Abstract
• Background and Aims The two closely related subtribes Bifrenariinae Dressler and Maxillariinae Benth. are easily distinguished on morphological grounds. Recently, however, molecular techniques have supported the inclusion of Bifrenariinae within a more broadly defined Maxillariinae. The present paper describes the diverse labellar micromorphology found amongst representatives of Bifrenariinae (Bifrenaria Lindl., Rudolfiella Hoehne, Teuscheria Garay and Xylobium Lindl.) and compares it with that found in Maxillaria Pabst & Dungs and Mormolyca Fenzl (Maxillariinae).
• Methods The labella of 35 specimens representing 22 species of Bifrenariinae were examined by means of light microscopy and scanning electron microscopy and their micromorphology compared with that of Maxillaria sensu stricto and Mormolyca spp. The labellar epidermis of representatives of Bifrenaria, Xylobium and Mormolyca was tested for protein, starch and lipids in order to ascertain whether this tissue is involved in the rewarding of pollinators.
• Key Results and Conclusions The labella of Bifrenaria spp. and Mormolyca spp. are densely pubescent but those of Xylobium, Teuscheria and Rudolfiella are generally papillose. However, whereas the trichomes of Bifrenaria and Mormolyca are unicellular, those found in the other three genera are multicellular. Hitherto, no unicellular trichomes have been described for Maxillaria, although the labella of a number of species secrete a viscid substance or bear moniliform, pseudopollen-producing hairs. Moniliform hairs and secretory material also occur in certain species of Xylobium and Teuscheria and these genera, together with Maxillaria, are thought to be pollinated by stingless bees (Meliponini). Differences in the labellar micromorphology of Bifrenaria and Mormolyca are perhaps related to Euglossine- and/ or bumble bee-mediated pollination and pseudocopulation, respectively. Although Xylobium and Teuscheria share a number of labellar features with Maxillaria sensu stricto, this does not necessarily reflect taxonomic relationships but may be indicative of convergence in response to similar pollinator pressures.
Keywords: Bifrenaria, Bifrenariinae, Maxillaria, Maxillariinae, Meliponini, papillae, pollination, pseudopollen, Rudolfiella, Teuscheria, trichomes, Xylobium
INTRODUCTION
In his treatment of the tribe Maxillarieae Pfitzer, Dressler (1990) assigned five genera, namely, Bifrenaria Lindl., Horvatia Garay, Rudolfiella Hoehne, Teuscheria Garay and Xylobium Lindl. to the newly erected subtribe Bifrenariinae Dressler since they share the following set of characters. They may be epiphytic or lithophytic with pseudobulbs consisting of a single node, sometimes covered with hard cataphylls. The leaves are articulate, terminal or distichous and convolute, plicate or subconduplicate and the inflorescence, which is lateral, bears one to several, spirally arranged, small or large flowers. These are generally resupinate with a column of variable length and usually have a pronounced foot. The anther is terminal, operculate with reduced partitions and the four pollinia are superposed with a prominent viscidium. The pollinia may be sessile or possess one, or more usually two, stipes. The stigma is entire (Dressler, 1990).
The distinct column-foot and mentum, the four rounded or ovoid pollinia and the broad, open stigma found in the Bifrenariinae are also shared by members of Maxillariinae Benth. and Lycastinae Schltr. However, members of Maxillariinae sensu stricto, are distinguished by their conduplicate leaves and usually crescent-shaped viscidium, whereas members of Lycastinae have plicate leaves and a strap-like viscidium (Whitten et al., 2000). Similarly, Xylobium is distinguished from Maxillaria Ruiz & Pav. by its plicate leaves and several-flowered, racemose inflorescences, the four pollinia arising from a transverse, scale-like viscidium (Bechtel et al., 1981). As a result, genera formerly assigned to Bifrenariinae and Lycastinae have recently been incorporated into Maxillariinae (Dressler, 1993; Ryan et al., 2000; Whitten et al., 2000; Koehler et al., 2002; Chase et al., 2003; Chase, 2005) thereby creating a Neotropical, species-rich assemblage that displays diverse vegetative morphology and growth patterns (Dressler, 1993; Atwood and Mora de Retana, 1999) whilst retaining a relatively conservative floral morphology (Dressler, 1993; Atwood and Mora de Retana, 1999; Ryan et al., 2000; Whitten et al., 2000, Koehler et al., 2002).
Both molecular and non-molecular evidence strongly agree that Maxillariinae is monophyletic (Dressler, 1993; Holtzmeier et al., 1998; Whitten et al., 2000; Dathe and Dietrich, 2006) but generic boundaries are poorly defined, particularly so with regard to the very morphologically diverse genus Maxillaria. This genus in its broader sense is polyphyletic (Singer and Koehler, 2004), whereas in its current, narrower circumscription, it is considered paraphyletic (Dathe and Dietrich, 2006). Parsimony analyses of combined nuclear ribosomal and plastid DNA sequence data strongly support the four clades Maxillariinae, Bifrenariinae, Lycastinae and Xylobium (Whitten et al., 2000) but, since there is little support for the position of Xylobium relative to Maxillariinae sensu stricto and Lycastinae, this would necessitate either the erection of a separate subtribe or entail that Xylobium be left incertae sedis (Whitten et al., 2000). Consequently, Whitten, Chase and co-workers (Whitten et al., 2000; Chase et al., 2003; Chase, 2005) favour the lumping of these four clades into a single, broadly defined Maxillariinae thereby reflecting their close relationships whilst avoiding the creation of a monogeneric subtribe.
Lindley (1832) based his genus Bifrenaria on the robust species B. atropurpureum Lindl. which has large flowers borne upon an inflorescence that does not exceed the pseudobulbs in height. Some years later, he erected the genus Stenocoryne Lindl. which he based upon S. longicornis (Lindl.) Lindl. (Lindley, 1843). This genus differed from Bifrenaria in that the small flowers were borne upon a relatively tall inflorescence. However, it would appear that Lindley, and all subsequent authors, had overlooked the fact that Rafinesque (1836) had already erected the genus Adipe Raf. based upon A. racemosa Raf., a species whose vegetative and inflorescence characters closely resemble those of S. longicornis, although florally the plants are very different (Koehler and Amaral, 2004, and references therein). Eventually, Wolff (1990) transferred S. longicornis to Adipe.
Meanwhile, Schlechter (1914) had described a new genus belonging to the Bifrenaria complex and named it Lindleyella (nom. illeg.). This was validated in 1944 by Hoehne under the name Rudolfiella. This genus shares a forked stipe and unifoliate pseudobulbs with Bifrenaria (Koehler et al., 2002) but can be distinguished from that genus by its compressed pseudobulbs, the strongly divided lobes to the labellum, the prominent claw at the back of the labellum and the conspicuous callus (Koehler and Amaral, 2004). Rudolfiella is monophyletic, and, like Teuscheria, is closely related to, but distinct from, Bifrenaria although its position within Bifrenariinae has hitherto not been satisfactorily resolved (Koehler et al., 2002).
Garay (1958) had erected the genus Teuscheria to accommodate a newly discovered orchid species, T. cornucopiae Garay, from the Ecuadorian Andes. Since then, a number of Teuscheria spp. have been discovered (Garay, 1970; Dressler, 1972; Dodson, 1978; Jenny and Braem, 1987) and these share the following features. They are epiphytic, caespitose or rhizomatous with unifoliate, conical, pyriform or ovate pseudobulbs with cataphylls. The leaves are petiolate, narrowly elliptic to oblong and plicate. The inflorescence is lateral and 1-flowered. Flowers are resupinate or not with a short to pronounced spur. The lateral sepals and tri-lobed labellum are fused to the column-foot. Column short; pollinia 4, unequal.
During his revision of the Bifrenaria complex, Hoehne (1944) considered a number of characters such as plant size, pseudobulb shape, length of inflorescence, flower size, shape of labellum and length of claw, presence and shape of spur, and pollinarium structure, and used these to separate Bifrenaria, Stenocoryne and Rudolfiella. Castro (1991a–c, 1996), in his treatment of the complex, recognized only the genus Bifrenaria excluding Rudolfiella and Senghas (1994) transferred B. tetragona (Lindl.) Schltr. and B. wittigii (Rchb.f.) Hoehne, on the grounds that these species possess several-flowered inflorescences, erect perianth segments and an entire pollinarium stipe, to Cydoniorchis Senghas. Likewise, Carnevali and Romero (2000) erected two new monotypic genera, Guanchezia G.A. Romero & Carnevali and Hylaeorchis Carnevali & G.A. Romero in which they placed B. maguirei C. Schweinf. and B. petiolaris (Schltr.) G.A. Romero & Carnevali, respectively.
More recently, morphological studies and phylogenetic analyses based on DNA sequence data (Koehler et al., 2002; Chase et al., 2003; Koehler and Amaral, 2004) concluded that Bifrenaria sensu lato constitutes a monophyletic group comprising Adipe, Cydoniorchis and Bifrenaria sensu stricto, but not Rudolfiella. These same studies showed that Cydoniorchis is monophyletic but that Adipe and Bifrenaria sensu stricto are not. Even so, since retaining Cydoniorchis as a separate genus would demand the erection of seven new genera for which there is little bootstrap support, Koehler and co-workers (Koehler et al., 2002; Koehler and Amaral, 2004) consider that the widening of the circumscription of Bifrenaria and the reduction of Adipe, Stenocoryne and Cydoniorchis to synonomy under Bifrenaria is the best way to maintain nomenclatural stability. Thus, according to the latest treatment (Koehler and Amaral, 2004), the South American genus Bifrenaria, as it is currently circumscribed, contains about 20 species. Morphological and molecular data indicate that there are two distinct clades. The first comprises species that occur mainly in the Atlantic Forest of south-eastern Brazil or less frequently as rupiculous plants in the Brazilian ‘campos rupestres’, whereas the other is represented by two species that grow exclusively in the Amazonian region. The genus is distinguished from other members of the Maxillariinae sensu lato by the four-angled pseudobulbs, the plicate leaves, the conspicuous floral spur and the forked pollinarium stipe.
By contrast, the genus Xylobium has been largely neglected since the revision of Schlechter (1913) in which he recognized 24 species most of which had previously been described as species of Maxillaria. Several of these are to be found in cultivation (Teuscher, 1974; Senghas, 1995; Röth, 2004) but are often wrongly labelled.
Since general morphology and molecular approaches currently support the incorporation of genera formerly assigned to Bifrenariinae into a broadly defined Maxillariinae (Whitten et al., 2000; Chase et al., 2003; Chase, 2005), it is reasonable to suppose that comparison of the labellar micromorphology of Bifrenaria and allied genera with that of Xylobium and Maxillaria sensu stricto could also yield useful information, especially since the labellar micromorphology of Maxillaria sensu stricto has already been extensively studied (Davies and Winters, 1998; Davies et al., 2000, 2003a, b; Davies and Turner, 2004a; Matusiewicz et al., 2004).
Mormolyca Fenzl is distinguished from Maxillaria sensu stricto on morphological grounds by the inflorescence, which is as long as the leaves, the absence of a foot and the lunate viscidium (Garay and Wirth, 1959; Bechtel et al., 1981) but Holtzmeier et al. (1998) and Dathe and Dietrich (2006) have shown by means of phylogenetic and maximum parsimony and Bayesian analysis that this genus, as represented by M. ringens (Lindl.) Schltr., is deeply embedded within the cladistic structure of Maxillaria and that, if M. ringens is to be accepted as a member of a distinct genus, then Maxillaria should be considered paraphyletic.
Consequently, the aim of this present paper is to describe the labellar micromorphology of Bifrenaria, Xylobium and representative species of Rudolfiella and Teuscheria and to compare it with that of Maxillaria sensu stricto and Mormolyca, with the intention of gaining greater insight into the evolution and pollination biology of these genera.
MATERIALS AND METHODS
Thirty-one spirit-preserved specimens, representing 20 taxa, were obtained from the herbarium of the Royal Botanic Gardens, Kew, UK and supplemented with living and preserved material from the first author's collection and from Swansea Botanical Complex, UK (Table 1). Their accession numbers are prefixed ‘K’, ‘KLD’ and ‘S’, respectively. The names by which these specimens were originally collected have been retained but recent changes in nomenclature have been noted. Preserved material was stored in ‘Copenhagen mix’ (70 cm3 industrial methylated spirit : 2 cm3 glycerol : 28 cm3 water) and the authorities for plant names follow Brummit and Powell (1992). Following preliminary examination by means of light microscopy, pieces of labellum were excised and prepared for scanning electron microscopy (SEM) as previously described (Stpiczyńska et al., 2003; Davies and Turner, 2004b) and examined by means of a JSM 5200 LV-SEM or TESLA BS-300 at an accelerating voltage of 20–25 kV.
TABLE 1.
Specimens studied and their provenance
| Taxon | Accession no. | Collector | Provenance | Date of Collection | Taxonomic notes |
|---|---|---|---|---|---|
| Bifrenaria aurea Barb. Rodr. | K54349 | Donated by Sander, F. | Brazil | Cult. 1998 | syn. B. harrisoniae (Hook.) Rchb.f. - Koehler & Amaral, 2004 |
| B. fuerstenbergiana Schltr. | K14273 | Purchased from Blossfield, R. | Brazil | Cult. 1944 | syn. B. inodora Lindl. - Pabst & Dungs 1977 |
| B. harrisoniae (Hook.) Rchb.f. | KLD200501 | Davies, KL. | Cult. 2005 | ||
| B. tetragona Lindl. | K50008 | Wyld Court Nursery | Brazil | Cult. 1986 | syn. Cydoniorchis tetragona (Lindl.) Senghas - Senghas, 1994 |
| B. tetragona Lindl. | KLD200502 | Davies, KL. | Cult. 2005 | syn. Cydoniorchis tetragona (Lindl.) Senghas - Senghas, 1994 | |
| B. tyrianthina (Lodd. ex Loudon) Rchb.f. | KLD200602 | Davies, KL. | Cult. 2006 | ||
| B. wendlandiana (Kraenzl.) Cogn. | K57074 | Warren, R. | Brazil | 1993 | syn. B. clavigera Rchb.f. - Koehler & Amaral, 2004 |
| Stenocoryne secunda (Vell.) Hoehne | K37726 | Gailer, J. 100 | Cult. 1975 | syn. B. aureofulva (Hook.) Lindl. - Koehler & Amaral, 2004 | |
| Rudolfiella aurantiaca (Lindl.) Hoehne | K61278 | Sothers, CA; Pereira, E.DA C. 601 | Manaus-Itacoatiara, Brazil | 1995 | |
| Rudolfiella aurantiaca (Lindl.) Hoehne | K57061 | da Silva, JBF. | Amazonas, Brazil | ||
| Teuscheria wageneri (Rchb.f.) Garay | K41855 | Dunsterville, GCK. 474 | Venezuela | 1958 | |
| Xylobium bractescens (Lindl.) Kraenzl. | K14421 | From N.B.G. Glasnevin | Cult. 1961 | ||
| X. colleyi (Bateman ex Lindl.) Rolfe | K37071 | Mason, LM. 1079 | Guyana | Cult. 1976 | |
| X. colleyi (Bateman ex Lindl.) Rolfe | K13833 | Donated by Mason, LM. | Guyana | Cult. 1957 | |
| X. corrugatum (Lindl.) Rolfe | K49592 | Hodgson, I. 264 | Ecuador | Cult. 1986 | |
| X. cf. corrugatum (Lindl.) Rolfe | S20030489 | Gregg, A. | Cult. 2004 | ||
| X. elongatum (Lindl.) Hemsl. | K14422 | Donated by Mason, LM. | Panama | Cult. 1959 | |
| X. foveatum (Lindl.) G. Nicholson | K14423 | Lankester, CH. | Costa Rica | Cult. 1934 | |
| X. latilabium C. Schweinf. | K45854 | Jenny, R. vo 178/82 | Peru | Cult. 1982 | syn. X. ornatum (Klotzsch) Rolfe |
| X. latilabium C. Schweinf. | K6811 | Donated by Mason, LM. | Peru | Cult. 1965 | syn. X. ornatum (Klotzsch) Rolfe |
| X. leontoglossum (Rchb.f.) Benth. ex Rolfe | KLD200601 | Davies, KL. | Cult. 2006 | ||
| X. pallidiflorum (Hook.) G. Nicholson | K47185 | Hodgson, IG. 280 | Ecuador | Cult. 1983 | |
| X. pallidiflorum (Hook.) G. Nicholson | K13835 | Donated by Lawrance, AE. 280 | Venezuela | Cult. 1931 | |
| X. palmifolium (Sw.) Fawc. | K37706 | Mason, LM. 1040 | West Indies | Cult. 1976 | syn. Maxillaria palmifolia (Sw.) Lindl. |
| X. powellii Schltr. | K8480 | Donated by Mahoney, LM. Mason | Panama | Cult. 1959 | |
| X. scabrilingue (Lindl.) Rolfe ex Gentil | K31551 | Mason, LM. 2146 | Peru | Cult. 1968 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 |
| X. squalens (Lindl.) Lindl. | K10492 | Donated by Sander, F. | Venezuela | Cult. 1956 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 |
| X. squalens (Lindl.) Lindl. | K12658 | Bought from Binot, J. | Cult. 1937 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 | |
| X. squalens (Lindl.) Lindl. | K13837 | Purchased at sale by Protheroe & Morris | Cult. 1937 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 | |
| X. squalens (Lindl.) Lindl. | K13838 | Donated by Lawrance, AE. | Venezuela | Cult. 1931 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 |
| X. squalens (Lindl.) Lindl. | K14424 | Mason, LM. | Guyana | 1960 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 |
| X. squalens (Lindl.) Lindl. | K14425 | Donated by Garnett, CS. | Cult. 1935 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 | |
| X. squalens (Lindl.) Lindl. | K32139 | Dunsterville, G. | Venezuela | Cult. 1968 | syn. X. variegatum (Ruiz & Pav.) Garay & Dunst. - Bechtel et al., 1981 |
| X. variegatum (Ruiz & Pav.) Garay & Dunst. | K46104 | Dunsterville, G. | Venezuela | Cult. 1982 | |
| X. variegatum (Ruiz & Pav.) Garay & Dunst. | K43855 | Storr, R. 077 | Brazil | Cult. 1980 | |
| Mormolyca ringens (Lindl.) Schltr. | S19980091 | Gregg, A. | Cult. 1998 | ||
| M. schweinfurthiana Garay & Wirth | K27543 |
On the basis of SEM results, labellum samples derived from representative specimens of the genera Bifrenaria (B. harrisoniae KLD200501), Xylobium (X. squalens K13837, K14424, X. leontoglossum KLD200601, X. cf. corrugatum S20030489) and Mormolyca (M. ringens S19980091) were subjected to histochemical analysis for protein, starch and lipids (Davies et al., 2000, 2002, 2003a–c; Davies and Turner 2004a, b) in order to determine whether particular labellar structures are involved in the production of pollinator rewards. Wherever possible, these tests were carried out on living tissue (KLD200501, S19980091, KLD200601). However, in the absence of living material, preserved tissue (K13837, K14424) was used reluctantly and only when SEM dictated that this was necessary. Although not ideal, there is evidence that these compounds are preserved and can be detected in plant material even after prolonged storage in ‘Copenhagen mix’ or formalin solution (Davies and Turner, 2004b).
RESULTS
The labellum of Bifrenaria is papillose and densely pubescent, the papillae and trichomes intergrading (Fig. 1A–D). The papillae are conical with pointed tips (Fig. 2A–D) and, in certain species such as B. tetragona, bear longitudinal, cuticular striations (Fig. 2E). At first, the epidermal cells have convex outer tangential walls. A small protrusion appears at its centre (Fig. 2C) and this forms a papilla which eventually develops into a trichome (Fig. 2D). The hairs are simple, unicellular and relatively long with rounded tips (40–620 μm; mean 322·4 μm in B. harrisoniae) (Figs 1B–D and 2F) but in B. tetragona, 2–3-celled trichomes were occasionally observed. The trichomes of B. aurea are shorter (36·3–131·6 μm; mean 69·5 μm) and much more closely resemble papillae (Fig. 2A, D) than those of the other Bifrenaria species studied. By contrast, the labellum of B. secunda [syn. B. aureofulva (Hook.) Lindl.], which was formerly assigned to Stenocoryne, differs from the other Bifrenaria spp. studied in that it is largely glabrous except for a few, sparsely arranged, relatively short, straight hairs (Fig. 3A, B).
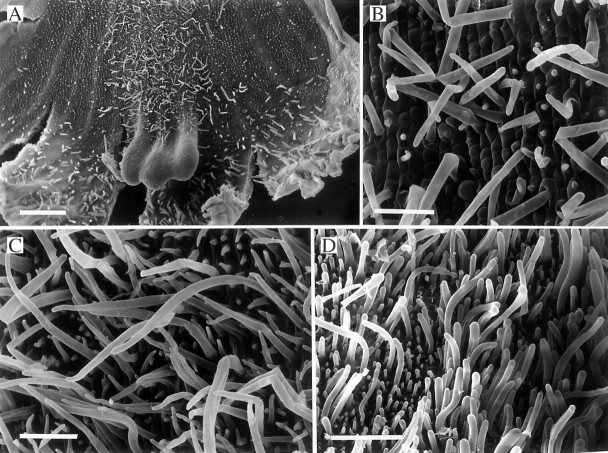
Fig. 1.
(A–C) Labellum of Bifrenaria wendlandianum (K57074) showing densely pubescent median region (A), stages in the development of short, unicellular trichomes from conical papillae (B) and fully formed, elongate trichomes (C). Scale bars: A = 1 mm; B and C = 100 μm. (D) Labellar surface of Bifrenaria harrisoniae (KLD200501) showing similar, unicellular trichomes. Scale bar = 500 μm.
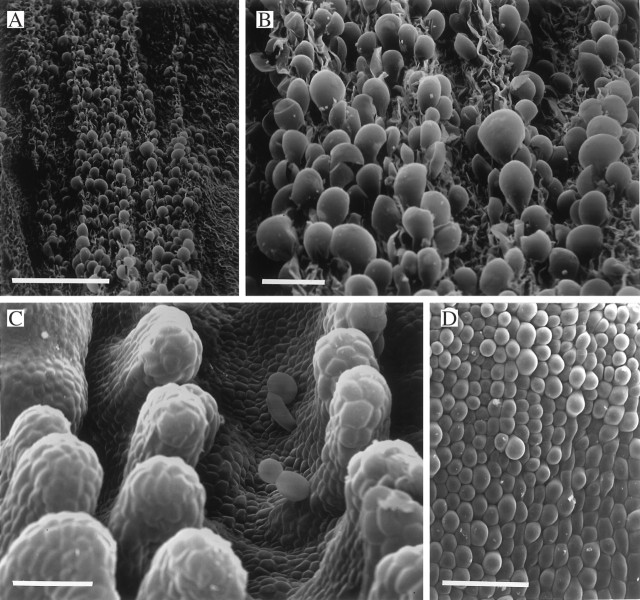
Fig. 2.
(A–D) Labellum of Bifrenaria aurea (K54349) showing short, conical papilla-like trichomes (A), conical to obpyriform papillae (B) and stages in trichome formation (C, D). The latter process commences with the development of a small protrusion upon the papilla (C). Further development of this protrusion results in the formation of a conical papilla and eventually a trichome (D). Scale bars: A = 500 μm; B = 100 μm; C = 25 μm; D = 100 μm. (E, F) Labellar surface of Bifrenaria tetragona (KLD200502) showing conical papillae with longitudinal, cuticular striations (E) and stages in the development of unicellular trichomes (F). Scale bars: E = 10 μm; F = 200 μm.
Fig. 3.
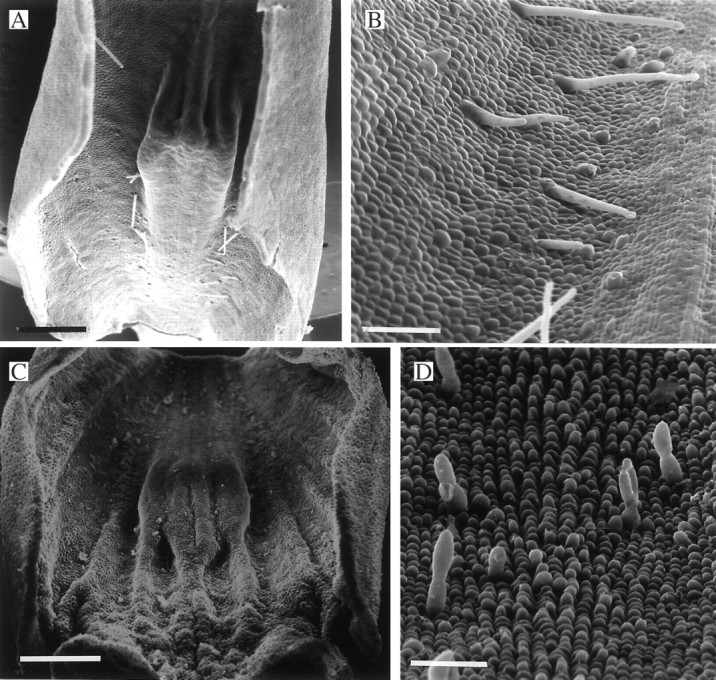
(A, B) Labellum of Stenocoryne secunda (syn. Bifrenaria aureofulva) (K37726) showing sparse (A), unicellular trichomes (B). Scale bars: A = 1 mm; B = 250 μm. (C, D) Papillose labellum of Xylobium foveatum (K14423) (C) showing obpyriform papillae and 2-celled trichomes with clavate terminal cells (D). Scale bars: C = 1 mm; D = 100 μm.
The labellum of Xylobium, however, shows greater micromorphological diversity. It is generally papillose (Figs 3C, D, 4A–F, 5A–F, 6A–D, 7A–F, 8A–F and 9A–D), although it may be glabrous or minutely papillose as in X. colleyi (Fig. 9E, F). In certain species such as X. squalens, even when papillae are present, hairs are usually absent.
Fig. 4.
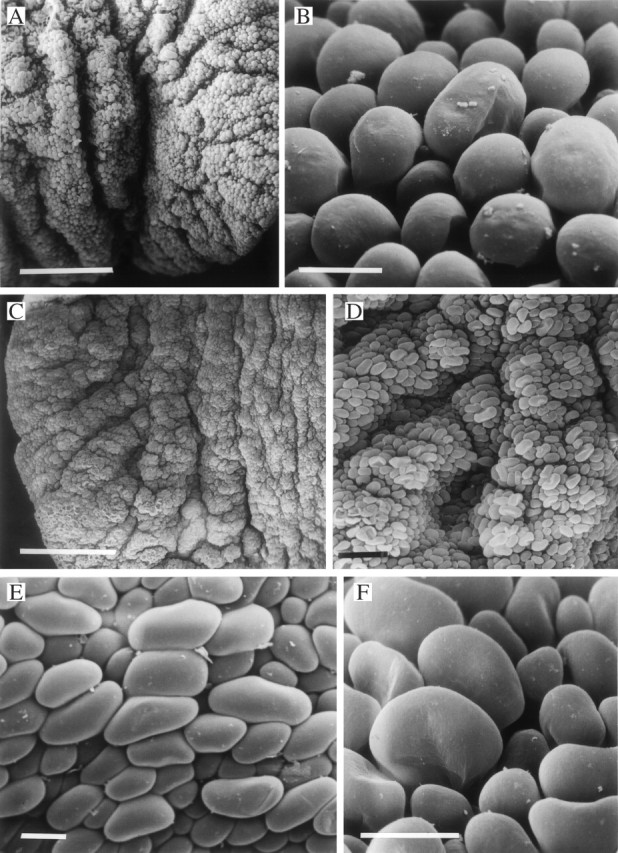
(A, B) Papillose labellum of Xylobium palmifolium (K37706) (A) with obpyriform papillae (B). Scale bars: A = 500 μm; B = 25 μm. (C–F) Labellum of Xylobium pallidiflorum (K47185) (C) showing laterally compressed papillae (D, E) that have a lollipop- or paddle-like profile (F). Scale bars: C = 500 μm; D = 100 μm; E and F = 25 μm.
Fig. 5.
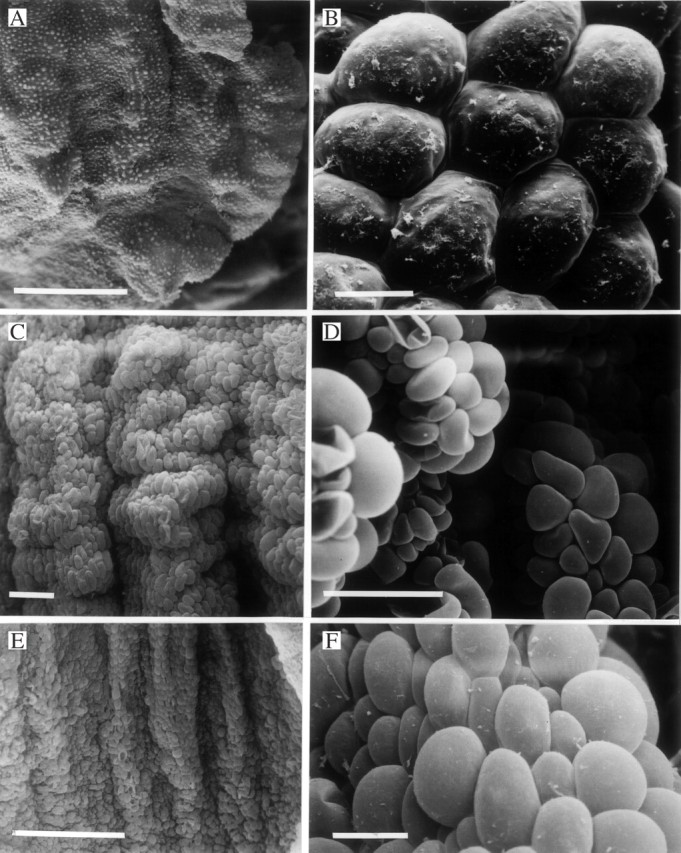
(A, B) Labellum of Xylobium corrugatum (K49592) (A) showing papillae with traces of secreted material (B). Scale bars: A = 500 μm; B = 10 μm. (C, D) Labellar surface of Xylobium bractescens (K14421) showing the arrangement (C) of laterally compressed, lollipop- or paddle-like papillae (D) along the carinae. Scale bars: C and D = 100 μm. (E, F) Labellum of Xylobium powellii (K8480) (E) showing laterally compressed papillae (F). Scale bars: E = 500 μm; F = 25 μm.
Fig. 6.
(A, B) Labellar surface of Xylobium powellii (K8480) showing arrangement (A) of laterally compressed, lollipop- or paddle-like papillae (B) along carinae. Scale bars: A = 500 μm: B = 100 μm. (C, D) Labellar surface of Xylobium elongatum (K14422) showing verrucae, simple, 2-celled trichomes (C) and obpyriform papillae (D). Scale bars: C and D = 200 μm.
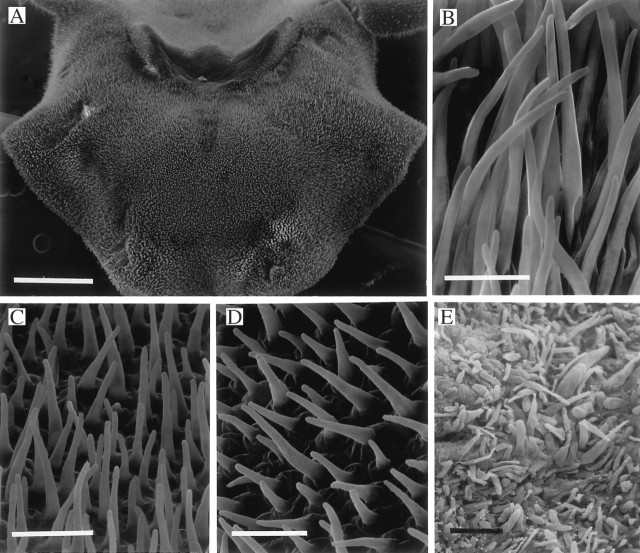
Fig. 7.
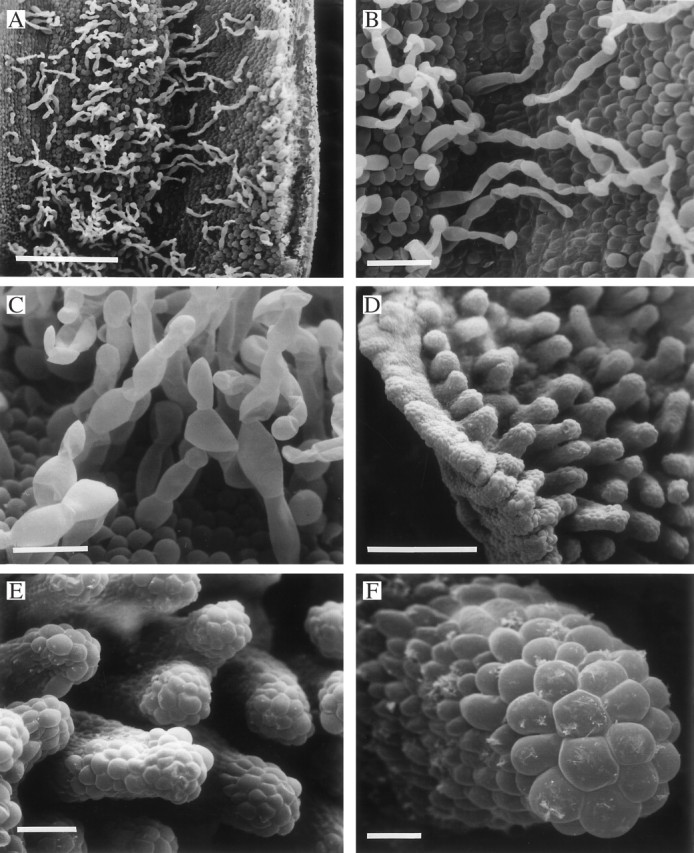
(A–C) Labellum of Xylobium latilabium (K6811) showing distribution (A) and detail (B, C) of uniseriate, moniliform trichomes and obpyriform papillae. Scale bars: A = 500 μm; B = 100 μm; C = 50 μm. (D–F) Labellum of Xylobium scabrilingue (K31551) showing verrucae (D, E) with well-defined cells at their tips (F). Scale bars: D = 500 μm; E = 100 μm; F = 25 μm.
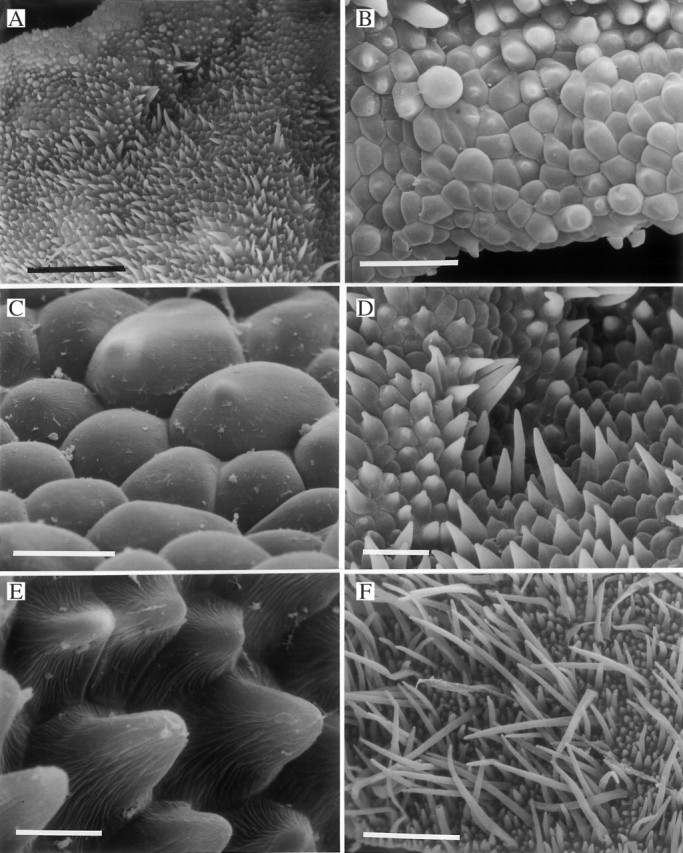
Fig. 8.
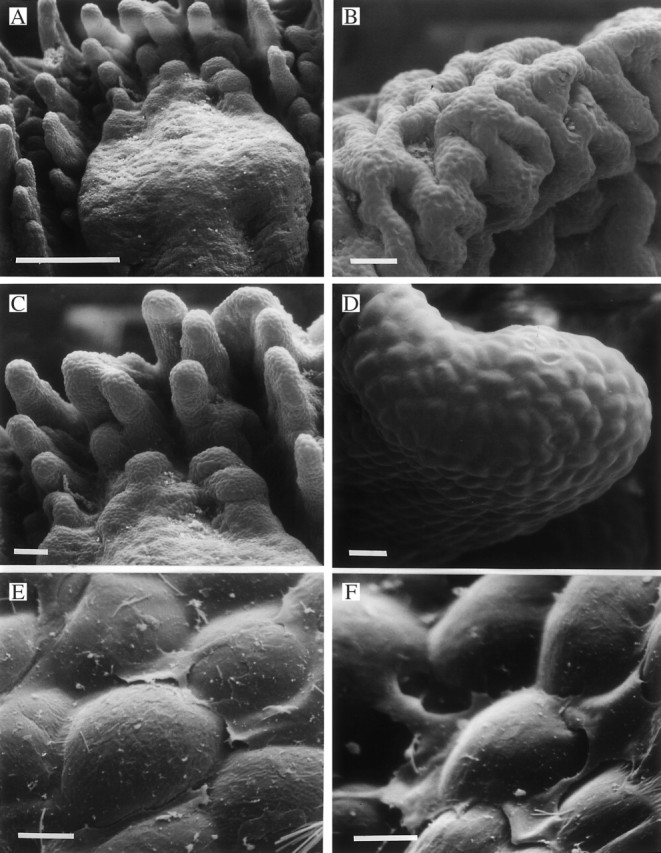
(A–F) Labellar surface of Xylobium squalens (K12658) showing callus (A) and verrucae (A–C) with poorly defined cells at their tips (D). This may be due to the presence of a film of secreted material, traces of which can be seen upon and between the labellar papillae (E, F). Scale bars: A = 500 μm; B and C = 100 μm; D = 25 μm; E and F = 5 μm.
Fig. 9.
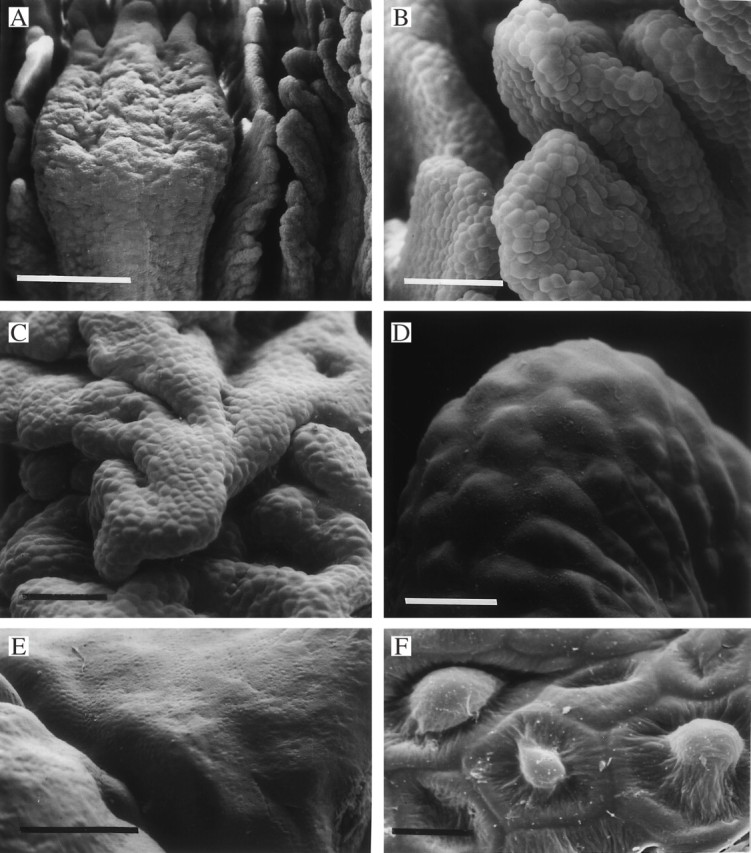
(A–D) Labellar surface of Xylobium variegatum (K46104) showing callus (A) and verrucae (A–C) whose tips appear to be coated with a film of secreted material, thus obscuring cell outlines (D). Scale bars: A = 500 μm; B and C = 100 μm; D = 25 μm. (E, F) Glabrous or minutely papillose labellum of Xylobium colleyi (K13833) (E) showing detail of papillae (F). Scale bars: E = 500 μm; F = 10 μm.
In X. squalens (considered by some authors, together with X. scabrilingue, conspecific with X. variegatum), the outlines of the labellar epidermal cells, especially those at the tips of the verrucae, like those of X. leontoglossum, are often indistinct (Fig. 8D). The epidermal, convex, outer tangential wall may bear traces of a film-like deposit (K12658), interpreted here as secreted material (Fig. 8E and F), and this may explain poor cell definition. Sparse, multicellular, 2–8-celled, uniseriate hairs with swollen, possibly glandular tips were observed on the proximal, adaxial surface of one specimen (K13837). Plants collected as X. squalens, however, may differ from those assigned to X. scabrilingue and X. variegatum in that the labellum of the two latter species often bear obpyriform to spherical papillae (Figs 7E, F and 9B). Again, in X. variegatum, unlike X. scabrilingue, these cells may be rather poorly defined towards the tips of the verrucae (Figs 7E, F and 9C, D) but no obvious secreted material was observed in X. variegatum nor in X. scabrilingue.
Obpyriform or spherical papillae also occur upon the labellum of X. corrugatum (Fig. 5A, B) and X. palmifolium (Fig. 4A, B). Moreover, there is some evidence that the papillae of X. corrugatum (K49592) produce a viscid secretion that accumulates at their bases (Fig. 5B). However, the rounded papillae of X. powellii, X. pallidiflorum and X. bractescens, which are arranged longitudinally along the labellar carinae, are laterally compressed and are paddle-like or resemble ‘lollipops’ (Figs 4C–F, 5C–F and 6A, B).
Unlike the other Xylobium spp. studied, hairs are present upon the labella of X. elongatum, X. foveatum and X. latilabium [syn. X. ornatum (Klotzsch) Rolfe] (Figs 3D, 6C and 7A–C). The trichomes of X. elongatum and X. foveatum are similar in that they are 1–2-celled but, whereas the hairs of the former species have rounded terminal cells (Fig. 6C), those of X. foveatum have clavate terminal cells (Fig. 3D). These hairs are interspersed between obpyriform to spherical papillae or conical to obpyriform papillae in X. elongatum and X. foveatum, respectively (Figs 3D and 6C, D) and the conical papillae have rounded tips. The trichomes of X. latilabium, however, are very different. They occur mainly on the mid-lobe of the labellum (Fig. 7A), arise from obpyriform to spherical papillae and are 4–8-celled, uniseriate and moniliform with rounded to clavate terminal cells (Fig. 7B, C).
Moniliform, 2–10-celled, uniseriate trichomes consisting of oval to rounded cells also occur in Teuscheria wageneri (Fig. 10A, C, D) but much of the epidermal surface is obscured by a film of secreted material (Fig. 10B, D). The epidermis, where visible, is papillose and consists of conical papillae with wide bases (Fig. 10B).
Fig. 10.
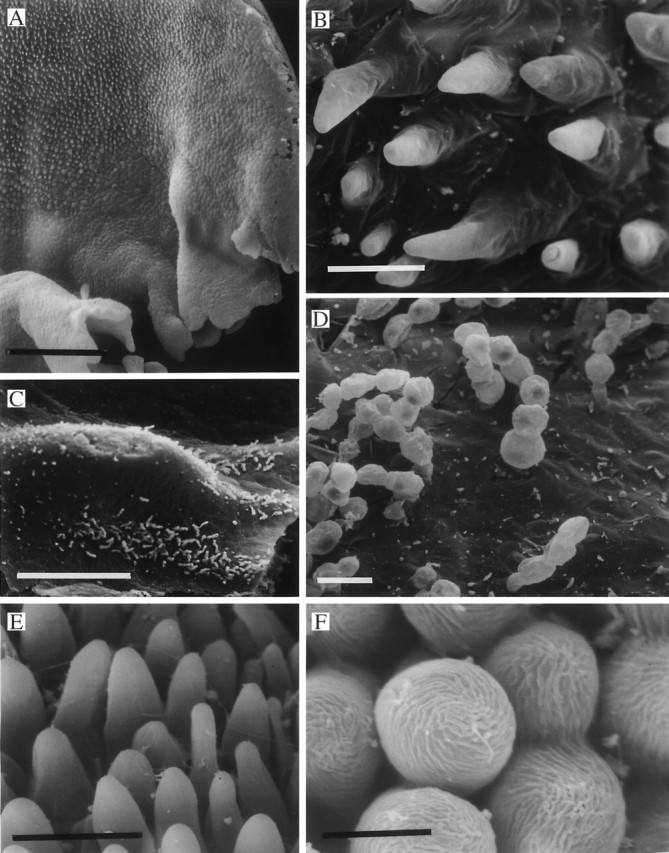
(A–D) Papillose labellum of Teuscheria wageneri (K41855) (A) showing conical papillae protruding through a thick layer of secreted material (B). Uniseriate, multicellular, moniliform trichomes also occur upon the labellum (C, D) and these too, penetrate the secreted material (D). Scale bars: A = 500 μm, B = 25 μm; C = 500 μm; D = 25 μm. (E, F) Labellar surface of Rudolfiella aurantiaca (K57061) showing conical papillae (E) with cuticular striations (F). Scale bars: E = 25 μm; F = 10 μm.
Similarly, the labellum of Rudolfiella aurantiaca is papillose with conical to obpyriform papillae (Figs 10E, F) bearing well-defined, cuticular striations (Fig. 10F). Uniseriate, 3–5-celled trichomes with pointed or rounded tips and cylindrical component cells are present at the point of attachment of the labellum.
The labella of both Mormolyca ringens (Figs 11A–D) and M. schweinfurthiana (Fig. 11E) are densely pubescent with conical papillae having pointed ends and narrow points of insertion intergrading to form simple, unicellular trichomes. These hairs measure 32–64 μm (mean 48 μm) and 25–92 μm (mean 53·2 μm) for M. ringens and M. schweinfurthiana, respectively.
Fig. 11.
(A–D) Densely pubescent labellum of Mormolyca ringens (S19980091) (A) showing unicellular trichomes with pointed tips and narrow points of insertion from proximal (B), median (C) and lateral (D) parts of lip. Scale bars: A = 1 mm; B–D = 50 μm. (E) Labellar surface of Mormolyca schweinfurthiana (K27543) showing similar unicellular trichomes to those found in M. ringens. Scale bar = 50 μm.
Since food materials also have a constitutive role, their presence alone does not necessarily indicate that they function as pollinator rewards. Only when they are present at elevated concentrations within secretions or structures such as hairs and papillae, and there is strong evidence that these are foraged and ingested by presumed pollinators or their larvae, can the role of these substances as pollinator rewards be established with any degree of certainty. In the absence of relevant field data, this was not possible, although histochemistry revealed that, in some cases, high concentrations of food materials were indeed present.
Histochemical analysis of B. harrisoniae (KLD 200501) revealed that the labellar trichomes did not contain starch or lipid nor were there higher concentrations of protein present here than in any other part of the labellum. In X. squalens (K13837 and K14424) and X. leontoglossum (KLD200601), treatment with an ethanolic solution of Sudan III revealed the presence of lipid along some of the epidermal cell walls and especially at those points where three (or more) adjacent cells meet. The labella of flowers of unknown provenance (S20030489), identical to those collected as X. corrugatum (K49592) but preserved in 5% formalin solution, stained even more intensely with Sudan III. In each case, the location of lipid-rich material corresponded to that of the presumed secretion observed using SEM (Figs 5B and 8E, F). The walls of some of the epidermal hairs of X. squalens (K13837) also stained with Sudan III. Histochemistry of Mormolyca ringens was frustrated by the presence of pigment in the epidermal hairs. At first, it appeared that the epidermis had stained more intensely for protein than underlying tissues but careful comparison of the extent of staining in non-pigmented trichomes with that of underlying parenchyma in hand-cut sections showed that the hairs did not contain greater quantities of protein, nor indeed starch and lipids, than other labellar tissues.
DISCUSSION
The labellar micromorphology of Maxillaria sensu stricto has been extensively studied (Davies and Winters, 1998; Davies et al., 2000, 2003a, b; Davies and Turner, 2004a; Matusiewicz et al., 2004). It may be glabrous and often rugose as in members of the M. cucullata Lindl. alliance but is generally papillose. The papillae are usually conical with wide bases and rounded or pointed tips but are frequently obpyriform or almost spherical, and some of the latter are modified and secrete wax or a resinous, viscid material (Davies et al., 2003a, b; Davies and Turner, 2004a; Matusiewicz et al., 2004). This contains aromatic amino acids, lipoidal compounds and triterpenoids (Davies et al., 2003a, b; Davies and Turner, 2004a; Flach et al., 2004; Singer et al., 2006) and it is thought that it may function as a pollinator reward. Trichomes, where present, tend to be simple, multicellular and uniseriate with pointed or rounded tips (Davies and Winters, 1998; Davies et al., 2003a; Davies and Turner, 2004a) and, although multiseriate hairs have been observed in this genus (Davies and Turner, 2004a), simple, unicellular hairs have not. Moniliform trichomes occur in certain Maxillaria spp., in particular those of the M. grandiflora (Humb., Bonpl. & Kunth) Lindl. complex and M. discolor (Lodd. ex Lindl.) Rchb.f. alliance (Davies and Winters, 1998; Davies et al., 2000, 2003a; Davies and Turner, 2004a; Matusiewicz et al., 2004) and their cells contain reserves of protein and, often, starch. They may fragment to form individual or short chains of cells (pseudopollen) and are gathered by stingless bees (Meliponini) that pollinate the flowers (Singer and Koehler, 2004), although, to date, there is no direct evidence that they are ingested by the pollinator. A small number of maxillarias secrete nectar and, although largely pollinated by stingless bees (Davies et al., 2005, and references therein), some are probably pollinated by hummingbirds (Stpiczyńska et al., 2003). In a study involving 100 species of Maxillaria, Davies et al. (2005) showed that some 13 % of species produce wax or viscid material, 16–23 % produce pseudopollen and 8 % produce nectar. The majority of species, some 56 %, however, do not produce any rewards and attract potential pollinators, mainly Trigona spp., solely by deceit (Singer and Cocucci, 1999; Singer and Koehler, 2004; Davies et al., 2005).
The labellum of Bifrenaria spp. resembles that of Maxillaria sensu stricto in that it bears conical papillae but the densely pubescent labellum of Bifrenaria at once distinguishes it from that genus. Moreover, the simple, unicellular type of trichome found in Bifrenaria has not been recorded for Maxillaria sensu stricto but these hairs are present, albeit sparsely, even in B. aureofulva, a species formerly placed in Stenocoryne. Generally, the distinction between papilla and trichome is simply a matter of degree, although usually, trichomes are more than twice as tall as they are wide with pointed or rounded tips and a narrow point of insertion. This distinction is much more clearly demarcated in Maxillaria than in Bifrenaria where there is a greater degree of intergrading. Differences in labellar micromorphology, the absence of secreted material upon the labellar surface of Bifrenaria and the absence of elevated concentrations of food substances in the labellar hairs of this genus make it unlikely that Bifrenaria and Maxillaria share the same pollinator. In fact, it would appear from the paucity of published data that the pollination of Bifrenaria has seldom been observed and much of the evidence available was arrived at indirectly. For example, Dressler (1990) reports Bifrenaria pollinaria on males of Eufriesia violacea (Euglossini) and Singer and Koehler (2004) cite the observation of I. Gajardo who reported seeing pollinaria of B. harrisoniae on Eufriesia violacea and Bombus brasiliensis (Bombini) in Paranapiacaba, São Paulo State. Smaller bees may be responsible for pollinating small-flowered species such as B. mellicolor Rchb.f. and it has even been suggested that B. aureofulva may be pollinated by hummingbirds which may explain the paucity of labellar hairs.
Koehler and Amaral (2004), in their review of Bifrenaria, concur with Castro and Campacci (2000) in reducing B. aurea to synonymy under B. harrisoniae and, although the present specimen of B. aurea generally conformed to the description of B. harrisoniae (Koehler and Amaral, 2004) in the dimensions, shape and proportions of all its floral parts, there were nonetheless, significant differences. For example, the callus was glabrous, the petal apices were not as rounded and the labellar hairs were much shorter and more papilla-like than those of B. harrisoniae. Unfortunately, it was not possible to examine the viscidium as the pollinarium was no longer present.
The labellar micromorphology of Xylobium more closely approaches that of Maxillaria than does Bifrenaria, in that the epidermis may be glabrous or papillose. A glabrous to minutely papillose labellum, however, was observed only for X. colleyi. Unlike other Brazilian species of Xylobium, which tend to have 2–3-leaved pseudobulbs and small flowers, X. colleyi has unifoliate pseudobulbs with relatively large flowers (Singer and Koehler, 2004). However, a number of Xylobium species such as X. corrugatum, X. latilabium, X. pallidiflorum and X. subintegrum C. Schweinf., from other parts of S. America, also have unifoliate pseudobulbs and flowers similar in size to those of X. colleyi (Dunsterville and Garay, 1965; Bennett and Christenson, 1993, 1995) but their labella, unlike that of X. colleyi, are distinctly papillose. In Xylobium, the papillose labellum bears conical, obpyriform or almost spherical papillae. The conical papillae have wide bases with pointed or rounded tips. Obpyriform to spherical papillae occur in X. palmifolium, a species retained in Maxillaria by some authorities. Indeed, in terms of labellar micromorphology alone, there is little to distinguish it from that genus, although it's several-flowered, racemose inflorescence and plicate leaves justify its inclusion in Xylobium. In some species of Xylobium, trichomes are present. As in Maxillaria, there is greater demarcation between the papillae and trichomes here than in Bifrenaria, and the trichomes of Xylobium, like those of Maxillaria, are multicellular rather than unicellular as in Bifrenaria. However, the laterally compressed, paddle- or lollipop-like papillae found in certain Xylobium spp. do not appear to occur in Maxillaria. Nevertheless, moniliform hairs, similar to those found in pseudopollen-forming species of Maxillaria, occur in X. latilabium, and lipoidal, secreted material, like that found in members of the Maxillaria acuminata Lindl., M. discolor and M. rufescens Lindl. alliances as well as in M. lepidota Lindl., M. reichenheimiana Endres & Rchb.f. and M. pseudoreichenheimiana Dodson (Davies et al., 2003a, b; Davies and Turner, 2004a; Flach et al., 2004; Matusiewicz et al., 2004; Singer et al., 2006), was detected in fresh X. leontoglossum and preserved specimens of X. corrugatum and X. squalens, in some cases, even after 69 years in spirit! Dunsterville and Garay (1965) described the lip of X. colleyi as having a ‘wet and “sticky” lustre’. Although this may be due to viscid, secreted material, none was observed for this species in the present study, although cell outlines were often indistinct, possibly due to the presence of an overlying film. Generally, most Xylobium spp., like the majority of Maxillaria spp., are nectarless and are visited and presumably pollinated by Meliponini such as species of Trigona (Dressler, 1990) including Trigona amalthea, T. silvestriana, Scaptotrigona postica, Partamona orizabaensis and P. musarum (Roubik, 2000) although, to date, no foraging activity has been recorded. Unfortunately, Roubik does not name the Xylobium spp. studied but does give locality data. However, van der Pijl and Dodson (1969) report that X. squalens is pollinated by Trigona postica (syn. S. postica). Pintaudi et al. (1990) have also observed the pollination of X. squalens by S. postica, and the nectarless flowers of X. latilabium are visited and pollinated by T. amalthea in Peru (van der Pijl and Dodson, 1969). Furthermore, it has been reported that smaller bees, also identified as T. amalthea, pollinate X. variegatum in Peru and Costa Rica. Interestingly, these bees did not visit flowers of X. latilabium even though they had to fly past them to reach X. variegatum (van der Pijl and Dodson, 1969). Thus, if X. squalens and X. variegatum truly are conspecific, then it would appear that the taxon is visited by at least two species of Meliponini. However, only when the transfer of pollinia has been unequivocally demonstrated in the field can it be claimed with certainty that pollination has taken place.
The labella of Teuscheria wageneri also bear conical papillae but profiles of the epidermal cells are obscured by a thick, viscid layer through which papilla tips protrude. Moniliform hairs are also present in this species and Dunsterville and Garay (1961) and Bennett and Christenson (1995) have described the labellar callus of T. venezuelana Garay (syn. T. wageneri) and T. dodsonii Dressler as ‘covered with golden farinose material’ and ‘covered with bright yellow farinaceous trichomes’, respectively. Although, in the absence of field observations and histochemical data, it cannot be unequivocally claimed that the moniliform hairs of T. wageneri function as pseudopollen, there is every indication from their morphology that this is the case. Indeed, Vogel (1979) and Kjellsson and Rasmussen (1987) have argued that hairs which fragment into rounded component cells, even when devoid of food reserves, still have the potential to function as pseudopollen and attract pollinators by deceit. Although relatively uncommon, the co-occurrence of both secreted material and food-hairs within a single species has already been recorded for members of the Maxillaria discolor alliance (Davies et al., 2003a; Singer et al., 2006) and M. lepidota (Matusiewicz et al., 2004). Unfortunately, the pollinator of Teuscheria wageneri has yet to be identified. Even so, the presence of moniliform hairs and secreted material indicate that the pollinator, as in entomophilous species of Maxillaria, is possibly a member of the Meliponini.
The labellum of Rudolfiella aurantiaca mainly bears conical to obpyriform papillae with well-defined cuticular striations, together with simple trichomes at its point of attachment. Unlike Bifrenaria spp., where unicellular trichomes predominate, those of Rudolfiella are multicellular with cylindrical cells, and there is clear demarcation between papillae and trichomes. This supports the view of Koehler and co-workers (Koehler et al., 2002; Koehler and Amaral, 2004) that Bifrenaria and Rudolfiella are phylogenetically distinct. Braga (1977) has proposed that the hymenopteran pollinator of R. aurantiaca feeds upon labellar hairs. However, the present study showed that, in this species, hairs occur only at the point of attachment of the labellum, and these are neither moniliform nor is there any indication whatsoever that they fragment or are easily detached. Furthermore, they appear not to contain food reserves. Singer and co-workers, however, have reported putative labellar elaiophores in this species as in certain oil-producing members of the Oncidiinae (van der Cingel, 2001; Singer et al., 2006). If this is confirmed, then the pollinator is likely to be one of the specialized oil-gathering bees (van der Cingel, 2001; Singer et al., 2006) rather than a member of Meliponini as the observations of Braga (1977) would imply. Until further field work is undertaken and the pollinator identified, the matter cannot be resolved.
The densely pubescent labella of Mormolyca ringens and M. schweinfurthiana bear conical papillae with pointed tips and narrow points of insertion, and these papillae intergrade to form unicellular trichomes that lack food reserves. Phylogenetic and molecular studies (Holtzmeier et al., 1998; Chase et al., 2003; Singer et al., 2004; Dathe and Dietrich, 2006) all indicate that Mormolyca is nested within Maxillaria. However, despite the fact that such labellar trichomes have never been reported from Maxillaria sensu stricto, this does not necessarily conflict with molecular data. Instead, the presence of this type of hair may simply reflect the occurrence of sexual mimicry in the genus. That pseudocopulation occurs in M. ringens is now well established (Singer et al., 2004; Flach et al., 2006). In this species, sexually excited drones of Nannotrigona testaceicornis and Scaptotrigona sp. (Meliponini) pollinate the flower when attempting to copulate with the labellum. The labellar indumentum is said to resemble hairy areas found on the insect, and the hairs are concentrated at the basal, lateral margins of the labellum and on a purple, triangular area just below the column (Singer et al., 2004). The densely trichromatic and insect-like shape of the flower of M. ringens parallels that of the Old World genus Ophrys L. (Kullenberg, 1961). Furthermore, the labellar papillae and trichomes of M. ringens closely resemble those of Ophrys spp. (Servettaz et al., 1994; Ascensão et al., 2005). The chemical composition of the fragrance which attracts the insect pollinator to M. ringens has been shown to resemble that of pheromones produced by virgin queens of Scaptotrigona sp. (Flach et al., 2006). Both types of bee hover for a few seconds in front of the flower before alighting. The pollinator is then guided along the flower by visual and tactile cues provided by the labellar hairs (Singer et al., 2004). Attempts at pseudocopulation are indicated by spasmodic abdominal movements and extrusion of the genitalia, and in Nannotrigona drones (but not Scaptotrigona) this is accompanied by an audible buzzing caused by the vibrating of wings (Singer et al., 2004). Nannotrigona visits are much more common than those of Scaptotrigona but both types of bee are able to dislodge and deposit pollinaria (Singer et al., 2004). Although the pollination of M. schweinfurthiana has not yet been described, the micromorphology of its labellum and the similar length of the labellar hairs would suggest a pollination mechanism close to that of M. ringens.
The genera Bifrenaria and Mormolyca are atypical in having unicellular hairs, whereas Xylobium, Teuscheria and Rudolfiella, in possessing multicellular hairs, more closely resemble Maxillaria sensu stricto. Moreover, Xylobium and Teuscheria share a number of other labellar features such as moniliform hairs and secretory papillae with Maxillaria sensu stricto and this, at first sight, would appear to support the case for the inclusion of these genera in Maxillariinae sensu lato. However, Stern et al. (2004) have shown that anatomical characters alone are of limited value in determining relationships within the Maxillarieae and, more recently, this has been reiterated by Dathe and Dietrich (2006) who claim that ‘the value of morphological characters in phylogenetic reconstruction of Maxillariinae is limited by the high degree of homoplasy’. Benzing (1986) has also warned that the use of pollination-related traits alone to infer relationships among species and groups of species may lead to erroneous conclusions because of convergence. Indeed, the unusual, unicellular hairs of Bifrenaria and Mormolyca can certainly be related to pollination biology since Bifrenaria, unlike the other genera studied here, is thought not to be pollinated by Meliponini, whereas Mormolyca alone displays pseudocopulation. Conversely, the occurrence of moniliform hairs or secreted substances in certain species of Maxillaria and Xylobium can perhaps be explained in terms of a shared pollinator (Meliponini). Although the occurrence of both these labellar features in Teuscheria suggests that this genus too is pollinated by Meliponini, this may not necessarily be the case, and taxa that share such features may not necessarily be closely related. For example, almost identical moniliform hairs to those found in Maxillaria, Xylobium and Teuscheria also occur amongst representatives of Polystachya Hook. sect. Polystachya, an unrelated genus largely pollinated by halictid bees (Davies et al., 2002, and references therein). It is thus probable that several labellar features, but in particular moniliform hairs, have arisen on a number of occasions in Maxillariinae sensu lato as a result of convergence in response to similar pollinator pressures. Likewise, it has been demonstrated that convergence is responsible for a number of other shared morphological characters, both vegetative and reproductive, in Maxillariinae (Dathe and Dietrich, 2006) thus, simultaneously highlighting the need for caution in determining taxonomic relationships based solely on morphological data and stressing the importance of a multidisciplinary approach to orchid phylogeny.
Acknowledgments
K.L.D. is grateful to the Stanley Smith (UK) Trust for their generous grant. The authors also acknowledge the help of the staff of the Royal Botanic Gardens, Kew, UK, M.P. Turner, School of Biosciences, Cardiff University, UK and Alan Gregg, Swansea Botanical Complex, Swansea, UK.
LITERATURE CITED
- Ascensão L, Francisco A, Cotrim H, Pais MS. (2005) Comparative structure of the labellum in Ophrys fusca and O. lutea (Orchidaceae). American Journal of Botany 921059–1067. [DOI] [PubMed] [Google Scholar]
- Atwood JT and Mora de Retana DE. (1999) Orchidaceae: tribe Maxillarieae: subtribes Maxillariinae and Oncidiinae. Fieldiana 401–182. [Google Scholar]
- Bechtel H, Cribb P, Launert E. (1981) The manual of cultivated orchid species.(Blandford Press, Poole).
- Bennett DE Jr and Christenson EA. (1993) In Christenson EA (Ed.). Icones Orchidacearum Peruviarum. 199–200 Sarasota, FL, pp. 198.
- Bennett DE Jr and Christenson EA. (1995) In Christenson EA (Ed.). Icones Orchidacearum Peruviarum. 389–399 Sarasota, FL.
- Benzing DH. (1986) The genesis of orchid diversity: emphasis on floral biology leads to misconceptions. Lindleyana 173–89. [Google Scholar]
- Braga P. (1977) Aspectos biologicos das Orchidaceae da Amazônica Central. Acta Amazonica, Manaus 71–89. [Google Scholar]
- Brummitt RK and Powell CE. (1992) Authors of plant names.(Royal Botanic Gardens, Kew, London).
- Carnevali G and Romero GA. (2000) Orchids of Venezuela, a field guide.(Armitano, Caracas).
- Castro VP. (1991a) Estudo taxonòmico de Bifrenarias seçào Stenocoryne no Brazil. 1a. parte. Boletim Coordenadoria Associações Orquidófilas do Brazil 36–5. [Google Scholar]
- Castro VP. (1991b) Estudo taxonòmico de Bifrenarias seçào Stenocoryne no Brazil. 2a. parte. Boletim Coordenadoria Associações Orquidófilas do Brazil 344–55. [Google Scholar]
- Castro VP. (1991c) Estudo taxonòmico de Bifrenarias seçào Stenocoryne no Brazil. 3a. parte. Boletim Coordenadoria Associações Orquidófilas do Brazil 339–45. [Google Scholar]
- Castro VP. (1996) Contribution to the study of the genus Bifrenaria Lindl section Harrisoniae Pabst. Proceedings of the 15th World Orchid Conference(Naturalia Publications, Rio de Janeiro. Turriers, France) pp. 376–383.
- Castro VP and Campacci M. (2000) In Castro VP (Ed.). Icones Orchidacearum Brasiliensis Vol. 1 São Paulo.
- Chase MW. (2005) Classification of Orchidaceae in the age of DNA data. Curtis's Botanical Magazine 222–7. [Google Scholar]
- Chase MW, Barret RL, Cameron KN, Freudenstein JV. (2003) DNA data and Orchidaceae systematics: a new phylogenetic classification. In Dixon KM (Ed.). Orchid conservation.(Natural History Publications, Kota Kinabalu, Sabah, Malaysia) pp. 69–89.
- van der Cingel NA. (2001) An atlas of orchid pollination: America, Africa, Asia and Australia.(A.A. Balkema, Rotterdam).
- Dathe S and Dietrich H. (2006) Comparative molecular and morphological studies in selected Maxillariinae orchids. Willdenowia 3689–102. [Google Scholar]
- Davies KL and Turner MP. (2004a) Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 9375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL and Turner MP. (2004b) Pseudopollen in Eria Lindl. section Mycaranthes Rchb.f. (Orchidaceae). Annals of Botany 94707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL and Winters C. (1998) Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126349–361. [Google Scholar]
- Davies KL, Winters C, Turner MP. (2000) Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85887–895. [Google Scholar]
- Davies KL, Roberts DL, Turner MP. (2002) Pseudopollen and food-hair diversity in Polystachya Hook. (Orchidaceae). Annals of Botany 90477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP, Gregg A. (2003a) Atypical pseudopollen-forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae). Botanical Journal of the Linnean Society 143151–158. [Google Scholar]
- Davies KL, Turner MP, Gregg A. (2003b) Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 91439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP, Gregg A. (2003c) Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): reward or deception? Annals of Botany 94129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M, Gregg A. (2005) Nectar-secreting floral stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae). Annals of Botany 96217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson CH. (1978) Two orchids from Rio Palenque, Ecuador. Selbyana 2289–290. [Google Scholar]
- Dressler RL. (1972) Una Teuscheria nueva del Ecuador. Orquideologia 73–6. [Google Scholar]
- Dressler RL. (1990) The orchids—natural history and classification.(Harvard University Press, London).
- Dressler RL. (1993) Phylogeny and classification of the orchid family.(Cambridge University Press, Cambridge).
- Dunsterville GCK and Garay LA. (1959–1966) Venezuelan Orchids illustrated(Andre Deutsch, London) Vols 1–4..
- Flach A, Dondon RC, Singer RB, Koehler S, Amaral MCE, Marsaioli AJ. (2004) The chemistry of pollination in selected Brazilian Maxillariinae orchids: floral rewards and fragrance. Journal of Chemical Ecology 301045–1056. [DOI] [PubMed] [Google Scholar]
- Flach A, Marsaioli AJ, Singer RB, Amaral MCE, Menezes C, Kerr WE, et al. (2006) Pollination by sexual mimicry in Mormolyca ringens: a floral chemistry that remarkably matches the pheromones of virgin queens of Scaptotrigona sp. Journal of Chemical Ecology 3259–70. [DOI] [PubMed] [Google Scholar]
- Garay LA. (1958) A new orchid genus from the Ecuadorian Andes. American Orchid Society Bulletin 27820–823. [Google Scholar]
- Garay LA. (1970) Orquideas Colombianas nuevas o criticas Decena IV. Orquideologia 515–22. [Google Scholar]
- Garay LA and Wirth M. (1959) On the genera Mormolyca Fenzl and Cyrtoglottis Schltr. Canadian Journal of Botany 37479–490. [Google Scholar]
- Hoehne FC. (1944) Revisào taxonòmica do gènero Bifrenaria Lindl. Arquivos de Botanica do Estado de São Paulo 217–20. [Google Scholar]
- Holtzmeier MA, Stern WL, Judd WS. (1998) Comparative anatomy and systematics of Senghas's cushion species of Maxillaria (Orchidaceae). Botanical Journal of the Linnean Society 12743–82. [Google Scholar]
- Jenny R and Braem GJ. (1987) Teuscheria horichiana, a new orchid from Costa Rica. Orchid Digest 51187–189. [Google Scholar]
- Kjellsson G and Rasmussen FN. (1987) Does the pollination of Dendrobium unicum Seidenf. involve pseudopollen? Die Orchidee 38183–187. [Google Scholar]
- Koehler S and Amaral MCE. (2004) A taxonomic study of the South American genus Bifrenaria Lindl. (Orchidaceae). Brittonia 56314–345. [Google Scholar]
- Koehler S, Williams NH, Whitten WM, Amaral MCE. (2002) Phylogeny of the Bifrenaria (Orchidaceae) complex based on morphology and sequence data from nuclear rDNA internal transcribed spaces (ITS) and chloroplast trnL-trnF region. International Journal of Plant Sciences 1631055–1066. [Google Scholar]
- Kullenberg B. (1961) Studies in Ophrys pollination. Zoologiska Bidrag Uppsala 341–340. [Google Scholar]
- Lindley J. (1832) The genera and species of orchidaceous plantsBifrenaria atropurpurea. (Ridgways, London) 152.
- Lindley J. (1843) Stenocoryne longicornis. Edwards's Botanical Register(James Ridgway, London) 29 (Misc.): 53.
- Matusiewicz J, Stpiczyńska M, Davies KL. (2004) Pseudopollen in the flowers of Maxillaria lepidota Lindl. (Orchidaceae). Proceedings of the 53rd Meeting of the Polish Botanical Society, Toruń pp. pp. 14.
- Pabst GFJ and Dungs F. (1977) Orchidaceae Brasilienses, Band II.(Brücke-Verlag Kurt Schmersow, Hildesheim).
- van der Pijl L and Dodson CH. (1969) Orchid flowers: their pollination and evolution.(University of Miami Press, Coral Gables, FL).
- Pintaudi CT, Stort MNS, Marin-Morales MA. (1990) Artificial and natural pollination of Xylobium squalens Lindl. (Orchidaceae). Naturalia 1567–80. [Google Scholar]
- Adipe racemosa. Rafinesque CS. (1836) Fl. Tell. 2: 101.
- Röth J. (2004) Xylobium foveatum (Lindl.) Nicholson. Die Orchidee 55592–593. [Google Scholar]
- Roubik DW. (2000) Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution 222271–279. [Google Scholar]
- Ryan A, Whitten WM, Johnson MAT, Chase MW. (2000) A phylogenetic assessment of Lycaste and Anguloa (Orchidaceae: Maxillarieae). Lindleyana 1533–45. [Google Scholar]
- von Schlechter R. (1913) Die Gattung Xylobium Lindl. Orchis 721–24. [Google Scholar]
- Schlechter FRR. (1914) Die Orchideen.(Verlagsbuchhandlung Paul Parey, Berlin).
- Senghas K. (1994) Cydoniorchis. Journal für den Orchideenfreund 111–15. [Google Scholar]
- Senghas K. (1995) Xylobium pallidiflorum (Hook.) Nichols. 1887. Die Orchidee 46789–790. [Google Scholar]
- Servettaz O, Bini Maleci L, Grünanger P. (1994) Labellum micromorphology in the Ophrys bertolonii agg. and some related taxa (Orchidaceae). Plant Systematics and Evolution 189123–131. [Google Scholar]
- Singer RB and Cocucci AA. (1999) Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 1447–56. [Google Scholar]
- Singer RB and Koehler S. (2004) Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae). Annals of Botany 9339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Flach A, Koehler S, Marsaioli AJ, Do Carmo E, Amaral M. (2004) Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae: Maxillariinae). Annals of Botany 93755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Marsaioli AJ, Flach A, Reis MG. (2006) The ecology and chemistry of pollination in Brazilian orchids: recent advances. In Teixeira da Silva J (Ed.). Floriculture, ornamental and plant biotechnology(Global Science Books, Isleworth, Middlesex) Vol. IV. pp. 569–582. [Google Scholar]
- Stern WL, Judd WS, Carlsward BS. (2004) Systematic and comparative anatomy of Maxillariaeae (Orchidaceae), sans Oncidiinae. Botanical Journal of the Linnean Society 144251–274. [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. (2003) Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae). Annals of Botany 9387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher H. (1974) The genus Xylobium. American Orchid Society Bulletin 43207–212. [Google Scholar]
- Vogel S. (1979) Evolutionary shifts from reward to deception in pollen flowers. In Richards AJ (Ed.). The pollination of flowers by insects.(Academic Press, London) pp. 89–96.
- Whitten WM, Williams NH, Chase MW. (2000) Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. American Journal of Botany 871842–1856. [PubMed] [Google Scholar]
- Wolff M. (1990) Adipe Raf, Ein ‘vergessener Name’. Die Orchidee 4135–37. [Google Scholar]