Abstract
• Background and Aims Broad surveys have detected inverse relationships between seed and adult longevity and between seed size and adult longevity. However, low and unpredictable precipitation is also associated with seed bank (SB) expression in semi-arid and arid areas. The relationship between adult longevity, SB formation, seed mass and aridity is examined in annual and perennial herbs of Chaetanthera (Asteraceae) from the Chilean Mediterranean-type climate and winter-rainfall desert areas over a precipitation range of one order of magnitude.
• Methods Seeds of 18 species and subtaxa (32 populations) were buried in field locations, and exhumed after two successive germination periods. Seeds not germinating in the field were tested in a growth chamber, and remnant intact seed tested for viability. Seed banks were classed as transient or persistent. The effect of life form, species, population and burial time on persistent SB size was assessed with factorial ANOVA. Persistent seed bank size was compared with the Martonne aridity index (shown to be a surrogate for inter-annual variation in precipitation) and seed size using linear regression. ANCOVA assessed the effect of life-form on SB size with aridity as covariate.
• Key Results Three species had a transient SB and 15 a persistent SB. ANOVA revealed a significant effect of life-form on SB size with annuals having larger SB size and greater capacity to form a persistent SB than perennials. Significant inter-population variation in SB size was found in 64 % of cases. Seed mass was negatively correlated with persistent SB size. Persistent seed bank size was significantly correlated with the Martonne aridity index in the perennial and annual species, with species from more arid areas having larger persistent SBs. However, when aridity was considered as a covariate, ANCOVA revealed no significant differences between the annual and perennial herbs.
• Conclusions Persistent seed bank size in Chaetanthera appears to reflect environmental selection rather than any trade-off with adult longevity.
Keywords: Experimental seed burials, seed bank, adult longevity, aridity, phylogenetic effects, Chaetanthera, Asteraceae
INTRODUCTION
Following dispersal, seeds may germinate immediately or remain viable in the soil forming a seed bank (SB) (Fenner, 1985). Seed banks have been classified as transient or persistent (Thompson and Grime, 1979) in accordance with the time that seeds remain viable in the soil. Transient seed banks (TSB) are those in which all seeds either germinate or lose viabilility within the same year of production. In persistent seed banks (PSB) none, or a variable, fraction of seeds germinate during the first year, with the remaining seeds retaining viability for additional years (Fenner and Thompson, 2005). The size of the PSB varies widely from one species to another. Persistent seed banks are ecologically important (Fenner, 1985) because they permit species to explore environmental variability in time (Venable and Lawlor, 1980; Venable and Brown, 1988). Persistent seed banks also allow maintenance of biodiversity in communities (Bakker et al., 1996; Ozinga et al., 2005) and genetic diversity in populations (Levin, 1990). Seed banks can protect populations from local extinction when above-ground vegetation is removed, and hence are important for restoration and conservation purposes (Bakker et al., 1996; Kalisz et al., 1997). In a recent study, Whittle (2006) claims to have found a positive correlation between seed persistence and molecular evolutionary rates.
Ecological theory predicts (and empirical data support) that the formation of PSBs may be expected in temporally variable environments such as deserts and other arid ecosystems where precipitation tends to be low and unpredictable (e.g. Cohen, 1966, 1967; Venable and Brown, 1988). When conditions for seed germination are limiting and vary between years, a species that forms a PSB can maximize its fitness by eliminating the risks of failed germination in those years in which environmental conditions restrict successful recruitment (‘bet-hedging strategy’; Philippi, 1993). In Mediterranean-climate and desert environments, the size of the PSB could be expected to be positively correlated with inter-annual variation in precipitation which is known to increase with decreasing annual precipitation or increasing aridity (Fuentes et al., 1988; Cowling et al., 2005).
In addition to the above ecological correlates, the propensity to form a PSB has been shown to be negatively correlated with life-history traits such as dispersal in space and adult longevity (Venable and Brown, 1988; Rees, 1994). Rees (1993, 1994) compiled data for a large number of species drawn from many plant genera and families and showed that an index of seed longevity (the number of seedlings that emerged in the first year of a burial experiment in relation to all seedlings that emerged in 5 years, which is highest when dormancy is limited or absent) tends to be inversely correlated with adult longevity expressed as a life-form category. Rees (1997) proposed that there is a trade-off between adult longevity and seed longevity whereby species with long-lived adults are buffered from temporal variation in the environment, such that there will be less selection for long-lived seeds. However, there are many exceptions to the relationship between adult and seed longevity (e.g. Guariguata and Azócar, 1988; Arroyo et al., 2004; Hill and Vander Kloet, 2005), as well as examples of inter-population variation within individual species (e.g. Cavieres and Arroyo, 2001). These exceptions could simply reflect greater or lesser tendencies (i.e. phylogenetic effects) to form a SB in particular plant taxa. However, they also raise the question as to the relative importance of intrinsic life-history selection and extrinsic factors in SB evolution.
Broad surveys have revealed a strong correlation between the propensity to form a PSB and seed size (e.g. Thompson et al., 1993; Funes et al., 1999). Rees (1997) showed that seed mass and seed longevity are negatively correlated and suggested that species with large seeds tend to have reduced dormancy because their seedlings can draw on larger food reserves and hence establish more readily. It is also known that longer-lived species have statistically larger seeds (which in turn are less likely to be found in the SB) (Salisbury, 1942; Baker, 1972; Thompson and Rabinowitz, 1989; Leishman and Westoby, 1994). However, there are exceptions to the relationship between adult longevity and seed size (Leishman and Westoby, 1998; Moles et al., 2000). Apart from life-history correlates, whereby selection for SB formation would be favoured when seeds are small, other factors may reinforce the seed size–seed longevity correlate. Consumption of large nutritious seeds by small mammals locally can reduce the size of the SB in large-seeded species (Fenner and Thompson, 2005). On the other hand, small seeds, which have a higher surface/volume ratio, are more likely to be incorporated into a PSB because of their capacity to move down vertically in the soil at a faster rate than larger seeds.
In the present paper, the relationship between adult longevity, SB formation, seed mass and degree of aridity is studied in 18 central Chilean species and subtaxa of the endemic South American genus Chaetanthera (Asteraceae: Mutisieae). Species of the same genus were deliberately chosen for the research so as to restrict deeply ingrained phylogenetic effects. The genus Chaetanthera was selected because PSB species have been reported in soil cores (Arroyo et al., 1999) and because it contains both annual and perennial herbs, representing two different and non-overlapping longevity classes. Species of Chaetanthera in central Chile are distributed in the winter rainfall deserts in north-central Chile, southward into the arid and humid Mediterranean-type climate areas. They may be found in relatively dry and warm coastal areas, in mid-elevation habitats, and in colder high alpine locations. The entire area of central Chile, including the high Andean Cordillera, is characterized by high variability in annual precipitation (Fuentes et al., 1988) as a result of the influence of the ENSO (El Niño Southern Oscillation) (Rutllant and Aceituno, 1991; Holmgren et al., 2001). During El Niño, precipitation can greatly exceed the average values, while during La Niña, the reverse situation prevails. For example, over the past 100 years, in Santiago (33°S) annual precipitation has fluctuated from close to 800 mm to <100 mm (Rutllant, 2004) with a mean of 328 mm. As has recently been shown in other mid-latitude Mediterranean-climate areas (Cowling et al., 2005), in central Chile, the coefficient of variation (CV) of annual precipitation increases as annual precipitation declines (Fuentes et al., 1988). In the southern, wetter areas occupied by species of Chaetanthera studied in this paper, where annual precipitation is >900 mm, the CV of variation for annual precipitation is in the order of 20 %. In the drier extreme in the winter rainfall deserts, where annual precipitation is <100 mm, the CV can be anywhere between 60 and 80 %. All these features make Chaetanthera appropriate for studying intrinsic life-history factors (such as the relationship between SB expression and adult longevity) and extrinsic factors (such as rainfall unpredictability) as they affect PSB size. Specifically, the following questions were addressed: (a) Do annual species of Chaetanthera have a greater tendency to form PSBs than perennial species? (b) Is PSB size in species of Chaetanthera related to degree of aridity (used as a surrogate for inter-annual variation in precipitation)? (c) Are seed size and size of the SB related in the genus Chaetanthera. The Martonne aridity index (di Castri and Hayek, 1976) was used to quantify aridity. Prior to using this index, it was ascertained that degree of aridity is strongly correlated with the CV of annual rainfall in the study area.
MATERIALS AND METHODS
Chaetanthera
Chaetanthera (Asteraceae: Mutisieae) is a moderately large, endemic South American genus (Cabrera, 1937) with over 40 currently valid species. Seven subgenera, with varying numbers of species have been recognized by Cabrera (1937). Chaetanthera is heavily concentrated at low and mid-elevations in the Mediterranean-type climate area and winter rainfall deserts of Chile where it occurs in open areas in woodlands, coastal and desert scrub, and in the alpine belt along the high Andes from Peru to Argentina where some species reach the upper limit of the vegetation at around 5000 m a.s.l.. Half of Chaetanthera species are annual herbs (hereafter annuals) of varying sizes. The remaining species range from small to moderate-sized perennial herbs (hereafter perennials), there being one small subshrub. Both annual and perennial species are found in lowland and high alpine habitats. The 18 annual and perennial herb species and subtaxa (hereafter species) considered in the present study are found between latitudes 29 and 37°S and 10 and 3400 m a.s.l. in central Chile, and cover six subgenera. In addition to assuring broad taxonomic representation, the final choice of species was determined by accessibility of the populations, availability of sufficient ripe seed and representation of a wide range of precipitation and temperature conditions. The particular species studied occur over a precipitation range of one order of magnitude (<100 mm to >1000 mm).
Experimental burials
To distinguish between a TSB and PSB, knowledge of the presence of ungerminated viable seeds in the soil after 1 year of burial is required (Brown and Veneable, 1986). The size of the remnant fraction of viable seeds can also be used as a rough measure of expected seed longevity. The larger the remnant viable fraction, the more likely it will be that some seeds will remain after several years (cf. Rees, 1993). The amount of seed that had not germinated and remained viable in the soil was determined after the first, as well as after the second, year of germination in the field using the experimental seed burial protocol of Arroyo et al. (2004). Bulk collections of ripe achenes were made in the field during the spring of 2002 (lowland species) and austral summer 2002–2003 (alpine species) and stored in brown paper bags in a dry place in the laboratory; for locality data and altitudes, see Appendix 1. Two populations per species were considered for 14 species. Over the period April–May 2003, several sets of six replicates containing 50 plump achenes per replicate (hereafter seeds) were prepared for burial at the original population locations. Concomitantly, samples of 50 seeds per species and population were weighed on a precision balance. The seeds for each replicate were introduced into fine grain, nylon mesh envelopes which were placed in small wire cages. The cages were filled with sieved local soil and buried so as that the seed envelopes were at a depth of 5 cm. The burial sites were the six vertices of a hexagon (5 m sides). The wire cages prevented small mammals uprooting the envelopes as well as consuming the seeds. Use of the nylon mesh envelopes assured natural aeration and humidity levels during the experiment, and hence representative field germination conditions.
Seed burial was performed in autumn so as permit natural stratification of seeds in the field over the coming winter. One set of replicates was exhumed within <1 year of seed production (Yr-1), while a second was retrieved around 12 months after the first date in 2004 (Yr-2). Other sets of replicates still remain in the ground, and will be used to model seed decay rates sometime in the future. Exhumation dates were planned so as to ensure that the first and second germination seasons following burial were completely over in each species. Because of altitudinal and latitudinal differences in the timing of germination in the field, the exact exhumation dates were spread out from spring (lowland species) to early to mid-summer (alpine species). Thus, the time that the seeds remained buried in the first year varied from 4 to 9 months, whereas the total burial time for seed exhumed in the second year was 16–21 months. Each retrieved nylon mesh envelope was examined for germinated seeds. Remaining intact ungerminated seeds were sown immediately in the light in a growth chamber and monitored for germination for 30 d (10/20 °C night/day). All seeds that failed to germinate in the growth chamber were then tested for viability with the standard tetrazolium chloride test. Precipitation in central Chile for the latitudes of the populations studied showed a moderate deficit in 2003 and a weak deficit in 2004 with respect to average precipitation (Climatic Bulletin 2003 and 2004, Department of Geophysics, University of Chile; online).
Aridity index and inter-annual variation in rainfall
For assessment of the relationship between rainfall unpredictability and PSB size, the Martonne aridity index (di Castri and Hajek, 1976) was used: A = P/(T + 10) where A = aridity, P = mean annual precipitation and T = mean annual temperature, which will be shown to be highly correlated with inter-annual variation in precipitation. Values of this index approaching zero indicate very high levels of aridity, while high values of the index indicate low levels of aridity. This simple index has the advantage of requiring nothing more than the mean annual temperature and the mean annual precipitation, and thus can be used when climatic data are poor or incomplete, as is the case for many of the specific locations of the populations of Chaetanthera studied. Prior to using the Martonne index, the CV for annual precipitation was compared with the Martonne index for 27 weather stations in central Chile using the following electronic database (http://www.cazalac.org/rp_paises.php?pais_seleccionado=chile). All stations in the database for the latitudinal range of the populations of Chaetanthera studied (29–36°S) with annual precipitation and temperature data individualized for at least 20–30 contiguous years were selected. For 20 stations, data were available for 30 contiguous years, for six, over 30 years, and for one, 20 years. For the populations studied, annual precipitation and temperature (required to calculate the Martonne index) were obtained from published weather station data for the nearest available stations (di Castri and Hajek, 1976; CEAZA database: www.ceaza.cl) or from published estimates in the absence of stations (Santibañez and Uribe, 1990; Teillier et al., 2005). For localities intermediate between two weather stations, average values were calculated. For alpine locations in the Farellones-La Parva-Valle Nevado area and Los Queñes, the amount of precipitation was estimated from a curve joining available weather stations and published precipitation amounts (di Castri and Hajek, 1976; Santibañez and Uribe, 1990; Arroyo et al., 1999). Temperatures in these last cases were derived from Cavieres et al. (2000) or from a standard 6 °C lapse rate. For two cordilleran localities close to weather stations, the precipitation data were used, but the temperature was adjusted on the basis of a 6 °C lapse rate.
Data analysis
Two response variables were established: (1) the SB, or viable seeds that had not germinated in the field, was obtained by summing the number of remnant seeds that germinated in the growth chamber to the number of viable remnant seeds as per the tetrazolium test; (2) dormant seeds, consisting of remnant ungerminated seeds following laboratory tests, that tested positive in the tetrazolium test. Variables were expressed as a percentage of initial buried seed number (n = 50 per replicate). Results from the first and second exhumations allowed species with a TSB and PSB to be detected, as well as variation in SB size after each exhumation date. Differences in the two response variables were first tested with a nested analysis of variance (SAS Institute, 1994) with life form, species, population and year of exhumation as factors. Species was nested in life form while population was nested in species.
The relationship between the two response variables and seed mass, and the relationship between degree of aridity and SB (= PSB) size were assessed through linear regression. Given that there were significant differences between most of the populations of the same species (see Results), the 35 individual population means were used for the aridity analysis. Seed bank size was arcsine transformed, and seed weight and the aridity index log-transformed. Finally, in order to determine whether differences in the size of the SB between annual and perennials was independent of level of aridity, an analysis of covariance (ANCOVA) was performed with the aridity index as covariate. The slopes of the two regression lines were previously tested for equality using the Test of Parallelism (Statistica Version 6·0).
RESULTS
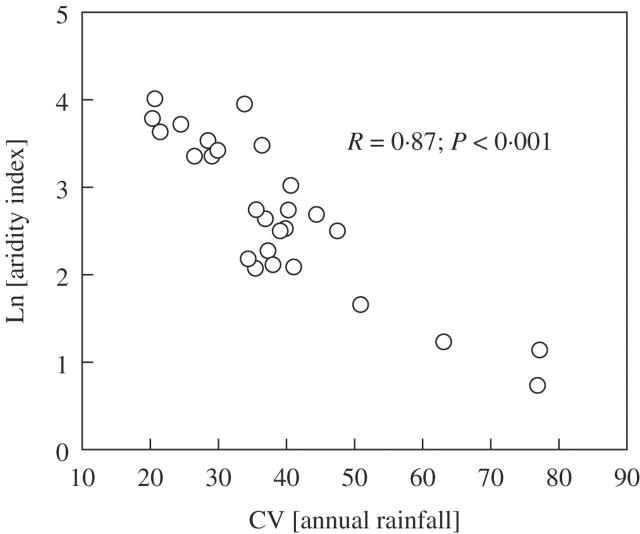
Figure 1 shows the relationship between the Martonne aridity index and the CV for annual precipitation for 27 weather stations in central Chile. Degree of aridity and the CV are closely correlated, indicating that the Martonne index is an adequate surrogate for inter-annual variation in precipitation or rainfall predictability. The highly significant linear relationship between the logarithm of the aridity index and the CV in Fig. 1 also shows that inter-annual variation (large values of the CV) increases logarithmically with increasing aridity (small values of the index) for this latitudinal range in Chile.
Fig. 1.
Relationship between ln Martonne aridity index and the coefficient of variation (CV) for annual rainfall in 27 weather stations drawn throughout the study area.
Notable variation in the percentage of field germinated seeds was observed among the 18 species and 32 populations studied (Table 1). In Yr-1, 100 %, or close to, of the seed had germinated in some species (e.g. C. pentacaenoides), whereas at the other extreme <2 % had germinated (e.g. one population of C. tenella var. tenella). In Yr-2, all species and populations showed an increase in the percentage of seeds germinated. Nevertheless, in a few species (C. tenella var. tenella and C. tenella var. taltalensis) <10 % of the seed had germinated by this time. In most species only small proportions of the retrieved seed germinated in the growth chamber (Table 1), indicating that precipitation conditions were sufficient to allow germination of most non-dormant seed. Following the first exhumation date, practically all the remaining seed (after subtracting germination obtained in the growth chamber) were viable, although this percentage decreased somewhat in Yr-2 (Table 1) due to a combination of physical seed decay and loss of viability in intact seeds.
Table 1.
Percentage of field-germinated seeds, growth chamber germination, dormant seeds and non-viable seeds in Yr-1 and Yr-2 in populations of species of Chaetanthera
| Year-1 |
Year.2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | Seed mass (g per 50 seeds) | Field-germinated seeds | Growth chamber-germinated seeds | Dormant seeds | Seed bank | Non-viable seeds | Field-germinated seeds | Growth chamber-germinated seeds | Dormant seeds | Seed bank | Non-viable seeds | |
| Annual species | ||||||||||||
| C. ciliata | R.N. Robleria de Cobre de Loncha | 0·02533 ± 0·00051 | 26·3 ± 7·3 | 6·3 ± 3·6 | 67·3 ± 5·5 | 73·7 ± 7·3 | 0·0 ± 0·0 | 89·0 ± 5·5 | 0·3 ± 0·7 | 7·0 ± 3·7 | 7·3 ± 3·2 | 3·7 ± 4·2 |
| Cuesta Lo Prado | 0·01752 ± 0·00059 | 30·0 ± 7·3 | 2·3 ± 1·9 | 67·7 ± 7·0 | 70·0 ± 7·3 | 0·0 ± 0·0 | 54·0 ± 10·5 | 0·3 ± 0·7 | 36·0 ± 7·1 | 36·3 ± 7·0 | 9·7 ± 4·4 | |
| C. euphrasioides | Valle Nevado | 0·08788 ± 0·00157 | 30·3 ± 13·2 | 0·0 ± 0·0 | 69·7 ± 13·2 | 69·7 ± 13·2 | 0·0 ± 0·0 | 80·3 ± 11·1 | 0·0 ± 0·0 | 6·3 ± 3·5 | 6·3 ± 3·5 | 13·3 ± 8·1 |
| Valle Nevado | 0·09681 ± 0·00327 | 63·7 ± 13·0 | 0·0 ± 0·0 | 36·3 ± 13·0 | 36·3 ± 13·0 | 0·0 ± 0·0 | 64·0 ± 13·0 | 0·0 ± 0·0 | 15·0 ± 6·4 | 15·0 ± 6·4 | 21·0 ± 12·8 | |
| C. flabellata | S.N. Yerba Loca | 0·02463 ± 0·00069 | 17·3 ± 6·7 | 0·3 ± 0·7 | 82·3 ± 7·2 | 82·7 ± 6·7 | 0·0 ± 0·0 | 24·0 ± 12·4 | 1·7 ± 1·9 | 64·7 ± 18·6 | 66·3 ± 17·1 | 9·7 ± 6·6 |
| Farellones | 0·02432 ± 0·00025 | 15·0 ± 7·1 | 0·0 ± 0·0 | 85·0 ± 7·1 | 85·0 ± 7·1 | 0·0 ± 0·0 | 37·3 ± 24·5 | 2·0 ± 2·1 | 55·7 ± 23·4 | 57·7 ± 24·9 | 5·0 ± 2·7 | |
| C. glabrata | S.N. Yerba Loca | 0·08009 ± 0·03196 | 32·0 ± 11·4 | 0·3 ± 0·7 | 67·7 ± 11·5 | 68·0 ± 11·4 | 0·0 ± 0·0 | 58·0 ± 18·3 | 0·3 ± 0·7 | 32·7 ± 15·4 | 33·0 ± 15·1 | 9·0 ± 4·5 |
| Puente Juan Soldado | 0·04176 ± 0·00090 | 13·3 ± 6·0 | 7·7 ± 7·5 | 78·7 ± 11·4 | 86·3 ± 5·6 | 0·3 ± 0·7 | 36·7 ± 15·5 | 11·3 ± 6·9 | 46·3 ± 14·9 | 57·7 ± 14·7 | 5·7 ± 6·9 | |
| C. incana | Los Vilos | 0·01752 ± 0·00059 | 34·4 ± 17·0 | 4·8 ± 5·0 | 60·8 ± 14·9 | 65·6 ± 17·0 | 0·0 ± 0·0 | 49·2 ± 14·2 | 0·4 ± 0·8 | 37·6 ± 12·2 | 38·0 ± 11·6 | 12·8 ± 8·9 |
| Los Molles | 0·02980 ± 0·00060 | 14·7 ± 5·4 | 0·7 ± 1·3 | 83·7 ± 5·9 | 84·3 ± 5·1 | 1·0 ± 1·4 | 37·7 ± 18·2 | 1·3 ± 1·3 | 49·7 ± 16·0 | 51·0 ± 16·4 | 11·3 ± 4·5 | |
| C. linearis var. albiflora | Los Vilos | 0·02980 ± 0·00060 | 18·7 ± 11·9 | 8·3 ± 2·8 | 73·0 ± 11·9 | 81·3 ± 11·9 | 0·0 ± 0·0 | 35·3 ± 12·8 | 16·7 ± 9·4 | 42·7 ± 7·6 | 59·3 ± 14·8 | 5·3 ± 2·9 |
| Punta Teatinos | 0·01075 ± 0·00032 | 23·2 ± 13·2 | 5·2 ± 4·1 | 71·6 ± 13·9 | 76·8 ± 13·2 | 0·0 ± 0·0 | 71·6 ± 16·8 | 7·2 ± 5·9 | 18·0 ± 14·5 | 25·2 ± 17·5 | 3·2 ± 3·0 | |
| C. linearis var. linearis | R.N. Río Clarillo | 0·03546 ± 0·00097 | 51·3 ± 11·2 | 9·0 ± 8·0 | 39·7 ± 8·4 | 48·7 ± 11·2 | 0·0 ± 0·0 | 84·7 ± 6·7 | 3·7 ± 2·6 | 5·0 ± 3·5 | 8·7 ± 2·9 | 6·7 ± 5·8 |
| S.N. Yerba Loca | 0·06115 ± 0·00119 | 65·7 ± 4·7 | 12·0 ± 5·8 | 22·3 ± 4·2 | 34·3 ± 4·7 | 0·0 ± 0·0 | 85·3 ± 8·5 | 2·0 ± 1·8 | 10·0 ± 5·9 | 12·0 ± 7·1 | 2·7 ± 2·7 | |
| C. microphylla | Cuesta La Dormida | 0·02190 ± 0·00025 | 25·0 ± 16·0 | 15·3 ± 3·2 | 59·7 ± 18·1 | 75·0 ± 16·0 | 0·0 ± 0·0 | 57·7 ± 13·5 | 16·7 ± 3·2 | 24·0 ± 12·1 | 40·7 ± 12·4 | 1·7 ± 2·6 |
| Los Queñes | 0·02281 ± 0·00106 | 81·0 ± 5·7 | 6·7 ± 3·7 | 12·3 ± 4·6 | 19·0 ± 5·7 | 0·0 ± 0·0 | 90·3 ± 7·2 | 6·0 ± 5·9 | 3·0 ± 3·2 | 9·0 ± 7·3 | 0·7 ± 1·3 | |
| C. moenchioides | Til-Til | 0·03018 ± 0·00122 | 11·0 ± 10·4 | 0·0 ± 0·0 | 88·7 ± 10·2 | 88·7 ± 10·2 | 0·3 ± 0·7 | 71·0 ± 15·1 | 0·3 ± 0·7 | 22·3 ± 17·0 | 22·7 ± 16·6 | 6·3 ± 3·2 |
| Vicuña | 0·04640 ± 0·00026 | 4·7 ± 2·9 | 1·3 ± 1·3 | 93·3 ± 2·7 | 94·7 ± 2·2 | 0·7 ± 1·3 | 28·7 ± 20·5 | 1·3 ± 0·8 | 68·7 ± 19·1 | 70·0 ± 19·4 | 1·3 ± 2·7 | |
| C. planiseta | Mirador Farellones | 0·04776 ± 0·00168 | 92·0 ± 2·3 | 0·0 ± 0·0 | 8·0 ± 2·3 | 8·0 ± 2·3 | 0·0 ± 0·0 | 98·7 ± 2·7 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 1·3 ± 2·7 |
| Valle Nevado | 0·07406 ± 0·00304 | 76·7 ± 9·2 | 0·0 ± 0·0 | 23·3 ± 9·2 | 23·3 ± 9·2 | 0·0 ± 0·0 | 89·7 ± 3·8 | 0·0 ± 0·0 | 3·3 ± 2·2 | 3·3 ± 2·2 | 7·0 ± 4·5 | |
| C. pusilla | Valle Nevado | 0·05007 ± 0·00147 | 46·7 ± 15·5 | 0·0 ± 0·0 | 53·3 ± 15·5 | 53·3 ± 15·5 | 0·0 ± 0·0 | 67·3 ± 9·8 | 0·0 ± 0·0 | 18·0 ± 7·7 | 18·0 ± 7·7 | 14·7 ± 10·4 |
| C. tenella var. taltalensis | Punta Teatinos | 0·02313 ± 0·00093 | 18·0 ± 9·6 | 13·3 ± 6·5 | 63·7 ± 10·9 | 77·0 ± 8·4 | 5·0 ± 5·2 | 28·0 ± 7·6 | 0·7 ± 1·3 | 41·3 ± 22·2 | 42·0 ± 22·7 | 30·0 ± 19·5 |
| Cuesta Buenos Aires | 0·03295 ± 0·00050 | 2·3 ± 1·6 | 3·0 ± 2·0 | 94·7 ± 3·2 | 97·7 ± 1·6 | 0·0 ± 0·0 | 5·0 ± 3·1 | 7·0 ± 7·3 | 82·3 ± 11·5 | 89·3 ± 6·3 | 5·7 ± 4·4 | |
| C. tenella var. tenella | Lagunillas | 0·02164 ± 0·00058 | 3·3 ± 2·2 | 2·7 ± 1·7 | 94·0 ± 3·4 | 96·7 ± 2·2 | 0·0 ± 0·0 | 7·0 ± 3·8 | 0·0 ± 0·0 | 83·0 ± 6·7 | 83·0 ± 6·7 | 10·0 ± 4·6 |
| Cuesta La Dormida | 0·00928 ± 0·00032 | 1·3 ± 1·3 | 0·7 ± 0·8 | 98·0 ± 1·5 | 98·7 ± 1·3 | 0·0 ± 0·0 | 9·3 ± 4·3 | 1·3 ± 1·3 | 87·3 ± 5·6 | 88·7 ± 5·0 | 2·0 ± 2·5 | |
| Perennial species | ||||||||||||
| C. chilensis | R.N. Río Clarillo | 0·11424 ± 0·00149 | 65·7 ± 8·1 | 1·0 ± 2·0 | 33·3 ± 6·4 | 34·3 ± 8·1 | 0·0 ± 0·0 | 75·7 ± 6·6 | 0·3 ± 0·7 | 17·3 ± 6·1 | 17·7 ± 5·8 | 6·7 ± 2·5 |
| Los Queñes | 0·07559 ± 0·00234 | 57·7 ± 12·1 | 4·7 ± 2·7 | 37·7 ± 11·6 | 42·3 ± 12·1 | 0·0 ± 0·0 | 81·0 ± 12·8 | 0·0 ± 0·0 | 6·7 ± 6·7 | 6·7 ± 6·7 | 12·3 ± 6·4 | |
| C. lycopodioides | Valle Nevado | 0·07061 ± 0·00126 | 97·0 ± 4·5 | 0·0 ± 0·0 | 3·0 ± 4·5 | 3·0 ± 4·5 | 0·0 ± 0·0 | 99·7 ± 0·7 | 0·0 ± 0·0 | 0·3 ± 0·7 | 0·3 ± 0·7 | 0·0 ± 0·0 |
| La Parva | 0·06971 ± 0·00134 | 100·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 100·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | |
| C. pentacaenoides | La Parva | 0·06593 ± 0·00039 | 96·3 ± 2·6 | 0·0 ± 0·0 | 3·7 ± 2·6 | 3·7 ± 2·6 | 0·0 ± 0·0 | 100·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 |
| C. renifolia | La Parva | 0·31154 ± 0·00544 | 99·0 ± 1·4 | 0·0 ± 0·0 | 1·0 ± 1·4 | 1·0 ± 1·4 | 0·0 ± 0·0 | 100·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 |
| C. serrata | Chillán | 0·06772 ± 0·00184 | 28·3 ± 3·2 | 2·0 ± 2·1 | 69·7 ± 3·2 | 71·7 ± 3·2 | 0·0 ± 0·0 | 66·7 ± 13·1 | 0·0 ± 0·0 | 20·0 ± 10·2 | 20·0 ± 10·2 | 13·3 ± 5·4 |
The seed bank is the sum of the greenhouse-germinated and viable seeds.
Means are shown with 2 s.e. for six burial replicates per population and year.
See Appendix 1 for geographical co-ordinates and altitudes of populations.
A wide range of SB expressions is evident in both life-forms (Table 1). In some of the perennial species all, or practically all, seed germinated in the first year following over wintering in the soil (C. pentacaenoides, C. lycopodioides, C. renifolia). In other perennials (C. chilensis and C. serrata) small SBs remained after the Yr-2 germination period. Considering the annuals, the range of SB expression was considerably greater than in the perennial species. In C. tenella var. taltalensis and C. tenella var. tenella between 77 and 99 % of the seed remained in the SB after Yr-1 and 42–89 % after Yr-2. In both species, few seeds germinated in the growth chamber and very high percentages of the ungerminated seeds remained dormant in both years (Table 1). In C. ciliata (one population), C. flabellata, C. glabrata, C. incana, C. linearis var. albiflora and C. moenchioides moderately large SBs and reserves of dormant seeds remained by the second year, although there was variation among populations of a species in some cases (Table 1). In C. linearis var. linearis, C. ciliata (one population) and C. euphrasioides, while considerable amounts of viable seeds remained after Yr-1, the proportion dropped to below 15 % after the Yr-2 germination period. In the annual C. planiseta only 0–3 % of the seeds remained in the SB after the Yr-2 with between 77 and 92 % germinating in Yr-1. Significant differences in SB size (t-tests) were found among different populations of the same species in nine out of 14 species (64·3 %) for which two populations were studied. Of particular note are annual C. microphylla and C. ciliata where 4- to 5-fold differences in SB size in Yr-2 were detected among populations. Considering the formal definition of a PSB as one where a fraction of viable ungerminated seeds remains in the soil after the first year, the great majority of the species of Chaetanthera (15 spp., 13 annual and 2 perennial) would be classed as having a PSB, although annual C. planiseta clearly has a very small PSB. The remaining three species (C. lycopodioides, C. pentacaenoides and C. renifolia – all perennial) for all intents and purposes have a TSB. The frequency of PSB formation is statistically larger in the annual species (Fisher's Exact Test, P = 0·012).
Analysis of variance (ANOVA) showed that life form, species, population and year of germination after seed burial had significant effects on the size of the SB and on the number of remnant dormant seeds (Table 2). The latter were both larger for annuals than for perennials in Yr-1 and Yr-2 (Tukey test, P < 0·05) (Fig. 1). In addition, the two response variables were significantly different for the two exhumation times (Tukey test, P < 0·05) (Fig. 2).
Table 2.
Analysis of variance evaluating the effect of life form, species, population and time of burial on seed bank size and size of the dormant seed fraction in species of Chaetanthera
| Effect | d.f. | MS | F | P |
|---|---|---|---|---|
| (A) Seed bank | ||||
| Life form | 1 | 15·678 | 9·40 | 0·007 |
| Species | 16 | 1·917 | 6·23 | 0·001 |
| Population | 14 | 0·312 | 2·90 | 0·027 |
| Time | 1 | 6·448 | 62·02 | 0·001 |
| Life form × burial time | 1 | 0·362 | 3·48 | 0·075 |
| Species × burial time | 16 | 0·102 | 0·91 | 0·541 |
| Population × burial time | 14 | 0·107 | 4·41 | 0·001 |
| Error | 316 | 0·024 | ||
| (B) Dormant seeds | ||||
| Life form | 1 | 18·455 | 11·06 | 0·004 |
| Species | 16 | 1·906 | 5·26 | 0·003 |
| Population | 14 | 0·371 | 3·37 | 0·015 |
| Time | 1 | 6·869 | 66·97 | 0·001 |
| Life form × burial time | 1 | 0·358 | 3·49 | 0·075 |
| Species × burial time | 16 | 0·100 | 0·91 | 0·574 |
| Population × burial time | 14 | 0·110 | 4·42 | 0·001 |
| Error | 316 | 0·024 | ||
Fig. 2.
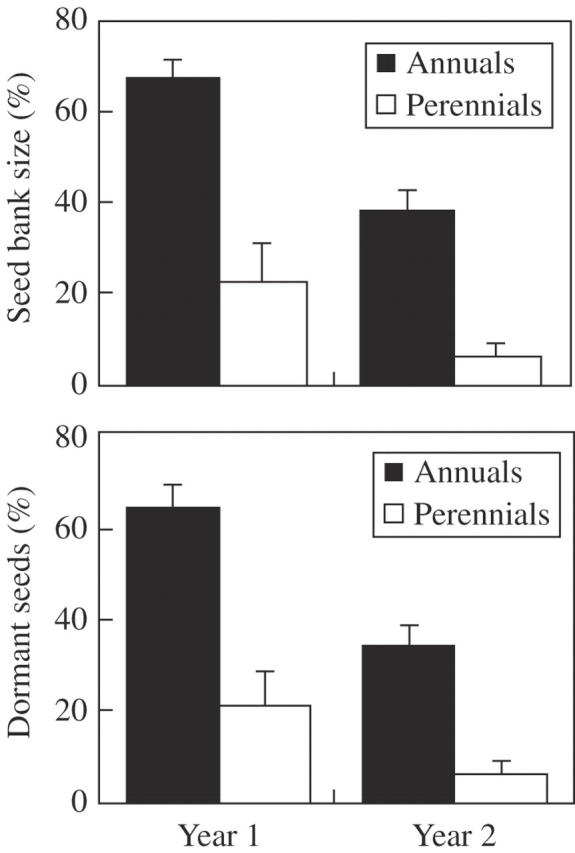
Remnant seed bank size and percentage of remnant dormant seeds after Yr-1 and Yr-2 in annual and perennial species of Chaetanthera (Asteraceae) in central Chile. Means ± 2 s.e. are shown. Differences between annuals and perennials are significant in all cases.
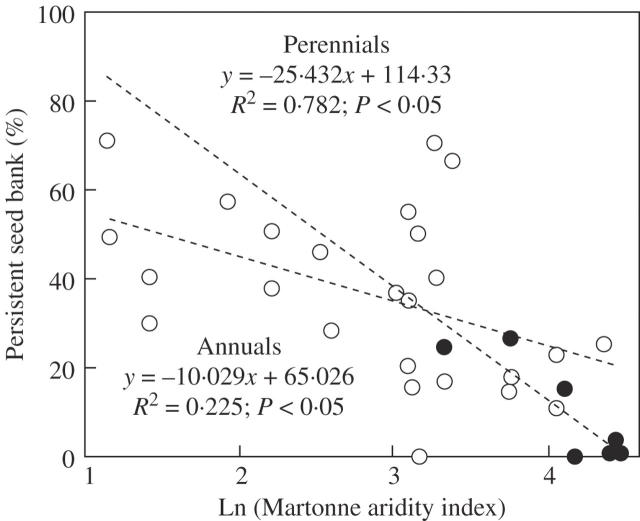
Figure 3 shows the results of the linear regressions between SB size (Yr-2 data) and the Martonne aridity index for the two life forms after Yr-2 germination. Significant regressions were obtained in both cases, with the size of the remnant SB tending to become larger with increasing aridity. However, the test of parallelism showed that the slopes of the lines were not significantly different [F(1,28) = 0·934, P = 0·342), justifying application of ANCOVA. When aridity was considered as a covariate, ANCOVA showed that life-form no longer had significant effect on PSB size (F(1,29) = 2·611, P = 0·12).
Fig. 3.
Relationship between PSB size and the Martonne aridity index in annual and perennial species of the genus Chaetanthera after Yr-2. Points correspond to the 35 populations studied. Open circles, annual herbs; closed circles, perennial herbs.
Seeds of most Chaetanthera species were small (Table 1). Seed mass of aliquots of 50 seeds ranged from 0·01075 ± 0·00032 g (mean ± 2 s.e.) in annual C. linearis var. albiflora (population 2) to 0·31154 ± 0·00544 g in perennial C. renifolia. Linear regression showed that seed mass (grams per 50 seeds) and SB size were significantly related (R2 = 0·39, P < 0·001 and R2 = 0·37, P < 0·001 for Yr-1 and Yr-2, respectively) (Fig. 4), with larger seeds being associated with smaller SBs. The corresponding determination coefficients for dormant seeds were also significant (SB: R2 = 0·35, P < 0·001 and R2 = 0·32, P < 0·001, for Yr-1 and Yr-2, respectively) (Fig. 4).
Fig. 4.
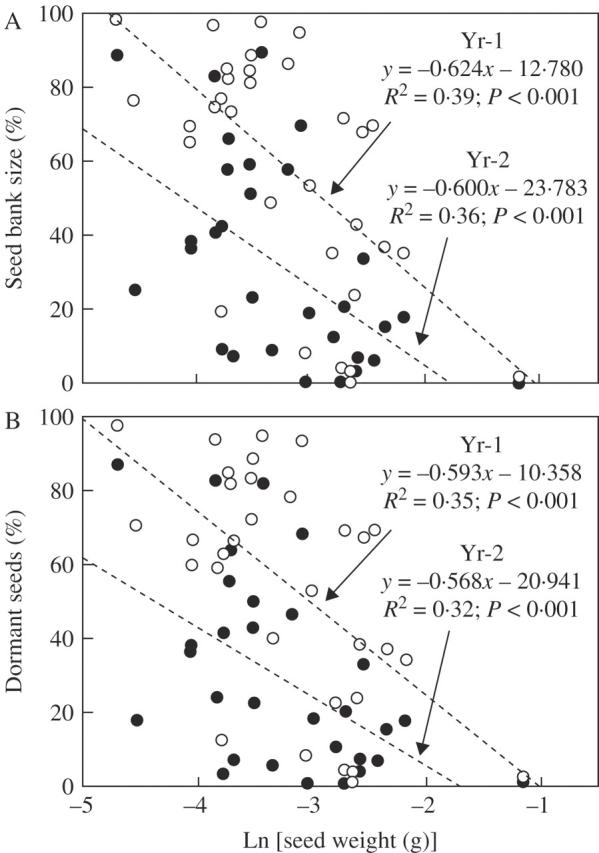
Relationship between seed mass and (A) remnant seed bank size and (B) remnant dormant seed fraction after Yr-1 and Yr-2 in Chaetanthera. Points correspond to the 35 populations studied. Open circles, Yr-1; closed circles, Yr-2. Annuals: n = 25 × 6 replicates; perennials: n = 7 × 6 replicates.
DISCUSSION
The present study of Chaetanthera species would appear to constitute the first experimental seed burial work in which SB expression has been compared using an identical experimental protocol for a large number of species drawn from a single genus, including two life forms representing non-overlapping longevity classes. This provided an ideal situation for evaluating the relative strength of life history correlates versus a relevant extrinsic factor (aridity) for a closely related set of species. Previously, the relationship between adult and seed longevity has been evaluated by comparing species derived from many different genera and families (e.g. Rees, 1993; Purvis and Rambaut, 1995). A similar approach was taken by Jurado and Flores (2005) in their recent study in which the relationship between seed dormancy type, adult longevity and certain environmental factors was investigated. Although these authors used phylogenetically independent contrasts (PICs) to eliminate the equivalent of pseudo-replication, results may be affected by deep-seated phylogenetic tendencies at the generic and familial levels impinging on individual traits. A growing body of evidence has demonstrated strong phylogenetic constraints in flowering phenology (Kochmer and Handel, 1986), seed number and mass (Mazer, 1989) and seed longevity (Thompson et al., 1998). By studying annual and perennial species of the same genus, confounding effects of making trait correlations between a taxonomically diverse set of species have been tightly controlled. Of course, no inter-specific study is totally devoid of phylogenetic effects. Molecular phylogenetic reconstruction completed after the present study was initiated shows that Chaetanthera forms two major clades (subgenera Oriastrum and Egania together, and subgenera Proselia, Euchaetanthera, Glandulosa, Tylloma and Carmelita together) (Hershkovitz et al., 2006). Some of the early-recognized subgenera are unnatural. Thus some low-level phylogenetic effects at the clade level cannot be excluded. Nevertheless, it is fair to say that studying species of the same genus is about as close as one can get to eliminating phylogenetic effects.
A remarkably wide range of SB expressions was found among species of Chaetanthera, with 83·3 % (15 of 18) of the species studied exhibiting a PSB. The size of the remnant PSB after Year-2 was not only variable between species, but also between populations of the same species. That most of the seeds in the remnant SB failed to germinate in the growth chamber where water was not limiting, but remained viable, suggests the presence of strong dormancy in Chaetanthera seeds. Given that species of the same genus were considered, it may be concluded that SB expression is a non-conservative, evolutionarily labile life-history trait subject to rapid local selection. This, of course, is not a new result, but it is one that tends to be overlooked when looking for general trends. For example, Cavieres et al. (2000) using reciprocal seed burials, demonstrated significant variation in seed longevity along a short, but steep, alpine gradient in central Chile. Such evolutionary lability suggests that species might be able to adjust more rapidly to global climatic change when it involves increased aridity, than generally assumed.
Annual species of Chaetanthera were seen to form PSBs more frequently than perennial species as well as larger PSBs, as has been reported in previous studies of taxonomically-diverse sets of species (e.g. Thompson et al., 1998). At the gross level of TSB versus PSB these findings, at face value, seem to support the idea of Rees (1993, 1994) that there is a trade off between adult and seed longevity, with perennial species having shorter-lived SBs. If the larger PSBs of annual species also turn out to be more long-lived, as expected, this trend is also seen at another level. In both longevity classes, SB size after Yr-2 was correlated with aridity, with species growing under more arid conditions having larger SBs. However, when aridity was considered as a covariate, the effect of life form on SB size disappeared. That is, in the genus Chaetanthera, the difference in SB expression between annual and perennial herbs, in at least as far as SB size is concerned, seems to be more a reflection of the fact that these two life-forms tend to occupy opposite ends of the aridity gradient, than of adult longevity as per Rees (1993).
Returning to the recently constructed molecular phylogeny of Chaetanthera (Hershkovitz et al., 2006), it may now be asked if any strong phylogenetic effects are evident. Two-thirds of the species of Chaetanthera in the largest clade (containing a total of 28 species) are annuals. Three-quarters of those in the second clade (containing a total of 16 species) are perennials. Of the five perennials studied, three belong to the first, and two to the second clade. Of the 13 annual taxa studied, 11 are from the first clade, and two from the second clade. Thus representation of the two clades, while not exactly in the same proportions with respect to life-form (due to inaccessibility of many high-elevation perennial species in the smaller clade), has been comprehensive. Persistent seed banks were found in annuals belonging to both clades, and TSB in perennial species representing both clades. However, the two perennial species with a PSB belong to the first clade, and the PSBs of the two annual species in the second clade tended to be smaller on average than for annuals belonging to the first clade. While some phylogenetic effects at the clade level cannot be ruled out, it turns out that the two perennial species with PSBs grow in more arid, lowland habitats than the perennial species studied from the smaller clade, while the two annual species with small PSBs from the second clade are alpine species growing under relatively low aridity conditions in comparison with the average annual in the first clade.
The positive relationship between degree of aridity (which proved to be closely related to inter-annual variation in precipitation) and PSB size in Chaetanthera supports the notion of the SB reflecting a ‘bet hedging’ strategy (Cohen, 1966, 1967; Venable and Brown, 1988). Nevertheless, variation in aridity explained a mere 23 % of the variance in SB size in the annuals. Insofar as extrinsic factors are concerned, the present study was limited to a single variable. However, many other external environmental factors are likely to influence final SB size and/or longevity. Hill and Vander Kloet (2005), in their study of perennial species of Vaccinium, found that annual temperature range in the centre of the species' distribution range accounted for 42 % of the variation in seed longevity, while reproductive variables such as testa hardness, time to seed set, germination time and others accounted for a further 55 %. Factors such as soil type, degree of compaction and rates of snow melt (in alpine areas) are likely to be relevant in Chaetanthera and would warrant study. Critical experiments are needed to determine the relative roles of such factors from an evolutionary point of view. Interesting differences in PSB size in pairs of annuals growing at the same location are also evident. At Cuesta Dormida, the PSB of robust annual C. microphylla was significantly smaller than that of the much smaller annual, C. tenella var. tenella. It might have been expected that larger annuals, which would have more difficulty establishing and attaining reproductive size in drier years in an arid climate, would have relatively larger SBs. Possibly this difference reflects selection for an optimal absolute number of seeds in the SB for population survival under the environmental conditions at these localities, with the smaller plants having to contribute proportionally more of their seeds to the PSB. Unfortunately there are no good data on total seed output for these species at this stage.
Remnant SB size after Yr-1 and Yr-2 was negatively correlated with seed size. These results are in agreement with several previous studies (e.g. Thompson et al., 1993; Rees, 1997; Funes et al., 1999) and with Jurado and Flores (2005) who recently showed that seeds with some form of dormancy (e.g. physical, physiological) tend to be lighter in weight. However, Hill and Vander Kloet (2005) failed to find a relationship between seed weight and seed longevity in 28 species of the genus Vaccinium, except for the short-term persistence of the three species with heaviest seeds. Although significant coefficients of determination for the regressions between seed size and SB size were obtained in Chaetanthera, they accounted for no more than 39 % of the variance. Differences in levels of competition, seed predation and conditions of seed storage experienced by Chaetanthera over its latitudinal and altitudinal range are likely to be additional selective factors affecting final seed size.
Although not the principal focus of the present research, it is worthwhile asking whether the burial experiments provide accurate estimates of SB size under non-experimental conditions. For example, protecting the experiments against small mammal damage might have prevented a higher proportion of the seeds of the species with larger seeds exiting the SB due to a known tendency for mammals to select larger seeds over small seeds (Fenner and Thompson, 2005). This effect is unlikely in Chaetanthera where the achenes are very small (means of 0·25–6·23 mg) and hence unattractive to mammals. Moreover, mammals have never been observed consuming Chaetanthera seeds during 5 years of field work on the genus. Because small seeds tend to move down vertically in the soil at a faster rate than large seeds (Fenner and Thompson, 2005) the real size of the SBs in Chaetanthera species with smaller seeds is possibly somewhat larger than revealed in the experiments where any vertical movement was prevented by the seed being contained in special envelopes. Since it was shown that the smaller-seeded species of Chaetanthera tend to have longer-lived SBs, this last scenario would tend to further strengthen the relationship between SB size and seed mass, and possibly also that between SB size and adult longevity. In any case, avoiding these kinds of effects is inevitable in an experimental situation where factors, other than those of interest, necessarily must be controlled for.
Finally, there is a surprising dearth of experimental SB studies considering the large number of species from the same genus using an identical experimental protocol. As far as is known, the only other genus besides Chaetanthera for which such information exists is Vaccinium (e.g. Hill and Vander Kloet, 2005), but in that study, in contrast to the present one, only perennial species were considered. Most previous SB work and meta-analyses depend on seeds found in soil cores taken from particular vegetation types, or on seed burial experiments on representative species of a given vegetation type. That is, the dominant tendency up to now in seed bank research has been to study the SB in species derived from a diverse array of genera growing under similar conditions, rather than studying the responses of closely related species to a range of environmental conditions. While site-specific studies provide valuable ecological information, consideration of large numbers of species from the same genus, as an attempt has been made to show here, is more appropriate for evaluating the relative importance of intrinsic versus extrinsic on SB expression, and for developing evolutionary hypotheses. Such studies could be profitably accompanied by comparisons of SB expression in different populations of single species growing in a wide range of environmental conditions.
Acknowledgments
This work was supported by FONDECYT Grant No. 1020956 (MTKA-LC), Chilean Millennium Science Initiative Grant No. P02-051 and the BBVA-2004 Prize in Conservation Biology. Paulina Chacón held an ICM-CMEB Postdoctoral Fellowship during 2005.
LITERATURE CITED
- Arroyo MTK, Cavieres LA, Castor C, Humaña AM. 1999. Soil seed pool, persistent seed bank and standing vegetation in a high alpine site in the central Chilean Andes. Oecologia 119: 126–132. [DOI] [PubMed] [Google Scholar]
- Arroyo MTK, Cavieres LA, Humaña AM. 2004. Experimental evidence of potential for persistent seed bank formation at a subantarctic alpine site in Tierra del Fuego, Chile. Annals of the Missouri Botanical Garden 91: 357–365. [Google Scholar]
- Baker HG. 1972. Seed weight in relation to environmental conditions in California. Ecology 53: 997–1010. [Google Scholar]
- Bakker JP, Poschlod P, Strykstra RJ, Bekker RM, Thompson K. 1996. Seed banks and seed dispersal: important topics in restoration ecology. Acta Botanica Neerland 45: 461–490. [Google Scholar]
- Brown JS, Venable DL. 1986. Evolutionary ecology of seed-bank annuals in temporally varying environments. American Naturalist 127: 31–47. [Google Scholar]
- Cabrera AL. 1937. Revisión del género Chaetanthera (Compositae). Revista del Museo de la Plata, Sec. Bot. 1: 87–120. [Google Scholar]
- di Castri F, Hajek R. 1976. Bioclimatología de Chile. Santiago: Universidad Católica de Chile.
- Cavieres LA, Arroyo MTK. 2001. Persistent soil seed banks in Phacelia secunda J.F. Gmel. (Hydrophyllaceae): experimental detection of variation along an altitudinal gradient in the Andes of central Chile. Journal of Ecology 81: 31–39. [Google Scholar]
- Cavieres LA, Peñaloza A, Arroyo MTK. 2000. Altitudinal vegetation belts in the high Andes of Central Chile (33°S). Revista Chilena de Historia Natural 73: 331–344. [Google Scholar]
- Cohen D. 1966. Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology 12: 119–129. [DOI] [PubMed] [Google Scholar]
- Cohen D. 1967. Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. Journal of Theoretical Biology 16: 1–4. [DOI] [PubMed] [Google Scholar]
- Cowling RM, Ojeda F, Lamont BB, Rundel PW, Lechmere-Oertel R. 2005. Rainfall reliability, a neglected factor in explaining convergence and divergence of plant traits in fire-prone Mediterranean-climate ecosystems. Global Ecology and Biogeography 14: 509–519. [Google Scholar]
- Fenner M. 1985. Seed ecology. London: Chapman and Hall.
- Fenner M, Thompson K. 2005. The ecology of seeds. Cambridge: Cambridge University Press.
- Fuentes ER, Espinoza GA, Hajek ER. 1988. Some consequences of rainfall variability for Mediterranean-type ecosystems in Chile. In: di Castri F, Floret C, Rambal S, Roy J, eds. Time scales and water stress. Proceedings of the 5th International Conference on Mediterranean Ecosystems. Paris: IUBS, 347–360.
- Funes G, Basconcelo S, Díaz S, Cabido M. 1999. Seed size and shape are good predictors of seed persistence in soil in temperate mountain grasslands of Argentina. Seed Science Research 9: 341–345. [Google Scholar]
- Guariguata MR, Azócar A. 1988. Seed bank dynamics and germination ecology in Espeletia timotensis (Compositae), an Andean giant rosette. Biotropica 20: 54–59. [Google Scholar]
- Hershkovitz M, Arroyo MTK, Bell C, Hinojosa F. 2006. The Phylogeny of Chaetanthera (Asteraceae: Mutisieae) reveals both ancient and recent origins of the high elevation lineages. Molecular Phylogeny and Evolution (in press). [DOI] [PubMed]
- Hill NM, Vander Kloet SP. 2005. Longevity of experimentally buried seed in Vaccinium: relationship to climate, reproductive factors and natural seed banks. Journal of Ecology 93: 1167–1176. [Google Scholar]
- Holmgren M, Scheffer M, Ezcurra E, Gutiérrez JR, Mohren GMJ. 2001. El Niño effects on the dynamics of terrestrial ecosystems. Trends in Ecology and Evolution 16: 89–94. [DOI] [PubMed] [Google Scholar]
- Jurado E, Flores J. 2005. Is seed dormancy under environmental control or bound to plant traits? Journal of Vegetation Science 16: 559–564. [Google Scholar]
- Kalisz S, Horth L, McPeek MA. 1997. Fragmentation and the role of seed banks in promoting persistence of Collinsia verna in isolated populations. In: Schwartz M, ed. Conservation in highly fragmented landscapes. New York, NY: Chapman and Hall, 268–312.
- Kochmer JP, Handel SN. 1986. Constraints and competition in the evolution of flowering phenology. Ecological Monographs 56: 303–325. [Google Scholar]
- Leishman MR, Westoby M. 1994. Hypotheses on seed size: tests using the semiarid flora of western New South Wales, Australia. American Naturalist 143: 890–906. [Google Scholar]
- Leishman MR, Westoby M. 1998. Seed size and shape are not related to dormancy in Australia in the same way as in Britain. Functional Ecology 12: 480–485. [Google Scholar]
- Levin DA. 1990. The seed bank as a source of genetic novelty in plants. American Naturalist 135: 563–572. [Google Scholar]
- Mazer S. 1989. Ecological, taxonomic and life history correlates of seed mass among Indiana dune angiosperms. Ecological Monographs 59: 153–175. [Google Scholar]
- Moles AT, Hodson DW, Webb CJ. 2000. Do seed size and shape predict presistence in soil in New Zealand? Oikos 89: 541–545. [Google Scholar]
- Ozinga WA, Schaminée JHJ, Bekker RM, Bonn S, Poschlod P, Tackenberg O, et al. 2005. Predictability of plant community composition from environmental conditions is constrained by dispersal limitation. Oikos 108: 555–561. [Google Scholar]
- Philippi T. 1993. Bet-hedging germination of desert annuals: beyond the first year. American Naturalist 142: 474–487. [DOI] [PubMed] [Google Scholar]
- Purvis A, Rambaut A. 1995. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analyzing comparative data. Computer Applications in the Biosciences (CABIOS) 11: 247–251. [DOI] [PubMed] [Google Scholar]
- Rees M. 1993. Trade-offs among dispersal strategies in the British flora. Nature 366: 150–152. [Google Scholar]
- Rees M. 1994. Delayed germination of seeds: a look at the effects of adult longevity, the timing of production, and population age/stage structure. American Naturalist 144: 43–64. [Google Scholar]
- Rees M. 1997. Evolutionary ecology of seed dormancy and seed size. In: Silovertown J, Franco M, Harper JL, eds. Plant life histories. Ecology, phylogeny and evolution. Cambridge: Cambridge University Press, 121–142.
- Rutllant JA. 2004. Aspectos de la circulación atmosférica de gran escala asociada al ciclo ENSO 1997–1999 y sus consecuencias en el régimen de precipitación en Chile central. In: Avaria S, Carrasco J, Rutllant J, Yañéz E, eds. El Niño-La Niña 1997–2000. Sus Efectos en Chile. CONA, Chile, Valparaíso, 61–76.
- Rutllant J, Aceituno P. 1991. Southern Hemisphere circulation signals in connection with winter rainfall forecasting in Central Chile. International Centre for Theoretical Physics, Trieste, Italy-Internal Report IC/91/64, 1–20.
- Salisbury EJ. 1942. The reproductive capacity of plants: studies in quantitative biology. London: G. Bell & Sons.
- Santibáñez F, Uribe M. 1990. Atlas agroclimático de la V región y Región Metropolitana. Santiago, Chile: Ministerio de Agricultura.
- SAS Institute. 1994. SAS/STAT user's guide. Cary, NC: SAS Institute Inc.
- Teillier S, Aldunate G, Riedermann P, Niemeyer H. 2005. Flora de la Reserva Nacional Río Clarillo. Santiago: Facultad de Ciencias, Universidad de Chile.
- Thompson K, Grime JP. 1979. Seasonal variation in seed banks of herbaceous species in ten contrasting habitats. Journal of Ecology 67: 893–921. [Google Scholar]
- Thompson K, Rabinowitz D. 1989. Do big plants have big seeds? American Naturalist 133: 722–728. [Google Scholar]
- Thompson K, Band SR, Hodgson JG. 1993. Seed size and shape predict persistence in soil. Functional Ecology 7: 236–241. [Google Scholar]
- Thompson K, Bakker JP, Bekker RM, Hodgson JG. 1998. Ecological correlates of seed persistence in soil in the north-west European flora. Journal of Ecology 86: 163–169. [Google Scholar]
- Venable DL, Brown JS. 1988. The selective interactions of dispersal, dormancy and seed size as adaptations for reducing risk in variable environments. American Naturalist 131: 360–384. [Google Scholar]
- Venable DL, Lawlor L. 1980. Delayed germination and dispersal in desert annuals: escape in space and time. Oecologia 46: 272–282. [DOI] [PubMed] [Google Scholar]
- Whittle CA. 2006. The influence of environmental factors, the pollen : ovule ratio and seed bank persistence on molecular evolutionary rates in plants. Journal of Evolutionary Biology 19: 302. [DOI] [PubMed] [Google Scholar]