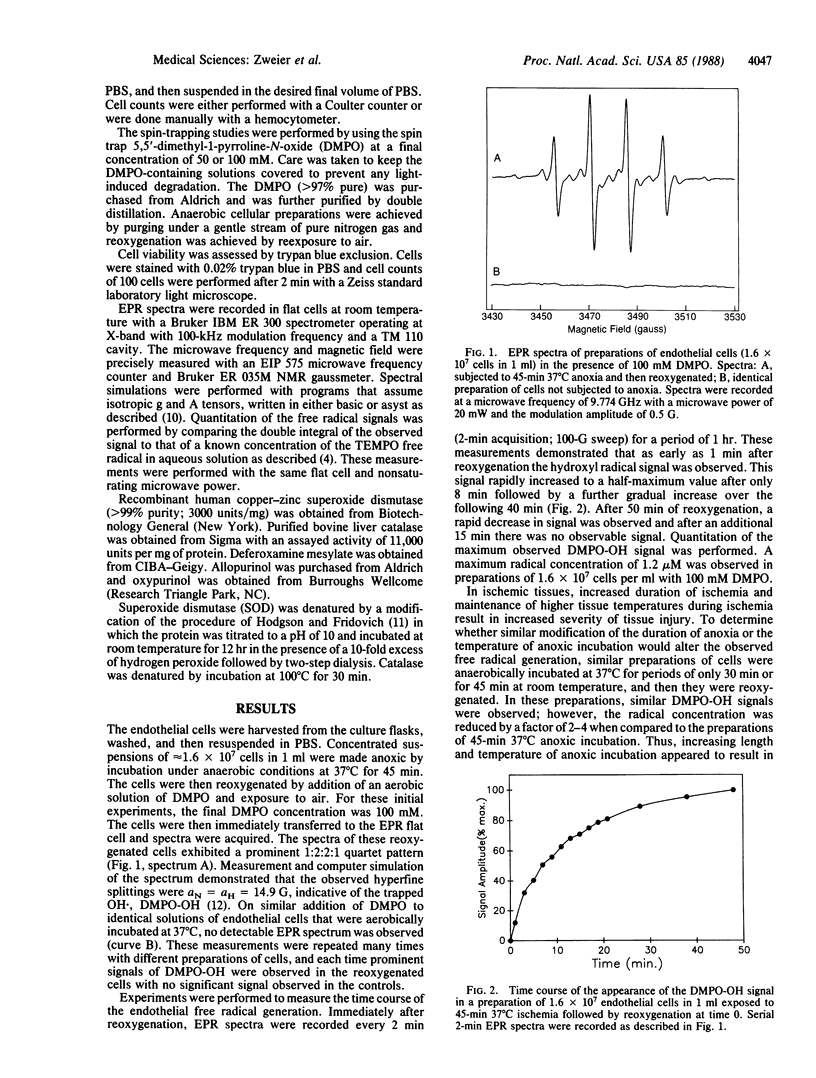
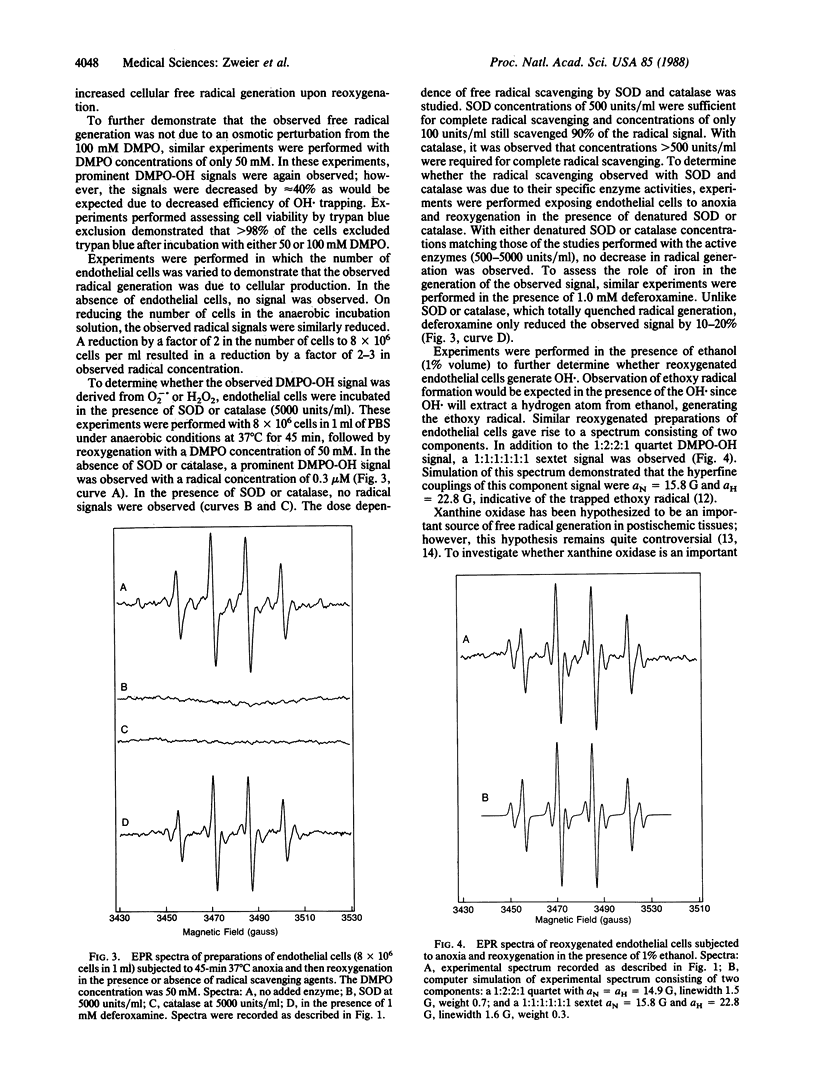
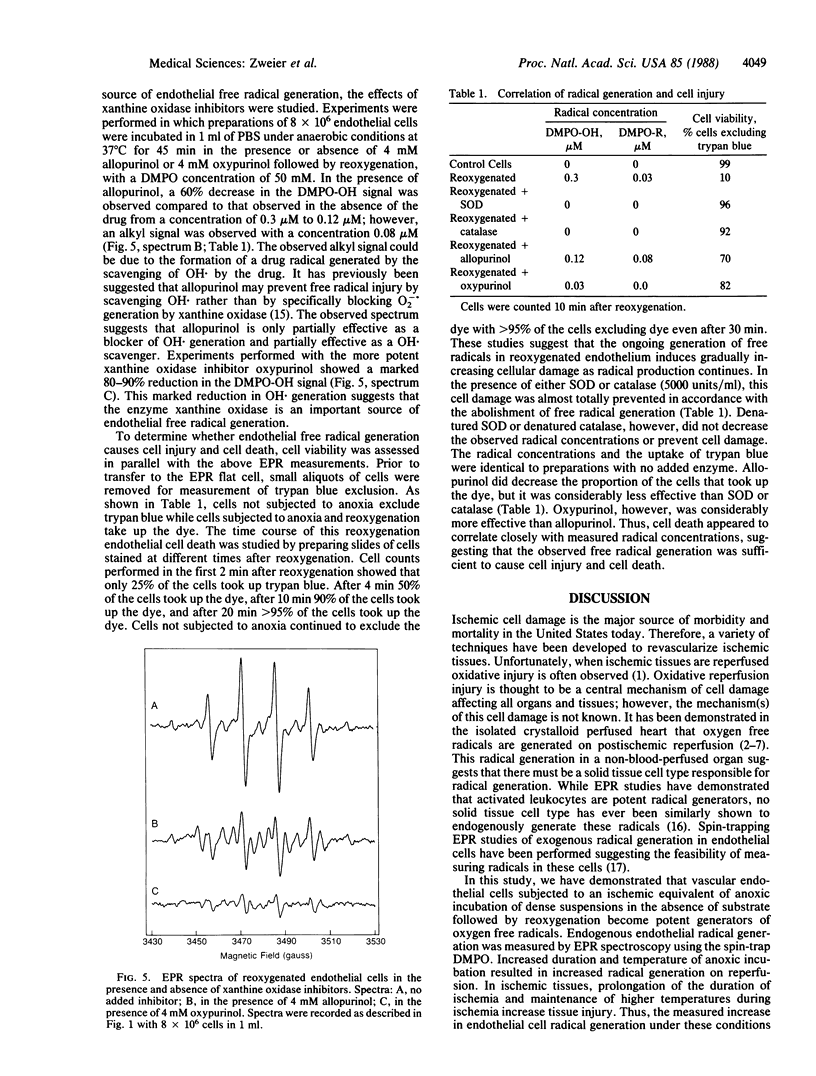
Abstract
Oxygen free radicals have been demonstrated to be important mediators of postischemic reperfusion injury in a broad variety of tissues; however, the cellular source of free radical generation is still unknown. In this study, electron paramagnetic resonance measurements with the spin trap 5,5'-dimethyl-1-pyrroline-N-oxide (DMPO) demonstrate that bovine endothelial cells subjected to anoxia and reoxygenation become potent generators of superoxide and hydroxyl free radicals. A prominent DMPO-OH signal aN = aH = 14.9 G is observed on reoxygenation after 45 min of anoxic incubation. Quantitative measurements of this free radical generation and the time course of radical generation are performed. Both superoxide dismutase and catalase totally abolish this radical signal, suggesting that O2 is sequentially reduced from O2-. to H2O2 to OH.. Addition of ethanol resulted in trapping of the ethoxy radical, further confirming the generation of OH.. Endothelial radical generation was shown to cause cell death, as evidenced by trypan blue uptake. Radical generation was partially inhibited and partially scavenged by the xanthine oxidase inhibitor allopurinol. Marked inhibition of radical generation was observed with the potent xanthine oxidase inhibitor oxypurinol. These studies demonstrate that endothelial cells subjected to anoxia and reoxygenation, conditions observed in ischemic and reperfused tissues, generate a burst of superoxide-derived hydroxyl free radicals that in turn cause cell injury and cell death. Most of this free radical generation appears to be from the enzyme xanthine oxidase. Thus, endothelial cell free radical generation may be a central mechanism of cellular injury in postischemic tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Khalidi U. A., Chaglassian T. H. The species distribution of xanthine oxidase. Biochem J. 1965 Oct;97(1):318–320. doi: 10.1042/bj0970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio G., Zweier J. L., Jacobus W. E., Weisfeldt M. L., Flaherty J. T. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: the role of iron in the pathogenesis of reperfusion injury. Circulation. 1987 Oct;76(4):906–915. doi: 10.1161/01.cir.76.4.906. [DOI] [PubMed] [Google Scholar]
- Arroyo C. M., Kramer J. H., Dickens B. F., Weglicki W. B. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987 Aug 31;221(1):101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3(4):259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- Chambers D. E., Parks D. A., Patterson G., Roy R., McCord J. M., Yoshida S., Parmley L. F., Downey J. M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985 Feb;17(2):145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Fenselau A., Mello R. J. Growth stimulation of cultured endothelial cells by tumor cell homogenates. Cancer Res. 1976 Sep;36(9 PT1):3269–3273. [PubMed] [Google Scholar]
- Gitlin J. D., D'Amore P. A. Culture of retinal capillary cells using selective growth media. Microvasc Res. 1983 Jul;26(1):74–80. doi: 10.1016/0026-2862(83)90056-0. [DOI] [PubMed] [Google Scholar]
- Hilton B. D., Misra R., Zweier J. L. Magnetic resonance studies of fredericamycin A: evidence for O2-dependent free-radical formation. Biochemistry. 1986 Sep 23;25(19):5533–5539. doi: 10.1021/bi00367a028. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Heid H. W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- Massey V., Komai H., Palmer G., Elion G. B. On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem. 1970 Jun 10;245(11):2837–2844. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B. Failure of the xanthine oxidase inhibitor allopurinol to limit infarct size after ischemia and reperfusion in dogs. Circulation. 1985 May;71(5):1069–1075. doi: 10.1161/01.cir.71.5.1069. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]
- Zweier J. L., Rayburn B. K., Flaherty J. T., Weisfeldt M. L. Recombinant superoxide dismutase reduces oxygen free radical concentrations in reperfused myocardium. J Clin Invest. 1987 Dec;80(6):1728–1734. doi: 10.1172/JCI113264. [DOI] [PMC free article] [PubMed] [Google Scholar]



