Abstract
• Background and Aims Flowering incidence in Bambusa tulda has a high socio-economic impact. The aim of the present study was to describe the species for morphological characters (vegetative and reproductive) as well as molecular markers in order to enable species identification at various stages of the life cycle.
• Methods Thirty-two key morphological characters (15 culm and 17 culm-sheath) were studied along with detailed inflorescence and floral characters. Incidence of sporadic flowering was recorded. Genomic DNA was isolated from leaves collected from 17 eco-geographical locations and RAPD profiles were generated.
• Key Results The description of culm, culm-sheath, inflorescence and floral morphology are in agreement with the prior taxonomic description by Gamble in 1896, but in this communication a more detailed description and illustrations are presented. No seed set was recorded following sporadic flowering, probably due to prezygoting isolating mechanisms (herkogamy or protandry). All 17 populations surveyed generated identical RAPD profiles.
• Conclusions Sporadic flowering may occur in B. tulda, but may not necessarily be followed by gregarious flowering, and does not result in seed production.
Keywords: Bambusa tulda, culm, culm-sheath, floral morphology, RAPD, sporadic flowering
INTRODUCTION
Bambusa tulda is a semelparous tropical bamboo, with a long period of vegetative growth followed by seeding and death of the clump. (The terminology used here follows that of McClure, 1966, who used ‘clump’ for genet and ‘culm’ for ramet.) Bambusa tulda is a tall, sturdy and quick growing bamboo suitable for the production of high quality paper (Upreti and Sundriyal, 2001) and furniture.
Due to the monocarpic nature and the unusually long flowering cycle, records of bamboo flowering is limited in the literature (Siefriz, 1950; Mohan Ram and Harigopal, 1981; Filgueiras and Pereira, 1988; Koshy and Pushpangadan, 1997; Ramanayake and Yakandawala, 1998; Ramanayake and Weerawardene, 2003; Singha et al., 2003).
In mast flowering, all members of a cohort (plants from seeds of common origin) or even a species enter the reproductive phase simultaneously and subsequently die. In India, this fascinating biological phenomenon in B. tulda was documented in the years 1880–1884 and 1928–1929 in its native habitat (Mohan Ram and Harigopal, 1981). Mohan Ram and Harigopal (1981) also reported sporadic flowering in the year 1976 in Mizoram, the north-eastern hilly region, followed by mast flowering until 1979. Accordingly, B. tulda appears to have an intermast period of about 48 years (Mohan Ram and Harigopal, 1981).
Bamboo seeds are highly palatable to a number of animals including rodents, deer, pigs and elephants (Troup, 1921; Soderstrom and Calderon, 1974; Janzen, 1976). Bamboo flowering is regarded as a bad omen in several States of India, especially where the flowering incidence is accompanied by an increase in rodents, especially Rattus rattus brunneusculus populations. There is a belief that flowering of bamboo harbingers disasters like famine and natural calamites, which has compelled the rural people to destroy the cohorts/clumps after or during blooming. Thus, the knowledge and information on this mesmerizing biological phenomenon is still incomplete. Furthermore, such societal reaction also enhanced the extinction rate of the bamboo species.
During field surveys over the last 9 years across different districts of West Bengal, India, sporadic incidence of flowering was observed in one of the sites surveyed. In this communication a detailed account of the reproductive organs of B. tulda is given along with other key morphological characters. Due to the unavailability of reproductive characters throughout most of the life cycle, the identification of B. tulda and other bamboo species is usually based on vegetative characters. However, in a separate study describing the phylogenetic relationships of bamboo species and genera, the shortcomings of vegetative characters to distinguish closely allied species have been noted (Das et al., 2006). For these reasons, populations of B. tulda were also chosen to be characterized for RAPD fingerprint profiles, and these profiles were investigated to see if they could be used as an alternative means of species identification.
MATERIALS AND METHODS
Scoring morphological data and collection of study material
Natural stands of Bambusa tulda Roxb. were surveyed at 17 eco-geographical locations in different districts of West Bengal, India (Fig. 1 and Table 1). Thirty-two key morphological culm (15) and culm-sheath (17) characters were studied. Culm characters, both qualitative and quantitative, were recorded at site. Dry, intact culm-sheaths were collected and preserved to enable more detailed examination of morphological characters. Quantitative morphological characters were scored from five randomly chosen culms at each of the 17 collection sites. Young leaves were collected separately for DNA isolation from one or several clumps at each of the 17 sites under study. The species-identity of each clump was confirmed by amplification of a B. tulda-specific SCAR marker, Tuldo609 (AY 684298) (see below).
Fig. 1.
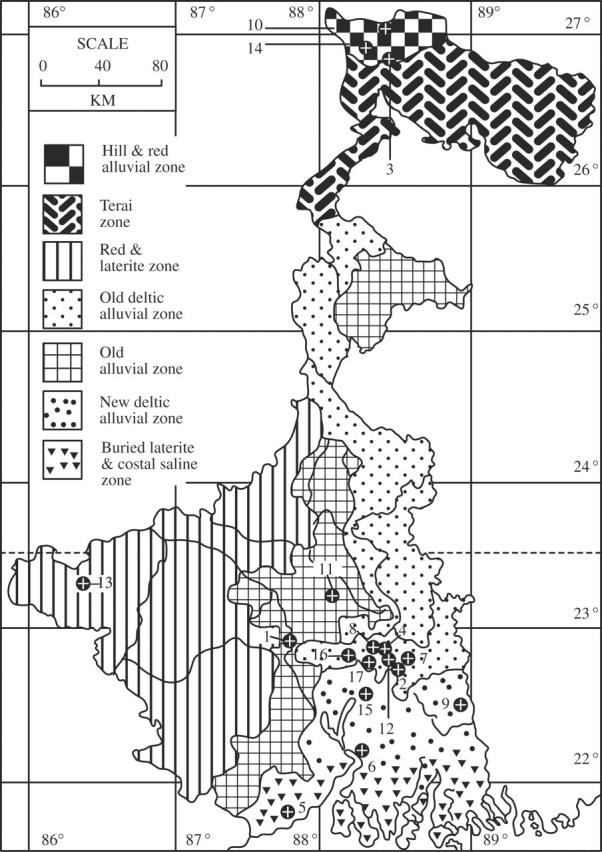
Map of West Bengal (India) depicting 17 different eco-geographical-collection sites of B. tulda populations: 1, Arambagh; 2, Badu; 3, Baromile; 4, Bhadreswar; 5, Contai; 6, Diamond Harbour; 7, Digberia; 8, Dighra; 9, Hasnabad; 10, Kalimpong; 11, Memari; 12, Nilganj; 13, Purulia; 14, Salugaura; 15, Sibpur; 16, Singur; 17, Srerampore. Sporadic flowering was observed at Dighra.
Table 1.
Principal eco-geographical features of the collection sites
| Collection number* | Place of collection† | Latitude | Longitude | Altitude (m) | Max. temp. (°C) | Min. temp. (°C) | Average annual rainfall (mm) | Soil type |
|---|---|---|---|---|---|---|---|---|
| MD/ARA/02/026 | Arambagh | 22°52′N | 87°46′E | 14·0 | 36·0 | 15·0 | 1605·0 | Old Alluvial |
| MD/BAD/02/040 | Badu | 22°41′N | 88°27′E | 14·0 | 30·5 | 19·2 | 1625·0 | Old Deltic Alluvial |
| AP/BAR/99/193 | Baromile | 26°46′N | 88°26′E | 280·0 | 29·0 | 8·0 | 2160·0 | Hill and Red Alluvial |
| SB/BHA/03/033 | Bhadreswara,b,c | 22°49′N | 88°21′E | 11·6 | 36·0 | 17·5 | 1523·0 | New Deltic Alluvial |
| MD/CON/01/009 | Contai | 21°46′N | 87°45′E | 5·0 | 36·3 | 19·4 | 1610·0 | Buried Laterite and Coastal Saline |
| MD/DIA/03/073 | Diamond Harbour | 22°11′N | 88°11′E | 0 | 35·0 | 19·0 | 1480·0 | New Deltic Alluvial |
| MD/DIG/03/090 | Digberia | 22°43′N | 88°31′E | 13·0 | 30·7 | 19·6 | 1600·8 | Old Deltic Alluvial |
| SB/DGA/03/035 | Dighraa | 22°54′N | 88°26′E | 11·0 | 36·0 | 17·2 | 1542·0 | New Deltic Alluvial |
| MD/HAS/02/065 | Hasnabad | 22°34′N | 88°55′E | 6·0 | 32·5 | 19·1 | 1630·0 | New Deltic Alluvial |
| AP/KAL/99/151 | Kalimpong | 27°40′N | 88°28′E | 787·0 | 25·0 | 7·0 | 2200·0 | Hill and Red Alluvial |
| SB/MEM/03/071 | Memarid | 23°11′N | 88°7′E | 24·0 | 36·0 | 13·2 | 1621·0 | Old Alluvial |
| MD/NIL/02/053 | Nilganj | 22°45′N | 88°22′E | 14·0 | 36·0 | 20·0 | 1500·0 | New Deltic Alluvial |
| MD/PUR/03/083 | Purulia | 23°19′N | 86°22′E | 227·0 | 42 | 5·0 | 1250·0 | Red and Laterite |
| AP/SAL/99/171 | Salugaura | 26°51′N | 88°16′E | 134·0 | 20·1 | 6·0 | 2500·0 | Hill and Red Alluvial |
| SB/SIB/02/014 | Sibpura, d | 22°34′N | 88°19′E | 10·0 | 30·7 | 19·8 | 1633·6 | New Deltic Alluvial |
| SB/SIN/03/059 | Singurd | 22°48′N | 88°13′E | 13·0 | 36·0 | 16·8 | 1445·0 | Old Alluvial |
| SB/SRE/03/048 | Sreramporec | 22°53′N | 88°24′E | 14·2 | 35·0 | 18·0 | 1580·0 | New Deltic Alluvial |
Collection number of representative individual of each population.
Locations under different districts of West-Bengal, India.
Yellow striation on culm.
bending of culm.
swelling of internodes.
swollen node absent.
Flowering
Natural stands were surveyed to record the incidence of flowering. During January 2003 sporadic flowering was noticed in only one out of four clumps of B. tulda at Dighra, Hooghly, India (SB/DGA/03/035; see Table 1 and Fig. 2A). Out of a total of ten culms in this clump, only two flowered. The number of fertile nodes recorded was six in one culm and eight in another. Inflorescences were collected and key morphological characters of fresh spikelets were recorded. Measurements were taken from at least five different fertile florets from each inflorescence.
Fig. 2.

(A) Capitate arrangement of distichous spikelets on rachis of B. tulda culm; (B) a portion of culm showing arrangement of culm sheaths; (C) clumps with prominent yellow striation on the culm at Bhadreswar; (D) drawing of culm-sheath showing hairs on the abaxial side and two unequal, fringed auricles with bristles (scale bar = 50 mm); (E) anthers protruding from the flower during anthesis; (F) a bent culm with swollen, curved internode at Bhadreswar.
Isolation of PCR-compatible genomic DNA
Genomic DNA was isolated from 200 mg of sterilized leaf tissue using a modified protocol of Dellaporta et al. (Basak et al., 2004). After removal of RNA by RNase treatment, the concentrations of DNA samples were determined by comparing band intensity with known concentrations of lambda DNA digest on ethidium bromide-stained 0·8 % agarose gel.
Fingerprinting through RAPD
Genomic DNA was isolated from at least one randomly selected culm from each site, including the culms showing morphological variation (Table 1) and each extract was independently screened with 30 RAPD primers. The amplification reactions were carried out in a 50 μL reaction mixture following the protocol of Das et al. (2005). The thermal cycler programme was: 4 min at 95 °C followed by 35 cycles (45 s at 94 °C, 45 s at 35 °C, 1 min at 72 °C) and finally 10 min at 72 °C for elongation. The amplified products were resolved by electrophoresis on 1·5 % agarose gel and were visualized by ethidium bromide staining. Amplification of the B. tulda-specific SCAR marker was performed with the primer pair Tuldo600F (GTGACGTAGGCGAACATGGC) and Tuldo600R (GTGACGTAGGGCATACCTTG) at an annealing temperature of 60 °C (Das et al., 2005).
Statistical analysis
Analysis of variance (ANOVA) was performed to describe variability between 17 populations of B. tulda surveyed. The quantitative characters considered for ANOVA analysis were height and diameter of culm, internode length, ratio of cavity to culm diameter, ratio of culm-sheath length to breadth at base and ratio of culm sheath length to blade length.
RESULTS AND DISCUSSION
Description of B. tulda based on data collected in the present study
Culms 17·7–21·3 m high, usually straight, bright to dark green (Fig. 2B), occasionally with vertical yellow striations (Fig. 2C), 50·0–88·0 mm in diameter; ratio of cavity diameter to culm diameter 0·28–0·4; internodes rarely swollen; nodes usually swollen with sheath scars and whitish rings. Culm-sheaths (modified leaves protecting young culms) with notably different sizes at different culm heights; adaxial surface glabrous; abaxial surface covered with profuse, black hairs (Fig. 2D); ratio of total length to breadth at the base 1·0–1·4; blade straight, ovate; ratio of total length to blade length 2·7–3·7; auricles conspicuous, continuous with blade, unequal, fringed; ligule with serrate margin.
Inflorescence a leafless panicle with a branching pattern similar to that of a vegetative culm (Fig. 2A). Rachis smooth with 3–5 spikelets in capitate-like clusters in the nodes (Figs 2E and 3A). Spikelets subtended by shining, chaffy bracts, distichous, 20–50 mm long; the 2–4 lowermost florets reduced to empty glumes, followed by 4–6 fertile florets (Fig. 3B). Florets bisexual, 15–22 mm long, 5–7 mm broad at the base; lemma broadly acuminate, mucronate, concave, glabrous, many nerved, bright green when fresh, overlapping with the palea (Fig. 3C); palea subtending a bisexual floret 9–15 mm long, 3–5 mm broad at the base, 5–7 nerved, membranous, penicillate, 2-keeled, bearing long, white ciliae (Fig. 3D); lodicules 3, 2·5–4·0 mm long, cuneate, oblong, hyaline, 5–6 nerved with upper part whitish fimbriate (Fig. 3E). Stamens 6; anthers 3·5–6·0 mm long, basifixed, purple, emarginate (Fig. 3F), with linear dehiscence; filaments thread-like, elongating to 8–10 mm during anthesis. Ovary ovate at anthesis, 1–2 mm long; style 2–3 mm long, hairy; stigmas 3, 2–3 mm long, golden yellow, plumose, wavy (Fig. 3G).
Fig. 3.
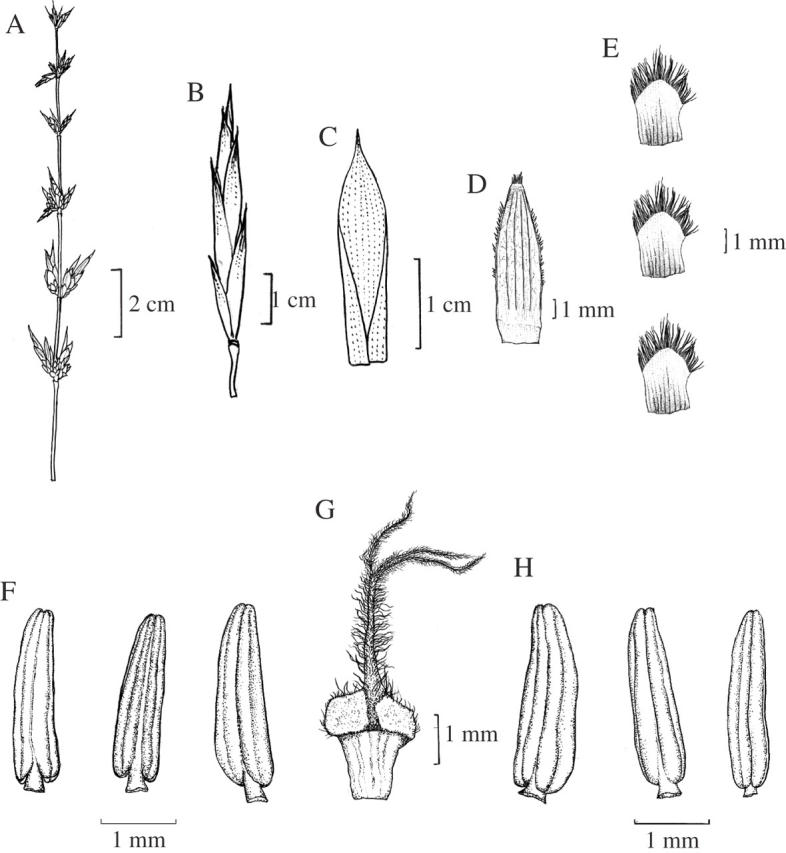
Different floral parts of B. tulda: (A) flowering branch bearing whorls of spikelets; (B) a single spikelet; (C) lemma; (D) palea; (E) lodicules; (F) stamens; (G) pistil.
Comments on morphometric data
According to Gamble (1896) B. tulda is not known to have swollen nodes. However, swollen nodes were observed in most of the populations, except the populations at Memari, Singur and Sibpur. Yellow striations on culms were noted at Bhadreswar (Figs 1 and 2C; SB/BHA/03/033) and at Dighra (Fig. 1; SB/DGA/03/035). Curved culms and swollen internodes were observed at Bhadreswar (Figs 1 and 2F; SB/BHA/03/033). Swollen internodes were also recorded in the population at Srerampore (Fig. 1; SB/SRE/03/048).
Analyses of variance revealed high levels of variation between populations in vegetative characters (Table 2). The most variable character was culm diameter with an F value of 13·6. The remaining characters were less variable: height of culm, 1·85; internode length, 2·4; ratio of culm cavity to culm diameter, 1·8; ratio of culm sheath length to breadth at base, 2·8; ratio of culm sheath length to length of blade, 3·44.
Table 2.
Analysis of variance estimated within and between 17 B. tulda populations for height of culm (H, in m), diameter of culm (D, in mm), internode length (I, in mm), ratio of culm cavity to culm diameter (CD), ratio of culm sheath length to breadth at base (LB) and ratio of culm sheath length to length of blade (LL)
| Source of variation | d.f. | H | D | I | CD | LB | LL |
|---|---|---|---|---|---|---|---|
| Sum of squares | |||||||
| Population | 16 | 105·0609 | 7200·588 | 92260·99 | 0·08556 | 0·583421 | 7·944209 |
| Error | 68 | 43·656 | 444 | 30551·2 | 0·03588 | 0·16768 | 1·85956 |
| Mean squares | |||||||
| Population | 16 | 6·566309 | 450·0368 | 5766·312 | 0·005348 | 0·036464 | 0·496513 |
| Error (within population) | 68 | 0·642 | 6·529412 | 449·2824 | 0·000528 | 0·002466 | 0·027346 |
| Computed F | 10·2279* | 68·92455* | 12·83449* | 10·13462* | 14·78733* | 18·15639* | |
| Error (between populations) | 4 | 1·184862 | 88·70148 | 1063·406 | 0·000964 | 0·0068 | 0·093833 |
P < 0·001 level.
Vegetative characters, mainly describing culm and culm-sheath, are widely used for bamboo species determination (Ohrnberger and Goerrings, 1986). However, Wu (1962) expressed concern on the reliability of vegetative characters due to potential influence by the environment. It is concluded that floral characters, if available, may assist in reaching correct species identification.
The inflorescence characters and floral morphology of B. tulda as described above are in agreement with Gamble (1896), but more comprehensive. According to Gamble (1896) it is difficult to distinguish B. tulda from B. nutans in the absence of flowers. Bambusa tulda also resembles B. auriculata and B. arundinacea in vegetative morphology. However, B. arundinacea differs from its close relatives in the presence of a dense network of thorny culm-branches. Bambusa auriculata is characterized by a pale-green culm, greyish nodal rings with hairs, ciliated culm sheath margins, dentate ligules with hairs and hairy margin on the blade.
Flowering incidence
During January 2003 sporadic flowering was noticed in one out of four clumps of B. tulda at Dighra, Hooghly, India (SB/DGA/03/035; see Table 1 and Fig. 2E). Out of a total of ten culms in this clump, only two flowered. One culm had six fertile nodes, and the other one eight.
Sporadic flowering in B. tulda was reported from Bengal in the 19th Century (Brandis, 1899). Mohan Ram and Harigopal (1981) observed sporadic flowering in B. tulda, which led to a gregarious outbreak within 3 years of incidence in Mizoram. In the population at Dighra studied here, no mast flowering has been noticed as yet (2005), and it has not been within the scope of the present report to confirm the 48 years of intermast period in B. tulda as reported earlier (Mohan Ram and Harigopal, 1981).
Janzen (1976) recorded infrequent flowering in bamboos, with a few clumps flowering in the year just before and after the main flowering cycle. Filgueiras and Pereira (1988) observed the incidence of sporadic flowering in a population of Actinocladum verticillatum which was superseeded by gregarious blooming by the end of the following year. These reports highlight the importance of recording the incidence of sporadic flowering, which may act as an indicator of gregarious flowering soon after.
Seed set
No seed production was recorded in the present study. This may be either due to protandry or due to the short height of the pistil coupled with tight overlapping of lemma and palea, which prevent the pistil from coming out, thereby reducing the chances of cross-pollination. The latter phenomenon has been reported previously in Bambusa vulgaris by Koshy and Harikumar (2000) and in B. cacharensis by Singha et al. (2003).
McClure (1966), with his vast experience of bamboos, concluded that introduced or cultivated bamboos often flower without any seed setting and those that set seeds are usually wild plants. It is reported from Taiwan that approximately 40 introduced bamboo species set very few seeds during flowering (Wang and Chen, 1971, 1972). Gamble (1904) and others (referred to in Janzen, 1976) were of the opinion that sporadic flowering in an isolated wild clump may set little or no seed. Janzen (1976) proposed that such off-set clumps are derived from synchronous populations due to the lack of selection pressure of seed-predators.
Molecular characterization through RAPD fingerprinting
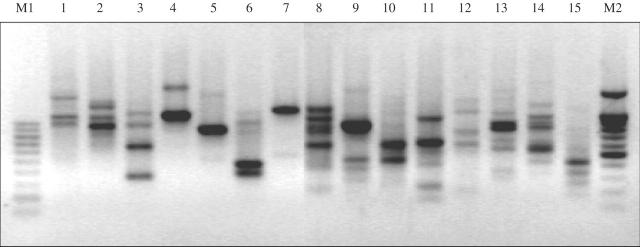
Differentiation between populations of B. tulda was studied by means of RAPD fingerprinting patterns. This technique was chosen for its simplicity, rapidity and cost-effectiveness. During this investigation 30 random oligomers were screened, out of which 15 generated reproducible fingerprinting patterns for B. tulda (Fig. 4). The approximate size range of the RAPD products was 300 bp to 1·5 kb (Table 3). The highest number (five) of bands was obtained with OPOJ-01 and the lowest (one) was scored with OPA-07 and PW-01 primers.
Fig. 4.
A representative profile of RAPD-based molecular cataloguing of B. tulda. Lane 1, OPA-02; lane 2, OPA-03; lane 3, OPA-05; lane 4, OPA-06; lane 5, OPA-07; lane 6, OPA-10; lane 7, PW-01; lane 8, OPOJ-01; lane 9, OPOJ-04; lane 10, OPOJ-12; lane 11, OPOJ-18; lane 12, OPOB-02; lane 13, OPOB-03; lane 14, OPOB-04; lane 15, OPOB-05. M1 = 100 bp DNA ladder marker; M2 = 1·5 kb + 100 bp DNA ladder marker.
Table 3.
The amplified products obtained using different RAPD primers from the genomic DNA of B. tulda
| Primers | Nucleotide sequence (5′–3′) | Total no. of amplified bands | Size ranges of bands (bp) |
|---|---|---|---|
| OPA-02 | TGCCGAGCTG | 3 | 1038–1350 |
| OPA-03 | AGTCAGCCAC | 3 | 1015–1263 |
| OPA-05 | AGGGGTCTTG | 4 | 432–1155 |
| OPA-06 | GGTCCCTGAC | 2 | 1133–1458 |
| OPA-07 | GAAACGGGTG | 1 | 963 |
| OPA-10 | GTGATCGCAG | 2 | 474–1068 |
| OPOB-02 | TGATCCCTGG | 3 | 793–1172 |
| OPOB-03 | CATCCCCCTG | 3 | 761–1145 |
| OPOB-04 | GGACTGGAGT | 4 | 747–1273 |
| OPOB-05 | TGCGCCCTTC | 2 | 503–594 |
| OPOJ-01 | CTGCTGGGAC | 5 | 785–1208 |
| OPOJ-04 | CCTTGACGCA | 3 | 629–1023 |
| OPOJ-12 | GGAGGGTGTT | 2 | 625–785 |
| OPOJ-18 | AGGGAACGAG | 3 | 322–1098 |
| PW-01 | TGGTCACTGA | 1 | 1200 |
However, no polymorphism could be detected between any of the different populations studied or between any of the phenotypic variants identified in B. tulda. This finding is in striking contrast to experience from other species of Bambusa in which levels of polymorphism generated by many of these random oligomers has been found to be reasonably high at inter and infra generic level (Das et al., 2006; see Appendix).
As far as is known, this is the first report of an integrated study at both the molecular and the morphological levels for complete characterization of a commercially important bamboo species.
Acknowledgments
We acknowledge Council of Scientific & Industrial Research, New Delhi, India for financial support [Grant No. 38 (1062)/03/EMR-II] and CSIR-NET fellowships to two of the authors (S.B. and R.B.). We also thank Kristina M. Sefc, S. M. S. D. Ramanayake and Mikael Hedrén for their helpful comments to improve the quality of the manuscript and E. Henry Lee, USEPA, Corvallis for his suggestions on statistical analysis.
LITERATURE CITED
- Basak J, Das M, Ghose TK, Pal A. 2004. An efficient and high-yielding method of PCR-compatible DNA isolation for biotechnological investigations from economically important plants. In: D'Souza L, Anuradha M, Shashikiran N, Hegde S, Rajendra K, eds. Biotechnology for a better future. Mangalore, India: SAC Publications, 99–105.
- Brandis D. 1899. Biological notes on Indian bamboos. Indian Forester 25: 1–25. [Google Scholar]
- Das M, Bhattacharya S, Pal A. 2005. Generation and characterization of SCARs by cloning and sequencing of RAPD products: a strategy for species specific marker development in bamboo. Annals of Botany 95: 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Bhattacharya S, Basak J, Pal A. 2006. Phylogenetic relationships among the bamboo species as revealed by morphological characters and polymorphism analyses. Biologia Plantarum (in press).
- Filgueiras TS, Pereira BAS. 1988. On the flowering of Actinocladum verticillatum (Gramineae: Bambusoideae). Biotropica 20: 164–166. [Google Scholar]
- Gamble JS. 1896. Bambuseae of British India. Calcutta, India: Annals of the Royal Botanical Garden.
- Gamble JS. 1904. The flowering of the bamboo. Nature 70: 423. [Google Scholar]
- Janzen DH. 1976. Why bamboos wait so long to flower. Annual Review of Ecology and Systematics 7: 347–391. [Google Scholar]
- Koshy KC, Harikumar D. 2000. Flowering incidences and breeding system in Bambusa vulgaris. Current Science 79: 1650–1651. [Google Scholar]
- Koshy KC, Pushpangadan P. 1997. Bambusa vulgaris blooms, a leap towards extinction? Current Science 72: 622–624. [Google Scholar]
- McClure FA. 1966. The bamboos, a fresh perspective. Cambridge, MA: Harvard University Press.
- Mohan Ram HY, Harigopal B. 1981. Some observations on the flowering of bamboos in Mizoram. Current Science 50: 708–710. [Google Scholar]
- Ohrnberger D, Goerrings J. 1986. The bamboos of the world. Odenthal, Germany.
- Ramanayake SMSD, Weerawardene TE. 2003. Flowering in a bamboo, Melocanna baccifera (Bambusoideae: Poaceae). Botanical Journal of the Linnean Society 143: 287–291. [Google Scholar]
- Ramanayake SMSD, Yakandawala K. 1998. Incidence of flowering, death and phenology of development in the giant bamboo Dendrocalamus giganteus Wall. Ex Munro. Annals of Botany 82: 779–785. [Google Scholar]
- Siefriz W. 1950. Gregarious flowering of Chusquea. Nature 22: 635–636. [DOI] [PubMed] [Google Scholar]
- Singha LB, Bhatt BP, Khan ML. 2003. Flowering of Bambusa cacharensis Mazumder in the southern part of North-East India: a case study. Journal of Bamboo and Rattan 2: 57–63. [Google Scholar]
- Soderstrom TR, Calderon CE. 1974. Primitive forest grasses and the evolution of bambusoideae. Biotropica 6: 141–153. [Google Scholar]
- Troup RS. 1921. Silviculture of Indian trees. Oxford: Clarendon Press.
- Upreti TC, Sundriyal RC. 2001. Bamboo and cane resources of Arunachal Pradesh: utilization pattern and implications for management. Bamboo Science and Culture 15: 20–34. [Google Scholar]
- Wang TT, Chen MY. 1971. Studies on bamboo flowering in Taiwan. Technical Bulletin of Exerimental Forest, Taiwan University. 87: 27. [Google Scholar]
- Wang TT, Chen MY. 1972. Flowering and seeding of giant bamboo (Sinocalamuslatiflorus). Silvae Genetica 21: 251–252. [Google Scholar]
- Wu MC-Y. 1962. Classification of Bambuseae based on leaf anatomy. Botanical Bulletin of Academia Sinica 3: 83–107.