Abstract
• Background and Aims The main stems of trees on forest slopes incline down the slope to various extents that are characteristic of the species. The inclination has been explained as an active response to a horizontally asymmetrical light environment, but the contributing physiological mechanisms are unknown. The present study tested the hypothesis that stem phototropism, gravitropism, or a combination of the two determines the inclination of tree stems on forest slopes.
• Methods Cryptomeria japonica, Pinus densiflora, Quercus myrsinaefolia and Q. serrata were studied. Measurements were made of stem inclination of mature trees on forest slopes in uniform plantations of each species, and changes in stem inclination of potted seedlings in response to illumination treatments (unilateral or overhead) and inclination treatments (artificially inclined or erect). Indices of phototropic and gravitropic responsiveness were evaluated for each species, calculated from the change in stem inclination in response to artificial inclination with unilateral or overhead illumination.
• Key Results Stem inclination on forest slopes varied significantly among species: Q. serrata inclined most in the down-slope direction, C. japonica inclined the least, and P. densiflora and Q. myrsinaefolia were intermediate. The change in stem inclination of seedlings in each treatment varied significantly among species. One-year-old stems of Q. serrata and 2-year-old stems of Q. myrsinaefolia bent toward the light source. Interspecific variation in the change in stem inclination in response to the unilateral illumination or that in the index of phototropic responsiveness was strongly correlated with the variation in stem inclination on forest slopes.
• Conclusions The orientation of woody stems that have finished elongation can be actively controlled by phototropism. Interspecific variation in phototropic responsiveness of trees is a possible significant determinant of interspecific variation in stem inclination on forest slopes.
Keywords: Phototropism, gravitropism, tree, woody stem, reorientation, stem inclination, forest slope, Cryptomeria japonica, Pinus densiflora, Quercus myrsinaefolia, Quercus serrata
INTRODUCTION
The main stems of trees growing on forest slopes are often inclined in the down-slope direction. Passive inclination caused by external forces, such as landslides (Shimada, 1994; Sakai et al., 1995) or snow pressure (Taira, 1987), has been proposed to be responsible for the down-slope inlincation.
However, stem inclination of trees on forest slopes can also be considered an active response to a horizontally asymmetrical light environment around each tree, because stem inclination has been observed under natural (Loehle, 1986) and experimental (Sierra-de-Grado et al., 1997) asymmetry in the light environment. The crown and stem of a tree growing on a forest slope may receive more incident light from the down-slope azimuth than from the up-slope azimuth; this occurs because neighbouring trees growing up the slope are at a higher elevation and thus block light from the up-slope azimuth, and because the slope itself intercepts some of this incident light. Active responses to the asymmetrical light environment around a tree on forest slopes have been proposed as the causes of stem inclination in understorey trees (Ishii and Higashi, 1997, 1998), of the asymmetrical crown display of canopy trees (Umeki, 1995; Olesen, 2001), probably accompanied by stem inclination, and of the existence of more branches in the down-slope direction (Sumida et al., 2002). However, mechanisms for how trees incline their main stems in response to the horizontally asymmetrical light environment were not examined in these studies, in which stem inclination or crown display was described at only one point in time.
Phototropism occurs in response to horizontal asymmetry in the light environment and is a possible mechanism contributing to stem inclination. The elongating portion of the stem is known to be able to bend phototropically or gravitropically as a result of differential elongation of cells on opposite sides of the stem. For gravitropism, woody stems that have finished elongation can also bend by asymmetrical generation of longitudinal growth stresses (Wilson and Archer, 1977; Yamamoto et al., 2002). Even mature trees with thick woody stems can become gravitropically erect if they can generate a sufficiently large bending moment by the generation of asymmetrical longitudinal growth stresses (Timell, 1986). It is possible that phototropism actively controls the orientation of radially growing woody stems as well as that of elongating stems.
The resulting angles of stem inclination of trees on forest slopes appear to vary among species. It was hypothesized that differences in the magnitudes of stem phototropism, gravitropism, or both factors in the trees would be significant in determining the observed stem inclination on forest slopes. Four tree species were chosen for the study: Japanese cedar (Cryptomeria japonica), Japanese red pine (Pinus densiflora), an evergreen oak (Quercus myrsinaefolia) and a deciduous oak (Q. serrata). These species form the forest canopy in warm-temperate regions of Japan. To find out if the stem inclination of mature trees of these species on forest slopes was a characteristic of each species, this variable was measured in uniform plantations on slopes with similar inclination and azimuth.
The magnitudes of the stem phototropism and gravitropism of each species were experimentally quantified in seedlings. To evaluate phototropic or gravitropic responses separately, conventional methods in which an erect stem is unilaterally illuminated or is inclined artificially under darkness were avoided; instead, unilateral or overhead illumination was applied to both erect and artificially inclined seedlings to apply phototropic and gravitropic stimuli in the same direction or in opposite directions. Changes in stem inclination in the two treatments were measured, and their sum and difference computed to evaluate phototropic and gravitropic responsiveness, respectively. In this manner, it was possible to separate passive deflection caused by the weight of the shoots from phototropic reorientation and to sustain photosynthesis during the treatment period to provide photosynthates needed to allow the reorientation of the main stems. Passive deflection beyond photogravitropic control resulting from excessive stem and crown weight may contribute to the stem inclination in mature trees on forest slopes. In addition, the separated phototropic response may not correspond to the observed reorientation in response to the asymmetrical light environment on forest slopes. Therefore, a treatment was also established with unilateral illumination of erect stems, where deflection resulting from each shoot's own weight could affect the reorientation. Deformation within and between portions of the main stem was evaluated to examine whether the stem bending in response to the treatments occurred in both woody portions and newly elongating portions.
The contributions of stem phototropism and gravitropism in each species to creating stem inclination on forest slopes are discussed on the basis of the empirically determined relationships between these various factors.
MATERIALS AND METHODS
Stem inclination on forest slopes
Study sites
Field surveys were carried out in January 2003 at the University of Tokyo's University Forest in Chiba, located in the Boso Peninsula, Japan (35°11′N, 140°9′E), where mean annual precipitation is 2282 mm (Kuraji et al., 1998) and mean annual temperature is 13·8 °C (Anon., 1997). Table 1 shows the characteristics of the study stands for each species. All sites were uniform forests planted in cleared areas that slope down toward the north or north-west, with similar slope angles. The Q. serrata and C. japonica sites were planted in 1897, and the undergrowth was removed every year until 1987 to prevent planted seedlings from being shaded by other vegetation and to facilitate travel on the forest floor. The Q. myrsinaefolia site was planted in 1955, and the undergrowth was removed every year until 1987. Grafted seedlings were planted at the P. densiflora site in 1985, where underbrush was removed every year until 1989.
Table 1.
Characteristics of the study stands
| Species | Stand age (years) | Slope direction | Slope inclination (degrees) | n | Mean d.b.h. (cm) | Mean height (m) |
|---|---|---|---|---|---|---|
| Cryptomeria japonica | 107 | N | 32 | 29 | 23·9 | 19·6 |
| Pinus densiflora | 19 | N60°W | 33 | 132 | 11·4 | 7·5 |
| Quercus myrsinaefolia | 49 | N1°W | 38 | 27 | 16·6 | 13·8 |
| Quercus serrata | 107 | N2°W | 35 | 24 | 33·4 | 14·3 |
d.b.h., Diameter at breast height.
Measurement of stem inclination
For every mature tree at each site, measurements were made of stem inclination, which represents the angle of inclination of a straight line connecting the base of the stem and the top of the tree perpendicularly projected on a vertical plane that passes through the base of the tree and runs parallel to the slope azimuth (Fig. 1).
Fig. 1.
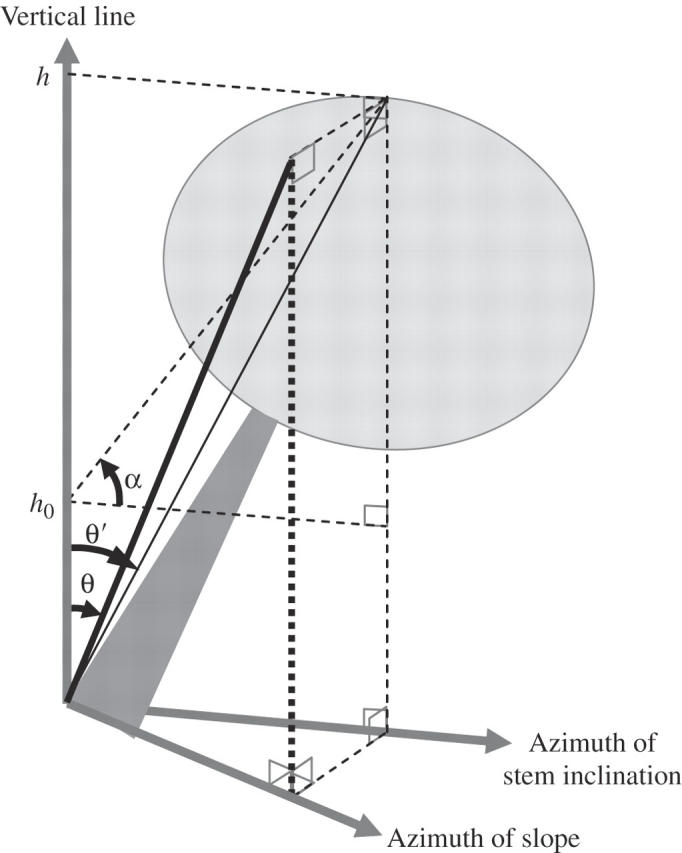
The geometry used to calculate stem inclination. α, Elevation angle; h, tree height; h0, eye level of the measurer; θ′, stem inclination with respect to the plane of inclination; θ, stem inclination.
First, the stem inclination was measured within a plane of inclination (θ′). Standing up-slope of the base of the stem of a tree, the angle of elevation was measured from eye-level to the tree top (α) with a clinometer (Vertex III, Haglöf, Långsele, Sweden), with a zenith prism set in front of the collimator when the elevation angle exceeded 85°. θ′ was calculated using the following equation, where h represents tree height and h0 represents the eye-level height of the measurer:
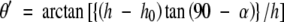 |
Secondly, the azimuth of the stem inclination was measured. To define the vertical plane that passes through the stem, a 1·3-m-long stick with a perpendicular attached to one end with thread was positioned along the basal 1·3 m of the stem. Then another stick was held horizontally so that it contacted the perpedicular's thread and the other stick, and the azimuth of this horizontal stick measured with a compass. It was assumed that the azimuth of the overall stem's inclination equals that of the inclination of the basal 1·3 m of the stem, and stem inclination (θ) was calculated on a vertical plane that passes through the base of the tree and runs parallel to the slope azimuth, using θ′ and the azimuth of inclination.
Evaluation of stem phototropism and gravitropism
Plant materials
One-year-old seedlings of C. japonica (mean length of main stem, 120 mm; mean diameter at base of stem, 3·0 mm), P. densiflora (177 and 3·4 mm) and Q. serrata (171 and 2·7 mm), and 2-year-old seedlings of Q. myrsinaefolia (328 and 6·6 mm) were used to study phototropism and gravitropism. The seedlings were transplanted into separate planters for each combination of species and treatment to make a single row of four or five seedlings of the same species in each treatment parallel to the long axis of the planter. The seedlings were grown outdoors under natural conditions until the experimental treatments began. The seedlings of Q. serrata, a deciduous species, began flushing in April.
Treatment
In June, two categories of treatment (illumination and inclination), each with two levels, were established for a total of four treatments (Fig. 2). The levels of the illumination treatment were unilateral (U) and overhead (O) illumination; the levels of inclination were erect (E) and inclined (I). The four treatments were thus denoted as U-E, O-E, U-I and O-I. The seedlings were illuminated laterally (for U) or from overhead (for O) in a shaded room using arrays of fluorescent tubes with an optimized spectrum for plant cultivation (Biolux-A, NEC, Tokyo, Japan) so that the photosynthetic photon flux density (PPFD) at the average position of the apices of the main stems was about 100 μmol m− 2 s−1 with 16 h of light and 8 h of dark. The planters were positioned so that their long axes were parallel to those of the fluorescent tubes. In the treatments with inclined stems, the planters were inclined by rotation around their long axis so that the soil surface formed an angle of 45° below the horizontal. In the U-I treatment, the illumination struck the underside of the inclined main stems of the seedlings and induced a phototropism that would counteract the gravitropism induced by the inclination.
Fig. 2.
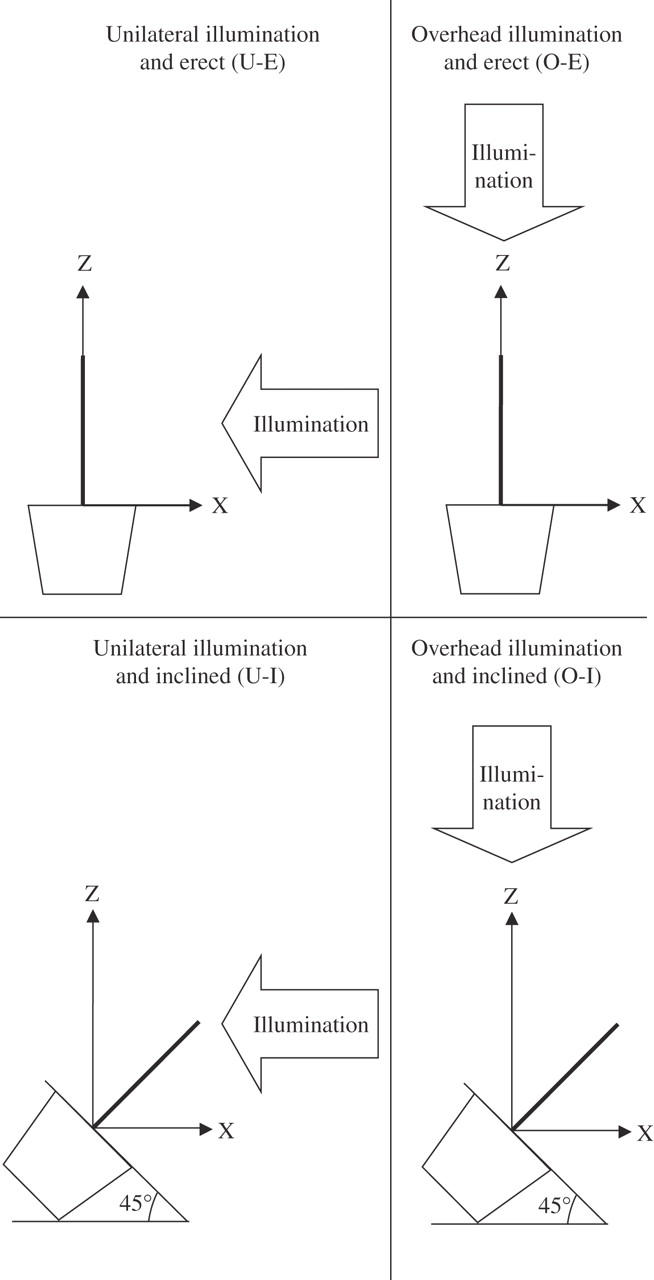
Schematic diagram of the four treatments and of the co-ordinate system used for the analyses.
The seedlings were provided with sufficient irrigation. The mean room temperature during the experiments was 28·7 °C. Treatments were continued until week 9, at which point little reorientation had been observed during the preceding 2 weeks.
Measurement of stem form
The three-dimensional form of the main stem was measured from its base to its apex at the beginning and end of the treatments using a three-dimensional digitizer (Microscribe G2L, Immersion, San Jose, CA, USA). If the apex of the leading shoot had not flushed by the beginning of the treatments, measurements were made from the base of the stem to the shoot whose flushed apex was nearest to the light source at the beginning of the treatment, and this was regarded as the ‘main stem’ henceforth. By moving the digitizer's probe along the stem axis, the co-ordinates of the stem were automatically logged at intervals of 2 mm. The co-ordinates of two points were measured along the long axis of each planter so that the line passing through these points could serve as a reference axis. A Cartesian co-ordinate system was used, with the z-axis pointing toward the zenith, the y-axis parallel to the long axis of the planter, and the x-axis perpendicular to the z- and y-axes in subsequent analyses (Fig. 2). Stem form was analysed to determine the x, z co-ordinates.
Change in stem inclination
The change in stem inclination during the treatment was computed to quantitatively evaluate tropic reorientation of the main stem as a whole. Stem inclination represented the angle of inclination of a straight line that connected the base and the apex of the main stem at the beginning of the treatments. A change in stem inclination away from the vertical is expressed as a positive value, whereas a change toward the vertical is expressed as a negative value.
Indices of phototropic and gravitropic responsiveness (Rp and Rg, respectively) were calculated from the change in stem inclination in the U-I and O-I treatments (RU-I and RO-I, respectively) to evaluate separately the magnitudes of the two tropic reorientations. Phototropism acted to increase the inclination of the stem in the U-I treatment and to make the stem more erect in the O-I treatment, whereas gravitropism acted to make the stems erect in both treatments. Because both the phototropic stimulus and the gravitropic stimulus acted at an angle of 45° to the main stem axis in both the inclined treatments, the intensities of the tropic stimuli were approximated as being the same in both treatments. An equilibrium state in which antagonistically acting phototropism and gravitropism counterbalance each other is called a photogravitropic equilibrium (Iino, 2001; Galland, 2002). On the assumption that stem inclination at the end of the treatment had reoriented toward photogravitropic equilibrium and was not affected by factors that limited the reorientation, such as the weight of each shoot, flexural rigidity of the main stem, or radial growth, it was considered that the change in stem inclination in the U-I and O-I treatments was equal to the sum and difference of Rg and Rp, respectively. Therefore, Rp and Rg can be calculated as follows:
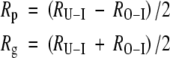 |
Rp and Rg were calculated not from the bending angle, but rather from changes in stem inclination under a limited intensity of phototropic and gravitropic stimuli (100 μmol m−2 s−1 PPFD and 45° inclination, respectively), but it is believed that these are adequate indices to separately evaluate interspecific variation in phototropic and gravitropic responsiveness.
Deformed portion of main stem
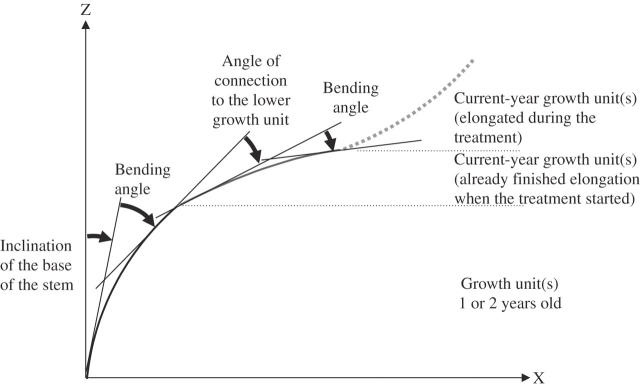
Shoots of P. densiflora, Q. serrata and Q. myrsinaefolia flush and elongate several times in a growth season. A stem portion that elongated within a given growth flush was denoted as a growth unit (GU) (Collet and Frochot, 1996; Fig. 3). A slight but abrupt difference in the orientation was often observed at the joint between two successive GUs. To examine the stem portions that contributed to the tropic reorientation of the main stems, curves were fitted to each GU and changes computed during the treatment period in the bending angle within a GU and in the angle of the connection between two successive GUs. The inclination of the curve fitted to the most basal GU, i.e. inclination of the base of the main stem, was also computed using the geometry defined in Fig. 3. The 1-year-old and 2-year-old stems were treated as a single GU because it was difficult to segment them into GUs based solely on stem morphology. Although C. japonica seedlings did not form a resting bud and elongated continuously during the experiment, the portions of the main stem that had elongated before and after the start of the treatment were treated as different GUs because they were expected to respond differently to the treatments.
Fig. 3.
Evaluation of deformation within and between growth units (GUs), which may contribute to a change in stem inclination. Thin lines denote tangents at the endpoints of each GU. When several GUs existed within a given age category, the data for each individual seedling were averaged.
Principal-components analysis was performed to standardize the axis direction, and subsequently a third-order polynomial was fitted to the points composing the GU, with the first principal component as the independent variable and the second principal component as the dependent variable. The inclination at both ends of the GU was calculated from the derivative of the fitted polynomial. The bending angle was computed as the difference between the inclinations at the top and base of the GU, and the angle of the connection at the joint between two successive GUs as the difference between the inclination at the basal end of the upper GU and the inclination at the top end of the lower GU. Changes in the bending angle, angle of connection and inclination of the base of the stem were computed as the differences between the values at the beginning and the end of the treatments to evaluate deformation within and between GUs.
RESULTS
Stem inclination on forest slopes
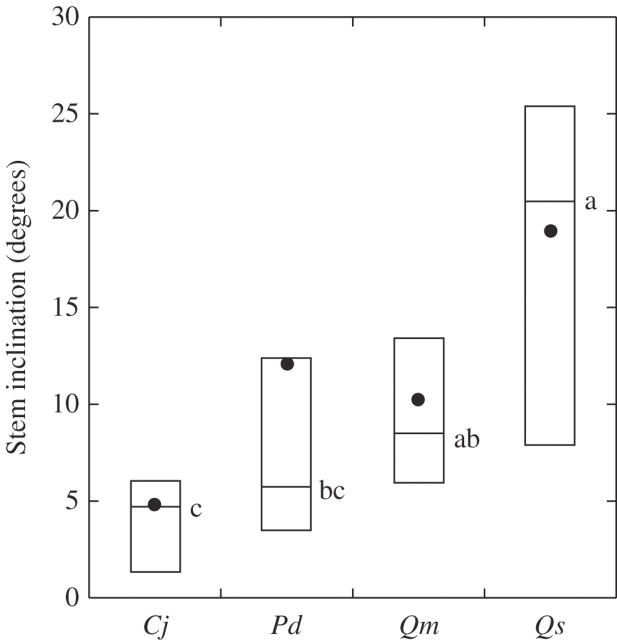
More than 90 % of the individuals of each species inclined toward the down-slope direction, and the degree of stem inclination varied significantly among the species (Fig. 4). The mean stem inclination was largest for Q. serrata, was intermediate and similar for P. densiflora and Q. myrsinaefolia, and was smallest for C. japonica.
Fig. 4.
Stem inclination of mature trees on forest slopes. Boxes, horizontal bars, and dots represent the quartiles, medians and mean values, respectively. Different letters denote significant differences (P < 0·05) between species based on Scheffé's multiple-comparison test following the Kruskal–Wallis test. Cj, Cryptomeria japonica; Pd, Pinus densiflora; Qm, Quercus myrsinaefolia; Qs, Q. serrata.
Interspecific variation in stem phototropism and gravitropism
Change in stem inclination
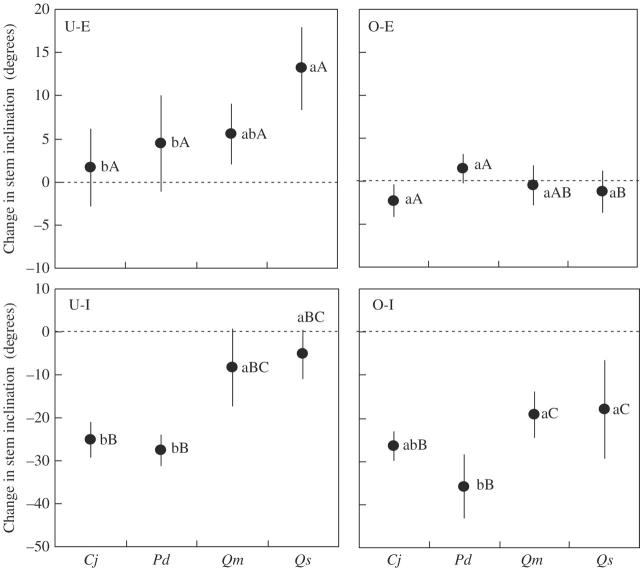
Figure 5 shows the change in stem inclination for each species in each treatment. Among the species in the U-E treatment, only Q. serrata showed a significantly larger reorientation toward the light source than in the O-E treatment. Quercus serrata reoriented the most toward the light source among the four species, Q. myrsinaefolia was intermediate, and P. densiflora and C. japonica showed significantly less reorientation toward the light source than Q. serrata. In the O-I treatment, all species showed significantly greater reorientation toward an erect posture than in the O-E treatment. Pinus densiflora reoriented most toward an erect posture, C. japonica was intermediate, and Q. myrsinaefolia and Q. serrata reoriented significantly less than P. densiflora. In the U-I treatment, C. japonica and P. densiflora showed significantly more reorientation toward an erect posture than in the O-E treatment, and the reorientation was significantly larger than that of Q. myrsinaefolia and Q. serrata.
Fig. 5.
Mean change in stem inclination during the treatment period. Reorientation away from the vertical is expressed as a positive value, and that toward the vertical is expressed as a negative value. Error bars represent standard deviation. Different letters denote significant differences (P < 0·05) between species within a treatment (lower-case letters) and between treatments within a species (capital letters) based on Spjotvoll and Stoline's multiple-comparison test. Cj, Cryptomeria japonica; Pd, Pinus densiflora; Qm, Quercus myrsinaefolia; Qs, Q. serrata.
Deformed portion of main stem
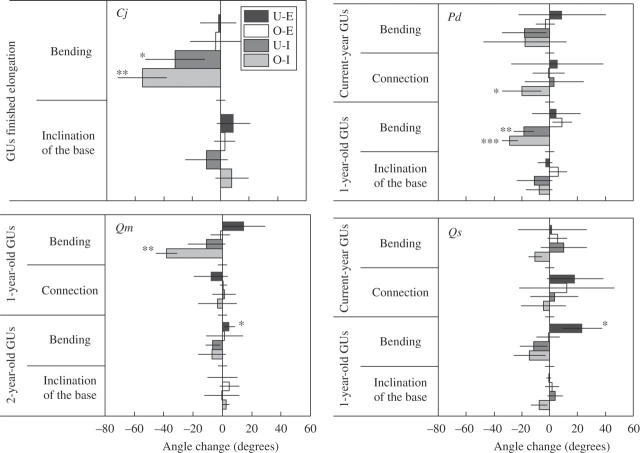
Figure 6 shows the changes in the inclination of the base of the stem, the bending angle, and the angle of connection to the lower GU for each category of GU. No significant change in the inclination of the base of the stem was detected in any species or treatment. The GUs of C. japonica that had finished elongation before the beginning of the U-I and O-I treatments showed significant bending toward an erect posture. The 1-year-old GUs of P. densiflora showed significant bending toward an erect posture in the U-I and O-I treatments, and current-year GUs showed significant changes in the angle of connection toward erection in the O-I treatment. The 1-year-old GUs of Q. myrsinaefolia also showed significant bending toward an erect posture in the O-I treatment. The 2-year-old GUs of Q. myrsinaefolia and the 1-year-old GUs of Q. serrata showed significant bending toward the light source in the U-E treatment. No other significant angle changes were detected.
Fig. 6.
Mean changes in the bending angle and the angle of connection of growth units (GUs) of each age class during the treatment period. Changes away from the vertical are expressed as positive values, and changes toward erection are expressed as negative values. Values for a given age have been averaged when there were multiple GUs for an individual. Error bars represent standard deviation. Asterisks denote the significance level of the change based on a pairwise t-test (*, P < 0·05; **, P < 0·01; ***, P < 0·001). The current-year GUs have been omitted for Qm because few current-year GUs had finished elongation by the beginning of the treatments. Cj, Cryptomeria japonica; Pd, Pinus densiflora; Qm, Quercus myrsinaefolia; Qs, Q. serrata.
Indices of phototropic and gravitropic responsiveness and their relationships with the stem inclination on forest slopes
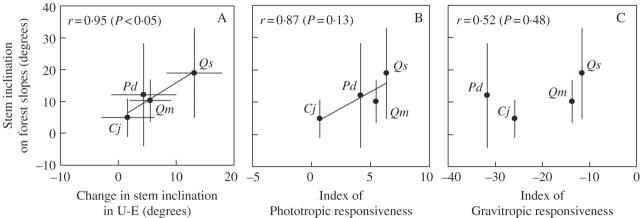
The observed change in stem inclination in the U-E treatment was significantly correlated with the stem inclination of mature trees on forest slopes (Fig. 7A).
Fig. 7.
Relationship between the mean observed stem inclination on forest slopes and (A) the change in stem inclination in the U-E treatment, (B) the index of phototropic responsiveness (Rp) or (C) the index of gravitropic responsiveness (Rg). Species with a larger absolute value of Rp or Rg are more responsive. Error bars represent standard deviation. Pearson's correlation coefficient (r) and its P-value are shown for tests of the correlation in the population. Cj, Cryptomeria japonica; Pd, Pinus densiflora; Qm, Quercus myrsinaefolia; Qs, Q. serrata.
The index of phototropic responsiveness (Rp) was largest for Q. serrata and was smallest for C. japonica (Fig. 7B). The magnitude of the index of gravitropic responsiveness (Rg) was smallest for P. densiflora and largest for Q. myrsinaefolia and Q. serrata (Fig. 7C). Interspecific variation in the stem inclination values of mature trees on forest slopes showed a strong but nonsignificant correlation with the index of phototropic responsiveness (Fig. 7B), and showed a weaker and nonsignificant correlation with the index of gravitropic responsiveness (Fig. 7C).
DISCUSSION
Possibility for active phototropic bending of woody stems
Stem reorientation corresponding to the direction of illumination and inclination was quantitatively evaluated by observing changes in stem inclination, and significant interspecific variation was found.
Stems can be inclined at the base as a result of deformation or failure of the root system and soil in response to an external force (Ennos, 2000). Since no significant change was found in the inclination of the base of the stem in any species or treatment (Fig. 6), we are confident that support of the aerial part of the seedlings by the root system and soil had no effect on the change in stem inclination in the experiment that evaluated tropisms.
Some 1-year-old GUs and the current-year GUs in the O-I and U-I treatment were observed to bend significantly (Fig. 6), and this contributed to the gravitropic reorientation toward erect posture in these treatments (Fig. 5). It is well known that radially growing woody stems that have finished elongation can actively bend through gravitropism by means of asymmetry in the longitudinal growth stresses generated by the asymmetrical formation of reaction wood (tension or compression wood) and normal wood (Wilson and Archer, 1977). The asymmetry in longitudinal growth stresses must contribute to the active gravitropic bending of these woody stems.
It is possible that the orientation of woody stems is actively controlled by phototropism as well as by gravitropism. The smaller stem erection in the U-I than in the O-I treatment (Fig. 5) might be due to suppression of gravitropic erection by phototropism. The tendency to less bending of the 1-year-old and current-year GUs in the U-I than in the O-I treatment (Fig. 6) contributed to the possible suppression of gravitropic bending by phototropism. Moreover, significant bending was observed in the 2-year-old GUs of Q. myrsinaefolia and the 1-year-old GUs of Q. serrata in the U-E treatment (Fig. 6), where the stems showed phototropic reorientation toward the light source (Fig. 5). Active phototropic bending is likely to contribute at least in part to the observed bending, but passive deflection as a result of the weight of the main stem and an asymmetrical load caused by the lateral shoots, which tended to grow toward the light source, may also contribute.
Active phototropic bending of these woody stems is likely to be caused by the generation of asymmetrical longitudinal growth stresses rather than by differential cell elongation. Fully differentiated secondary xylem with thick secondary cell walls was observed in transverse sections of each GU for P. densiflora and Q. serrata in the U-E and O-E treatments (data not shown). Fully differentiated secondary xylem cannot elongate because these cells mainly consist of dead cells that lack cytoplasm (Shimaji, 1985). Because differentiating secondary xylem and fully differentiated secondary xylem adhere to each other, it is possible that turgor pressure in the differentiating secondary xylem may also cause elongation of the fully differentiated xylem. However, this effect is not likely to be significant because of the rigidity of the lignified secondary walls in fully differentiated xylem. Expansion of xylem cells is known to cease by the time lignification is initiated (Kozlowski, 1971). Moreover, the 2-year-old and 1-year-old GUs that showed apparent phototropic bending were covered with a thin but achlorophyllous mature periderm (data not shown), which is generally initiated only after stem elongation is complete (Dickison, 2000). Therefore, it is unlikely that the observed phototropic bending was due to differential cell elongation, as has been reported for phototropism in elongating stems. In the present study, these GUs were radially growing woody stems that had apparently finished elongation. The active phototropic bending of these woody stems is more likely to have resulted from asymmetry in longitudinal growth stresses, as is well known to occur in gravitropism.
Environmental and physiological factors determining stem inclination on forest slopes
The comparison of stem inclination in uniform plantations growing on similar forest slopes revealed significant interspecific variation (Fig. 4). The crown and the stem of trees growing on forest slopes may receive more incident light from the down-slope azimuth than from the up-slope azimuth in the uniform plantations in the present study, as has been proposed for forest slopes on which researchers have observed down-slope inclination of the main stems (Ishii and Higashi, 1997, 1998) or asymmetrical crown display toward the down-slope azimuth (Umeki, 1995; Olesen, 2001; Sumida et al., 2002). The significant correlation between the interspecific variation in change in stem inclination in the U-E treatment and that in stem inclination on forest slopes (Fig. 7A) strongly suggests that the stem inclination observed on forest slopes represents a response to the horizontally asymmetrical light environment. It was also found that the interspecific variation in stem reorientation in response to the horizontally asymmetrical light environment is a significant factor in determining the interspecific variation in stem inclination on forest slopes.
Two processes can contribute to the reorientation of the main stem in response to the horizontally asymmetrical light environment. One is passive deflection as a result of the weight of the main stem and the asymmetrical load caused by asymmetrical crown development toward the down-slope azimuth, in which direction the optimal light environment exists. The asymmetrical crown development can result from growth, branching and death of shoots in response to the local light environment, which determines gross photosynthetic production of the shoot (branch autonomy; Sprugel et al., 1991). The other process is active phototropic reorientation of elongating stems or radially growing woody stems that have finished elongation.
To examine the contribution of phototropism to the stem inclination on forest slopes, the relationships between indices of phototropic and gravitropic responsiveness (Rp and Rg, respectively) and the stem inclination on forest slopes were evaluated. The strong correlation of the stem inclination values of mature trees on forest slopes with the index of phototropic responsiveness (Fig. 7B) rather than with the index of gravitropic responsiveness (Fig. 7C) suggests that phototropism contributes to the stem inclination of mature trees on forest slopes. Although more investigation is needed to reach a convincing conclusion because of the differences in tree size and age among the stands of each species and the limited conditions under which phototropic and gravitropic responsiveness were evaluated, it is quite likely that interspecific variation in stem phototropism of trees is a significant factor in determining stem inclination on forest slopes.
Conclusions
Orientation of woody stems that have finished elongation can be actively controlled by phototropism. Interspecific variation in stem inclination on forest slopes is likely to be determined mainly by phototropic responsiveness of the species. As well as phototropic orientation of elongating stems, phototropic reorientation of woody stems may contribute to asymmetrical development of tree form in response to an asymmetrical light environment.
Acknowledgments
We thank the Experimental Station at Tanashi for providing facilities and plant materials, the University Forest in Chiba for providing study sites and assistance in the fieldwork, and the Arboriculture Research Institute of the University of Tokyo for providing plant materials. We also thank the Laboratory of Forest Botany and Forest Health and the Laboratory of Forest Zoology of the University of Tokyo for providing appliances for the fluorescent tubes and a room for the experiment, respectively. We thank Drs Y. Saito, M. Norisada, K. Kojima and T. Hogetsu of the University of Tokyo for instructive discussion of our study. We thank Dr M. Iino, Osaka City University, and two anonymous reviewers for valuable comments on earlier drafts. This study was supported in part by a grant from the 21st Century COE Program ‘Biodiversity and Ecosystem Restoration Research Project’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
LITERATURE CITED
- Anon. 1997. The 11th management and experiment plan of the Tokyo University Forest in Chiba (1997–2004). Miscellaneous Information The Tokyo University Forests 37: 1–82 [in Japanese, with English title]. [Google Scholar]
- Collet C, Frochot H. 1996. Effects of interspecific competition on periodic shoot elongation in oak seedlings. Canadian Journal of Forest Research 26: 1934–1942. [Google Scholar]
- Dickison WC. 2000. Integrative plant anatomy. New York, NY: Academic Press.
- Ennos AR. 2000. The mechanics of root anchorage. Advances in Botanical Research incorporating Advances in Plant Pathology 33: 133–157. [Google Scholar]
- Galland P. 2002. Tropisms of Avena coleoptiles: sine law for gravitropism, exponential law for photogravitropic equilibrium. Planta 215: 779–784. [DOI] [PubMed] [Google Scholar]
- Iino M. 2001. Phototropism in higher plants. In: Häder D-P, Jori G, eds. Photomovement. Amsterdam: Elsevier, 659–790.
- Ishii R, Higashi M. 1997. Tree coexistence on a slope: an adaptive significance of trunk inclination. Proceedings of the Royal Society of London, Series B 264: 133–140. [Google Scholar]
- Ishii R, Higashi M. 1998. The adaptive significance of trunk inclination: a further thought. Proceedings of the Royal Society of London, Series B 265: 175–177. [Google Scholar]
- Kozlowski TT. 1971. Growth and development of trees, Vol. II. New York, NY: Academic Press.
- Kuraji K, Yamanaka C, Nagashima T, Karukome T, Norisada M. 1998. Rainfall characteristics in the Kiyosumi Range of the Boso Peninsula (I). Bulletin of the Tokyo University Forests 99: 235–243 [in Japanese, with English summary]. [Google Scholar]
- Loehle C. 1986. Phototropism of whole trees: effects of habitat and growth form. American Midland Naturalist 116: 190–196. [Google Scholar]
- Olesen T. 2001. Architecture of a cool-temperate rain forest canopy. Ecology 82: 2719–2730. [Google Scholar]
- Sakai A, Ohsawa T, Ohsawa M. 1995. Adaptive significance of sprouting of Euptelea polyandra, a deciduous tree growing on steep slopes with shallow soil. Journal of Plant Research 108: 377–386. [Google Scholar]
- Shimada K. 1994. Topographical distribution of five pioneer tree species and significance of their tree forms in natural forests on Mt. Takao, central Japan. Japanese Journal of Ecology 44: 293–304 [in Japanese, with English summary]. [Google Scholar]
- Shimaji K. 1985. Mokuzai no keisei. In: Shimaji K, Saeki H, Harada H, Shiokura T, Ishida S, Shigematsu Y, et al., eds. Mokuzai no kouzou. Tokyo: Bun'eidou, 101–124 [in Japanese].
- Sierra-de-Grado R, Moulia B, Fournier M, Alía R, Díez-Barra R. 1997. Genetic control of stem form in Pinus pinaster Ait. seedlings exposed to lateral light. Trees—Structure and Function 11: 455–461. [Google Scholar]
- Sprugel DG, Hinckley TM, Schaap W. 1991. The theory and practice of branch autonomy. Annual Review of Ecology and Systematics 22: 309–334. [Google Scholar]
- Sumida A, Terazawa I, Togashi A, Komiyama A. 2002. Spatial arrangement of branches in relation to slope and neighbourhood competition. Annals of Botany 89: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira H. 1987. The study of mechanism of Sugi basal bending and its control methods. Bulletin of Toyama Prefectural Forest Experiment Station 12: 1–80 [in Japanese, with English summary]. [Google Scholar]
- Timell TE. 1986. Compression wood in gymnosperms, Vol. 3. Berlin: Springer-Verlag.
- Umeki K. 1995. Modelling the relationship between the asymmetry in crown display and local environment. Ecological Modelling 82: 11–20. [Google Scholar]
- Wilson BF, Archer RR. 1977. Reaction wood: induction and mechanical action. Annual Review of Plant Physiology 28: 23–43. [Google Scholar]
- Yamamoto H, Yoshida M, Okuyama T. 2002. Growth stress controls negative gravitropism in woody plant stems. Planta 216: 280–292. [DOI] [PubMed] [Google Scholar]