Abstract
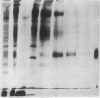
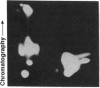
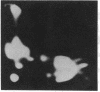
A Mr 32,000 integral membrane protein has previously been identified on erythrocytes bearing the Rh(D) antigen and is thought to contain the antigenic variations responsible for the different Rh phenotypes. To study it on a biochemical level, a simple large-scale method was developed to purify the Mr 32,000 Rh protein from multiple units of Rh(D)-positive and -negative blood. Erythrocyte membrane vesicles were solubilized in NaDodSO4, and a tracer of immunoprecipitated 125I surface-labeled Rh protein was added. The Rh protein was purified to homogeneity by hydroxylapatite chromatography followed by preparative NaDodSO4/PAGE. Approximately 25 nmol of pure Rh protein was recovered from each unit of Rh(D)-positive and -negative blood. Rh protein purified from both Rh phenotypes appeared similar by one-dimensional NaDodSO4/PAGE, and the N-terminal amino acid sequences for the first 20 residues were identical. Rh proteins purified from Rh(D)-positive and -negative blood were compared by two-dimensional iodopeptide mapping after 125I-labeling and alpha-chymotrypsin digestion. The peptide maps were very similar; however, at least two additional iodopeptides were consistently noted in the Rh proteins purified from Rh(D)-positive erythrocytes. These data indicate that a similar core Rh protein (or group of related proteins) exists in both Rh(D)-positive and -negative erythrocytes, and the Rh proteins from erythrocytes with different Rh phenotypes contain distinct structural polymorphisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Saboori A. M., Asimos A., Smith B. L. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem. 1987 Dec 25;262(36):17497–17503. [PubMed] [Google Scholar]
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- Bloy C., Blanchard D., Lambin P., Goossens D., Rouger P., Salmon C., Cartron J. P. Human monoclonal antibody against Rh(D) antigen: partial characterization of the Rh(D) polypeptide from human erythrocytes. Blood. 1987 May;69(5):1491–1497. [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Gahmberg C. G., Karhi K. K. Association of Rho(D) polypeptides with the membrane skeleton in Rho(D)-positive human red cells. J Immunol. 1984 Jul;133(1):334–337. [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular characterization of the human red cell Rho(D) antigen. EMBO J. 1983;2(2):223–227. doi: 10.1002/j.1460-2075.1983.tb01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular identification of the human Rho (D) antigen. FEBS Lett. 1982 Apr 5;140(1):93–97. doi: 10.1016/0014-5793(82)80528-0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kuypers F., van Linde-Sibenius-Trip M., Roelofsen B., Tanner M. J., Anstee D. J., Op den Kamp J. A. Rhnull human erythrocytes have an abnormal membrane phospholipid organization. Biochem J. 1984 Aug 1;221(3):931–934. doi: 10.1042/bj2210931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauf P. K., Joiner C. H. Increased potassium transport and ouabain binding in human Rhnull red blood cells. Blood. 1976 Sep;48(3):457–468. [PubMed] [Google Scholar]
- Moore S., Green C. The identification of specific Rhesus-polypeptide-blood-group-ABH-active-glycoprotein complexes in the human red-cell membrane. Biochem J. 1987 Jun 15;244(3):735–741. doi: 10.1042/bj2440735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Woodrow C. F., McClelland D. B. Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy. Nature. 1982 Feb 11;295(5849):529–531. doi: 10.1038/295529a0. [DOI] [PubMed] [Google Scholar]
- Ridgwell K., Tanner M. J., Anstee D. J. The Rhesus (D) polypeptide is linked to the human erythrocyte cytoskeleton. FEBS Lett. 1984 Aug 20;174(1):7–10. doi: 10.1016/0014-5793(84)81066-2. [DOI] [PubMed] [Google Scholar]
- Sturgeon P. Hematological observations on the anemia associated with blood type Rhnull. Blood. 1970 Sep;36(3):310–320. [PubMed] [Google Scholar]
- Wiener A. S. THE Rh SERIES OF ALLELIC GENES. Science. 1944 Dec 29;100(2609):595–597. doi: 10.1126/science.100.2609.595. [DOI] [PubMed] [Google Scholar]