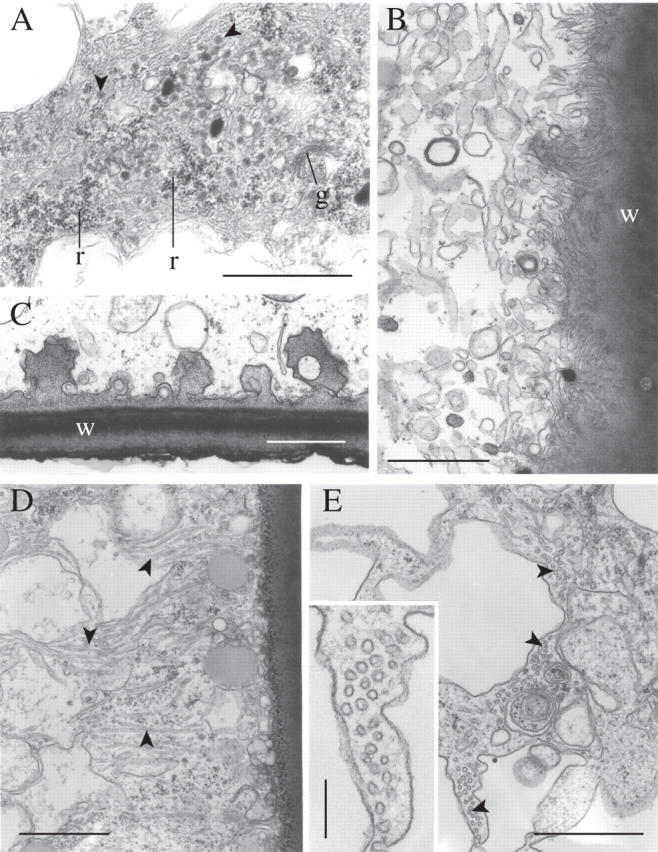
TEMs of desiccated leptoids: (A) aggregates of free ribosomes, a Golgi body, numerous coated vesicles (arrowed) and sheets of smooth endoplasmic reticulum; (B) labyrinthine tubular extensions of the cell wall closely followed by the plasma membrane; (C) conspicuous ingrowths of electron-opaque wall material; (D) membrane-bounded tubules lying at right angles to the long axis of the cell (arrowed); (E) transverse section of tubules (arrowed). The insert is a higher magnification of (E) showing the range of size in the tubule diameters. g, Golgi body; r, ribosomes; w, cell wall. Scale bars: A–E = 1 µm; insert in E = 0·2 µm.
The plasmalemma at both ends of the cells forms numerous tiny invaginations containing cell wall material (Fig. 4B; see also Fig. 10A). Coarser sac-like invaginations of the plasmalemma are also visible along the longitudinal walls (Fig. 4C).
The plasmodesmata in the end and lateral walls of desiccated leptoids are occluded with plugs of electron-opaque material (Fig. 5A, B; see also Fig. 10A, B) that are absent in the hydrated condition (Fig. 5C).
Fig. 5.
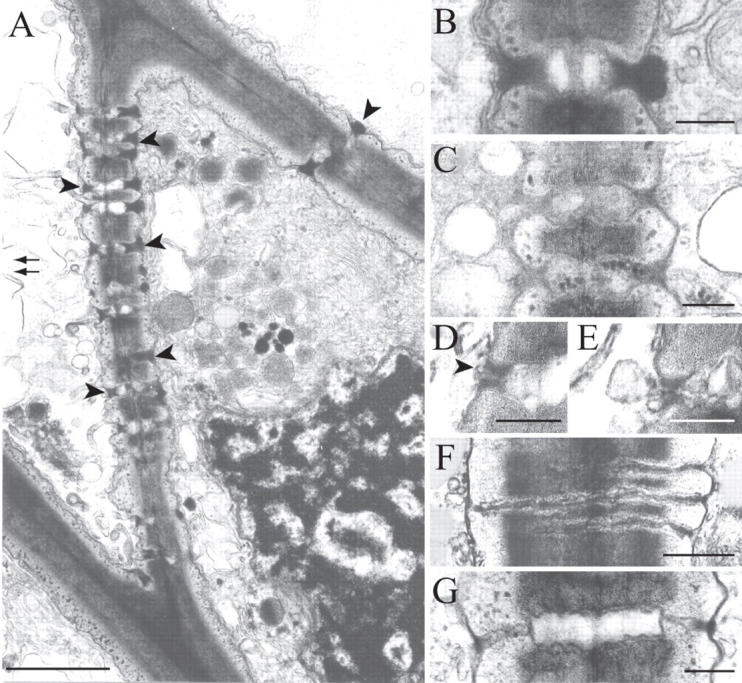
TEM details of leptoids and parenchyma cells. (A) Leptoids in desiccated shoot showing retention of cytoplasmic polarity. The plasmodesmata in the end and lateral walls are occluded with plugs of electron-opaque material (arrowed). The nucleus near the end wall has an irregular shape and contains massive amounts of condensed chromatin. The double arrow points to the direction of the stem apex. (B) Higher magnification of a plugged plasmodesma in a dehydrated leptoid. (C) Plasmodesmata in the end wall between two fully hydrated leptoids. Note the enlarged lumina. (D and E) Plasmodesmata in leptoids after 2-h rehydration: (D) the plugs of electron-opaque material have nearly completely disappeared (arrowed); (E) desmotubule showing continuity with tubular endoplasmic reticulum. (F and G) Plasmodesmata in desiccated parenchyma cells. The constricted extremities are free of electron-opaque deposits regardless of the absence (F) or presence (G) of a median enlargement. Scale bars: A = 10 µm;B–E = 0·2 µm; F and G = 0·5 µm.
It has been argued that the use of aqueous chemical fixatives might allow partial rehydration of dehydrated tissues leading to osmotic swelling of cells and organelles prior to chemical stabilization (Platt et al., 1997; Thomson and Platt, 1997; Wesley-Smith, 2001); hence the use of cryofixation and freeze-substitution has been advocated as a more reliable technique especially when dealing with dried tissues (Thomson and Platt, 1997). Attempts to carry out high pressure cryofixation and freeze-substitution of leptoids in P. formosum shoots (Schmid, 1998) were met with substantial difficulties probably inherent to the position of these cells deep in the stem (Schmid, 1998) and were abandoned. As an alternative procedure, desiccated material was prepared by anhydrous fixation via heptane. The results obtained (Fig. 6A–C) were fully consistent with those from standard aqueous fixation (Fig. 3A, B), thus confirming that the cytological alterations observed in desiccated leptoids with the latter protocol are not artefacts.
Fig. 6.
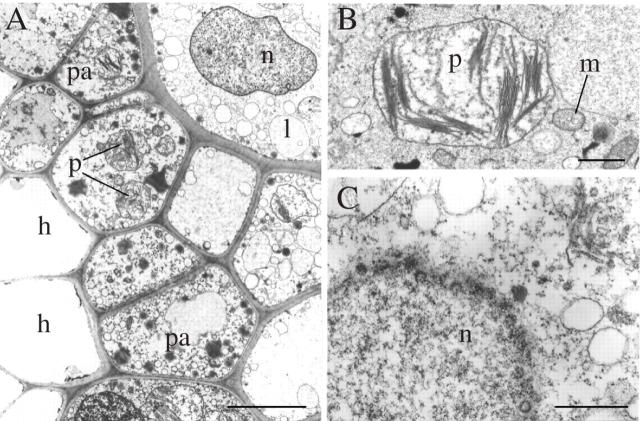
TEM details of desiccated shoots after anhydrous fixation via heptane. (A) Transverse section showing leptoids adjacent to parenchyma cells and hydroids. Note that cellular integrity remains intact. (B) Starch-free rounded plastid with a normal thylakoid system in a parenchyma cell. (C) Grazing section through the nuclear envelope in a leptoid. Pores are clearly visible, endoplasmic microtubules are absent. h, Hydroids; l, leptoids; m, mitochondrion; n, nuclei; p, plastids; pa, parenchyma cells. Scale bars: A = 10 µm; B = 1 µm; C = 5 µm.
Cytology of rehydration
The water content of the leafy shoots of P. formosum rose to a value close to that of the controls as soon as 30 min after rewetting, but thereafter it continued to increase slowly up to values slightly higher than in controls (Table 1). After 30 min of rehydration, the vacuoles in the leptoids are larger (2 µm vs. 1 µm) than in the dry state (Fig. 7A, B). The chloroplasts remain approximately spherical but now have somewhat undulating profiles. The nucleus is still ovoid but the nucleolus is more clearly defined and masses of condensed chromatin are generally absent (Fig. 7A). The membranous tubular structures are still visible but are now aligned mostly longitudinally (Fig. 7B).
Fig. 7.
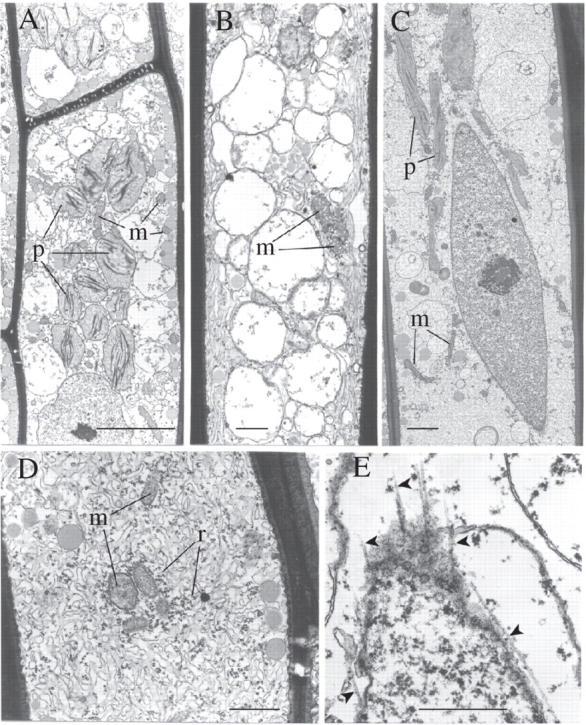
Effects of rehydration in shoot leptoids (TEMs). (A) 30-min rehydration: note separation of the organelles in the rehydrating cytoplasm. Plastids are approximately spherical but their margins are undulate (cf. Fig. 3C). Note the large vacuoles. (B) 30-min rehydration: longitudinally aligned membranous tubules in peripheral cytoplasm (cf. Fig. 4D). (C and D) 2-h rehydration: (C) spindle-shaped nucleus, elongate plastids and mitochondria—the labyrinthine plasma membrane/wall interface cannot be distinguished; (D) ribosome aggregates beginning to disperse as polysomes. (E) Grazing profile through the extremity of a spindle-shaped nucleus. Note the longitudinally aligned microtubules extending from its envelope (arrowed). m, Mitochondria; p, plastids; r, ribosomes. Scale bars: A = 5 µm; B, D and E = 1 µm; C = 2 µm.
Two hours after rehydration, the majority of the organelles have regained their shapes as seen in hydrated controls; the nucleus is spindle-shaped, plastids and mitochondria are elongate and longitudinally aligned (Fig. 7C). The labyrinthine cell wall elaborations observed in the desiccated state are no longer present. The ribosome aggregates have begun to disperse and reorganize as polysomes associated with swollen, irregular ER profiles (Fig. 7D). The endoplasmic microtubule cytoskeleton reappears in the form of short microtubules extending from the nuclear envelope (Fig. 7E).
The plasmodesmatal plugs start disappearing 2 h after rehydration; in samples rehydrated for 12 h plugs are no longer visible and continuity between desmotubules and tubular ER is again clearly visible (Fig. 5D, E; see also Fig. 10C). Although microtubules become visible after 2 h rehydration, the full extent of the longitudinal arrays typical of hydrated controls is not regained until 12–24 h from rewetting (Fig. 8A, B). The longitudinal alignment of the organelles is fully restored 24 h after rehydration and at this stage abundant starch is again visible in the plastids of the parenchyma cells adjoining leptoids (Fig. 8C).
Fig. 8.
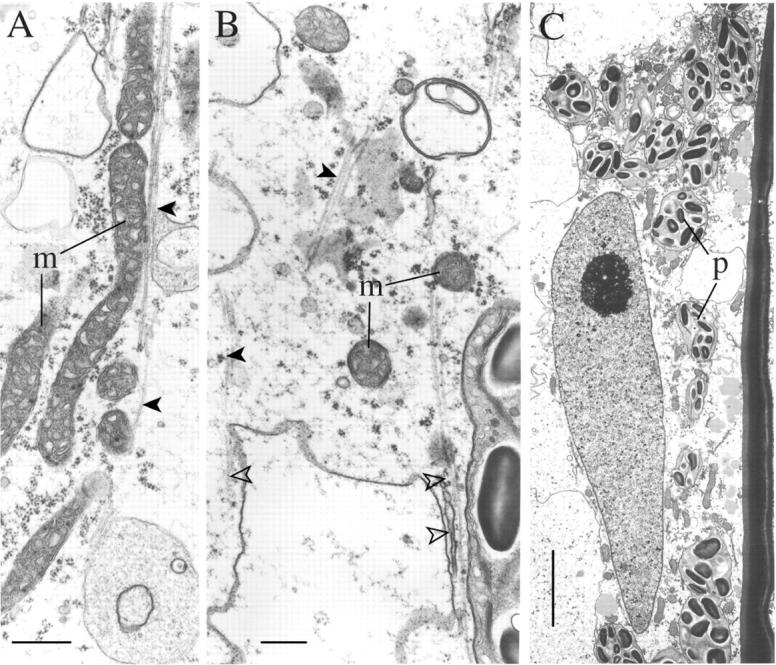
Effects of 12–24 h rehydration on leptoids (A and B) and parenchyma cells (C) in the leafy shoot (TEMs). (A) 12-h rehydration: longitudinal arrays of endoplasmic microtubules (arrowed) associated with elongate mitochondria and a plastid. (B) Endoplasmic microtubules associated with rough endoplasmic reticulum (closed arrow) and with tubules and vesicles (open arrow). (C) Abundant starch reserves in the plastids of parenchyma cells. p, Plastids. Scale bars: A and B = 0·5 µm; C = 2 µm.
Rehydration in the presence of oryzalin
In plants rehydrated in the presence of 10 µm oryzalin for 4 h (Figs 2C, D and 9A, B) and 12 h (Figs 2D and 9C, D) leptoid nuclei remain spherical or irregularly shaped. Short lengths of microtubules are occasionally visible along the nuclear envelope (Fig. 9B, C) or associated with elongate mitochondria (Fig. 9B), although none were observed following rehydration in the presence of 100 µm oryzalin (data not shown). Ovoid (Fig. 9A, C) or elongate plastids (Fig. 9D) are either scattered through the cell or aggregated near the nucleus (Fig. 9D). The bulk of the lumina of the leptoids are occupied by spherical vacuoles. No further changes from this condition were observed in plants rehydrated in oryzalin for 24 h.
Fig. 9.
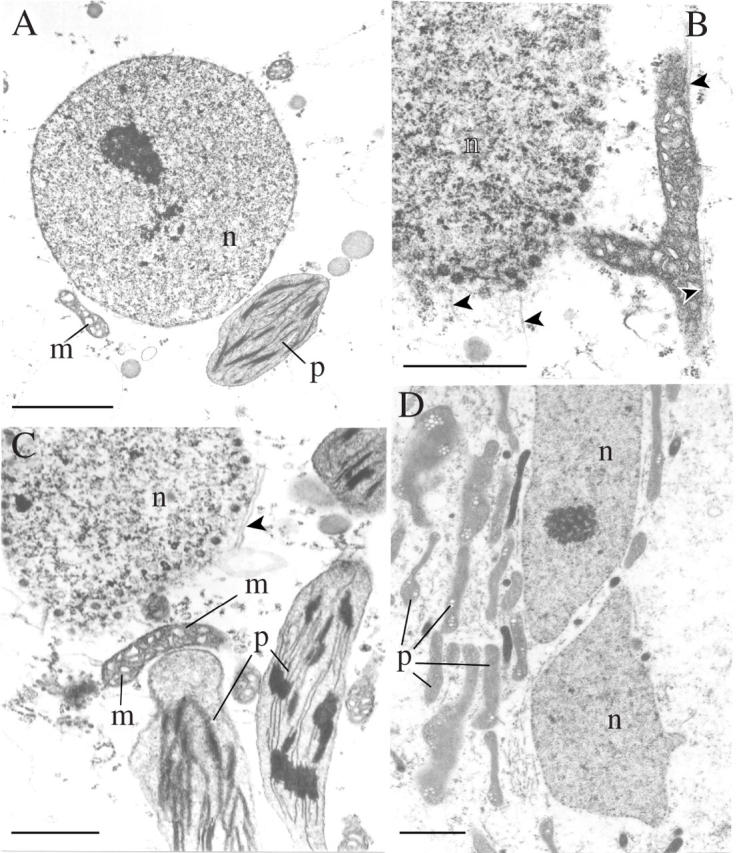
Rehydration of leptoids for 4 h (A and B) and 12 h (C and D) in the presence of 10 µm oryzalin (TEMs). Note the roundish (A) or irregularly shaped nucleus (D) and the plastids either scattered (C) or aggregated near the nucleus (D). Occasional microtubules (arrowed) are visible in association with the nuclear envelope (B and C) and an elongate mitochondrion in (B). Note the absence of starch accumulation in plastids in parenchyma cell after 12 h rehydration in the presence of oryzalin (C). m, Mitochondria; n, nucleus; p, plastids. Scale bars: A and D = 2 µm; B and C = 1 µm.
Scanning electron microscopy
SEM observations of desiccated and rehydrated leptoids in P. formosum (Fig. 10) corroborate the TEM results. In the dry state, the cell wall ingrowths and the plugs occluding plasmodesmata are clearly visible (Fig. 10A, B). The fact that these structural changes persist after removal of the cytoplasm during sample preparation is a further indication that they are not artefacts and, in the case of plasmodesmatal plugs, suggests that these consist of highly resistant material. In accordance with TEM observations, the plugs start disappearing 2 h after rewetting (Fig. 10C) and are no longer present in 12-h rehydrated samples (Fig. 10D).
Fig. 10.
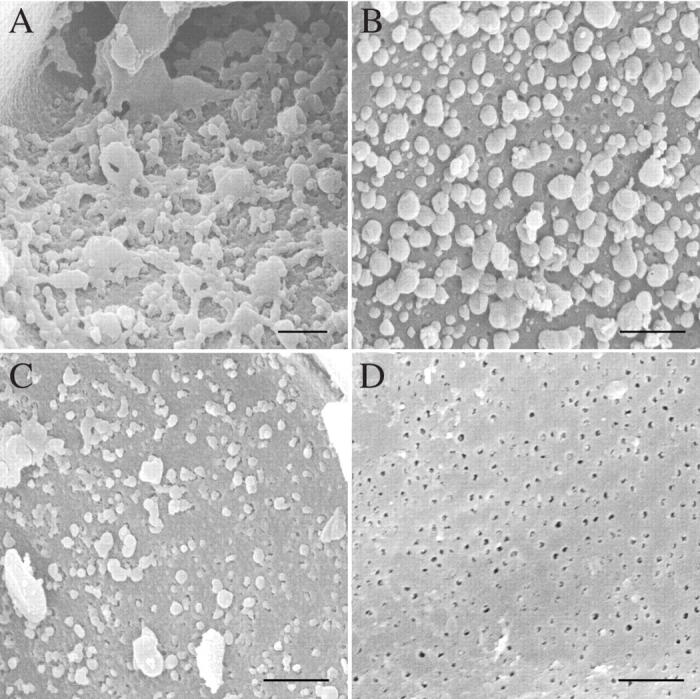
Scanning electron micrographs of leptoids from desiccated (A and B) and rehydrated shoots (C and D) in P. formosum: (A) detail of labyrinthine cell wall material; (B) detail of an end wall showing plasmodesmatal plugs; (C) 2 h after rehydration plugs are much reduced; (D) 12–24 h after rehydration leptoid plasmodesmata are again free from occlusions. Scale bars = 2 µm.
Meristematic cells of the shoot apex
The plasmodesmata in meristematic cells of the dehydrated shoot apex, about 40 nm in diameter, show no plugs and no structural alteration relative to the controls (Fig. 11A, B). The conspicuous arrays of cortical microtubules typical of interphase meristematic cells are absent from desiccated cells (Fig. 11A), but are re-assembled within 2 h of starting rehydration (Fig. 11C, D). Dehydration also appears to affect the cell wall structure, as the loose fibrillar texture (Fig. 11A) typical of the hydrated state is no longer visible in the dry state (Fig. 11C).
Fig. 11.
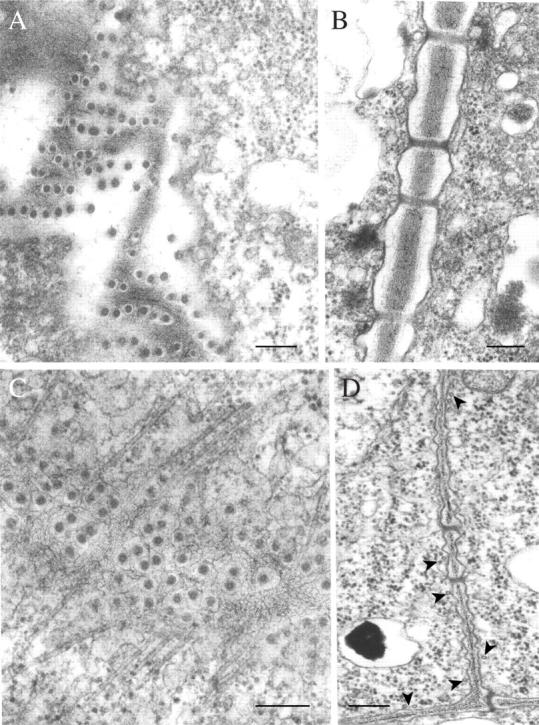
Transmission electron micrographs; details of apical meristematic cells in P. formosum in the dry state (A and B) and after 2-h rehydration (C and D). (A and C) Grazing sections of cell walls showing numerous plasmodesmata; cortical microtubules (arrowed in D), absent in the dry state (A), are abundant after 2-h rehydration (C and D); the cell wall in rehydrated cells show a loose fibrillar texture not visible in the dry state. (B) Longitudinal section of the plasmodesmata. Note absence of electron-opaque plugs (cf. Fig. 5A and B). Scale bars = 0·2 µm.
DISCUSSION
This study shows that desiccation causes major structural alterations in leptoids in the leafy shoot of the moss P. formosum, with the loss of the complex cytoplasmic organization that characterizes these cells in the hydrated condition. Besides the complete disappearance of the endoplasmic microtubules and longitudinal alignment of major organelles, structural changes include the ‘plugging' of plasmodesmata, changes in the ER organization, the appearance of numerous small vacuoles and the laying down of new labyrinthine wall material. The hydrated-state cytoplasmic organization is fully resumed within 12–24 h after rewetting. Cellular integrity is maintained both in the desiccated state and during rehydration, with no evidence of plasmolysis or membrane disruption. Thus, there appears to be no evidence for a repair-based mechanism involved in the recovery of leptoids during rehydration, as suggested in studies of desiccation-tolerance in assimilatory cells of bryophytes (for a review, see Oliver et al., 1998). Heptane fixation rules out artefacts in the dehydrated and rehydrating leptoids ascribable to the use of aqueous fixatives (Platt et al., 1997; Wesley-Smith, 2001).
Among the first events observed in leptoids during rehydration is the reestablishment of the endoplasmic microtubular cytoskeleton. The failure of leptoids to recover in the presence of oryzalin points to a key role of the endoplasmic microtubules in the control of the elaborate cytoplasmic architecture typical of these cells in the hydrated condition.
Previous studies using the antimicrotubular drug oryzalin (Ligrone and Duckett, 1996) have shown that depolymerization of the cytoskeletal microtubule system in moss leptoids caused substantial cellular disruption, resulting in the loss of organelle longitudinal alignment, displacement of the nuclei and profound changes in the endomembrane system. Based on these findings, Ligrone and Duckett (1996) concluded that microtubules not only affect the spatial arrangement of organelles but also the intrinsic stability of the endomembrane system. Because cellular polarity was barely affected by oryzalin, they concluded that microtubules do not have a direct role in the maintenance of polarity. The observation that dehydrated leptoids retain cellular polarity in the absence of endoplasmic microtubules supports this conclusion.
The disappearance of starch in the parenchyma cells of desiccated shoots of P. formosum and its prompt re-synthesis on rehydration is likely to reflect reversible conversion of starch into soluble sugars. Disappearance of starch from plastids in the dry state has been reported previously, e.g. in the moss Sphagnum (Gerdol et al., 1996) and the grass Sporobolus stapfianus (Quartacci et al., 1997). It is common for desiccation-tolerant angiosperms to hydrolyse starch as they dry (Gaff, 1997). Mounting evidence indicates that accumulation of protective proteins and sugars is a major metabolic change associated with desiccation-tolerance (Alpert and Oliver, 2002). Sucrose appears to be the major carbohydrate stored during dehydration, although accumulation of the non-reducing sugar trehalose has also been implicated in desiccation-tolerance (Zantella et al., 1999). Platt et al. (1994) suggested that sucrose was in part responsible for the maintenance of intact membranes in the dry leaves of Selaginella lepidophylla and Tortula ruralis.
Also in line with previous studies of desiccated vegetative tissues is the highly vesiculate nature of the cytoplasm of leptoids in the dehydrated condition (Fig. 3A). Gaff et al. (1976) observed that the vacuole in leaf chlorenchyma cells of Borya nitida became fragmented during dehydration. Similarly Thomson and Platt (1997) observed many small vacuoles with an intact tonoplast in dry cells of Selaginella lepidophylla, prepared by both conventional fixation and freeze substitution, and postulated that ‘the retention of vacuolar compartmentation and tonoplast integrity with dehydration could play a critical role in desiccation tolerance by retaining a possible complement of hydrolytic enzymes’. The nature and role of the vacuolar structures in the leptoids of P. formosum require further investigation; the possibility that they accumulate soluble sugars involved in protection against desiccation is an intriguing, albeit yet untested, hypothesis. The starch fluctuations observed during the dehydration/rehydration cycle essentially involve starch reserves in parenchyma cells adjoining leptoids. Considering that the plugging of plasmodesmata presumably interrupts symplasmic continuity between the two cell types in the dried condition, it appears possible that the development of labyrinthine structures on longitudinal walls of desiccated leptoids is a mechanism to enhance uptake of soluble sugars from the apoplast. Scheirer (1983) described irregular wall ingrowths, lined by the plasma membrane, similar to those described in this study, in leaf parenchyma cells of Polytrichum commune. He stated that these cells are ‘modified with transfer cell-like characteristics by virtue of their wall-membrane apparatus’ and suggested an apoplastic route for the movement of photoassimilates from the photosynthetic lamellae (Scheirer, 1983). On the other hand, the labyrinthine walls may also strengthen the cell wall–plasmalemma adhesion, thus preventing protoplast shrinkage during desiccation.
Mitochondria and plastids become rounded in the dry state, though their internal structures remain unchanged (in line with respiratory and photosynthetic machineries remaining intact in vegetative cells of the leafy shoot in P. formosum; M. C. F. Proctor, R. Ligrone and J. Duckett, unpubl. res.). The nucleus becomes ovoid and the chromatin forms condensed areas (also a feature of seeds during dehydration). Although it is possible that these changes in organelle appearance in dehydrated leptoids are a consequence of the disappearance of microtubules, it seems likely that they more directly reflect a mechanism of minimalization of organellar surface areas related to the withdrawal of water during dehydration.
Substantial reorganization of the endomembrane system is also evident in the dry state. ER cisternae and long tubular ER elements disappear from leptoids and membranous tubules are formed at right angles to the long axis of the cell. The available evidence suggests that the latter are a desiccation-induced storage form of ER membrane that on rehydration is reconverted into the ER system typical of hydrated leptoids.
The origins and nature of the plasmodesmatal plugs is unknown, although previous experiments using a variety of enzymes, including lysozyme, driselase, cellulase, pectinase and protease (Edwards et al., 2003) are indicative of a composition involving material particularly recalcitrant to enzymatic digestion. The fact that plugs were still clearly present in samples prepared for SEM following complete removal of the cytoplasm confirms a highly resistant nature. Ehlers et al. (2000) reported occlusion of sieve pores after injury in Vicia faba leaves and Lycopersicon esculentum internodes. These authors found that filamentous aggregates derived from P-proteins rapidly occluded sieve pores after injury, thus effectively sealing them. The occlusion of plasmodesmata and the consequent ‘sealing off’ of leptoids in desiccated shoots is probably functionally related to the concomitant reorganization of the ER. In the hydrated state this forms a prominent system extending from cell to cell through the desmotubules (Ligrone and Duckett, 1994, 1998), with remarkable similarities with the ‘vacuolar-tubular continuum’ reported in fungi (Ashford, 1998) and in the trichomes of an angiosperm (Lazzaro and Thomson, 1996).
Both these possible protective devices, i.e. the cell wall ingrowths and plasmodesmatal plugs, begin to disappear within 2 h from rehydration. It is also about at this time that the distinctive cytoplasmic organization of leptoids becomes again recognizable. However, it takes 12–24 h for the cells to recover fully, this being also the time necessary for the full complement of endoplasmic microtubule arrays to reassemble. These findings, coupled to the discovery that desiccation also causes reversible disassembly of the cortical microtubule arrays in meristematic cells, suggest a central role of the microtubule cytoskeleton in desiccation-tolerance in mosses.
In the context of the present study it is surprising that the cytoskeleton has hardly ever figured in studies of cytological effects of desiccation. Rocha Faria et al. (2004) in their studies of desiccation sensitivity in seeds of Inga vera reported a strong correlation between the disappearance of microtubules and the loss of viability of these recalcitrant seeds during drying and suggested that the failure of severely damaged cells to recovery on rehydration might reflect the loss of the ability to reassemble the microtubule cytoskeleton (Rocha Faria et al., 2004). Brown and Lemmon (1980, 1982, 1987) described the disappearance of microtubules during maturation of moss spores and their subsequent reassembly (repolymerization) during spore germination. As far as is known, no similar studies of vegetative tissues are available. The most likely explanation for this lack of data is that, to date, most of the research on vegetative desiccation-tolerance has focused on cellular metabolism and has therefore looked at mature assimilatory cells with abundant plastids and mitochondria but very few or inconspicuous microtubules.
The discovery of an intimate relationship between desiccation-tolerance and microtubule dynamics offers exciting prospects for future research, not the least of which being a novel non-invasive technique for exploring the latter. However, it must be underlined that whilst food-conducting cells and meristems in bryophytes are excellent materials for electron microscope studies, they cannot be used for direct observations because of their situation within complex tissues. They have proved unsuitable for freeze-substitution protocols, and thus will always be prone to possible artefacts generated by aqueous fixation. Against these constraints, techniques are now being developed for studying the desiccation biology of moss caulonema, the cytology and function of which closely mirror those of leptoids (Duckett et al., 1998). The protonemal system allows direct observations of living cells with the possible application of fluorescent probes and therefore might open the way to research on the functional genomics of the cytoskeleton in desiccation biology. Particularly interesting questions include elucidation of the molecular mechanisms underlying microtubule dynamics and possible links between the endoplasmic microtubules and dehydrins [Group II late embryogenesis abundant (LEA) proteins] and rehydrins (Close, 1996; Oliver et al., 2005).
Acknowledgments
Silvia Pressel thanks NERC for financial support from a CASE studentship with the Royal Botanic Gardens, Kew. The authors thank K. Pell for technical assistance. Thanks are also due to Dr K. C. Vaughn (USDA-ARS, Stoneville, MS, USA) for information on the anhydrous fixation protocol.
LITERATURE CITED
- Abdrakhamanova A, Wang QY, Khokhlova L, Nick P. 2003. Is microtubule disassembly a trigger for cold acclimation? Plant Cell Physiology 44: 676–686. [DOI] [PubMed] [Google Scholar]
- Alpert P. 2000. The discovery, scope, and puzzle of desiccation tolerance in plants. Plant Ecology 151: 5–17. [Google Scholar]
- Alpert P, Oliver MJ. 2002. Drying without dying. In: Black M, Pritchard HW, eds. Desiccation and survival in plants. Cambridge: Cambridge University Press, 3–31.
- Ashford AE. 1998. Dynamic pleiomorphic vacuoles: are they endosomes and transport compartments in fungal hyphae? Advances in Botanical Research 28: 119–159. [Google Scholar]
- Beckett RP. 2001. ABA-induced tolerance to ion leakage during rehydration following desiccation in the moss Atrichum androgynum. Plant Growth Regulation 35: 131–135. [Google Scholar]
- Berjak P, Pammenter NW. 2000. What ultrastructure has told us about recalcitrant seeds. Revista Brasileira de Fisiologia Vegetal 12: 22–55. [Google Scholar]
- Bewley JD. 1979. Physiological aspects of desiccation-tolerance. Annual Review of Plant Physiology 30: 195–238. [Google Scholar]
- Brown RC, Lemmon BE. 1980. Ultrastructure of sporogenesis in a moss, Ditrichum pallidum: spore wall formation. American Journal of Botany 67: 918–934. [Google Scholar]
- Brown RC, Lemmon BE. 1982. Ultrastructure of sporogenesis in the moss Amblystegium riparium. I. Meiosis and cytokenesis. American Journal of Botany 69: 1096–1107. [Google Scholar]
- Brown RC, Lemmon BE. 1987. Division, polarity, development and configuration of microtubule arrays in bryophyte meiosis. I. Meiotic prophase to metaphase I. Protoplasma 137: 84–99. [Google Scholar]
- Canny MJ. 2000. Water transport at the extreme—restoring the hydraulic system in a resurrection plant. New Phytologist 148: 187–189. [Google Scholar]
- Carter JV, Wick SM. 1984. Irreversible microtubule depolymerisation associated with freezing injury in Allium cepa root tip cells. Cryo-Letters 5: 373–382. [Google Scholar]
- Clausen E. 1952. Hepatics and humidity, a study on the occurrence of hepatics in a Danish tract and the influence of relative humidity on their distribution. Dansk Botanisk Arkiv 15: 1–80. [Google Scholar]
- Close T. 1996. Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiologia Plantarum 97: 795–803. [Google Scholar]
- Crowe JH, Heokstra FA, Crowe LM. 1992. Anhydrobiosis. Annual Review of Physiology 54: 579–599. [DOI] [PubMed] [Google Scholar]
- Duckett JC, Schmid AM, Ligrone R. 1998. Protonemal morphogenesis. In: Bates JW, Ashton NW, Duckett JG, eds. Bryology for the twenty-first century. London: British Bryological Society, 223–246.
- Edwards D, Axe L, Duckett JG. 2003. Diversity in conducting cells in early land plants and comparisons with extant bryophytes. Botanical Journal of the Linnean Society 141: 297–347. [Google Scholar]
- Ehlers K, Knoblauch M, van Bel AJE. 2000. Ultrastructural features of well-preserved and injured sieve elements: minute clamps keep the phloem transport conduits free for mass flow. Protoplasma 214: 80–92. [Google Scholar]
- Gaff DF. 1997. Mechanisms of desiccation tolerance in resurrection vascular plants. In: Basra AS, Basra RK, eds. Mechanisms of environmental stress resistance in plants. London: Harwood Academic Publishers, 43–58.
- Gaff DF, Zee FY, O'Brien TP. 1976. The fine structure of dehydrated and reviving leaves of Borya nitida Labill.—a desiccation tolerant plant. Australian Journal of Botany 24: 225–236. [Google Scholar]
- Gerdol R, Bonora A, Gualandri R, Pancaldi S. 1996. CO2 exchange, photosynthetic pigment composition, and cell ultrastructure of Sphagnum mosses during dehydration and subsequent rehydration. Canadian Journal of Botany 74: 726–734. [Google Scholar]
- Hearnshaw GF, Proctor MCF. 1982. The effect of temperature on the survival of dry bryophytes. New Phytologist 90: 221–228. [Google Scholar]
- Hébant C. 1976. Comparative anatomy of the gametophytes in Dawsonia (Polytrichales, Musci). Journal of the Hattori Botanical Laboratory 40: 221–246. [Google Scholar]
- Hébant C. 1977. The conducting tissue of bryophytes. Vaduz: J Cramer.
- Hill M, Preston CD, Smith AJE. 1992. Atlas of the bryophytes of Britain and Ireland. Mosses (except Diplolepidae), Vol. 2. Colchester: Harley Books.
- Hinshiri HM, Proctor MCF. 1971. The effect of desiccation on subsequent assimilation and respiration of the bryophytes Anomodon viticulosus and Porella platyphylla. New Phytologist 70: 527–538. [Google Scholar]
- Höfler K. 1946. Über Trockenhärtung und Härtungsgrenzen einiger Lebermoose. Anzeiger der Akademie der Wissenschaften in Wien. Mathematische-Naturwissenschaftliche Klasse 1945: 5–8. [Google Scholar]
- Lazzaro MD, Thomson WW. 1996. The vacuolar-tubular continuum in living trichomes of chickpea (Cicer arietinum). Physiologia Plantarum 94: 291–297. [Google Scholar]
- Ligrone R, Duckett JG. 1994. Cytoplasmic polarity and endoplasmic microtubules associated with the nucleus and organelles are ubiquitous features of food-conducting cells in bryoid mosses (Bryophyta). New Phytologist 127: 601–614. [Google Scholar]
- Ligrone R, Duckett JG. 1996. Polarity and endoplasmic microtubules in food-conducting cells of mosses: an experimental study. New Phytologist 134: 503–516. [Google Scholar]
- Ligrone R, Duckett JG. 1998. The leafy stems of Sphagnum (Bryophyta) contain highly differentiated polarized cells with axial arrays of endoplasmic microtubules. New Phytologist 140: 567–579. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. 2000. Conducting tissues and phyletic relationships of bryophytes. Philosophical Transactions of the Royal Society of London, Series B 355: 795–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall M, Proctor MCF. 1999. Desiccation tolerance and recovery of the leafy liverwort Porella platyphylla (L.) Pfeiff.: chlorophyll-fluorescence measurements. Journal of Bryology 21: 261–267. [Google Scholar]
- Mycock DJ, Berjak P, Finch-Savage WE. 2000. Effects of desiccation on the subcellular matrix of the embryonic axes of Quercus robur. In: Black M, Bradford KJ, Vazquez-Ramos J, eds. Seed biology: advances and applications. Wallingford: CABI Publishing, 197–203.
- Oliver MJ, Wood AJ, O'Mahony P. 1998. ‘To dryness and beyond’—preparation for the dried state and rehydration in vegetative desiccation-tolerant plants. Plant Growth Regulation 24: 193–201. [Google Scholar]
- Oliver MJ, Tuba Z, Mishler BD. 2000a. The evolution of vegetative desiccation tolerance in land plants. Plant Ecology 151: 85–100. [Google Scholar]
- Oliver MJ, Velten J, Wood AJ. 2000b. Bryophytes as experimental models for the study of environmental stress tolerance: Tortula ruralis and desiccation-tolerance in mosses. Plant Ecology 151: 73–84. [Google Scholar]
- Oliver MJ, Velten J, Mishler BD. 2005. Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats? Integrative and Comparative Biology 45: 788–799. [DOI] [PubMed] [Google Scholar]
- Platt KA, Oliver MJ, Thomson WW. 1994. Membranes and organelles of dehydrated Selaginella and Tortula retain their normal configuration and structural integrity: freeze fracture evidence. Protoplasma 178: 57–65. [Google Scholar]
- Platt KA, Oliver MJ, Thomson WW. 1997. Importance of fixative for reliable ultrastructural preservation of poikilohydric plant tissue: observations on dry, partially and fully hydrated tissues of Selaginella lepidophylla. Annals of Botany 80: 599–610. [Google Scholar]
- Proctor MCF. 2003. Experiments on the effect of different intensities of desiccation on bryophyte survivals, using chlorophyll fluorescence as an index of recovery. Journal of Bryology 25: 201–210. [Google Scholar]
- Proctor MCF, Smirnoff N. 2000. Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments. Journal of Experimental Botany 51: 1695–1704. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Tuba Z. 2002. Poikilohydry and homoiohydry: antithesis or spectrum of possibilities. New Phytologist 156: 327–349. [DOI] [PubMed] [Google Scholar]
- Quartacci MF, Forli M, Rascio N, Dalla Vecchia F, Bochicchio A, Navari-Izzo F. 1997. Desiccation-tolerant Sporobolus stapfianus: lipid composition and cellular ultrastructure during dehydration and rehydration. Journal of Experimental Botany 48: 1269–1279. [Google Scholar]
- Rocha Faria JM, van Lammeren AAM, Hilhorst HWM. 2004. Desiccation sensitivity and cell cycle aspects in seeds of Inga vera subsp. affinis. Seed Science Research 14: 165–178. [Google Scholar]
- Scheirer DC. 1983. Leaf parenchyma with transfer cell-like characteristics in the moss Polytrichum commune Hedw. American Journal of Botany 70: 987–992. [Google Scholar]
- Schmid A. 1998. A new photoassimilate translocation mechanism in the Polytrichales? PhD Dissertation, University of London.
- Schneider H, Manz B, Westhoff M, Mimietz S, Szimtenings M, Neuberger T. 2003. The impact of lipid distribution, composition and mobility on xylem water refilling of the resurrection plant Myrothamnus flabellifolia. New Phytologist 159: 487–505. [DOI] [PubMed] [Google Scholar]
- Schwarzerova K, Pokorna, J, Petrasek J, Zelenkova S, Capkova V, Janotova I, et al. 2003. The structure of cortical cytoplasm in cold-treated tobacco cells: the role of the cytoskeleton and the endomembrane system. Cell Biology International 27: 263–265. [DOI] [PubMed] [Google Scholar]
- Thomson WW, Platt KA. 1997. Conservation of cell order in desiccated mesophyll of Selaginella lepidophylla ([Hook and Grev.] Spring). Annals of Botany 79: 439–447. [Google Scholar]
- Tuba Z, Csintalan Z, Proctor MCF. 1996. Photosynthetic responses of a moss, Tortula ruralis, ssp. ruralis, and the lichens Cladonia convoluta and C. furcata to water deficit and short periods of desiccation, and their ecophysiological significance: a baseline study at present CO2 concentration. New Phytologist 133: 353–361. [DOI] [PubMed] [Google Scholar]
- Tuba Z, Proctor MCF, Csintalan Z. 1998. Ecophysiological responses of homoiochlorophyllous and poikilochlorophyllous desiccation-tolerant plants: a comparison and ecological perspective. Plant Growth Regulation 24: 211–217. [Google Scholar]
- Wagner H-J, Schneider H, Mimietz S, Wistuba N, Rokitta M, Krohne C. 2000. Xylem conduits of a resurrection plant contain a unique lipid lining and refill following a distinct pattern after desiccation. New Phytologist 148: 239–255. [DOI] [PubMed] [Google Scholar]
- Wang QY, Nick P. 2001. Cold acclimation can induce microtubular cold stability in a manner distinct from abscisic acid. Plant Cell Physiology 42: 999–1005. [DOI] [PubMed] [Google Scholar]
- Werner O, Ros Espin RM, Bopp M, Atzorn R. 1991. Abscisic-acid-induced drought tolerance in Funaria hygrometrica Hedw. Planta 186: 99–103. [DOI] [PubMed] [Google Scholar]
- Wesley-Smith J. 2001. Freeze-substitution of dehydrated plant tissues: artifacts of aqueous fixation revisited. Protoplasma 218: 154–167. [DOI] [PubMed] [Google Scholar]
- Zantella R, Mascorro-Gallardo JO, Van Kijck P, Folch-Mallol J, Bonini B, Van Vaeck C. 1999. A Selaginella lepidophylla trehalose-6-phosphate synthase complements growth and stress-tolerance defects in a yeast tps1 mutant. Plant Physiology 119: 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]