Abstract
• Background and Aims Loasaceae subfam. Loasoideae are mostly distributed in South America (sea level to over 4500 m) with a wide range of animals documented as pollinators. The aim was to investigate correlations between nectar parameters, flower morphology, pollination syndrome and phylogeny.
• Methods Nectar was collected from 29 species from seven genera in the subfamily. Concentration and volumes were measured and the amount of sugar calculated. Correlations of nectar data were plotted on a ternary graph and nectar characteristics compared with flower visitors, floral morphology and phylogenetic data.
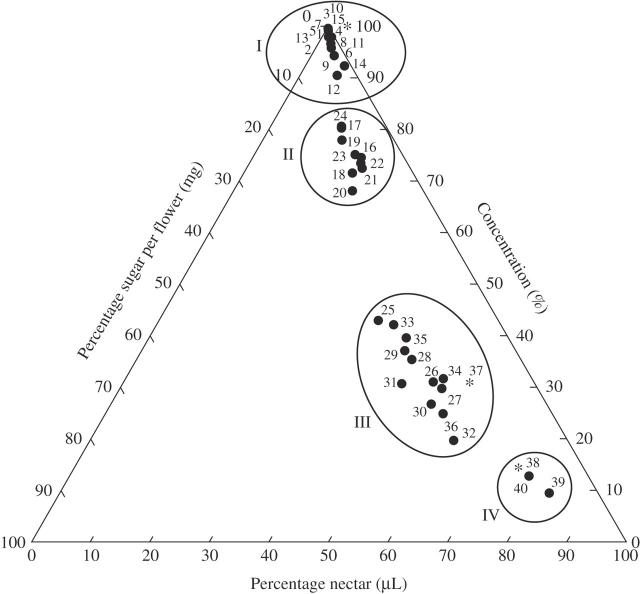
• Key Results Sugar concentrations are generally higher than reported for most plant families in the literature. The species investigated can be roughly grouped as follows. Group I: plants with approx. 1·5(–3·5) µL nectar with (40–)60–80 % sugar and 0·19–2 mg sugar flower−1; with small, white, star-shaped corollas, pollinated by short-tongued bees. Groups II, III and IV: plants with mostly orange, balloon-, saucer-, bowl- or bell-shaped corollas. Group II: plants with approx. 9–14 µL nectar with 40–60 % sugar and 4–10 mg sugar flower−1; mostly visited by long-tongued bees and/or hummingbirds. Group III: plants with 40–100 µL nectar with 30–40 % sugar and 14–36 mg sugar flower–1, mostly visited by hummingbirds. Group IV: geoflorous plants with 80–90 µL with 10–15 % sugar and 8·5–12 mg sugar flower–1, presumably visited by small mammals. Groups II and III include species visited by bees and/or hummingbirds.
• Conclusions Pollinator switches from short-tongued bees via long-tongued bees to hummingbirds appear to have taken place repeatedly in the genera Nasa, Loasa and Caiophora. Changes in nectar amount and concentration appear to evolve rapidly with little phylogenetic constraint.
Keywords: Nectar, pollination, Caiophora, Loasa, Nasa, Loasaceae, short-tongued bees, long-tongued bees, Colletidae, Apidae, Anthophoridae, rodents, ornithophily
INTRODUCTION
Nectar production and composition are understood to be crucial factors influencing flower visitation and consequently pollinator preferences for particular plant species (e.g. Baker and Baker, 1982; Endress, 1994). Certain pollinator species show a distinct preference for particular nectar types (e.g. Baker, 1975; Bolten and Feinsinger, 1978; Bolten et al., 1979; Baker and Baker, 1983; Heyneman, 1983; Zimmermann, 1983; Blem et al., 2000; McDade and Weeks, 2004). Thus, there is general agreement, that sugar concentration in hummingbird-pollinated flowers is generally lower (20–26 % sugar; Hainsworth and Wolf, 1972; Baker, 1975; Cruden et al., 1983) than in insect-pollinated flowers (>30 % sugar; rarely up to 80 %). Further differences have been reported between ‘lowland hummingbird nectar’ and ‘highland hummingbird nectar’, with highland nectar less concentrated and hence less viscous, but present in higher volumes such that these flowers present roughly the same caloric value (Hainsworth and Wolf, 1972; Baker, 1975). Heinrich and Raven (1972) and Forcone et al. (1997) argue that energetic reward for pollinators in habitats with low temperatures is higher than in areas with high temperatures. Cruden et al. (1983) claim that the nectar volume of flowers pollinated by ‘large bees’ (e.g. Bombus, Xylocopa, Centris) has to be significantly higher than that of flowers pollinated by ‘small bees’ (e.g. Colletes, Apis). Because bee pollination seems to phylogenetically precede hummingbird pollination in most plant groups [e.g. Scrophulariaceae: tribe Antirrhineae (Elisens and Freeman, 1988; Ghebrehiwet et al., 2000); Penstemon (Wilson et al., 2006); Mimulus (Fishman et al., 2002; Beardsley et al., 2003); Gesneriaceae: tribe Sinningieae (Perret et al., 2001, 2003)], scientists have variously addressed the question as to how the transition from ‘typical’ bee nectar to ‘typical’ hummingbird nectar took place. Bolten and Feinsinger (1978) argue that the relatively low sugar concentration in hummingbird nectar is not due to a preference for lower sugar concentrations by hummingbirds, but rather serves to render the flowers less attractive to bees. One crucial problem of many of the data sets published on the relationship between nectar and pollination is the comparison of nectar and pollinator data including taxa from distantly related plant groups. Differentiating between adaptive responses and possible phylogenetic constraints is thus difficult. There have been two major studies attempting to elucidate the evolution of nectar characteristics and pollination syndromes within presumably monophyletic plant groups, albeit without an explicit phylogenetic framework. The study on Scrophulariaceae (now Plantaginaceae) Tribe Antirrhineae (20 North American species; Elisens and Freeman, 1988) concentrated on sugar composition, i.e. the relative percentages of different sugars in the nectar, while giving no data on absolute nectar volumes, sugar concentrations or absolute sugar amounts. The study on Gesneriaceae Tribe Sinningieae (45 Neotropical species; Perret et al., 2001) provides data on sugar concentration, and sugar composition, but not on overall nectar production. Both studies show correlations between pollination syndromes and nectar composition, but in neither case is an explicit correlation of pollination syndrome to quantitative nectar features clarified.
The present study intends to compare nectar and pollination syndromes in Loasaceae subfam. Loasoideae, a monophyletic, largely Neotropical plant group of approx. 200 species, with its centre of diversity in the Central Andes (Weigend, 2004a) and with considerable variability in their floral morphology and pollination biology (Urban, 1886, 1889, 1892; Urban and Gilg, 1900; Brown and Kaul, 1981; Weigend, 2004a; Weigend et al., 2004). Representatives of this group are found in many different ecosystems ranging from tropical to temperate rainforests, from coastal lomas formations in the Atacama desert up to 4500 m in the Andes. The phylogeny of this group has been largely clarified (Hufford et al., 2003, 2005; Weigend et al., 2004). Taxa of subfamily Loasoideae share a complex floral morphology: the heterochlamydeous, polyandrous flowers have a highly differentiated androecium with antesepalous stamina modified into staminodial complexes alternating with antepetalous fascicles of (10–28) fertile stamens (Urban, 1886; Weigend, 2004a, Weigend and Gottschling, 2006). The staminodial complexes typically consist of two free, inner staminodia, and three outer, fused staminodia forming the so-called nectar scale. All flowers of Loasoideae are primarily nectar flowers, and pollen presentation is typically triggered by the manipulation of the nectar scale during nectar extraction by the flower visitor (Schlindwein, 2000). Nectar is secreted from the receptacle through antesepalous, inframarginal stomata into the nectar scales, where nectar is stored (Urban, 1886, 1892; Weigend and Rodriguez, 2003; Weigend, 2004b). The nectar is thus hidden from the flower visitor and only accessible through the opening between the apex of the floral scale and the free staminodia, by manipulating the floral scale and tilting it outwards. This functional floral morphology has been described as ‘tilt-revolver flower’ (Weigend and Gottschling, 2006). While this general pattern is fairly universal in Loasoideae, there are major differences in the size and coloration of the overall flower and also in the shape and size of the nectar scales (Weigend et al., 1998, 2003, 2004; Dostert and Weigend, 1999; Rodriguez and Weigend, 1999; Weigend, 2000a, b, 2001, 2004b; Weigend and Rodriguez, 2002, 2003; Weigend and Ackermann, 2003; Weigend and Gottschling, 2006). In some taxa the opening of the much larger floral scales is widened and nectar can be accessed without moving the floral scale. This flower type has recently been described as ‘funnel-revolver flower’ (Weigend, 2004b). Tilt-revolver flowers are characterized by producing very small amounts of very viscous nectar from very small nectaries (Weigend and Rodriguez, 2003), whereas funnel-revolver flowers produce larger amounts of less viscous nectar from much larger nectaries (Weigend, 2004b). Some functional morphological aspects have thus been clarified, but both pollination data and nectar analysis are still scarce for the family.
Pollinator observations have been published for 29 species (from eight genera: Aosa, Blumenbachia, Caiophora, Eucnide, Loasa, Mentzelia, Nasa and Scyphanthus) from the USA, Chile, Argentina and Brazil (Linsley and Hurd, 1959; Thompson and Ernst, 1967; Brown and Kaul, 1981; Keeler, 1981; Arroyo et al., 1982; Stiles and Freeman, 1993; Harter, 1995; Harter et al., 1995; Schlindwein, 1995, 2000; Wittmann and Schlindwein, 1995; Forcone et al., 1997; Schlindwein and Wittmann, 1997; Cocucci and Sérsic, 1998; Medan et al., 2002; Villagrán et al., 2003; Sargent and Otto, 2004; Troncoso and Vargas, 2004), and the reports include various groups of bees (long-tongued bees: Anthophoridae, Apidae, Megachilidae, Mellitidae; short-tongued bees: Colletidae, Halictidae), wasps (Ichneumonidae), flies (Syrphidae), moths (Sphingidae), hummingbirds (Trochilidae), passerines (Emberizidae, Tyrannidae) and small mammals (Muridae), i.e. a considerable range of very different pollinator groups. However, a large proportion of the taxa in Loasoideae are apparently primarily visited by short-tongued bees of a particular group (Colletidae; see Wittmann and Schlindwein, 1995; Weigend, 2004a; Weigend et al., 2004). Ornithophilous taxa in Nasa and Caiophora can be shown to represent derived and largely high Andean clades in originally melittophilous genera from intermediate elevations (Weigend et al., 2004; Weigend and Gottschling, 2006). Nectar analyses had so far been published for only three species of Loasoideae from Argentina and Costa Rica (Stiles and Freeman, 1993; Forcone et al., 1997; Cocucci and Sérsic, 1998). However, Loasaceae subfam. Loasoideae have their centre of diversity, both in terms of taxic richness and morphology, in the Central Andes (Weigend, 2000b, 2002, 2004a–c), and no data sets on either pollinators or nectar have been published from that region.
The present paper intends to fill this gap and provide an overview of nectar composition in subfam. Loasoideae. Nectar composition was studied in cultivated plants under flower visitor exclusion. In Caiophora there are several taxonomically unresolved species complexes comprising closely allied species with differences in floral colour and size (Weigend and Ackermann, 2003). Multiple accessions from these groups, representing different floral morphologies, were studied to investigate possible differences in pollination and nectar composition. In Andean South America the main pollinator groups for Loasoideae are long-tongued and short-tongued bees and hummingbirds, with a single report of small mammals. Assuming that nectar composition correlates with pollinator taxon, a wide range of different nectar types would be expected. The observations on nectar composition are also compared with phylogenetic data compiled from published phylogenies (Weigend et al., 2004; Hufford et al., 2005; Weigend and Gottschling, 2006) to investigate whether there have been multiple convergent changes of nectar composition in the evolution of subfam. Loasoideae. The aims are: (a) to clarify the characteristics of nectar produced by Loasoideae; (b) to provide flower visitor data for additional groups in Loasaceae subfam. Loasoideae from the full range of habitats from the Pacific coast to the high Andean region; (c) to correlate quantity and quality of nectar with overall floral morphology and flower visitors; and (d) to investigate a possible phylogenetic constraints versus adaptive responses on the basis of published systematic and phylogenetic data
MATERIALS AND METHODS
Plant material
Field studies were carried out in Argentina, Chile, Colombia, Ecuador, Peru and Venezuela, where pollinator observations were realized and habitat, growth habit and morphological data were obtained (approx. 200 collections of Nasa; approx. 200 collections of Caiophora; several collections of Blumenbachia, Loasa, Presliophytum and Xylopodia). Approx. 60 species of Loasaceae subfam. Loasoideae were brought into cultivation in the greenhouses at the Institut für Biologie, Freie Universität Berlin (February 2003 to December 2005). Seeds were sown into standard soil for seedlings and later potted into clay pots (potting soil: 2 parts mature leaf compost, 1 part peat). In winter (October–April) artificial light was used in the greenhouses (12 h, high pressure sodium lamps: Philips SON-T AGRO® 400 W). High Andean and south temperate taxa Caiophora, Nasa dillonii, N. macrothyrsa and Loasa sclareifolia were cultivated with night-time temperatures of 5–15 °C and daytime temperatures of 15–25 °C; all other species were grown at night-time temperatures of 18–20 °C and daytime temperatures of 20–25 °C. Cultivation in the greenhouses permitted nectar samples to be obtained under fairly standardized conditions eliminating possible effects of, for example, altitudinal differences, water stress, ambient air humidity (Corbet et al., 1979a, b; Plowright, 1981; Bertsch, 1983; Zimmermann, 1988; Carroll et al., 2001; Pacini et al., 2003). The measurements obtained document the amount and composition of nectar produced in the absence of flower visitors. There are several lines of evidence that argue that the samples obtained from the plants cultivated for this research represent a good proxy to natural conditions:
Published nectar data based on samples collected in nature agree with the present analysis of closely allied species: The field data on Caiophora coronata (Cocucci and Sérsic, 1998) are similar to greenhouse data on the closely allied C. pentlandii (Table 1 and Fig. 1). Field data on Nasa speciosa (Stiles and Freeman, 1993) and Caiophora nivalis (investigated by A. Wertlen) are also close to data for allied taxa from the greenhouse.
The few measurements of nectar volume that were taken in nature [Caiophora carduifolia (3), 20·0–23·5 µL; C. carduifolia (4), 10·5–21·5 µL; C. carduifolia (5), 11·0–60·0 µl; C. carduifolia (6), 13·5–20·0 µL; C. chuquitensis, 10–18·5 µL; C. pentlandii (2), 21·0–49·0 µL; Nasa urens, 0·2–2·0 µL] are close to those obtained from cultivation, but generally lower, probably due to pollinator visits (excluded in the greenhouse).
There is close agreement between floral morphology, pollinator spectra documented in the wild and nectar composition, so that there is no reason to believe that nectar data are grossly aberrant.
Kaczorowski et al. (2005) found that Nicotiana L. section Alatae, species with hummingbird-pollinated flowers show similar nectar composition under greenhouse conditions and in the field.
Several studies of nectar composition across a larger group of closely allied species used greenhouse experiments (Elisens and Freeman, 1988; Perret et al., 2001).
Table 1.
Nectar parameters (means ± s.d.), pollinators, morphological pattern and elevational distribution of Loasaceae subfam. Loasoideae from South America
| Group | Species | No. (in Fig. 1) | Elevation (m) | PL (mm) | Corolla shape | FT | Pollinator | n | Nectar amount (μL) | Concentration (%) | Sugar amount (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | A. rupestris | 1 | 0–2500 (*–3000) | 5 | Star-shaped | T | Co! | 16 | 0·75 ± 0·64 | 51·25 ± 17·05 | 0·34 ± 0·22 |
| B. hieronymi | 2 | 12 | Star-shaped | T | Co! + Le! + Be! + Hu! | 36 | 0·91 ± 0·70 | 72·95 ± 13·03 | 0·68 ± 0·57 | ||
| B. insignis | 3 | 15 | Star-shaped | T | Co! + Meg! + Hal! | 7 | 0·34 ± 0·19 | 66·76 ± 18·35 | 0·24 ± 0·18 | ||
| C. nivalis3 | 4 | 11 | Star-shaped | T | Be | 15 | 0·71 ± 0·51 | 40·18 ± 7·57 | 0·26 ± 0·16 | ||
| L. gayana | 5 | 16 | Star-shaped | T | Be | 16 | 0·74 ± 0·65 | 63·55 ± 15·27 | 0·41 ± 0·25 | ||
| N. moroensis | 6 | 14 | Star-shaped | T | Co! | 17 | 1·70 ± 0·99 | 57·23 ± 13·83 | 0·99 ± 0·63 | ||
| N. picta* | 7 | 15 | Star-shaped | T | Co! + Bo! | 6 | 0·55 ± 0·28 | 83·00 ± 4·10 | 0·46 ± 0·24 | ||
| N. poissoniana | 8 | 14 | Star-shaped | T | Co | 16 | 1·47 ± 0·82 | 66·51 ± 10·21 | 0·95 ± 0·54 | ||
| N. triphylla ssp. flavipes | 9 | 15 | Star-shaped | T | Co | 7 | 2·14 ± 1·45 | 51·40 ± 22·05 | 1·03 ± 0·70 | ||
| N. triphylla ssp. triphylla | 10 | 18 | Star-shaped | T | Co | 6 | 0·67 ± 0·15 | 68·62 ± 12·24 | 0·45 ± 0·13 | ||
| N. triphylla spec nov. ined. | 11 | 15 | Star-shaped | T | Co | 6 | 1·62 ± 0·70 | 62·30 ± 8·17 | 0·98 ± 0·40 | ||
| N. urens | 12 | 18 | Star-shaped | T | Co! | 9 | 3·66 ± 2·03 | 51·13 ± 4·07 | 1·87 ± 1·02 | ||
| N. vargasii | 13 | 16 | Star-shaped | T | Co! | 16 | 1·04 ± 1·23 | 65·94 ± 11·84 | 0·68 ± 0·79 | ||
| P. arequipensis | 14 | 16 | Star-shaped | T | Le! + Xy! + Hu! | 1 | 1·20 | 16·30 | 0·20 | ||
| X. klaprothioides* | 15 | 11 | Star-shaped | T | Co! | 18 | 0·28 ± 0·19 | 69·67 ± 7·90 | 0·19 ± 0·13 | ||
| II | N. dyeri ssp. australis | 16 | 0–1500 | 20 | Star-shaped | T | Co! | 11 | 9·88 ± 6·04 | 39·77 ± 9·45 | 3·85 ± 2·17 |
| L. sclareifolia | 17 | 18 | Saucer-shaped | T | Co | 11 | 9·36 ± 1·28 | 62·16 ± 5·77 | 5·79 ± 0·77 | ||
| P. incanum | 18 | 0–2500 | 17 | Star-shaped | T | Co! + Le! + Be! + Hu! | 22 | 14·34 ± 4·60 | 55·50 ± 7·05 | 7·94 ± 2·58 | |
| C. cirsiifolia (1) | 19 | 2500–3500 | 19 | Saucer-shaped | T | Ce! + Bo! | 7 | 11·43 ± 7·34 | 65·71 ± 3·15 | 7·35 ± 4·31 | |
| C. cirsiifolia (2) | 20 | 21 | Bowl-shaped | T | Ce! | 6 | 17·33 ± 11·31 | 58·58 ± 6·18 | 10·27 ± 7·02 | ||
| C. cirsiifolia (3) | 21 | 17 | Bowl-shaped | T | Ce! + Bo! | 29 | 11·97 ± 6·51 | 44·14 ± 9·52 | 4·97 ± 2·30 | ||
| C. grandiflora (1) | 22 | 18 | Balloon-shaped | T | Hu! + Bo! | 10 | 11·41 ± 7·98 | 44·00 ± 22·93 | 4·70 ± 3·00 | ||
| C. grandiflora (2) | 23 | 18 | Balloon-shaped | T | Hu! + Bo! | 11 | 11·36 ± 4·65 | 49·68 ± 18·05 | 5·30 ± 2·25 | ||
| C. lateritia | 24 | 20 | Bowl-shaped | F | Hu | 5 | 9·70 ± 5·37 | 63·40 ± 2·38 | 6·09 ± 3·28 | ||
| III | L. acanthifolia | 25 | 500–1000 (–1500) | 18 | Bowl-shaped | T | Bo! + Co! | 6 | 46·83 ± 17·57 | 54·00 ± 9·52 | 25·41 ± 11·35 |
| N. dillonii | 26 | 29 | Bell-shaped | F | Hu? | 40 | 53·18 ± 23·35 | 31·40 ± 6·28 | 17·10 ± 8·98 | ||
| N. olmosiana | 27 | 25 | Balloon-shaped | F | ? | 65 | 54·17 ± 21·38 | 29·38 ± 5·89 | 15·88 ± 7·12 | ||
| P. heucheraefolium | 28 | 25 | Star-shaped | T | Xy | 17 | 50·06 ± 13·38 | 38·06 ± 8·04 | 19·71 ± 9·05 | ||
| C. canarinoides | 29 | (**2000–) 3000–4000 | 40 | Bell-shaped | F | Hu! | 14 | 50·32 ± 24·02 | 41·73 ± 12·97 | 21·15 ± 11·75 | |
| C. carduifolia (1) | 30 | 21 | Bowl-shaped | T | Hu! | 11 | 69·59 ± 35·21 | 34·14 ± 14·46 | 24·96 ± 20·09 | ||
| C. carduifolia (2) | 31 | 23 | Bowl-shaped | T | Hu! | 7 | 75·86 ± 32·18 | 49·00 ± 7·30 | 35·91 ± 12·75 | ||
| C. chuquitensis | 32 | 22 | Balloon-shaped | F | Hu! | 6 | 101·83 ± 28·47 | 32·47 ± 9·74 | 31·62 ± 8·79 | ||
| C. cirsiifolia (4) | 33 | 30 | Saucer-shaped | T | Ce! + Bo! | 19 | 42·53 ± 14·12 | 44·47 ± 10·14 | 19·02 ± 7·37 | ||
| C. cf. superba | 34 | 26 | Balloon-shaped | F | Ce! | 19 | 51·03 ± 24·32 | 29·91 ± 9·67 | 14·08 ± 6·00 | ||
| C. cf. madrequisa | 35 | 18 | Bell-shaped | F | Hu | 8 | 45·44 ± 22·03 | 41·19 ± 8·62 | 17·90 ± 7·56 | ||
| N. macrothyrsa** | 36 | 32 | Star-shaped | T | Xy! + Co! | 64 | 75·13 ± 28·98 | 32·39 ± 10·61 | 24·04 ± 11·51 | ||
| N. speciosa2 | 37 | 55 | Bell-shaped | F | Hu! | 8 | 36·00 | 18·83 | 6·78 | ||
| IV | C. coronata1 | 38 | 3500–4500 | 30 | Bowl-shaped | F | Ma! + Be! + Hu! + Pa! | 79·90 ± 39·65 | 14·88 | 11·90 | |
| C. pentlandii (1) | 39 | 30 | Bowl-shaped | F | Ma? | 18 | 87·39 ± 25·53 | 9·78 ± 2·05 | 8·61 ± 3·23 | ||
| C. pentlandii (2) | 40 | 30 | Bowl-shaped | F | Ma? | 9 | 79·78 ± 54·21 | 12·90 ± 3·00 | 10·19 ± 6·66 |
PL, petal length; FT, floral type (see also Fig. 3); n, number of investigated flowers. Groups: I, short-tongued bee-pollinated; II, long-tongued bee and hummingbird-pollinated; III, hummingbird-pollinated; IV, mammal-pollinated. Genus names: A., Aosa; B., Blumenbachia; C., Caiophora; L., Loasa; N., Nasa; P., Presliophytum; X., Xylopodia. Floral type: T, tilt-revolver flower sensu Weigend and Gottschling (2006); F, funnel-revolver flowers sensu Weigend (2004b). Pollinator: Be, bee; Bo, Bombus; Ce, Centris; Co, colletids; Hal, Halictidae; Hu, Hummingbird; Le, Lepidoptera; Ma, Mammals; Meg, Megachilidae; Pa, Passerines; Xy, Xylocopa. !, direct observation in the field; abbreviation + ?, doubtful; ?, unknown. Literature data: 1, Cocucci and Sérsic (1998); 2, Stiles and Freeman (1993); 3, unpublished data from A. Wertlen, 2003. Asterisks next to species correspond to those next to elevation.
Fig. 1.
Ternary plot illustrating the relationships between nectar production (NP), sugar concentration (SC) and sugar production (SP) of some species of Loasaceae subfam. Loasoideae (data set from Table 1 is plotted in percentages, numbers and groups I–IV in diagram correspond to numbering and grouping in Table 1); e.g. Blumenbachia hieronymi, no. 2 in Table 1: original data: 0·91 NP + 72·95 SC + 0·68 SP = 100 %, thus calculated percentages = 1·22 % (NP) + 97·87 % (SC) + 0·91 % (SP). Four groups are recognizable: (I) SC high, NP and SP low; (II) SC, NP and SP between groups I and III; (III) SC lower than in group II, NP and SP high; IV) SC very low, NP high and SP lower than in group III. *, published data from Cocucci and Sérsic (1998), no. 38; Stiles and Freeman (1993), no. 37; and unpublished data from A. Wertlen (2003), no. 4.
Total nectar amount of individual flowers
The entire amount of nectar present in each flower was harvested by inserting micro-capillaries between the two staminodia and the floral scale (micro capillaries: 1- and 2-µL Microcaps; Drummond Scientific Co., Broomall, PA, USA; 5, 10 and 25-µL Duran Ringcaps; Hirschmann Laborgeräte, Eberstadt, Germany). Nectar was harvested twice from each floral scale within 5 min to obtain the full amount of nectar. Brix measurements were then made with a handheld refractometer (neoLab-Handrefraktometer Universal; 10–80 % Brix). Small amounts of nectar (mostly highly concentrated and therefore highly viscous as in all species of group I) was pipetted into 1 µL distilled water on the refractometer for measurements and the concentration was calculated for the original amount.
Nectar from 607 flowers (15·97 flowers mean per species, 14·23 s.d.) from 31 species (including three subspecies, 37 accessions in total) of seven genera (Aosa, Blumenbachia, Caiophora, Loasa, Nasa, Presliophytum and Xylopodia) was analysed, including multiple accessions of heterogeneous species complexes such as the Caiophora cirsiifolia- and C. carduifolia-aggregates. A complete list of the accessions used for the nectar analysis including all authors of plant names is given in the Appendix. Multiple accessions of individual species are differentiated by Arabic numerals in brackets behind the species and name throughout the text and in the appendix. Nectar data were all taken during the first half of the staminate phase to ensure that the data are comparable. Sugar concentration (%) and nectar volume (µL) were measured and total sugar production (mg) calculated for the individual flowers. Mean values and standard deviations were calculated for all flowers of one accession. To visualize the correlation between the three data sets (total amount of nectar, total amount of sugar and sugar concentration) the percentage of each value (mean value) was calculated relative to the total amount of nectar data (µL nectar + % sugar + mg sugar = 100 %) and these data then plotted, with Sigmaplot (for windows vers. 8·0, SPSS Inc. 2002) in a ternary plot. This plot is here favoured over a two-dimensional plot, since it pulls the individual data sets apart much more clearly and is thus better suited to illustrate the divergence of nectar characteristics. Two data sets published elsewhere were included in the ternary plot (Nasa speciosa = as ‘Loasa spectabilis’, Stiles and Freeman, 1993; Caiophora coronata, Cocucci and Sérsic, 1998) and also the unpublished data set of Caiophora nivalis, analysed in Argentina by Anna Wertlen (Institut für Biologie, Neurobiologie, Freie Universität Berlin, Germany).
Pollinator observations
Qualitative data on flower visitors were obtained in Peru and Ecuador (see Table 1 for observations and Appendix for dates and localities), observation times typically ranged from 60 to 90 min per species and location and were performed in clear weather only (typically between 0900–1200 h and 1600–1800 h). Hymenopteran flower visitors were captured and determined by D. Wittmann (Institut für Landwirtschaftliche Zoologie und Bienenkunde der Universität Bonn, Germany) and C. Schlindwein (Universidade Federal de Pernambuco, Departamento de Botânica, Brazil), where the insects are also deposited. Determination to species was usually not possible, but the taxonomy of the visitors is given to family rank. Hummingbird observations were noted in the field book as means of documentation without identification to species.
Correlates of nectar production
Floral morphology, elevational distribution and pollinator observations are summarized in Table 1 to permit a direct comparison of nectar composition to the other data sets. Figure 2 provides a consensus diagram of Loasaceae subfam. Loasoideae based on various published phylogenies (Weigend et al., 2004; Hufford et al., 2005; Weigend and Gottschling, 2006) where each species analysed is assigned to a ‘nectar group’ and gross floral morphology (based on Weigend, 2004b; Weigend and Gottschling, 2006).
Fig. 2.
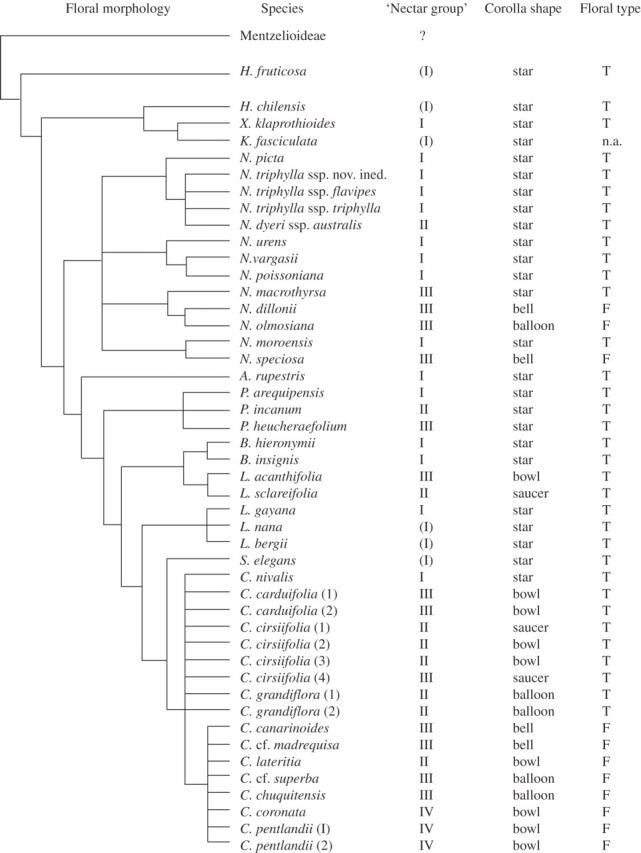
Consensus phylogeny of Loasaceae subfam. Loasoideae (based on Weigend et al., 2004; Hufford et al., 2005; Weigend and Gottschling, 2006) with nectar groups, corolla shape (corresponding to Table 1 and Fig. 3) and flower type (T, tilt-revolver flowers, F, funnel-revolver flowers; see also Table 1). Clades with <50 bs in these three studies collapsed. Genus names: A., Aosa; B., Blumenbachia; C., Caiophora; H., Huidobria; K., Klaprothia; L., Loasa; N., Nasa; P., Presliophytum; S., Scyphanthus; X., Xylopodia. Nectar groups I–IV (see Table 1 and Fig. 1): ?, many Mentzelia species with pollen flowers and no nectar, representative data on nectariferous taxa not available; (I), tiny amounts of highly viscose nectar observed, but no measurements available. n.a., not applicable.
RESULTS
Total nectar amount
Table 1 summarizes the data on nectar quantities, concentrations and sugar amounts. The amounts of nectar secreted per flower range from 0·3 to 100 µL, the concentrations from 10–83 % and the total amounts of sugar provided per flower from 0·19 to 36 mg. These widely variable data can be roughly grouped into four classes (correlation of the data visualized in Fig. 1 in the form of a ternary plot). The amounts of nectar are discontinuously distributed and measurements mostly fall into the following ranges: group I, 0·3–1·5(–3·5) µL (1·19 µL mean, 0·87 s.d.); group II, 9–14(–17) µL (11·86 µL mean, 2·54 s.d.); groups III and IV, 40–100 µL (III, 57·84 µL mean, 17·99 s.d.; IV, 82·36 µL mean, 4·36 s.d.). Roughly the same groups are retrieved from sugar concentration [group I, (40–)60–80 % (59·12 % mean, 15·89 s.d.); group II, 40–60(–65) % (53·66 % mean, 9·57 s.d.)] but those taxa with 50–100 µL of nectar per flower fall into two subgroups with widely different concentrations [group III, 30–40(–55) % (36·69 % mean, 9·35 s.d.); group IV, 10–15 % (12·52 % mean, 2·57 s.d.)]. These patterns are reflected in the overall amount of sugar offered by the flowers, which falls into group I, 0·19–2 mg (0·65 mg mean, 0·46 s.d.); group II, 4–10 mg (6·25 mg mean, 1·97 s.d.); group III, 14–36 mg (22·23 mg mean, 6·51 s.d.); and group IV, 8·5–12 mg (9·40 mg mean, 1·12 s.d.).
Altitudinal distribution, floral morphology and pollinators
The four nectar groups retrieved from nectar data roughly correspond to the morphological and ecological data. The corolla shapes as here defined are illustrated in Fig. 3. Floral display depends on both flower shape and petal length (Fig. 3 and Table 1; mean value and s.d. for petal lengths: group I, 14·07 ± 3·28; group II, 18·67 ± 1·41; group III, 28·00 ± 10·11; group IV, 30·00). Bowl-shaped flowers have a much larger floral display than star-shaped flowers with the same petal length. Petal size in combination with corolla shape is therefore here used as a proxy for display size. In general terms there is more nectar in (a) more closed flower types (versus more open), and flowers with (b) larger (versus smaller) petals. Also, highly concentrated nectar in small amounts (group I) is found only in tilt-revolver flowers and very dilute nectar in huge amounts (group IV) only in funnel-revolver flowers. Group II nectar is more often found in tilt-revolver flowers than in funnel-revolver flowers (six versus three taxa) and group III nectar is found roughly as often in tilt-revolver as in funnel-revolver flowers (six versus seven taxa).
Group I: mainly low-elevation plants (mostly <2500 m) with relatively small, typically white, star-shaped flowers [petals approx. (5–)12–18 mm long; Table 1 and Fig. 3A–D]. Flowers of this group are predominantly visited and pollinated by short-tongued bees, mostly colletid bees. Only Nasa picta and Xylopodia klaprothioides range into higher elevations.
Group II: mid-elevation plants (2500–3500 m) often with larger, more closed, mostly orange or red flowers (petals approx. 17–21 mm long; Table 1 and Fig. 3E, F, bell-, balloon-, saucer- or bowl-shaped). Both long-tongued bees and hummingbirds have been documented as flower visitors of that group. The only taxa which are aberrant in pollinator visitor (colletid bees) and elevational distribution (0–1500 m) for this group are Loasa sclareifolia, N. dyeri ssp. australis and Presliophytum incanum.
Group III: mostly high elevation plants (3000–4000 m) and a few species from low elevations (500–1500 m), with some of the largest flowers in the subfamily, flowers are largely closed and orange, red or rarely yellow (petals up to 55 mm long; Table 1 and Fig. 3G–I, L, bell-, bowl- or balloon-shaped). Only exceptions are Nasa macrothyrsa and Presliophytum heucheraefolium from lower elevations with white, star-shaped flowers. Hummingbirds are likely to be the most important flower visitors of this group, but long-tongued bees (Centris, Bombus and Xylocopa) have also been observed, often on the same plant species.
Group IV: only two decumbent high elevation taxa (>3500 m) with large petals (approx. 30 mm long; Table 1 and Fig. 3J, K), either white or orange-red bowl-shaped flowers (similar to types also found in group III). While it has not been possible to document flower visitors in the field, there is one publication indicating that small rodents may be the principal pollinators for one of the two taxa (C. coronata; Cocucci and Sérsic, 1998).
Fig. 3.

Flower morphology in Loasaceae subfam. Loasoideae. (A–F) Tilt-revolver flowers: (A–D) star-shaped flowers (A, B, Nasa moroensis; C, Presliophytum incanum; D, Blumenbachia insignis); (E, F) saucer-shaped flower of Caiophora cirsiifolia (1). (G–L) Funnel-revolver flowers (G, H, balloon-shaped-flower of Caiophora chuquitensis; I, L, bell-shaped flower of Caiophora canarinoides; J, K, bowl-shaped flower of Caiophora pentlandii).
DISCUSSION
Overall nectar and sugar production in relation to pollination syndrome
Sugar concentrations here reported are generally higher than most literature data (both for bee- and hummingbird-pollinated flowers; Baker, 1975; Bolten and Feinsinger, 1978; Bolten et al., 1979; Pyke and Waser, 1981; Cruden et al., 1983; Heyneman, 1983; Forcone et al., 1997; Galetto et al., 1998; Bernardello et al., 2000; Blem et al., 2000; Chalcoff et al., 2006; Wilson et al., 2006) and this may be an idiosyncratic phenomenon of Loasaceae. Group III may be predominantly hummingbird-pollinated, but the hummingbird-pollinated taxa studied here have unusually high sugar concentrations in the nectar (Table 1 and Fig. 1, nos 29, 31, 33 and 35, 30–55 % as compared with the ‘typical’ 20–26 %; Baker, 1975; Cruden et al., 1983). Heinrich and Raven (1972) and Forcone et al. (1997) argue that ‘highland hummingbird nectar’ should be less viscous and less concentrated, but this is apparently not true in Loasaceae. Higher than typical sugar concentrations in flowers pollinated by hummingbirds have also been found for hummingbird nectar by Kaczorowski et al. (2005) in Nicotiana sect. Alatae. This might be due to the high Andean habitat and the therefore high energy requirements of the birds: Heinrich and Raven (1972) and Forcone et al. (1997) argue that, in low temperatures, energetic rewards for hummingbirds must be higher than in high temperatures. Higher concentrations in this group of taxa may also be due to the fact that the flowers of at least some of the taxa concerned [e.g. Caiophora cf. superba, C. cirsiifolia (4), Loasa acanthifolia, Nasa macrothyrsa and Presliophytum heucheraefolium] are often also visited by long-tongued bees and there may be no reason for the plant to exclude them as flower visitors (Bolten and Feinsinger, 1978). Interestingly, there is a single data set from an ornithophilous species of Nasa from Costa Rica (N. speciosa; Stiles and Freeman, 1993) which has the typical, relatively low sugar concentration of hummingbird nectar (Heinrich and Raven, 1972; Forcone et al., 1997).
Pollination, nectar and elevation
Comparing the four groups defined above, it becomes apparent that there is a trend towards higher nectar volume and higher total amount of sugar per flower at increasing elevations, i.e. bird- and mammal-pollinated taxa are largely high-Andean (groups III and IV), whereas the taxa pollinated by short-tongued bees are found at low and intermediate elevations (group I). At elevations above approx. 3500 m only the two genera Nasa and Caiophora are present in the Andes, and both with species where hummingbird pollination predominates among the taxa.
Evolution of nectar characteristics and pollination syndromes
Figure 2 shows a phylogeny of Loasoideae together with the assignment of terminal taxa to nectar group and gross floral morphology. It appears that group I nectar represents the plesiomorphic condition and this agrees with the previously published hypothesis that pollination by short short-tongued bees (especially colletid bees) is the plesiomorphic condition in the subfamily (Weigend et al., 2004; Weigend and Gottschling, 2006). Evolution towards higher amounts of more dilute nectar appears to have happened several times: (a) at least twice in Nasa (in the Nasa triphylla group and at least once in the N. macrothyrsa–N. speciosa clade); (b) in Presliophytum; (c) in the Blumenbachia–Loasa acanthifolia clade; and (d) at least once in the Loasa gayana–Caiophora clade.
The transitions towards more dilute nectar took place without a transition towards funnel-revolver flowers in the Blumenbachia–Loasa acanthifolia clade and Presliophytum. It seems to be phylogenetically correlated with the transition from tilt-revolver flowers to funnel-revolver flowers in the Loasa gayana–Caiophora clade and in the Nasa macrothyrsa–N. speciosa clade. The nectar and pollinator data here presented show that the repeated morphological transformations of Loasoideae flowers from small, bee-pollinated flowers to large, bird-pollinated flowers (Weigend et al., 2004; Weigend and Gottschling, 2006; see also corolla shapes and petal lengths in Table 1) were likely preceded by changes in nectar composition. The evolution of a different nectar type (‘nectar group’) as a means of recruiting different pollinators seems to be a rapid process in relative terms. There are considerable differences in nectar production between closely allied taxa with morphologically very similar flowers (Nasa triphylla group, C. carduifolia complex, C. cirsiifolia complex). Vastly different forms of nectar production can apparently evolve with relative ease and nectar production (in terms of both absolute amounts and concentration) appears to evolve more rapidly than functional floral morphology in Loasaceae subfam. Loasoideae.
Acknowledgments
We would like to express out sincere gratitude to M. Achatz, P. Beckers, G. Brokamp, N. Cusimano, G. Fröhlich, S. Grossmann, T. Henning, P. Kramer, B. Nordt, N. Nürk, N. Poser, E. Scherer, C. Schneider, C. Schwarzer and A. Tais (Berlin, Germany) for obtaining nectar from flowers and measuring sugar concentration. We would like to thank Natalie Cusimano (Berlin, Germany) for help with calculating the ternary graphs with Sigma Plot, Anna Flügge (Berlin, Germany) for the data set from Caiophora nivalis, as well as Natalie Hempel de Ibarra (Berlin, Germany) for methodological advice and helpful discussions. We thank A. Cano and M. I. La Torre (Lima, Peru), Eric Rodriguez (Trujillo, Peru), N. Salinas (Cuzco, Peru), G. Vobis and C. Ezcurra (Bariloche, Argentina), H. Förther (München, Germany), N. Dostert, T. Henning, D. Kollehn, O. Mohr, C. Schwarzer and K. Weigend (Berlin, Germany) for help in the field and collecting seeds. We thank D. Wittmann (Institut für Landwirtschaftliche Zoologie und Bienenkunde der Universität Bonn, Germany) and C. Schlindwein (Universidade Federal de Pernambuco, Departamento de Botânica, Brazil) for identifying the captured hymenopteran pollinators. The funds kindly provided by the following institutions at various stages of the project are gratefully acknowledged: Studienstiftung des Deutschen Volkes (1992–1997), Deutscher Akademischer Austauschdienst (1999–2000), Lewis B. and Dorothy Cullman Laboratory for Molecular Systematics Studies at the New York Botanical Garden (1999–2000), Deutsche Forschungsgemeinschaft (Grant-nr.WE 2330/1, 2001–2003), botconsult GmbH (1999–present).
APPENDIX 1
Voucher data (multiple accessions of species are differentiated by Arabic numeral in brackets in list and throughout the text)
Aosa rupestris (Hook.) Weigend—cultivated from seeds collected in Bahía, Brazil by S. Vogel, Vienna, M. Weigend 7138 (B, M, BM).
Blumenbachia hieronymi Urb.—cultivated plants from Botanical Garden Berlin-Dahlem, 27 September 2004, M. Ackermann 601 (BSB).
Blumenbachia insignis Schrad.—cultivated plants from Botanical Garden Berlin-Dahlem, 27 September 2004, M. Weigend 7475 (BSB).
Caiophora canarinoides (Lenné & C.Koch) Urb. & Gilg—Peru, Depto. Puno, Prov. Sandia, road from Cuyocuyo passing Banos de Cuyocuyo, old Inca trail, 14°28′S, 69°32′W, 3550 m, 25 September 2002, M. Ackermann 395 (BSB, HUSA, M, USM).
Caiophora carduifolia C.Presl (1)—Peru, Depto. Apurimac, Prov. Andahuaylas. road from Abancay to Andahuaylas, 86 km, 13°41′S, 73°8′W, 3700 m 16 February 2000, M. and K. Weigend 2000/326 (HUSA, NY).
Caiophora carduifolia C.Presl (2)—Peru, Depto. Cuzco, Prov. Paucartambo, SE from Cuzco, from Saylla to Paucartambo, village of Huancarani, 13°30′S, 071°38′W, 3880 m, 17 September 2002, M. Ackermann & N. Salinas, 333 (BSB, HUSA, M, USM).
Caiophora carduifolia C.Presl (3)—Peru, Depto. Cuzco, Prov. Calca, road from Calca to Lares, after Rancal, 13°12′S, 71°56′W, 4310 m, 11 September 2002, M. Ackermann et al. 554 (BSB, HUSA, M).
Caiophora carduifolia C.Presl (4)—Peru, Depto. Cuzco, Prov. Urubamba, road from Urubamba to Quillabamba, between Ollantaytambo and Abra Malaga, 13°12′S, 72°17′W, approx. 3500 m, 12 September 2002, M. Ackermann and D. Kollehn 288 (BSB, HUSA, M, USM, NY, F).
Caiophora carduifolia C.Presl (5)—Peru, Depto. Cuzco, Prov. Cuzco, SE from Cuzco, from Saylla on small road to the ruins of Tipon, 13°34′S, 71°47′W, 3440 m, 17 September 2002, M. Ackermann and N. Salinas 329 (BSB, HUSA, M).
Caiophora carduifolia C.Presl (6)—Peru, Depto. Cuzco, Prov. Cuzco, road from San Jeronimo to Huacoto (small street to the east), fields near Huacoto, 13°30′S, 71°51′W, 4130 m, 13 September 2002, M. Ackermann and N. Salinas 296 (BSB, HUSA, M, USM, NY, F).
Caiophora cf. madrequisa Killip—Peru, Depto. Puno, Prov. Paucartambo, road from Paucartambo to Tres Cruces, Parque Nacional Manu, 13°10′S, 71°36′W, 3050 m, 18 September 2002, M. Ackermann 356 (BSB, HUSA, M, NY,USM).
Caiophora cf. superba Phil.—Peru, Depto. Moquegua, Prov. General Sanchez Cerro, between Puwuina and Omate, last road bends before the descent to Omate, near Charijon, approx. 3000 m, 21 May 2003, M. Weigend et al. 7761 (BSB).
Caiophora chuquitensis (Meyen) Urb. & Gilg (1)—Peru, Depto. Cuzco, Prov. Calca, road from Calca to Lares, after Rancal, 13°10′S, 71°57′W, 4000 m, 11 September 2002, M. Ackermann et al. 274 (BSB, HUSA, M).
Caiophora cirsiifolia C.Presl (1)—Peru, Depto. Arequipa, Prov. Arequipa, environment of Chiquata, east from Arequipa, 16°24′S, 71°22′W, 3100 m, 1 October 2002, M. Ackermann et al. 420 (BSB, HUSA, M, USM, NY, F).
Caiophora cirsiifolia C.Presl (2)—Peru, Depto. Lima, Prov. Yauyos, Road from Yauyos to Jauja, after Tomas, 12°17′S, 75 48′W, approx. 2300 m, 7 October 2002, M. Weigend et al. 7260 (BSB, HUSA, USM, M, NY).
Caiophora cirsiifolia C.Presl (3)—Peru, Depto. Cajamarca, Prov. Santa Cruz, La Florida, above Monteseco, 1200–1500 m, 5 May 2003, M. Weigend et al. 7559 (BSB).
Caiophora cirsiifolia C.Presl (4)—Peru, Depto. Ancash, Prov. Huarez, Rio Grande/Rio Chaccan, towards Pariacoto, 18L 0200571 UTM 8942645, 2999m, 16 May 2003, M. Weigend et al. 7697 (BSB, USM).
Caiophora grandiflora (G.Don) Weigend & Mark. Ackermann (1)—Peru, Depto. Cajamarca, Prov. San Miguel, road San Miguel to Tongad (Sta. Rosa—Hualgayoc), 6°46′S, 78°38′W, 3986 m, 2 May 2003, M. Weigend et al. 7509 (BSB, USM).
Caiophora grandiflora (G.Don) Weigend & Mark. Ackermann (2)—Peru, Depto. Cajamarca, Prov. Hualgayoc, 6°48′S, 78°57′W, 3600 m, 2 May 2003, M. Weigend et al. 7510 (BSB, USM).
Caiophora lateritia Benth.—cultivated plants from Botanical Garden Berlin Dahlem, 1 August 2004, M. Ackermann 603 (BSB).
Caiophora nivalis Lillo, Argentina, Prov. Mendoza, Vallecitos. 2826 m, 32°58′S 69°21′W, 8–18 January 2003, A. A. Cocucci et al. 2219 (CORD).
Caiophora pentlandii (Paxton) G.Don ex Loudon (1)—Peru, Depto. Puno, Prov. Melgar, road from Sicuani to Nunoa, approx. 3 km before Nunoa, 14°31′S, 70°37′W, 4000 m, 20 September 2002, M. Ackermann 360 (BSB, F, HUSA, M, NY, USM).
Caiophora pentlandii (Paxton) G.Don ex Loudon (2)—Peru, Depto. Puno, Prov. Puno, road from Puno to Juliaca, Ruins of Sillustani, 15°43′S, 70°9′W, 3880 m, 23 September 2002, M. Ackermann 366 (BSB, F, HUSA, M, NY, USM).
Loasa acanthifolia Desr. var. albomaculata Gunckel—Argentina, Prov. Neuquen, Depto. Aluminé, road N of Lago Quillén towards Lago Hui Hui, 39°22′S, 71°14′W, 1050 m, 17 January 2002, M. Weigend et al. 6925 (BRCO, BSB, M).
Loasa gayana Urb. & Gilg—Chile, X. Región, Los Lagos, road entre Lagos and Osorno, 25 km E of Osorno, approx. 2 km N of road, entrance to Fundo Los Pellines, 40°35′S, 72 50′W, 132 m, 3 February 2002, M. Weigend et al. 7057 (B, M, NY).
Loasa sclareifolia Juss.- Chile, VIII Región del Bío Bío, Prov. de Ñuble, east of San Fabián de Alico, orig. collection J. Grau, July 2005, M. Weigend 8183 (BSB, M).
Nasa dillonii Weigend—Peru, Depto. Cajamarca, Prov. Santa Cruz: La Florida, above Monteseco, 1200–1500 m, 5 May 2003, M. Weigend et al. 7556 (B, USM).
Nasa dyeri (Urb. & Gilg) Weigend ssp. australis Dostert & Weigend—Peru, Depto. Amazonas, Prov. Bagua, trail from La Peca to El Arenal, just above El Arenal, 1200 m, April 1998, N. Dostert 98/80 (M, USM).
Nasa macrothyrsa (Urb. & Gilg ) Weigend—Peru, Depto Cajamarca, Prov. San Miguel, one of the last road bends before San Miguel, 7°0′S, 78°51′W, 2517 m, 30 April 2003, M. Weigend et al. 7471 (BSB, HUT, USM).
Nasa moroensis Weigend—Peru, Depto. Ancash, Prov. Huaylas, Rio Grande/Río Chacchan, 2143 m, 16 May 2003, M. Weigend et al. 7694 (BSB, HUT, M, USM).
Nasa olmosiana (J.F.Macbr.) Weigend—Depto. Cajamarca, Prov. Santa Cruz, road from Monte Seco to Espinal, close to turn off to La Florida, 600–800 m. 7 March 1998 to 9 May 1998, Nicolas Dostert 98/163-C (BSB, M).
Nasa picta (Hook.f.) Weigend—Peru. Depto. Cajamarca. Prov. Chota, Huambos, 93 km from Chota on road Huambos, Llama Chiclayo, 2300 m. 14 May 1998, M. Weigend and N. Dostert 98/158 (M, USM).
Nasa poissoniana (Urb. & Gilg) Weigend—Peru, Depto. La Libertad, Prov. Pataz, road Buldibuyo to Tayabamba, 8°07′S, 77°23′W, 3163 m, 24 April 2004, M. Weigend and Ch. Schwarzer 8007 (B, USM).
Nasa triphylla (Juss.) Weigend ssp. flavipes Weigend & Dostert—Peru, Depto. Piura, Prov. Huancabamba, due west of town, 1700–1900 m, May 1998, M. Weigend and N. Dostert 98/203 (M, USM).
Nasa triphylla (Juss.) Weigend ssp. triphylla—cultivated plants from Botanical Garden Berlin Dahlem, 1 August 2004, M. Ackermann 602 (BSB).
Nasa triphylla (Juss.) Weigend ssp. nov. ined.—Peru, Depto. La Libertad, Prov. Sanchez Carrion, road Huamachuco to Chagual—Pataz, after Chugay and between Molino Viejo and Aricapampa, 7°48′S, 77°41′W, 2389 m, 20 April 2004, M. Weigend & Ch. Schwarzer 7913 (B, USM).
Nasa urens (Jaq.) Weigend—Peru, Depto. Lima, Prov. Yauyos, road from Quilmana to Panamericana, 122 km on Panamericana, Lomas de Quilmana, 12°57′S, 76°26′W, approx. 320 m, 8 October 2002, M. Weigend et al. 7327 (BSB, HUSA, USM, M, NY).
Nasa vargasii (Macbr.) Weigend—Peru, Depto. Huánuco. Prov. Ambo. Road from Huánuco to Cerro de Pasco, 27·3 km from Ambo. 2300 m. 10°11′S, 76°10′W, 3 April 2001, M. Weigend et al. 5463 (HUT, B, M, USM).
Presliophytum arequipensis Weigend—Peru, Depto. Moquegua, Moquegua on road to Torata, 1855 m, 13 October 1997, M. Weigend and H. Förther 97/848 (M, USM).
Presliophytum heucheraefolium (Killip) Weigend—Peru, Depto. Ancash, Prov. Huaylas, Rio Grande/Río Chacchan, 18L 0181840 UTM 8941740, 16 May 2003, M. Weigend et al. 7691 (BSB, USM).
Presliophytum incanum (Graham) Weigend—Peru, Depto. Lima, Prov. Huarochiri, Matucana, 2400 m, M. Weigend and N. Dostert 97/12 (M, USM).
Xylopodia klaprothioides Weigend—Peru, Depto. Cajamarca, Prov. Contumazá, road Contumaza to Chilete, first road bend after highest point of pass. 2900 m. April 1997, M. Weigend et al. 97/450 (M, USM).
LITERATURE CITED
- Arroyo M, Primack R, Armesto J. 1982. Community studies in pollination ecology in the high temperate Andes of Central Chile. I. Pollination mechanisms and altitudinal variation. American Journal of Botany 69: 82–97. [Google Scholar]
- Baker HG. 1975. Sugar concentrations in nectars from hummingbird flowers. Biotropica 7: 37–41. [Google Scholar]
- Baker HG, Baker I. 1982. Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. In: Nitecki HM, ed. Biochemical aspects of evolutionary biology. Chicago, IL: Chicago University Press, 131–171.
- Baker HG, Baker I. 1983. Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York, NY: Van Nostrand Reinhold, 117–141.
- Beardsley PM, Yen A, Olmstead RO. 2003. AFLP phylogeny of Mimulus section Erythranthe and the evolution of hummingbird pollination. Evolution 57: 1397–1410. [DOI] [PubMed] [Google Scholar]
- Bernardello G, Galetto L, Anderson GJ. 2000. Floral nectary structure and nectar chemical composition of some species from Robinson Crusoe Island (Chile). Canadian Journal of Botany 78: 862–872. [Google Scholar]
- Bertsch A. 1983. Nectar production of Epilobium angustifolium L. at different air humidities: nectar sugar in individual flowers and the optimal foraging theory. Oecologia 59: 40–48. [DOI] [PubMed] [Google Scholar]
- Blem CR, Blem LB, Felix J, van Gelder J. 2000. Rufous hummingbird sucrose preference: precision of selection varies with concentration. Condor 102: 235–238. [Google Scholar]
- Bolten AB, Feinsinger P. 1978. Why do hummingbird flowers secrete dilute nectar? Biotropica 10: 307–308. [Google Scholar]
- Bolten AB, Feinsinger P, Baker HG, Baker I. 1979. On the calculation of sugar concentration in flower nectar. Oecologia 41: 301–304. [DOI] [PubMed] [Google Scholar]
- Brown DK, Kaul RB. 1981. Floral structure and mechanism in Loasaceae. American Journal of Botany 68: 361–372. [Google Scholar]
- Carroll AB, Pallardy SG, Galen C. 2001. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae). American Journal of Botany 88: 438–446. [PubMed] [Google Scholar]
- Chalcoff VR, Aizen MA, Galetto L. 2006. Nectar concentration and composition of 26 species from the temperate forest of South America. Annals of Botany 97: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci AA, Sérsic AN. 1998. Evidence of rodent pollination in Cajophora coronata (Loasaceae). Plant Systematics and Evolution 211: 113–128. [Google Scholar]
- Corbet SA, Unwin DM, Prys-Jones OE. 1979a. Humidity, nectar and insect visits to flowers, with special reference to Crataegus, Tilia and Echium. Ecological Entomology 4: 9–22. [Google Scholar]
- Corbet SA, Willmer PG, Beament JWL, Unwin DM, Prys-Jones OE. 1979b. Post-secretory determinants of sugar concentration in nectar. Plant, Cell and Environment 2: 293–308. [Google Scholar]
- Cruden RW, Herman SM, Petterson S. 1983. Patterns of nectar production and plant–pollination coevolution. In: Bentley B, Elias T, eds. The biology of nectaries. New York, NY: Columbia University Press, 80–125.
- Dostert N, Weigend M. 1999. A synopsis of the Nasa triphylla complex (Loasaceae), including some new species and subspecies. Harvard Papers in Botany 4: 439–467. [Google Scholar]
- Elisens WJ, Freeman CE. 1988. Floral nectar sugar composition and pollinator type among New World genera in tribe Antirrhineae (Scrophulariaceae). American Journal of Botany 75: 971–978. [Google Scholar]
- Endress PK. 1994. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press.
- Fishman L, Kelly AJ, Willis JH. 2002. Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution 56: 2138–2155. [DOI] [PubMed] [Google Scholar]
- Forcone A, Galetto L, Bernardello G. 1997. Floral nectar chemical composition of some species from Patagonia. Biochemical Systematics and Ecology 25: 395–402. [Google Scholar]
- Galetto L, Bernardello G, Sosa CA. 1998. The relationship between floral nectar composition and visitors in Lycium (Solanaceae) from Argentina and Chile: what does it reflect? Flora 193: 303–314. [Google Scholar]
- Ghebrehiwet M, Bremer B, Thulin M. 2000. Phylogeny of the tribe Antirrhineae (Scrophulariaceae) based on morphological and ndhF sequence data. Plant Systematics and Evolution 220: 223–239. [Google Scholar]
- Hainsworth FR, Wolf LL. 1972. Energetics of nectar extraction in small, high altitude, tropical hummingbird, Selasphorus flammula. Journal of Comparative Physiology 80: 377–387. [Google Scholar]
- Harter B. 1995. Blütenökologie einiger von Bienen und Kolibris bestäubter Cajophora-Arten (Loasaceae). Unpublished thesis, University of Tübingen, Germany.
- Harter B, Schlindwein C, Wittmann D. 1995. Bienen und Kolibris als Bestäuber von Blüten der Gattung Cajophora (Loasaceae). Apidologie 26: 356–357. [Google Scholar]
- Heinrich B, Raven PH. 1972. Energetics and pollination ecology. Science 176: 597–602. [DOI] [PubMed] [Google Scholar]
- Heyneman AJ. 1983. Optimal sugar concentration of floral nectars—dependence on sugar intake efficiency and foraging costs. Oecologia 60: 198–213. [DOI] [PubMed] [Google Scholar]
- Hufford L, McMahon MM, Sherwood AM, Reeves G, Chase MW. 2003. The major clades of Loasaceae: phylogenetic analysis using the plastid matK and trnL-trnF regions. American Journal of Botany 90: 1215–1228. [DOI] [PubMed] [Google Scholar]
- Hufford L, McMahon MM, O'Quinn R, Poston MS. 2005. A phylogenetic analysis of Loasaceae subfamily Loasoideae based on plastid DNA sequences. International Journal of Plant Sciences 166: 289–300. [Google Scholar]
- Kaczorowski RL, Gardener MC, Holtsford TP. 2005. Nectar traits in Nicotiana section Alatae (Solanaceae) in relation to floral traits, pollinators, and mating system. American Journal of Botany 92: 1270–1283. [DOI] [PubMed] [Google Scholar]
- Keeler KH. 1981. The nectaries of Mentzelia nuda: from pollinator attraction to seed protection. American Journal of Botany 68: 295–299. [Google Scholar]
- Linsley EG, Hurd PD. 1959. Ethological observations on some bees of southeastern Arizona and New Mexico. Entomology News 70: 63–68. [Google Scholar]
- McDade LA, Weeks JA. 2004. Nectar in hummingbird-pollinated neotropical plants. I. Patterns of production and variability in 12 species. Biotropica 36: 196–215. [Google Scholar]
- Medan D, Montaldo NH, Devoto M, Mantese A, Vasellati V, Roitman GG, et al. 2002. Plant–pollinator relationships at two altitudes in the Andes of Mendoza, Argentina. Arctic, Antarctic and Alpine Research 34: 233–241. [Google Scholar]
- Pacini E, Nepi M, Vesprini JL. 2003. Nectar biodiversity: a short review. Systematics and Evolution 238: 7–21. [Google Scholar]
- Perret M, Chautems A, Spichiger R, Peixoto M, Savolainen V. 2001. Nectar sugar composition related to pollination syndromes in Sinningieae (Gesneriaceae). Annals of Botany 87: 267–273. [DOI] [PubMed] [Google Scholar]
- Perret M, Chautems A, Spichiger R, Kite G, Savolainen V. 2003. Systematics and evolution of tribe Sinningieae (Gesneriaceae): evidence from phylogenetic analyses of six plastid DNA regions and nuclear NCPGS1. American Journal of Botany 90: 445–460. [DOI] [PubMed] [Google Scholar]
- Plowright RC. 1981. Nectar production in the boreal forest lily Clintonia borealis. Canadian Journal of Botany 59: 156–160. [Google Scholar]
- Pyke GH, Waser NM. 1981. The production of diluted nectar by hummingbird and honeyeater flowers. Biotropica 13: 260–270. [Google Scholar]
- Rodriguez E, Weigend M. 1999. Nasa umbraculifera (Loasaceae: Loasoideae), una nueva especies con hojas peltadas para el Perú. Arnaldoa 6: 49–56. [Google Scholar]
- Sargent RD, Otto SP. 2004. A phylogenetic analysis of pollination mode and the evolution of dichogamy in angiosperms. Evolutionary Ecology Research 6: 1183–1199. [Google Scholar]
- Schlindwein C. 1995. Wildbienen und ihre Trachtpflanzen in einer südbrasilianischen Buschlandschaft: Fallstudie Guaritas, Bestäubung bei Kakteen und Loasaceen. Eberhard-Karls-Universität Tübingen, Fakultät für Biology. Ulrich E. Grauer Verlag, Tübingen, Germany.
- Schlindwein C. 2000. Verhaltensanpassungen oligolektischer Bienen an synchrone und an kontinuierliche Pollenpräsentation. In: Breckle SW, Schweizer B, Arndt U, eds. Ergebnisse weltweiter ökologischer Forschung. Stuttgart, Germany: Verlag Günter Heimbach, 235–250.
- Schlindwein C, Wittmann D. 1997. Micro-foraging routes of Bicolletes pampeana (Colletidae) and bee-induced pollen presentation in Cajophora arechavaletae (Loasaceae). Botanica Acta 110: 177–183. [Google Scholar]
- Stiles FG, Freeman CE. 1993. Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica 25: 191–205. [Google Scholar]
- Thompson HJ, Ernst WR. 1967. Floral biology and systematics of Eucnide (Loasaceae). Journal of the Arnold Arboretum 48: 56–88. [Google Scholar]
- Troncoso AJ, Vargas RR. 2004. Efecto del vecindario floral sobre la tasa de visitas por insectos a Loasa triloba Domb. ex A.J. Juss. y Loasa tricolor Ker-Gawl en la Reserva Nacional de Río Clarillo, Región Metropolitana, Chile. Chloris Chilensis 7 (1). http://www.chlorischile.cl/loasa/Loasaalejandra.htm (24 March 2006).
- Urban I. 1886. Die Bestäubungseinrichtungen der Loasaceen. Jahrbuch des Botanischen Gartens zu Berlin 4: 364–388. [Google Scholar]
- Urban I. 1889. Loasaceae. In: Martius CFP, ed. Flora Brasilienis 13 (3). München, Germany: Verlag Beck F, Fleischer F, 205–222.
- Urban I. 1892. Blüten- und Fruchtbau der Loasaceen. Berichte der Deutschen Botanischen Gesellschaft 10: 259–265. [Google Scholar]
- Urban I, Gilg W. 1900. Monographia Loasacearum. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germanicae Naturae Curiosorum 76: 1–368. [Google Scholar]
- Villagrán C, Romo M, Castro V. 2003. Etnobotánica del Sur de los Andes de la Primera Región de Chile: un Enlace entre las Culturas Altiplánicas y las de Quebradas Altas del Loa Superior. Chungara Revista de Antropología Chilena 35: 73–124. [Google Scholar]
- Weigend M. 2000a. A revision of the Peruvian species of Nasa ser. Alatae in Peru. Nordic Journal of Botany 20: 15–32. [Google Scholar]
- Weigend M. 2000b. Loasaceae. In: Andersson L, Harling G, eds. Flora of Ecuador 132. Stockholm, Sweden: University of Goteborg and the Section for Botany, Riksmuseum, 1–92.
- Weigend M. 2001. Loasaceae. In: Bernal R, Forero E, eds. Flora de Colombia 22. Santa Fé de Bogotá: Instituto de Ciencias Naturales, 1–100.
- Weigend M. 2002. Observations on the biogeography of the Amotape-Huancabamba Zone in northern Peru. In: Young K, Ulloa UC, Luteyn JL, Knapp S, eds. Plant Evolution and Endemism in Andean South America—Botanical Review 68: 38–54. [Google Scholar]
- Weigend M. 2004a. Loasaceae. In: Kubitzki K, Bayer C, eds. The families and genera of the vascular plants. Vol. 6. Köln, Germany: Springer Verlag, 239–254.
- Weigend M. 2004b. Four new species of Nasa ser. Alatae (Loasaceae) in the Amotape-Huancabamba Zone of Peru. Novon 14: 134–146. [Google Scholar]
- Weigend M. 2004c. Additional observations on the biogeography of the Amotape-Huancabamba Zone in Northern Peru—defining the south-eastern limits. Revista Peruana de Biología 11: 127–134. [Google Scholar]
- Weigend M, Ackermann M. 2003. Los nombres antiguos en el género Caiophora (Loasáceas subfam. Loasoídeas) y una clasificación infragenérica preliminar. Arnaldoa 10: 75–94. [Google Scholar]
- Weigend M, Gottschling M. 2006. Evolution of funnel-revolver flowers and ornithophily in Nasa (Loasaceae). Plant Biology 8: 120–142. [DOI] [PubMed] [Google Scholar]
- Weigend M, Rodriguez E. 2002. Las espécies arbustivas de Nasa Ser. Grandiflorae en el Norte de Perú, con la descripción de una espécie nueva de la Abra de Barro Negro (Callacalla). Arnaldoa 9: 7–20. [Google Scholar]
- Weigend M, Rodriguez E. 2003. A revision of the the Nasa stuebeliana group [Nasa ser. Saccatae (Urb. and Gilg) Weigend, Loasaceae] with notes on morphology, ecology, and distribution. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 124: 345–382. [Google Scholar]
- Weigend M, Henning T, Schneider C. 2003. Notes on the systematics, morphology, distribution and pollination of Nasa Ser. Carunculatae (Loasaceae subfam. Loasoideae). Systematic Botany 29: 765–781. [Google Scholar]
- Weigend M, Gottschling M, Hoot S, Ackermann M. 2004. A preliminary phylogeny of Loasaceae subfam. Loasoideae (Angiospermae: Cornales) based on trnL(UAA) sequence data and its relation to systematics and historical biogeography. Organisms, Diversity and Evolution 4: 73–90. [Google Scholar]
- Weigend M, Rodriguez E, Dostert N. 1998. Nasa insignis y Nasa glandulosissima, dos especies nuevas de Nasa con hojas peltadas. Arnaldoa 5: 151–157. [Google Scholar]
- Wilson P, Castellanos MC, Wolfe A, Thomson JD. 2006. Shifts between bee- and bird-pollination among Penstemon. In: Waser N, Ollerton J, eds. Plant–pollinator interactions: from specialization to generalization. Chicago. IL: University of Chicago Press, 47–68. www.csun.edu/biology/grad/faculty/wilsonpdfs/chapter.pdf (24 March 2006).
- Wittmann D, Schlindwein C. 1995. Melittophilous plants, their pollen and flower visiting bees in southern Brazil. 1. Loasaceae. Biociências 3 (2): 19–34.
- Zimmermann M. 1983. Plant reproduction and optimal foraging: experimental nectar manipulations in Delphinium nelsonii. Oikos 41: 57–63. [Google Scholar]
- Zimmermann M. 1988. Nectar production, flowering phenology, and strategies for pollination. In: Doust Jl, Doust LL, eds. Plant reproductive ecology, patterns and strategies. Oxford: Oxford University Press, 157–178.