Abstract
• Aims This botanical briefing examines how molecular systematics has contributed to progress in understanding the history of Tertiary relict genera, i.e. those that that now occur disjunctly in parts of Eurasia and N America, and how progress in understanding Southern Hemisphere biogeography paradoxically makes unravelling Northern Hemisphere biogeography more complex.
• Scope Tertiary relict floras comprise genera of warm wet climates that were once circumboreal in distribution but are now confined to E Asia, south-eastern and western N America, and SW Eurasia. The intercontinental disjunctions among these genera have long been believed to result from land connections between Eurasia and N America, across Beringia and the N Atlantic. This view is reassessed in the light of new evidence for long dispersal of propagules across oceans being responsible for many plant disjunctions involving southern continents. The impact of molecular dating, which has been very different in Southern and Northern Hemisphere biogeography, is discussed.
• Conclusions For N America–Eurasia disjunctions involving Tertiary relict floras, land connections remain the more likely cause of disjunctions but data from fossils or infraspecific variation will be required to exclude long-dispersal explanations for disjunctions in any individual genus. Molecular dating of divergence between disjunctly distributed Tertiary relict floras can tell us which palaeoclimatic or palaeogeographic events impacted on them, and how, but only if migration over land and vicariance can be proved and molecular dating is sufficiently accurate.
Keywords: Tertiary relict floras, plant disjunctions, biogeography, Bering land bridge, dispersal, vicariance, molecular dating, Northern Hemisphere, paleoclimate
INTRODUCTION: TERTIARY RELICT FLORAS AND LAND BRIDGES
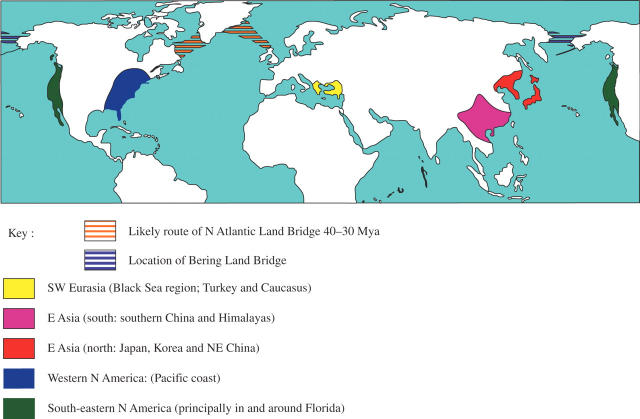
Climate change threatens to change and contract radically the distributions of many plant species and communities, but this will not be the first time it has happened. The response of plants to natural changes in climate over the past 65 million years (Myr) can be used to predict the effects of future climate change (Pennington et al., 2004). Cooling climates in the latter part of the Tertiary (65–2 Myr ago) period forced large assemblages of warm temperate to subtropical biotas to retreat from medium to high latitude circumboreal distributions southwards to large refugial regions that preserved the warm wet climate that they needed. These refugia are in E Asia, south-eastern N America, western N America, and SW Eurasia (around Turkey) (Figs 1 and 2), and the floras concerned are hence termed Tertiary relict floras (Tiffney, 1985a, b; Wen, 1999; Milne and Abbott, 2002). Tertiary relict floras have long attracted interest because over 100 genera occur disjunctly between two or more of these regions (i.e. there and nowhere in between; Wood, 1972, and references therein; Wen, 1999; Milne and Abbott, 2002), with disjunctions between south-eastern N America and E Asia being much the commonest (Xiang et al., 2000; Donoghue and Smith, 2004). The last Tertiary land connection between Eurasia and N America was the Bering land bridge (BLB), which lay close to the Arctic Circle (Tiffney, 1985a, b; Tiffney and Manchester, 2001), and is known to have experienced significant climate cooling from 15 Myr ago onwards (Wolfe, 1994; White et al., 1997; Tiffney and Manchester, 2001). This cooling may have initiated divergence and speciation between N American and Eurasian species of many Tertiary relict genera by driving them from Beringia (Milne and Abbott, 2002) before the bridge sundered 5·4–5·5 Myr ago. By comparison of palaeoclimatic data with molecular data indicating the time of divergence for many E Asia–N America disjunct species pairs, it may be possible to examine the effects of Beringian climate change in some detail.
Fig. 1.
Principal areas where Tertiary relict floras occur, and Tertiary land bridges between Eurasia and N America. The route shown for the N Atlantic land bridge is the ‘Greenland–Faeroes bridge’ (Xiang et al., 2005), the only connection that might have persisted after 40 Myr ago (see Milne and Abbott, 2002). Before this time, a second more northerly connection existed between Greenland and Scandinavia (for a detailed description, see Tiffney, 1985b). The Bering land bridge existed for most of the tertiary period but moved progressively southwards throughout this period, and was well within the Arctic Circle in the early to middle Tertiary (Milne and Abbott, 2002). Many references treat E Asia as a single area, but this figure follows Donoghue et al. (2001) and Milne and Abbott (2002) in separating northern and southern parts of this refugial area.
Fig. 2.

Rhododendron smirnowii (subgenus Hymenanthes; pink flowers) and R. luteum (subgenus Pentanthera; yellow flowers) growing together in the Pontus mountains on the Black Sea coast of Turkey, part of the SW Eurasia refugial region. Both these subgenera have independently developed Tertiary relict distributions, with species in SW Eurasia, south-eastern and western N America, and Japan (Kron, 1993; Milne, 2004). Subgenus Hymenanthes additionally has species in other parts of E Asia, most notably the Himalayas where rapid radiation appears to have occurred (Milne, 2004).
When the Tertiary period began, N America and Eurasia were each separated into western and eastern portions by epicontinental seaways (Tiffney, 1985a; Tiffney and Manchester, 2001). At the same time western N America was connected to E Asia across the BLB, while eastern N America was connected via Greenland to north-eastern Europe—a connection known as the N Atlantic land bridge (NALB; Tiffney, 1985b). Pollen records confirm that the Northern Hemisphere was divided into two biogeographic regions of which one was western N America plus most of Asia, and the other eastern N America plus Europe (Tiffney, 1985a). Climates at high latitudes were warm and wet, and a forest community developed here mixing warm temperate, subtropical and, possibly, also tropical taxa. In fact, the NALB, which lay further south than the BLB (McKenna, 1983; Tiffney, 1985b; Tiffney and Manchester, 2001), may also have served as part of a land connection between Africa and America for tropical taxa (Lavin et al., 2000; Xiang et al., 2005). During the early Tertiary, the epicontinental seaways on N America and Eurasia receded, removing or reducing barriers between the western and eastern portions of each continent, perhaps allowing some taxa to develop unbroken circumboreal distribution patterns (Tiffney, 1985a; Tiffney and Manchester, 2001). Between then and now, four processes combined to break up this distribution. First, the opening Atlantic Ocean broke up the NALB, although the timing of this is far from certain. Greenland was last in direct contact with Scotland around 50 Myr ago and Scandinavia around 40 Myr ago (Tiffney, 1985b), but the activity of the Iceland hotspot may have formed a continuous land connection from Greenland to Scotland for some time after this, which sank piece by piece, becoming an island chain with progressively larger gaps until only Iceland and the Faeroes remained (McKenna, 1983; Milne and Abbott, 2002; Xiang et al., 2005). Biotic connections across the NALB were therefore probably lost very gradually up until some time in the middle Tertiary, perhaps 25 or possibly even 15 Myr ago (Milne and Abbott, 2002). The second and third processes were climate cooling and aridification. The climate cooled gently from 50 to 35 Myr ago, then fluctuated until 15 Myr ago, after which the climate cooled progressively, culminating in the Quaternary (2–0 Myr ago) glaciations (Milne and Abbott, 2002). Linked to this was the development of belts of relatively arid climates in the centres of both continents, so that floras of wetter climates were once again separated into eastern and western provinces on both Eurasia and N America (Tiffney and Manchester, 2001). In addition, an east–west orientated belt of arid conditions existed in eastern Asia for much of the Tertiary period, separating the E Asia refugial region into northern and southern portions (Tiffney, 1985b; Tiffney and Manchester, 2001; Fig. 1), causing these portions to exhibit separate biogeographic affinities (Donoghue et al., 2001; Milne and Abbott, 2002). The fourth and final event was the sundering of the BLB, known with some precision to have occurred 5·5–5·4 Myr ago (Gladenkov et al., 2002). Between them, these events fragmented the previously continuous Tertiary forest communities and confined them to the warm wet regions they now occupy (Figs 1 and 2). However, the relative significance of these events is hotly debated; for example, it is far from clear whether climatic cooling or the actual sundering of the BLB was the more important event in breaking biotic connections between Tertiary floras of E Asia and N America (Milne and Abbott, 2002).
Molecular systematic work has been used to investigate when and how Tertiary relict genera moved between N America and Eurasia (Wen, 1999; Xiang et al., 2000, 2005; Donoghue et al., 2001; Milne and Abbott, 2002). A phylogeny can indicate trans-Atlantic migration by resolving a N American species as sister to one from SW Eurasia, as in Liquidambar (Li and Donoghue, 1999) and Styrax (Fritsch, 1999), and the likely history of more complex phylogenetic patterns can be postulated by use of analytical methods such as dispersal–vicariance analysis (Ronquist, 1997; for examples, see Xiang et al., 2005; Feng et al., 2005). However, extinctions of Tertiary relict taxa, which occurred disproportionately frequently in Europe (Milne and Abbott, 2002), may render phylogenies based on extant taxa misleading, except where fossil data can be incorporated (Xiang et al., 2005). Molecular dating can also indicate which route was used to move between continents, most commonly via divergence dates that are too recent for the NALB, and therefore favour the BLB (Xiang et al., 2000; Milne, 2004). Tracing the flora's history back further, i.e. identifying centres of origin, has been attempted, but the true pattern is likely to be complex. Early workers considered E Asia to be the area of origin of Tertiary relict floras, but the higher diversity there is largely due to lower extinction rates (Wen, 1999; Milne and Abbott, 2002) and probably also higher speciation rates (Xiang et al., 2004). There is no reason to assume that all Tertiary relict genera share a common area of origin, or that this must be somewhere that they currently occur.
VICARIANCE AND LONG DISPERSAL
The above account implies that most or all disjunctions among tertiary relict floras arose via vicariance, i.e. the splitting of one large population into two by the formation of a physical or climatic barrier between them, e.g. severance of the BLB or NALB, or through local extinction due to climate change. Where vicariance has occurred, molecular dating of divergence between two disjunct species provides a date indicating when the barrier that initially separated them might first have arisen—hence it allows dating of abiotic events by use of biotic data, especially when similar divergence times are found for many unrelated but similarly distributed species pairs.
There is, however, an alternative means by which plants can move between landmasses. Long dispersal occurs when a single propagule is carried across a barrier (usually an ocean), from an existing population on one side to found a new population on the other. Accidental dispersal across oceans happens frequently among birds—with British birdspotters flocking to see ‘vagrant’ individuals that have accidentally arrived from America every year—but for plant genera the successful establishment and reproduction by a single seed carried between continents needs only to happen once every few tens of millions of years to have a large impact on biogeography. Long dispersal has been an unpopular hypothesis among biogeographers because it is highly random in nature, almost impossible to falsify, and unlike vicariance cannot normally be linked to specific abiotic events (McGlone, 2005). Recently, however, a dramatic change in opinion has come about, the principal cause of which has been molecular dating.
MOLECULAR DATING
Molecular dating is the process by which an age is placed on a divergence event, represented by a node on a phylogeny, indicating when two populations, species or clades of species diverged from one another. In most modern molecular dating studies there are three stages, reflecting contributions from the fields of molecular systematics, mathematics and palaeobotany, respectively. First, a phylogeny is generated, normally by use of data from one or more DNA sequences. Every branch in a phylogeny has a length measured in number of substitutions for the sequence(s) examined. It is not possible to convert branch length data directly into an estimate of time because substitution rates vary between lineages, a consequence of rate heterogeneity (Gaut, 1998; Soltis et al., 2002; Welch and Bromham, 2005). Therefore, the second part of the process is to convert substitution numbers on branches into measures of relative time within the phylogeny, a process known as rate smoothing for which many methods exist (Sanderson et al., 2004; Welch and Bromham, 2005). For any node in the phylogeny, before rate smoothing the total sum of branch lengths between it and each species descended from it will vary; afterwards they will be equal. Effectively, the oldest node is assigned an arbitrary age and every other node an age that is a proportion of that. Therefore, the third process is the conversion of relative ages into actual ages, which begins with assigning an actual age to at least one node of the phylogeny. Nodes assigned actual ages are known as calibration points, and the method normally used is dated fossils. A fossil is identified whose morphology proves that it must belong to a specific branch or clade within a phylogeny. The node representing the first divergence of said clade or branch from its sister lineage represents the earliest point on the phylogeny at which that fossil could have existed, so this becomes the calibration node. The minimum age of that fossil is determined from its stratigraphic position, and that (minimum) age is assigned to the node. Then, the (minimum) age of every other node is calculated as a proportion of that of the calibration node.
In practice, molecular dating is an inexact process. A divergence time for the same two taxa may vary according to which molecule, calibration method, fossil calibration point(s), and rate smoothing method is used, the accuracy of phylogenetic relationships recovered, and the number of taxa sampled in the phylogeny (Milne and Abbott, 2002; Bell and Donoghue, 2004; Sanderson et al., 2004; Linder et al., 2005; Magallon and Sanderson, 2005). Moreover, stochastic variation in substitution rates (‘noise’ in the data set) means that even if these factors are controlled for, there will be an error range on any estimate (Wikstrom et al., 2001). However, although any node may by definition be older than the minimum age calculated, in practice this does not mean the ages are potentially infinite. Furthermore, the greater the number of fossil calibration points employed, the closer some of them will be to the actual age of the calibration nodes to which they are assigned, and the more accurate the calculated node ages will therefore be (Near and Sanderson, 2004).
THE RISE OF DISPERSAL BIOGEOGRAPHY AND THE FADING OF GONDWANA
The advent of plate tectonics provided an extremely attractive explanation for the numerous examples of plant species that occurred disjunctly on two or more landmasses. Disjunctions involving Southern Hemisphere land-masses could be explained in terms of prior contact between them as the super-continent Gondwana. The shared ancestors of disjunct taxa were assumed to have been present on this supercontinent as it broke up, leaving species on separate landmasses as they drifted apart. However, when molecular dates for such intercontinental disjunctions started to become available for plants, these were consistently far younger than the dates at which the relevant landmasses parted (Renner et al., 2001; Givnish and Renner, 2004; Pennington et al., 2004; Renner, 2004; Sanmartin and Ronquist, 2004; de Queiroz, 2005; McGlone, 2005). Most strikingly, Africa and S. America were last in contact around 110 Myr ago (Sanmartin and Ronquist, 2004), with a possible link via the Northern Hemisphere across the NALB when the Tertiary period was at its hottest, around 50 Myr ago (see above). However, many tropical plant species and a few animals crossed the Atlantic more recently than this (Renner, 2004; de Queiroz, 2005), with crossings within Rapateaceae, Bromeliaceae and Melastomataceae each dated within the past 15 Myr ago (Renner, 2004). Molecular dating had the effect of forcing the scientific community to accept other evidence for dispersal explanations that previously had been overlooked or avoided (McGlone, 2005), leading to a recent consensus among most scientists that most Southern Hemisphere plant disjunctions arose by dispersal (Givnish and Renner, 2004; Pennington et al., 2004; Renner, 2004; Sanmartin and Ronquist, 2004; de Queiroz, 2005; McGlone, 2005). Gondwanan vicariance remains a prominent pattern among animals (Sanmartin and Ronquist, 2004), and a few dramatic plant examples also exist (e.g. Rutschmann et al., 2004), but generally the signatures of Gondwanan vicariance events among plants are overlain by subsequent dispersal events (de Queiroz, 2005).
The frequency of long dispersal in the tropics could be much greater than that in temperate zones, as more effective long-dispersal mechanisms appear to exist there (Renner, 2004). More data are required to test rigorously whether temperate Southern Hemisphere disjunctions are older than their tropical counterparts, indicating a lesser contribution from dispersal in the former. If long dispersal between, for example, S America and Africa were proved to be rarer in their temperate than their tropical regions, then its likely relevance in north temperate regions would also diminish. Overall, however, Southern Hemisphere disjunctions demonstrate clearly that long dispersal across oceans is a regular occurrence in geological time.
Dispersal explanations for disjunctions cause two severe methodological problems for biogeographers: (1) the hypothesis that a single seed was carried across a wide ocean once in a period of tens of millions of years is very hard to prove or falsify (McGlone, 2005); (2) if long dispersal cannot be falsified, it follows that any alternative explanation, i.e. land bridge migration and vicariance, cannot be proved absolutely. These are reasonable points for explaining the unpopularity of the concept; however, one cannot reject a hypothesis simply because one dislikes it—that is the prerogative of Intelligent Design supporters, not scientists. Long dispersal does occur, and the methodology of biogeography must adapt to this fact (Cook and Crisp, 2005; McGlone, 2005).
DISPERSAL, VICARIANCE AND THE NORTHERN HEMISPHERE
Dispersal between Southern Hemisphere continents is easily proved because they have been unconnected by land for so long; however, this is not the case between Eurasia and N America, which had land connections probably continuously between 65 and 5·5 Myr ago. Hence most proven examples of long dispersal between them occurred too long ago to be anthropogenic but much more recently than 5·5 Myr ago (e.g. Coleman et al., 2003). Although vicariance may be the more likely hypothesis among genera that diverged ≥5·5 Myr ago, Southern Hemisphere data indicate that dispersal might have played a significant role and that data must be interpreted accordingly (Cook and Crisp, 2005).
There is plenty of circumstantial evidence that favours vicariance over long dispersal for the means by which most Tertiary relict disjunctions arose. First, the congruent distributions of so many unrelated genera argue for a shared history. Though genera with identical distributions did not necessarily achieve them simultaneously or via identical routes, the modern pattern is nonetheless still more consistent with sharing of biota between landmasses via one or more land connections than it is with individual species travelling one by one in unpredictable dispersal events. Secondly, among the regions where Tertiary relict floras occur, disjunctions between E Asia and eastern N America are disproportionately common compared with those involving western N America or SW Eurasia (Donoghue and Smith, 2004), indicating a specific biogeographic link between the former two regions, which again argues for a shared pattern of movement over land. If oceanic dispersal was important, links would be expected between south-eastern N America and SW Eurasia, or western N America and E Asia. Thirdly, those genera with good fossil records are known to have been widespread at fairly high latitudes within Eurasia, as the vicariance hypothesis predicts (Tiffney, 1985a; Milne and Abbott, 2002; Xiang et al., 2005). Fourthly, the large seeds of some genera involved, notably the chestnuts Aesculus and Castanea, would be difficult to disperse across oceans. Fifthly, many of the genera involved have undergone very little morphological change since divergence (i.e. ‘stasis’; Wen, 1999, 2001; Milne and Abbott, 2002), which is more consistent with vicariance of one large population into two than it is with long dispersal, because the latter involves a founder effect which is often the driver for rapid morphological change (Milne and Abbott, 2002).
While these points together indicate that vicariance was almost certainly far more important than dispersal overall, none of them constitutes hard evidence to reject a dispersal hypothesis for any given genus. For rejecting dispersal, hard evidence is required to support the vicariance hypothesis, above and beyond indicating that a land connection existed around the time of divergence. Analytical methods for reconstructing biogeographic histories, such as dispersal–vicariance analysis (Ronquist, 1997), can be adapted to allow a higher likelihood for long dispersal (de Queiroz, 2005), but are not perfect for examining a scenario that involves long dispersal (Cook and Crisp, 2005). Moreover the output of such methods only indicates the most likely history for the group concerned, and is itself a hypothesis; other explanations are not excluded. Where it is available, fossil data can be combined with molecular data to provide very strong evidence for long dispersal (e.g. Symphonia; Dick et al., 2003), or migration over land connections (e.g. Cornus; Xiang et al., 2005); palaeoclimatic data can further improve such analyses (Feng et al., 2005). However, where fossil data are absent or scant, another approach will be required.
INFRASPECIFIC HAPLOTYPE VARIATION: EVIDENCE FOR VICARIANCE?
American tertiary relict species, when examined, have been found to exhibit considerable infraspecific (within a species) variation for genetic markers (e.g. Liriodendron; Sewell et al., 1996), including multiple haplotypes (plastid types distinguishable by sequence data) for the slow-evolving gene rbcL (R. Milne, unpubl. res.). These results demonstrate that recent range contractions due to climatic cooling and glacial maxima have not expunged all the pre-existing genetic variation; in particular, if multiple refugia were occupied during glacial maxima, then some of this variation might predate the divergence of the species and could be highly informative. Nuclear markers have larger effective population sizes than plastid markers because two alleles are contained in each individual rather than one, so these could provide still more data on the history of disjunct species.
Long dispersal from one continent to another will create a daughter population (later, species) with (initially) a single haplotype, while the mother species will normally have several. If more than one haplotype from the mother species survives to the present day that species will be paraphyletic for cpDNA haplotypes, with respect to the daughter species (Fig. 3A). The initial divergence event among haplotypes will be older for the mother than the daughter species. Vicariance of one large population into two, however, will create two daughter populations (later, species) with similar numbers of haplotypes, and neither species will show divergence of haplotypes much earlier than the other (Fig. 3B). Moreover, if more than one haplotype was shared between the daughter populations at the time of vicariance, and both haplotypes persisted in both species, then a pattern of multiple shared haplotypes between disjunct species will be detected (Fig. 3C), which would provide strong evidence for vicariance, because such a pattern would otherwise require two long-dispersal events—an exceptionally unlikely scenario.
Fig. 3.
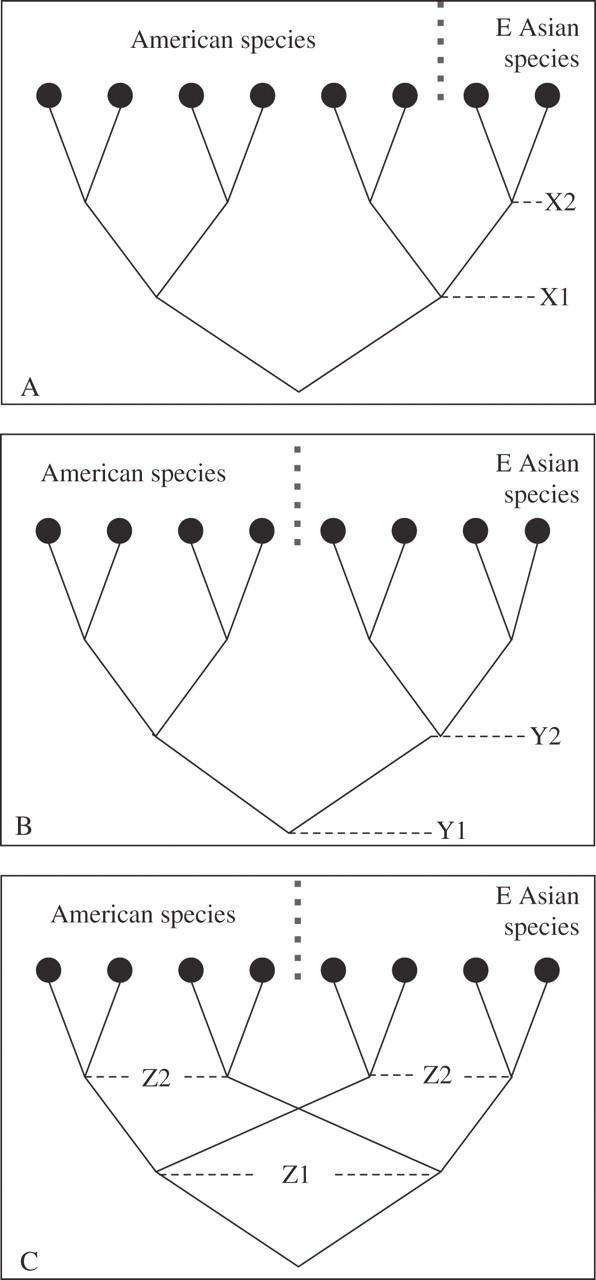
Patterns of haplotype relationships within a disjunct species pair, and what can be interpreted from them. (A) Paraphyly, i.e. one species' haplotypes derived from within a clade of the other's, indicates that the American species existed first, and that the E Asian species was derived from it between points X1 and X2. This pattern fits a hypothesis that the Asian species was founded by long dispersal. (B) Reciprocal monophyly, indicating divergence at point Y1. This pattern is consistent with vicariance between points Y1 and Y2, but does not exclude long dispersal, as it could be derived from pattern A by loss of the clade on the left. (C) Two clades of haplotypes shared between both species, strongly indicates that the two species were derived from a single large population that contained two or more widespread haplotypes, and so would provide hard evidence for vicariance having occurred between points Z1 and Z2. Note that in examples (A) and (C), divergence time calculations based on a single haplotype per species will not necessarily obtain the correct value, and may calculate the age of the basal node, not that at which the two species actually diverged.
IN SEARCH OF A UNIVERSAL MOLECULAR DATING METHOD
If vicariance can be proved, at least in some cases, the problem of accurate molecular dating still remains. The science of dating is advancing rapidly, but nonetheless different methods can still produce different, and sometimes highly conflicting, node ages (Milne and Abbott, 2002; Bell and Donoghue, 2004; Linder et al., 2005). Ultimately, if these problems are to be avoided, molecular systematists must move towards a universal dating method rather than forever finding new genes to sequence, or inventing new rate-smoothing methods. Comparability between results for unrelated genera is valuable as it allows a test of a hypothesis that all might have diverged owing to the same event (e.g. Xiang et al., 2000); however, little can be concluded from comparing results acquired by different methods for different genera (Milne and Abbott, 2002). Part of the problem is that direct fossil calibration is desirable, but not all families have a suitable fossil record. This problem can be overcome by use of deeper phylogenies (i.e. going to the order level and above); indeed, probably the most reliable calibration point for angiosperms is the widespread appearance of tricolpate pollen in the fossil record 124 Myr ago (e.g. Anderson et al., 2005), marking a fairly precise date for the appearance of Eudicots. Hence a phylogeny that incorporates this calibration point can be used to place initial dates on any node within the angiosperms, following the methods of Wikstrom et al. (2001). Furthermore, the use of multiple fossils also increases the accuracy of fossil dating for a phylogeny (Soltis et al., 2002; Near and Sanderson, 2004; Anderson et al., 2005; Xiang et al., 2005), and allows more recent fossils that clarify dates within specific families to be used alongside older ones. By use of multiple fossils and several gene sequences together to reduce the effects of stochastic variation (e.g. Wikstrom et al., 2001), a universal method of dating can be developed, so that accurate dates are not restricted to fossil-rich families, and allowing cross-comparison of dates between many unrelated disjunct pairs, permitting a holistic view of when Tertiary relict disjuncts diverged from one another. However, the number of taxa sampled in a phylogeny can profoundly affect node ages calculated (Linder et al., 2005), and angiosperm lineages vary dramatically in the number of species they contain (APG II, 2003). Therefore work is required to determine how best the effects of uneven taxon numbers between clades might be dealt with without introducing biases to the node ages calculated.
CONCLUSIONS
Vicariance of populations previously connected across land bridges is almost certainly the principal means by which the disjunct distributions exhibited by Tertiary relict floras has arisen; however, hard evidence is thus far only available for those genera that have a useful fossil record. For other genera, it may be necessary to re-examine the evidence for vicariance in the light of the surprising frequency of long-dispersal events between southern continents, by use of data such as infraspecific molecular variation. However, the discovery that long dispersal has been the principal cause of Southern Hemisphere plant disjunctions should re-invigorate work on Tertiary relict floras, as the latter now appear to represent the best example of a large-scale biotic disjunction that came about primarily via vicariance. Molecular dating has a vital role to play in understanding the history of Tertiary relict floras, especially as phylogenies based on extant species might give misleading results. However, to make meaningful comparisons between divergence times for unrelated genera, a universal method of molecular dating must be developed and adhered to. The goal must be to date divergence events to within a few million years, and simultaneously to provide strong evidence for vicariance in individual disjunct genera. Once this has been achieved, the time at which specific genera disappeared from the Beringia region can be calculated based on molecular data, and used to determine how they responded to the progressive changes in climate that were happening there in the late Tertiary period.
Acknowledgments
I thank Paul Manos for constructive comments on the manuscript. My work on Tertiary relict floras is supported by NERC fellowship NE/B500658/1.
LITERATURE CITED
- Anderson CL, Bremer K, Friis EM. 2005. Dating phylogenetically basal Eudicots using rbcL sequences and multiple fossil reference points. American Journal of Botany 92: 1737–1748. [DOI] [PubMed] [Google Scholar]
- APG II. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436. [Google Scholar]
- Bell CD, Donoghue MJ. 2005. Dating the Dipsacales: comparing models, genes, and evolutionary implications. American Journal of Botany 92: 284–296. [DOI] [PubMed] [Google Scholar]
- Coleman M, Liston A, Kadereit JW, Abbott RJ. 2003. Repeat intercontinental dispersal and Pleistocene speciation in disjunct Mediterranean and desert Senecio (Asteraceae). American Journal of Botany 90: 1446–1454. [DOI] [PubMed] [Google Scholar]
- Cook LG, Crisp MD. 2005. Directional asymmetry of long-distance dispersal and colonization could mislead reconstructions of biogeography. Journal of Biogeography 32: 741–754. [Google Scholar]
- Dick CW, Abdul-Salim K, Bermingham E. 2003. Molecular systematic analysis reveals cryptic tertiary diversification of a widespread tropical rain forest tree. American Naturalist 162: 691–703. [DOI] [PubMed] [Google Scholar]
- Donoghue MJ, Smith SA. 2004. Patterns in the assembly of temperate forests around the Northern Hemisphere. Philosophical transactions of the Royal Society of London Series B—Biological Sciences 359: 1633–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ, Bell CD, Li J. 2001. Phylogenetic patterns in Northern Hemisphere plant geography. International Journal of Plant Science 162: S41–S52. [Google Scholar]
- Feng Y, Oh S-H, Manos PS. 2005. Phylogeny and historical biogeography of the genus Platanus as inferred from nuclear and chloroplast DNA. Systematic Botany 30: 786–799. [Google Scholar]
- Fritsch P. 1999. Phylogeny of Styrax based on morphological characters, with implications for biogeography and infrageneric classification. Systematic Botany 24: 356–378. [Google Scholar]
- Gaut BS. 1998. Molecular clocks and nucleotide substitution rates in higher plants. Evolutionary Biology 30: 93–120. [Google Scholar]
- Givnish TJ, Renner SS. 2004. Tropical intercontinental disjunctions: Gondwana breakup, immigration from the boreotropics, and transoceanic dispersal. International Journal of Plant Science 165 (Suppl. S): S1–S6.
- Gladenkov AY, Oleinik AE, Marincovich L, Barinov KB. 2002. A refined age for the earliest opening of Bering Strait. Palaeogeography Palaeoclimatology Palaeoecology 183: 321–328. [Google Scholar]
- Kron KA. 1993. A taxonomic revision of Rhododendron sect. Pentanthera (Ericaceae). Edinburgh Journal of Botany 50: 249–364. [Google Scholar]
- Lavin M, Thulin M, Labat JN, Pennington RT. 2000. Africa, the odd man out: molecular biogeography of dalbergioid legumes (Fabaceae) suggests otherwise. Systematic Botany 25: 449–467. [Google Scholar]
- Li J, Donoghue MJ. 1999. More molecular evidence for interspecific relationships in Liquidambar (Hamamelidaceae). Rhodora 101: 87–91. [Google Scholar]
- Linder HP, Hardy CR, Rutschmann F. 2005. Taxon sampling effects in molecular clock dating: an example from the African Restionaceae. Molecular Phylogenetics and Evolution 35: 569–582. [DOI] [PubMed] [Google Scholar]
- McGlone MS. 2005. Goodbye Gondwana. Journal of Biogeography 32: 739–740. [Google Scholar]
- McKenna MC. 1983. Cenozoic paleogeography of North Atlantic land bridges. In Bott MHP, Saxov S, Talwani M, Thiede J, eds. Structure and development of the Greenland–Scotland bridge: new concepts and methods. New York, NY: Plenum, 351–395.
- Magallon SA, Sanderson MJ. 2005. Angiosperm divergence times: the effect of genes, codon positions, and time constraints. Evolution 59: 1653–1670. [DOI] [PubMed] [Google Scholar]
- Milne RI. 2004. Phylogeny and biogeography of Rhododendron subsection Pontica, a group with a Tertiary relict distribution. Molecular Phylogenetics and Evolution 33: 389–401. [DOI] [PubMed] [Google Scholar]
- Milne RI, Abbott RJ. 2002. The origin and evolution of Tertiary relict floras. Advances in Botanical Research 38: 281–314. [Google Scholar]
- Near TJ, Sanderson MJ. 2004. Assessing the quality of molecular divergence time estimates by fossil calibrations and fossil-based model selection. Philosophical Transactions of the Royal Society of London Series B—Biological Sciences 359: 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington RT, Cronk QCB, Richardson JA. 2004. Introduction and synthesis: plant phylogeny and the origin of major biomes. Philosophical Transactions of the Royal Society of London series B—Biological Sciences 359: 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Queiroz A. 2005. The resurrection of oceanic dispersal in historical biogeography. Trends in Ecology and Evolution 20: 68–73. [DOI] [PubMed] [Google Scholar]
- Renner SS. 2004. Plant dispersal across the tropical Atlantic by wind and sea currents. International Journal of Plant Sciences 165: S23–S33. [Google Scholar]
- Renner SS, Clausing G, Meyer K. 2001. Historical biogeography of Melastomataceae: the roles of tertiary migration and long-distance dispersal. American Journal of Botany 88: 1290–1300. [PubMed] [Google Scholar]
- Ronquist F. 1997. Dispersal–vicariance analysis: a new approach to the quantification of historical biogeography. Systematic Biology 46: 195–203. [Google Scholar]
- Rutschmann F, Eriksson T, Schonenberger J, Conti E. 2004. Did Crypteroniaceae really disperse out of India? Molecular dating evidence from rbcL, ndhF, and rpl16 intron sequences. International Journal of Plant Sciences 165 (Suppl. S): S69–S83. [Google Scholar]
- Sanderson MJ, Thorne JL, Wikstrom N, Bremer K. 2004. Molecular evidence on plant divergence times. American Journal of Botany 91: 1656–1665. [DOI] [PubMed] [Google Scholar]
- Sanmartin I, Ronquist F. 2004. Southern Hemisphere biogeography inferred by event-based models: plant versus animal patterns. Systematic Biology 53: 216–243. [DOI] [PubMed] [Google Scholar]
- Sewell MM, Parks CR, Chase MW. 1996. Intraspecific cpDNA variation and biogeography of North American Liriodendron L. (Magnoliaceae). Evolution 50: 1147–1154. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE, Savolainen V, Crane PR, Barraclough TG. 2002. Rate heterogeneity among lineages of tracheophytes: integration of molecular and fossil data and evidence for molecular living fossils. Proceedings of the National Academy of Sciences of the USA 99: 4430–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffney BH. 1985a. Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. Journal of the Arnold Arboretum 66: 73–94. [Google Scholar]
- Tiffney BH. 1985b. The Eocene North Atlantic land bridge: its importance in Tertiary and modern phylogeography of the Northern Hemisphere. Journal of the Arnold Arboretum 66: 243–273. [Google Scholar]
- Tiffney BH, Manchester SR. 2001. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the northern hemisphere tertiary. International Journal of Plant Sciences 162: S3-S17. [Google Scholar]
- Welch JJ, Bromham L. 2005. Molecular dating when rates vary. Trends in Ecology and Evolution 20: 320–327. [DOI] [PubMed] [Google Scholar]
- Wen J. 1999. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annual Review of Ecology and Systematics 30: 421–455. [Google Scholar]
- Wen J. 2001. Evolution of eastern Asian and eastern North American biogeographic disjunctions: a few additional issues. International Journal of Plant Sciences 162: S117–S122. [Google Scholar]
- White JM, Ager TA, Adam DP, Leopold EB, Liu G, Jette H, et al. 1997. An 18 million year record of vegetation and climate change in north-western Canada and Alaska: tectonic and global climatic correlates. Palaeogeography Palaeoclimatology Palaeoecology 130: 293–306. [Google Scholar]
- Wikstrom N, Savolainen V, Chase MW. 2001. Evolution of the angiosperms: calibrating the family tree. Proceedings of the Royal Society of London 268: 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JA. 1994. An analysis of Neogene climates in Beringia. Palaeogeography Palaeoclimatology Palaeoecology 108: 207–216. [Google Scholar]
- Wood Jr CE. 1972. Morphology and phytogeography: the classical approach to the study of disjunctions. Annals of the Missouri Botanical Garden 59: 107–124. [Google Scholar]
- Xiang QY, Soltis DE, Soltis PS, Manchester SR, Crawford DJ. 2000. Timing the eastern Asian–eastern North American floristic disjunction: molecular clock corroborates paleontological estimates. Molecular Phylogenetics and Evolution 15: 462–472. [DOI] [PubMed] [Google Scholar]
- Xiang QY, Zhang WH, Ricklefs RE, Qian H, Chen ZD, Wen J, et al. 2004. Regional differences in rates of plant speciation and molecular evolution: a comparison between eastern Asia and eastern North America. Evolution 58: 2175–2184. [DOI] [PubMed] [Google Scholar]
- Xiang QY, Manchester SR, Thomas DT, Zhang WH, Fan CZ. 2005. Phylogeny, biogeography, and molecular dating of Cornelian cherries (Cornus, Cornaceae): tracking Tertiary plant migration. Evolution 59: 1685–1700. [PubMed] [Google Scholar]