Abstract
• Background and Aims Glycinebetaine (GB), a quaternary ammonium compound, is a very effective compatible solute. In higher plants, GB is synthesized from choline (Cho) via betaine aldehyde (BA). The first and second steps in the biosynthesis of GB are catalysed by choline monooxygenase (CMO) and by betaine aldehyde dehydrogenase (BADH), respectively. Rice (Oryza sativa), which has two genes for BADH, does not accumulate GB because it lacks a functional gene for CMO. Rice plants accumulate GB in the presence of exogenously applied BA, which leads to the development of a significant tolerance to salt, cold and heat stress. The goal in this study was to evaluate and to discuss the effects of endogenously accumulated GB in rice.
• Methods Transgenic rice plants that overexpressed a gene for CMO from spinach (Spinacia oleracea) were produced by Agrobacterium-mediated transformation. After Southern and western blotting analysis, GB in rice leaves was quantified by 1H-NMR spectroscopy and the tolerance of GB-accumulating plants to abiotic stress was investigated.
• Key Results Transgenic plants that had a single copy of the transgene and expressed spinach CMO accumulated GB at the level of 0·29–0·43 μmol g−1 d. wt and had enhanced tolerance to salt stress and temperature stress in the seedling stage.
• Conclusions In the CMO-expressing rice plants, the localization of spinach CMO and of endogenous BADHs might be different and/or the catalytic activity of spinach CMO in rice plants might be lower than it is in spinach. These possibilities might explain the low levels of GB in the transgenic rice plants. It was concluded that CMO-expressing rice plants were not effective for accumulation of GB and improvement of productivity.
Keywords: Oryza sativa, glycinebetaine, choline monooxygenase, transgenic rice, tolerance to abiotic stress
INTRODUCTION
Rice (Oryza sativa) is one of the most important cereals in the world and is a popular model plant for studies of monocots. Improvements in the tolerance of cereal plants to abiotic stress are important if the efficiency of food production is to be increased. Furthermore, information on the modification of rice plants would be applicable to other cereal crops, such as wheat (Triticum aestivum), barley (Hordeum vulgare) and maize (Zea mays), and the converse would also be true. Many loci for genes that control tolerance to abiotic stress in plants have been identified by genetic analysis (e.g. Lanceras et al., 2004; Saito et al., 2004). However, many genes that control agronomically important traits remain to be identified and modified to generate new varieties with desirable traits. There is evidence that transgenic plants in which the expression of a single gene has been modified have enhanced tolerance to abiotic stress (Bajaj and Mohanty, 2005). Ideally, modification of a single gene should confer tolerance to more than one form of abiotic stress. Alternation in the pattern of expression of the gene for DREB1A, a transcription factor, improves the tolerance to drought, salt and freezing of Arabidopsis thaliana (Kasuga et al., 1999). Introduction of genes that are involved in the synthesis of compatible solutes, such as betaines, polyols and proline, into plants also contributes to tolerance to multiple forms of abiotic stress (Rathinasabapathi, 2000; Chen and Murata, 2002).
Glycinebetaine (GB), a quaternary ammonium compound, is a very effective compatible solute (Rathinasabapathi, 2000; Chen and Murata, 2002) and is found in a wide range of foods (de Zwart et al., 2003). In plants that synthesize GB, which are known as GB-accumulators, e.g. spinach (Spinacia oleracea), maize and barley, this compatible solute accumulates in leaves in response to a water deficit and salt stress, as well as during acclimation to cold (McCue and Hanson, 1990; Rhodes and Hanson, 1993; Kishitani et al., 1994). Moreover, GB has been shown in vitro to stabilize membranes of the oxygen-evolving photosystem II complex (Murata et al., 1992; Papageorgiou and Murata, 1995). GB also stabilizes the activity of ribulose 1,5-bisphosphate carboxylase/oxygenase in a transgenic cyanobacterium in vivo (Nomura et al., 1998). In higher plants, GB is synthesized from choline (Cho) via betaine aldehyde (BA). The first and second steps in the biosynthesis of GB are catalysed by choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH), respectively (Rhodes and Hanson, 1993). In Arthrobacter globiformis, choline oxidase (COD), encoded by codA, catalyses the conversion of Cho to GB in a single step (Ikuta et al., 1977). Choline dehydrogenase (CDH) and BADH, encoded by both betA and betB, catalyse the conversion of Cho to GB, via BA, in Escherichia coli (Landfald and Strom, 1986). Yet another pathway, namely, the three-step methylation of glycine, for the biosynthesis of GB is catalysed by glycine sarcosine methyltransferase and sarcosine dimethylglycine methyltransferase in Aphanothece halophytica and Ectothiorhodospira halochloris (Nyyssola et al., 2000).
Rice is the only important cereal crop that does not accumulate GB. The rice genome includes a non-functional gene for CMO (P0545E05·33 on chromosome 6), which is probably a pseudo-gene, as well as two copies of a gene for BADH (OSJNBa0060P14·8 on chromosome 4 and P0456B03·101 on chromosome 8), both of which encode a signal sequence for targeting of the gene product to peroxisomes (International Rice Genome Sequencing Project, 2005). Transgenic rice plants that express COD accumulate GB and exhibit enhanced tolerance to salt and/or cold stress (Sakamoto et al., 1998; Mohanty et al., 2002), even though the concentrations at which GB accumulates are lower than those in stressed GB-accumulating plants, such as maize (Yang et al., 1995). Rice transformants that overexpress barley BADH and even wild-type rice plants have been shown to accumulate a considerable amount of GB, as compared with rice plants that express COD, when they are supplied with exogenous BA, and such plants develop significant tolerance to salt, cold and heat stress (Kishitani et al., 2000). Enhancement of the synthesis of GB improves drought and chilling tolerance in maize, a crop plant that naturally accumulates GB (Quan et al., 2004a, b). Therefore, an attempt was made to enhance the accumulation of GB in rice by introducing the gene for the enzyme that catalyses the first step in the synthesis of GB. A gene for CMO from spinach was the first such gene to be isolated from a higher plant (Rathinasabapathi et al., 1997), and it has been expressed in tobacco and Arabidopsis, neither of which normally accumulates GB (Nuccio et al., 1998, Hibino et al., 2002). As far as is known, however, there have been no reports of rice plants that express CMO. In the present study, the effects of endogenously accumulated GB were evaluated in transgenic rice plants that expressed a gene for CMO from spinach and the tolerance to temperature stress and salt stress of transgenic seedlings and the productivity of mature transgenic plants was investigated.
MATERIALS AND METHODS
Transgenic plant materials
For the construction of an expression vector for spinach CMO, a DNA fragment was isolated from the plasmid pPCMO (Hibino et al., 2002) that encoded CMO and signal peptides for targeting of proteins to chloroplasts (Rathinasabapathi et al., 1997) using SacI. This fragment was ligated into the corresponding site of the binary vector pBI101 (Ariizumi et al., 2002), which was constructed for overexpression of individual genes under the control of the promoter of a gene for ubiquitin from maize (Cornejo et al., 1993). This resultant construct was named Ubi::CMO (Fig. 1A). This plasmid was introduced into the japonica rice cultivar ‘Sasanishiki’ by Agrobacterium-mediated transformation, as described previously (Yokoi et al., 1997). Hygromycin-resistant plants were selected on MS medium (Murashige and Skoog, 1962) that contained 50 mg L−1 hygromycin and examined the synthesis of CMO in the resultant transformants by western blotting analysis.
Fig. 1.
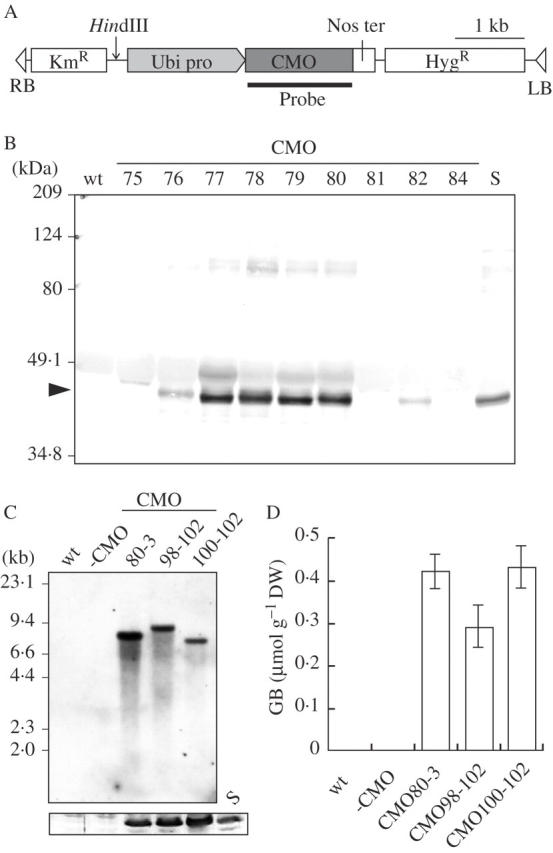
(A) A schematic diagram of part of the T-DNA region of the transforming vector Ubi::CMO (see Ariizumi et al., 2002). The arrow and the solid bar indicate the HindIII site and the region corresponding to the probe, respectively, that were used for Southern blotting analysis. (B) Western blotting analysis of the extracts of spinach leaves (S), as a positive control, and wild-type (wt) and transgenic rice (75–84) with the CMO-specific antibody. The arrowhead indicates the predicted mobility of CMO at a position that corresponds to 43 kDa. (C) Southern blotting analysis of genomic DNA (upper panel) and western blotting analysis of the extracts (lower panel) of wild-type leaves (wt), spinach leaves (S), -CMO leaves (-CMO) and leaves of transgenic rice (80–3, 98–102 and 100–102), respectively. (D) Concentrations of GB in wild-type plants (wt), -CMO plants (-CMO) and transformants (CMO80-3, 98–102 and 100–102). The values are the means ± standard errors of the results from three independent T2 plants in each case.
Western blotting analysis
Young leaves of rice plants and of spinach, as a control, were ground in liquid nitrogen and extracted in 150 ml of soluble-protein buffer [100 mm Tris–HCl (pH 8·0), 1 mm EDTA and 2 mm DTT]. After centrifugation of these extracts (600 g, 10 min, 4 °C), supernatants were subjected to western blotting analysis. Sixty micrograms of protein per extract, as estimated by Bradford's assay (with a kit from BIO-RAD, USA) were fractionated on SDS–polyacrylamide gel (10 % polyacrylamide), and bands of protein were electroblotted onto a polyvinylidene difluoride membrane. As the primary antibody, a CMO-specific antibody raised against CMO from spinach in rabbit was used (Hibino et al., 2002) at a dilution of 1 : 2000. Immunoreactive proteins were detected as previously described by Okada et al. (2003).
Southern blotting analysis
A digoxigenin-labelled probe was prepared by PCR with the binary vector Ubi::CMO and the primer pair SpPCMO-F and SpPCMO-R described by Hibino et al. (2002), using the PCR DIG-Labeling Mix from Roche Diagnostics (Switzerland). The thermal-cycling conditions were as follows: 1-min denaturation at 94 °C; 30 cycles of 1-min denaturation at 94 °C, 1-min annealing at 58 °C, 1-min extension at 72 °C and a final 3-min extension at 72 °C. Genomic DNA was isolated with a DNeasy Plant Mini Kit (QIAGEN, Germany) from young leaves. Genomic DNA (2 μg) was digested with HindIII (TAKARA BIO, Japan) and subjected to electrophoresis on 1 % agarose gel. Southern blotting analysis was performed as described previously by Shirasawa et al. (2004).
Quantitation of glycinebetaine and choline
Levels of GB and Cho in the leaves were quantified as described previously by Arakawa et al. (1990) with minor modifications. Quaternary ammonium compounds were precipitated overnight as periodides (Wall et al., 1960) and analysed by 1H-NMR spectroscopy (Jones et al., 1986) in a Fourier-transform NMR spectrometer (JMN-600; JEOL, Japan). Cho was applied to T1 plants that were growing on MS-medium (Murashige and Skoog, 1962) by adding 5 mm choline chloride to the growth medium.
Examination of stress tolerance
Ten T2 plants per line were used for each stress test. All tests were simultaneously repeated three times. Plants were cultured in 750-mL containers (50 cm2 × 15 cm) filled with synthetic soil for rice seedlings (Kumiai Gousei Baido 3; Sanken Soil, Japan) in a greenhouse at 23/18 °C (day/night) under natural light for 4 weeks as the control conditions. Since extreme differences in temperature between daytime and night-time are critically stressful to plants and sometimes occur under natural conditions, groups of plants were grown at 28/13 °C for 5 weeks to subject them to temperature stress. For the salt-stress test, plants were treated by adding 25 mL of 100 mm NaCl daily to the soil after growth for 10 d at 23/18 °C. Then 25 mL of 150 mm NaCl were added daily for 5 d and finally 50 mL of water were added daily for 3 d. After each treatment, plants were dried at 75 °C for 2 d. The resultant dry weights were evaluated as a measure of stress tolerance. An allocation index ( %) was calculated for each plant, as follows: [dry weight of shoot (mg)/#{dry weight of shoot (mg) + dry weight of root (mg)#}] × 100. Four plants per line were grown to maturity in 1/5000-a pots in a greenhouse for examination of plant height, sink and source size, panicle number and 1000-grain weight.
RESULTS
Characterization of transgenic rice plants
Eighty-six independent transgenic rice plants were generated by Agrobacterium-mediated transformation. In western blotting analysis of extracts of leaves, 12 of the 86 T0 transformants that expressed greater amounts of CMO than that of spinach (Fig. 1B) were selected. Four transformants, each of which carried a single transgene by Southern blotting analysis, were selected, and the T1 progeny of three (CMO80, CMO98 and CMO100) of the four lines chosen. The T1 plants of the three lines were tested for resistance to hygromycin. Each T1 line (CMO80-3, CMO98-102 and CMO100–102) that was selected for further analysis was homozygous for Ubi::CMO (all 20 tested progeny survived on MS medium that contained 50 mg L−1 hygromycin). A T1 plant derived from a CMO80 T0 plant that lacked the transgene as the control plant, namely, -CMO was used. The appropriate presence or absence of the transgene and its expression was confirmed by Southern and western blotting analysis, respectively (Fig. 1C) and GB was quantified in leaves of three T2 plants per line. The mean concentration of GB was 0·42, 0·29 and 0·43 μmol g−1 d. wt in leaves from the CMO80-3, CMO98-102 and CMO100–102 plants, respectively, and no GB was found in wild-type and -CMO plants (Fig. 1D). The concentrations of GB were determined after supplying exogenous Cho to transgenic plants. Upon application of Cho, the level of GB that accumulated in transformants reached approximately ten times that in plants to which Cho was not supplied (data not shown), suggesting a shortage of Cho in transformants.
Stress tolerance of seedlings that accumulated glycinebetaine
Under control conditions, the averages of growth parameters (dry weight of shoots and roots) of three transgenic lines, namely, CMO100–102, CMO80-3 and CMO98-102, were more vigorous than those of wild type. The allocation index, namely, the shoot dry weight as a percentage of the total dry weight of the three lines, increased (Table 1), while the index for -CMO was similar to that for the wild type. In subsequent analyses, -CMO plants were omitted because there was little difference between them and the wild type under control conditions. The shoot dry weight of transformants tended to increase, as compared with that of the wild type, under salt stress. Conversely, the root dry weight of transformants decreased, respectively, compared with that for the wild type. As the result, the allocation indices of three CMO lines under salt stress were significantly elevated (Table 1). Similarly, shoot dry weight and allocation indices were elevated in the CMO lines after exposure to temperature stress (Table 1). The numbers of tillers on the three transgenic lines were elevated under both control and stress conditions, in particular, under temperature stress (Table 1). The aerial parts of transformant seedlings that accumulated GB grew more vigorously and the underground parts grew less vigorously than those of wild-type plants, in which no GB accumulated.
Table 1.
Various parameters related to the growth of seedling after exposure to salt stress and temperature stress*
| Control |
Salt stress |
Temperature stress |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Line | Organ | Dry weight (mg plant−1) | Allocation (%) | No. of tillers | Dry weight (mg plant−1) | Allocation (%) | No. of tillers | Dry weight (mg plant−1) | Allocation (%) | No. of tillers |
| wt | Shoot | 149 ± 5c | 76·7 ± 0·5bc | 2·0 ± 0·0b | 127 ± 3bc | 80·9 ± 0·1c | 1·5 ± 0·0ab | 123 ± 2b | 77·9 ± 0·2b | 2·5 ± 0·0b |
| Root | 45 ± 1z | 30 ± 1y | 35 ± 1y | |||||||
| CMO80-3 | Shoot | 195 ± 2ab | 78·4 ± 0·3ab | 2·3 ± 0·1ab | 123 ± 3c | 82·7 ± 0·3b | 1·3 ± 0·1b | 121 ± 3b | 80·1 ± 0·5a | 2·8 ± 0·1b |
| Root | 54 ± 1y | 26 ± 1yz | 30 ± 2z | |||||||
| CMO98-102 | Shoot | 193 ± 9ab | 77·6 ± 0·4b | 2·1 ± 0·0b | 137 ± 5ab | 85·0 ± 0·5a | 1·5 ± 0·1ab | 145 ± 4a | 80·8 ± 0·5a | 3·4 ± 0·1a |
| Root | 56 ± 2y | 24 ± 2z | 35 ± 1y | |||||||
| CMO100–102 | Shoot | 211 ± 9a | 78·9 ± 0·4a | 2·4 ± 0·1a | 146 ± 4a | 84·3 ± 0·8ab | 1·7 ± 0·1a | 154 ± 6a | 81·1 ± 0·1a | 3·6 ± 0·2a |
| Root | 57 ± 3y | 27 ± 2z | 36 ± 2y | |||||||
| -CMO | Shoot | 181 ± 11b | 75·6 ± 0·1c | 2·0 ± 0·1b | – | – | – | – | – | – |
| Root | 58 ± 3y | – | – | |||||||
Each value is given as the mean ± standard error (n = 30). Within columns, means followed by the same letter are not significantly different at P < 0·05 (LSD test).
* See text for details.
Productivity of mature transgenic plants
The agronomical traits, such as biomass, length of stems, weight of spikelets as sink size, weight of leaves and stems as source size, number of panicles and weight of 1000 grains, of control and transgenic plants, were examined to evaluate the effects of the accumulation of GB in mature plants. While stems of CMO98-102 plants were shorter than those of wild-type plants, the other traits did not differ significantly among the five lines examined (Table 2). Thus, GB in rice plants did not affect grain production but did have a negative effect on stem length.
Table 2.
Productivity of mature plants
| Line | Stem length (cm) | Sink size (g d. wt) | Source size (g d. wt) | No. of panicles | 1000-grain weight (g) |
|---|---|---|---|---|---|
| wt | 96·5 ± 1·3b | 45·0 ± 0·6a | 38·8 ± 1·6a | 22·5 ± 1·2a | 20·3 ± 0·3ab |
| CMO80-3 | 93·0 ± 2·6b | 39·1 ± 1·8a | 35·7 ± 1·5a | 22·0 ± 0·7a | 19·4 ± 0·2b |
| CMO98-102 | 86·8 ± 1·2c | 40·4 ± 4·6a | 32·2 ± 4·7a | 22·5 ± 2·2a | 20·9 ± 0·4a |
| CMO100-102 | 91·8 ± 1·1b | 41·3 ± 5·2a | 36·4 ± 3·7a | 23·5 ± 2·3a | 20·4 ± 0·4ab |
| -CMO | 102·3 ± 1·5a | 39·0 ± 2·9a | 38·7 ± 3·2a | 20·0 ± 1·1a | 20·9 ± 0·1a |
Each value is given as the mean ± standard error (n = 4). Within columns, means followed by the same letter are not significantly different at P < 0·05 (LSD test).
DISCUSSION
In transgenic rice plants that expressed cDNA for spinach CMO with a transit peptide for targeting of the product to chloroplasts, the levels of GB were very low, ranging from 0·29 to 0·43 μmol g−1 d. wt, in spite of the expression of CMO. However, there was a 10-fold increase in levels of GB when Cho was supplied to the rice plants that expressed CMO (abbreviated as CMO-rice, the format used for other combinations of enzyme and plant name, hereafter). Similar results, with limited accumulation of GB, have been reported in CMO-Arabidopsis, with levels increasing upon the addition of exogenous Cho (Hibino et al., 2002), as well as in CMO-tobacco (Nuccio et al., 1998). Levels of GB in CMO-tobacco increased upon enhancement of the synthesis of Cho (McNeil et al., 2001), showing that endogenous Cho is a limiting factor in this non-GB-accumulator. However, GB accumulated at levels of approx. 1–5 μmol g−1 f. wt and 1 μmol g−1 d. wt in COD-rice (Sakamoto et al., 1998; Mohanty et al., 2002) without a supply of exogenous Cho. Moreover, COD-Arabidopsis accumulated much more GB than did CMO-Arabidopsis (Hayashi et al., 1997; Hibino et al., 2002), and COD-tobacco also accumulated much more GB than did CMO-tobacco (Nuccio et al., 1998; Huang et al., 2000). Cho can be transported into chloroplasts, but the levels of GB in CMO-expressing plants were still lower than those in COD- or CDH-expressing plants. There are at least two possible explanations for the observation that the amounts of GB that accumulate in plants differ so much between plants that express enzymes derived from higher plants and those that express enzymes derived from bacteria. The first possible explanation is that the localization of spinach CMO and that of endogenous BADHs differ in CMO-rice. The cDNA for spinach CMO that was used in the present study encoded the precursor to mature CMO and included a transit peptide, namely, chloroplast stromal targeting peptide (Rathinasabapathi et al., 1997). Thus, it was predicted that the mature CMO would be localized in the chloroplasts. By contrast, each BADH of rice contains SKL as the carboxy-terminal tripeptide, which delivers proteins to peroxisomes. It is likely that CMO and both BADHs were almost completely localized in chloroplasts and peroxisomes, respectively, in CMO-rice. In spinach, both CMO and BADH are targeted to chloroplasts (Nakamura et al., 1997; Rathinasabapathi et al., 1997). In barley, the two types of BADH, namely, BBD1 and BBD2, are localized in peroxisomes and the cytosol, respectively, with different patterns of expression (Nakamura et al., 2001). The compartment in which BA is converted to GB in monocotyledonous GB-accumulators has not been identified. Thus, at present, it is advantageous to use enzymes derived from bacteria that do not to need to co-operate with BADH-like enzymes in specific cellular compartments because such enzymes can catalyse the conversion of Cho to GB in a single step. The second possible explanation for the above-mentioned observations is that the catalytic activity of CMO is lower than that of COD and CDH. The activity of purified CMO is extremely low, 393 pkat mg−1 (Burnet et al., 1995). Even in E. coli, cells that express spinach CMO have been found to accumulate about one-third as much betaine as E. coli that express CDH encoded by betA from E. coli under salt stress (Hibino et al., 2002). Certain trans-acting factors, modifiers or post-translational regulators, that are absent from non-GB-accumulators, such as rice, Arabidopsis and tobacco, might activate CMO in plants that do accumulate GB.
In the present study, the accumulation of GB in transgenic rice seedlings enhanced their tolerance to salt stress and temperature stress (Table 1). The aerial mass (shoot weight and tiller number) was greater than that of wild-type plants, while the underground mass (root weight) was slightly lower after salt stress and temperature stress. Thus, allocation of assimilated carbons to aerial parts was elevated in the transgenic plants. The mechanism responsible for this difference is unknown. In maize, there is a positive correlation between the concentration of GB, and the extent of tolerance to salt stress and drought stress of transgenic plants (Quan et al., 2004a, b). In rice seedlings, tolerance to salt stress and cold stress is enhanced by the accumulation of GB even if levels of GB are lower than those in GB-accumulators (Sakamoto et al., 1998). In the present study, productivity of the mature transgenic rice plants was unaffected by the accumulation of GB (Table 2). Moreover, a preliminary investigation indicated that tolerance to high as well as low temperature at the reproductive stage, which is sensitive to such stresses, was not enhanced. The amount of GB in these transgenic rice plants might have been too low to improve productivity, as similarly reported in maize (Quan et al., 2004b). Mature COD-rice plants, which accumulated more GB than CMO-rice, did, however, exhibit increased productivity after salt stress (Mohanty et al., 2002). Our CMO-rice had a tendency towards shorter stems (Table 2). Shorter shoots were also observed in wild-type rice that accumulated GB upon exposure to exogenous BA (Kishitani et al., 2000). GB might cause semi-dwarfism in non-GB-accumulators. By contrast to the present results in rice, the stem length of maize that expresses CaMV35S::betA was increased by the accumulation of GB (Quan et al., 2004b). The effects of GB might differ among GB-accumulators and non-GB-accumulators, even if both belong to Poaceae and express active BADHs. One of the rice isozymes for BADH, P0456B03·101, has been reported to be a candidate gene for fragrance (Bradbury et al., 2005). Certainly, BADH has been shown to have broad substrate-specificity with respect to amino acids and related compounds (Trossat et al., 1997).
In the present study, transgenic CMO-rice plants accumulated GB at lower levels than those reported previously (Sakamoto et al., 1998, Mohanty et al., 2002). Although, in the present study, the transformants also exhibited tolerance to salt stress and temperature stress in the seedling stage, not enough GB is accumulated in the plants to improve their productivity. The CMO from a higher plant, spinach, has proved to be less effective for the accumulation of GB in non-GB-accumulating rice plants than bacterial COD and CDH. Recently, Waditte et al. (2005) have reported that Arabidopsis plants expressing genes for N-methyltransferase from A. halophytica accumulated a higher level of GB into roots, stems, leaves and flowers than COD-plants, and showed improved seed yield under stress conditions. This, rather than the introduction of CMO, may be more effective for non-GB-accumulating plants to produce GB. Even though a shortage of substrates has been observed in the plants expressing N-methyltransferase (Waditte et al., 2005), this is a much less serious problem than that posed by Cho of COD- and CDH-plants. By introduction of the gene for N-methyltransferase into rice plants to allow accumulation of a high level of GB, it is expected that resistance to abiotic stresses and hence productivity can be enhanced. It was concluded that this approach for accumulation of GB in rice can be expected to be useful in efforts to improve abiotic stress tolerance and productivity, though CMO-plants were less effective for accumulation of GB and improvement of productivity.
Acknowledgments
The authors are grateful to Dr T. Takabe of Meijo University for providing the plasmid pPCMO and the CMO-specific antibody and to Dr K. Toriyama and Ms J. Sasaki of Tohoku University for producing the transformants. K. Shirasawa received a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists.
LITERATURE CITED
- Arakawa K, Katayama M, Takabe T. 1990. Levels of glycinebetaine and glycinebetaine aldehyde dehydrogenase activity in the green leaves, and etiolated leaves and roots of barley. Plant and Cell Physiology 31: 797–803. [Google Scholar]
- Ariizumi T, Kishitani S, Inatsugi R, Nishida I, Murata N, Toriyama K. 2002. An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings. Plant and Cell Physiology 43: 751–758. [DOI] [PubMed] [Google Scholar]
- Bajaj S, Mohanty A. 2005. Recent advances in rice biotechnology—towards genetically superior rice. Plant Biotechnology Journal 3: 275–307. [DOI] [PubMed] [Google Scholar]
- Bradbury LMT, Fitzgerald TL, Henry RJ, Jin Q, Waters DLE. 2005. The gene for fragrance in rice. Plant Biotechnology Journal 3: 363–370. [DOI] [PubMed] [Google Scholar]
- Burnet M, Lafontaine PJ, Hanson AD. 1995. Assay, purification, and partial characterization of choline monooxygenase from spinach. Plant Physiology 108: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Murata N. 2002. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current Opinion in Plant Biology 5: 250–257. [DOI] [PubMed] [Google Scholar]
- Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE. 1993. Activity of a maize ubiquitin promoter in transgenic rice. Plant Molecular Biology 23: 567–581. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Alia, Mustardy L, Deshnium P, Ida M, Murata N. 1997. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. The Plant Journal 12: 133–142. [DOI] [PubMed] [Google Scholar]
- Hibino T, Waditee R, Araki E, Ishikawa H, Aoki K, Tanaka Y, et al. 2002.. Functional characterization of choline monooxygenase, an enzyme for betaine synthesis in plants. Journal of Biological Chemistry 277: 41352–41360. [DOI] [PubMed]
- Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA, Selvaraj G. 2000. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiology 122: 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S, Imamura S, Misaki H, Horiuti Y. 1977. Purification and characterization of choline oxidase from Arthrobacter globiformis. Journal of Biochemistry 82: 1741–1749. [DOI] [PubMed] [Google Scholar]
- International Rice Genome Sequencing Project. 2005. The map-based sequence of the rice genome. Nature 436: 793–800. [DOI] [PubMed] [Google Scholar]
- Jones GP, Naidu BP, Starr RK, Paleg LG. 1986. Estimation of solutes accumulating in plants by 1H nuclear magnetic resonance spectroscopy. Australian Journal of Plant Physiology 13: 649–658. [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. 1999. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnology 17: 287–291. [DOI] [PubMed] [Google Scholar]
- Kishitani S, Watanabe K, Yasuda S, Arakawa K, Takabe T. 1994. Accumulation of glycinebetaine during cold acculimation and freezing tolerance in leaves of winter and spring barley plants. Plant, Cell and Environment 17: 89–95. [Google Scholar]
- Kishitani S, Takanami T, Suzuki M, Oikawa M, Yokoi S, Ishitani M, et al. 2000.. Compatibility of glycinebetaine in rice plants: evaluation using transgenic rice plants with a gene for peroxisomal betaine aldehyde dehydrogenase from barley. Plant, Cell and Environment 23: 107–114. [Google Scholar]
- Lanceras JC, Pantuwan G, Jongdee B, Toojinda T. 2004. Quantitative trait loci associated with drought tolerance at reproductive stage in rice. Plant Physiology 135: 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfald B, Strom AR. 1986. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. Journal of Bacteriology 165: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue KF, Hanson AD. 1990. Drought and salt tolerance: towards understanding and application. Trends in Biotechnology 8: 358–362. [Google Scholar]
- McNeil SD, Nuccio ML, Ziemak MJ, Hanson AD. 2001. Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proceedings of the National Academy of Sciences of the USA 98: 10001–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi AK. 2002. Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theoretical and Applied Genetics 106: 51–57. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assay with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Murata N, Mohanty PS, Hayashi H, Papageorgiou GC. 1992. Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Letters 296: 187–189. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yokota S, Muramoto Y, Tsutsui K, Oguri Y, Fukui K, Takabe T. 1997. Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. The Plant Journal 11: 1115–1120. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nomura M, Mori H, Jagendorf AT, Ueda A, Takabe T. 2001. An isozyme of betaine aldehyde dehydrogenase in barley. Plant and Cell Physiology 42: 1088–1092. [DOI] [PubMed] [Google Scholar]
- Nomura M, Hibino T, Takabe T, Sugiura T, Yokota A, Miyake H, et al. 1998. Transgenically produced glycinebetaine protects ribulose 1,5-bisphosphate carboxylase/oxygenase from inactivation in Synechococcus sp. PCC7942 under salt stress. Plant and Cell Physiology 39: 425–432. [Google Scholar]
- Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson AD. 1998. The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. The Plant Journal 16: 487–496. [DOI] [PubMed] [Google Scholar]
- Nyyssola A, Kerovuo J, Kaukinen P, von Weymarn N, Reinikainen T. 2000. Extreme halophiles synthesize betaine from glycine by methylation. Journal of Biological Chemistry 275: 22196–22201. [DOI] [PubMed] [Google Scholar]
- Okada A, Okada T, Ide T, Itoh M, Tanaka F, Toriyama K. 2003. Accumulation of Japanese cedar pollen allergen, Cry j 1, in the protein body I of transgenic rice seeds using the promoter and signal sequence of glutelin GluB-1 gene. Molecular Breeding 12: 61–70. [Google Scholar]
- Papageorgiou GC, Murata N. 1995. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynthesis Research 44: 243–252. [DOI] [PubMed] [Google Scholar]
- Quan R, Shang M, Zhang H, Zhao Y, Zhang J. 2004a. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Science 166: 141–149. [Google Scholar]
- Quan R, Shang M, Zhang H, Zhao Y, Zhang J. 2004b. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnology Journal 2: 477–486. [DOI] [PubMed] [Google Scholar]
- Rathinasabapathi B, Burnet M, Russell BL, Gage DA, Liao PC, Nye GJ, et al. 1997.. Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: prosthetic group characterization and cDNA cloning. Proceedings of the National Academy of Sciences of the USA 94: 3454–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinasabapathi B. 2000. Metabolic engineering for stress tolerance: installing osmoprotectant synthesis pathways. Annals of Botany 86: 709–716. [Google Scholar]
- Rhodes D, Hanson AD. 1993. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 44: 357–384. [Google Scholar]
- Saito K, Hayano-Saito Y, Maruyama-Funatsuki W, Sato Y, Kato A. 2004. Physical mapping and putative candidate gene identification of a quantitative trait locus Ctb1 for cold tolerance at the booting stage of rice. Theoretical and Applied Genetics 109: 515–522. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Alia, Murata N. 1998. Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Molecular Biology 38: 1011–1019. [DOI] [PubMed] [Google Scholar]
- Shirasawa K, Kishitani S, Nishio T. 2004. Conversion of AFLP markers to sequence-specific markers for closely related lines in rice by use of the rice genome sequence. Molecular Breeding 14: 283–292. [Google Scholar]
- Trossat C, Rathinasabapathi B, Hanson AD. 1997. Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and ω-aminoaldehydes. Plant Physiology 113: 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waditee R, Bhuiyan MN, Rai V, Aoki K, Tanaka Y, Hibino T, et al. 2005.. Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proceedings of the National Academy of Sciences of the USA 102: 1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JS, Christianson DD, Dimler RJ, Senti FR. 1960. Spectrophotometric determination of betaines and other quaternary nitrogen compounds as their periodides. Analytical Chemistry 32: 870–874. [Google Scholar]
- Yang WJ, Nadolska-Orczyk A, Wood KV, Hahn DT, Rich PJ, Wood AJ, et al. 1995.. Near-isogenic lines of maize differing for glycinebetaine. Plant Physiology 107: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Tsuchiya T, Toriyama K, Hinata K. 1997. Tapetum-specific expression of the Osg6B promoter-β-glucuronidase gene in transgenic rice. Plant Cell Reports 16: 363–367. [DOI] [PubMed] [Google Scholar]
- de Zwart FJ, Slow S, Payne RJ, Lever M, George PM, Gerrard JA, et al. 2003. Glycine betaine and glycine betaine analogues in common foods. Food Chemistry 83: 197–204. [Google Scholar]


