Abstract
• Background and Aims Drought causes a decline of root hydraulic conductance, which aside from embolisms, is governed ultimately by aquaporins. Multiple factors probably regulate aquaporin expression, abundance and activity in leaf and root tissues during drought; among these are the leaf transpiration rate, leaf water status, abscisic acid (ABA) and soil water content. Here a study is made of how these factors could influence the response of aquaporin to drought.
• Methods Three plasma membrane intrinsic proteins (PIPs) or aquaporins were cloned from Phaseolus vulgaris plants and their expression was analysed after 4 d of water deprivation and also 1 d after re-watering. The effects of ABA and of methotrexate (MTX), an inhibitor of stomatal opening, on gene expression and protein abundance were also analysed. Protein abundance was examined using antibodies against PIP1 and PIP2 aquaporins. At the same time, root hydraulic conductance (L), transpiration rate, leaf water status and ABA tissue concentration were measured.
• Key Results None of the treatments (drought, ABA or MTX) changed the leaf water status or tissue ABA concentration. The three treatments caused a decline in the transpiration rate and raised PVPIP2;1 gene expression and PIP1 protein abundance in the leaves. In the roots, only the drought treatment raised the expression of the three PIP genes examined, while at the same time diminishing PIP2 protein abundance and L. On the other hand, ABA raised both root PIP1 protein abundance and L.
• Conclusions The rise of PvPIP2;1 gene expression and PIP1 protein abundance in the leaves of P. vulgaris plants subjected to drought was correlated with a decline in the transpiration rate. At the same time, the increase in the expression of the three PIP genes examined caused by drought and the decline of PIP2 protein abundance in the root tissues were not correlated with any of the parameters measured.
Keywords: Abscisic acid, drought, methotrexate, Phaseolus vulgaris, plasma membrane aquaporins, root hydraulic conductance, transpiration rate
INTRODUCTION
Plants in the field are exposed to several stressful conditions, and water deficit is one of the most common. Soils too dry for crop production cover 28 % of the Earth's land surface (Bray, 2004). Generally, water deficit causes a reduction in both stomatal and root hydraulic conductance, compromising the water status of the plant (Siemens and Zwiazeck, 2004). While the mechanisms of stomatal closure during water deficit are well understood (Wilkinson and Davies, 2002; Mori and Schroeder, 2004), the mechanisms that lead to changes in root hydraulic conductance during water deficit remain unknown (Javot and Maurel, 2002; Luu and Maurel, 2005). The effects of drought on root hydraulic conductance depend on stress intensity (Siemens and Zwiazek, 2004) and on plant genotype (Saliendra and Meinzer, 1992). For example, under moderate water deficit, root hydraulic conductance sometimes increases (Siemens and Zwiazek, 2004). Root hydraulic conductance generally rises again after re-watering, and such recovery also depends on stress intensity and plant genotype (Saliendra and Meinzer, 1992; Martre et al., 2002).
Root hydraulic conductance is governed by several external and internal factors such as embolism in the xylem elements, root anatomy, water availability, salts in the soil aqueous phase, temperature and root cellular properties, among others (Saliendra and Meinzer, 1992; Martre et al., 2002; Siemens and Zwiazek, 2004; Aroca et al., 2005; Boursiac et al., 2005). Many of these factors regulate root hydraulic conductance mainly by affecting water channel protein (aquaporin) activity and/or abundance (Javot and Maurel, 2002; Luu and Maurel, 2005).
Aquaporins are proteinaceous pores that facilitate the passive diffusion of water across membranes of living cells. Plant aquaporins are divided into four groups or clades based on amino acid sequence similarities: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin-like intrinsic proteins (NIPs) and small basic intrinsic proteins (SIPs). Some groups may be subdivided again (for a recent review, see Luu and Maurel, 2005), as is the case with PIP proteins (divided into PIP1 and PIP2). Plants transformed with antisense constructs and with low levels of PIP proteins have lower root hydraulic conductance than wild-type plants (Martre et al., 2002; Siefritz et al., 2002). However, the role of PIPs in drought tolerance is not clear. Rice and tobacco plants overexpressing a PIP1 gene increased their drought tolerance (Lian et al., 2004; Yu et al., 2005), whereas tobacco plants overexpressing a different PIP1 gene decreased their drought tolerance (Aharon et al., 2003). Water deficit may also cause changes in the post-transcriptional regulation of aquaporin abundance, since aquaporin activity has been shown to be regulated by phosphorylation, divalent cations and pH (see Luu and Maurel, 2005).
It is well documented that water deficit results in an increase in the abscisic acid (ABA) content of plant tissues (Davies and Zhang, 1991; Pospíšilová, 2003). At the same time, ABA can modulate root hydraulic conductance in plants. In most studies, exogenously applied ABA increased root hydraulic conductance, but in some cases it had no effect or even decreased it (Hose et al., 2000; Wan and Zwiazek, 2001; Aroca et al., 2003). Such different findings are attributed to differences in concentrations, times of exposure, temperature, nutrient status and plant species used (see Aroca et al., 2003). Also, it is known that ABA modulates the expression of some PIP genes in roots and leaves (Suga et al., 2002; Jang et al., 2004; Zhu et al., 2005). However, there are no studies in which the effects of ABA on root hydraulic conductance and PIP expression have been measured at the same time.
The common bean (Phaseolus vulgaris) is the most important grain legume for direct human consumption in the world, especially in Latin America and East Africa (Broughton et al., 2003). Only 7 % of the bean-growing area is well watered, and water deficit is the abiotic stress that is most limiting for bean production. Therefore, physiological and molecular studies of drought tolerance in the common bean are needed to find the traits and genes involved in drought tolerance (see Broughton et al., 2003).
Here, leaf and root changes in aquaporin mRNA and protein abundance during several days after drought and during subsequent re-watering in common bean plants are reported. Osmotic root hydraulic conductance, transpiration rate, leaf osmotic potential and relative water content, and root and leaf ABA contents were also analysed. Finally, the same parameters were measured in bean plants to which ABA or methotrexate (MTX), an inhibitor of stomatal opening (Klein et al., 2004), were applied as foliar sprays. Thus, correlations were sought between the factors (e.g. decrease of transpiration rate, leaf dehydration and ABA) that regulate root hydraulic conductance and aquaporin expression during drought.
MATERIALS AND METHODS
Plant material and experimental design
Common bean (P. vulgaris L. ‘Pinto’) seeds were germinated in wet Perlite for 7 d. Then the seedlings were placed in 500 mL pots filled with Perlite. The pots were watered to field capacity (draining excess water) with modified full Hoagland solution (Downs and Helmers, 1975). Three days after transplanting, some pots were not watered for 4 d and then were re-watered for 1 d. The conditions inside the growth chamber were: 23 °C/21 °C (day/night), 200 µmol m−2 s−1 photosynthetic photon flux density (PPFD), 16 : 8 h (light : dark) and 60 % relative humidity (RH).
In another set of experiments, the plants were sprayed with a 100 μm ABA solution (Aroca et al., 2003), or with 200 μm MTX. MTX was prepared as a stock solution of 10 mm dissolved in MES-KOH, pH 5·8 (Klein et al., 2004). In each experiment, the working solution was freshly prepared. Plants were sprayed 2 h after the lights were turned on, and the samples were collected 24 h later. Since the effects at both leaf and root levels were being looked for, and knowing that ABA can be transported from leaves to roots via the phloem (Jeschke et al., 1997a, b), and without any knowledge about the possible transport of MTX from roots to shoot, it was decided to apply both solutions (ABA and MTX) by spraying the leaves.
Perlite water potential
The Perlite water potential was measured using a dew point microvoltimeter model HR33T (Wescor Inc., Logan, UT, USA).
Leaf transpiration rate
The leaf transpiration rate was determined by a gravimetric method (Aroca et al., 2003). The surfaces of the pots were covered with aluminium foil. The pot–plant system was weighed and is referred to as W0. The pot–plant system was weighed again after 2 h and is referred to as Wf. The leaf transpiration rate was calculated as: (W0 − Wf)/t × A, where t is the time in seconds, and A is the leaf area in m2. Leaf area was calculated as follows: leaves of a whole plant were detached and scanned (hp scanjet 5550c, Hewlett Packard, Palo Alto, CA, USA). The corresponding images were analysed with Adobe Photoshop CS (Adobe Systems Incorporated, San Jose, CA, USA). Measurements started 2 h after the lights were turned on.
Leaf relative water content and osmotic potential
Leaf relative water content (RWC) was measured as described by Aroca et al. (2003). Leaf osmotic potential at full turgor (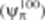 ) was calculated as described by Augé et al. (2004). Leaflets were rehydrated at 4 °C. The leaflets were then placed in 1·5 mL Eppendorf tubes with a small hole in the bottom, immersed in liquid N2 and stored at −80 °C for at least 1 week; this procedure of killing leaf cells has been applied previously by other authors (Augé et al., 2004; Liu et al., 2005). The tubes were allowed to thaw, placed inside another tube and centrifuged at maximum speed in the Eppendorf centrifuge (Centrifuge 5415C, Eppendorf). The osmotic potential of the collected sap was measured with a cryoscopic osmometer (Osmomat 030, Gonotec Gmbh, Berlin, Germany). Thereafter, the actual osmotic potential (ψπ) of each sample was calculated knowing its RWC using the following formula (Irigoyen et al., 1996):
) was calculated as described by Augé et al. (2004). Leaflets were rehydrated at 4 °C. The leaflets were then placed in 1·5 mL Eppendorf tubes with a small hole in the bottom, immersed in liquid N2 and stored at −80 °C for at least 1 week; this procedure of killing leaf cells has been applied previously by other authors (Augé et al., 2004; Liu et al., 2005). The tubes were allowed to thaw, placed inside another tube and centrifuged at maximum speed in the Eppendorf centrifuge (Centrifuge 5415C, Eppendorf). The osmotic potential of the collected sap was measured with a cryoscopic osmometer (Osmomat 030, Gonotec Gmbh, Berlin, Germany). Thereafter, the actual osmotic potential (ψπ) of each sample was calculated knowing its RWC using the following formula (Irigoyen et al., 1996): 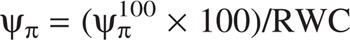 .
.
Osmotic root hydraulic conductance (L)
Osmotic root hydraulic conductance was measured in detached root systems exuding under atmospheric pressure (Aroca et al., 2003, 2005). Pots were immersed in aerated nutrient solution. Plants were cut below the cotyledons and a pipette with a silicone tube was attached to the stem. The liquid exuded in the first 15 min was discarded. The exudate of the following 1 h was collected with a syringe and weighed. The osmolarities of the exuded sap and the nutrient solution were determined using a cryoscopic osmometer (Osmomat 030, Gonotec Gmbh, Berlin, Germany). Osmotic root hydraulic conductance (L) was calculated as L = Jv/Δψ, where Jv is the exuded sap flow rate and Δψ the osmotic potential difference between the exuded sap and nutrient solution. Measurements started 2 h after the lights were turned on.
Cloning of PIP aquaporin genes
Total RNA from root tissues was extracted using the RNeasy Plant Mini Kit (Qiagen, Chatsworth, CA, USA) according to the manufacturer's protocol, but a DNase step was included before washing the column with ethanol. One hundred units of DNase I (Invitrogen, San Diego, CA, USA) were applied to the column in 0·1 m sodium acetate (pH = 5·0) containing 5 mm MgCl2, and incubated at room temperature for 30 min.
cDNA was synthesized from 2 µg of total RNA using oligo(dT)12–18 as a primer and M-MLV reverse transcriptase (Invitrogen). For the identification of PIP aquaporins, cDNA was amplified by polymerase chain reaction (PCR) using degenerate primers (Weig et al., 1997). The degenerated PCR primers used were 5′-GG[AT]CC[ACT][AG]CCCA[AG][AT]A[AGC]A[CT]CCA-3′ and 5′-CA[CT][AG]T[GTC]AA[CT]CC[AT]GC[ATG]GT[GT][AT]C. Amplification was done for 40 cycles at 94 °C for 60 s, 50 °C for 60 s and 72 °C for 60 s. Amplified products were separated on a 2 % agarose gel, and visible bands of the expected size (approx. 400 kb) were eluted with a QIAquick Gel Extraction Kit (Qiagen) and cloned into a PCR 2·1-TOPO vector using the TOPO TA cloning kit (Invitrogen).
For determination of the sequences of the full-length cDNAs, 5′- and 3′-RACEs (rapid amplification of cDNA ends) were carried out on total RNA using the 5′/3′ RACE Kit, 2nd Generation (Roche, Basel, Switzerland). After the 3′- and 5′-RACEs, PCR primers were newly designed to cover the full genes and PCR was performed with high fidelity Pfu DNA polymerase (Stratagene, La Jolla, CA, USA). Specific primers were also designed to produce cDNAs of the 3′-untranslated region (UTR) of each gene obtained before to be used as probes in the northern blots.
Northern blot analysis
Total RNA was extracted from bean roots and leaves as described in Aroca et al. (2005) using Trizol reagent (Invitrogen). Total RNA samples (15 µg each) were fractionated by electrophoresis on a MOPS–formaldehyde–formamide 1·5 % agarose gel, and transferred by capillary action overnight to Hybond-N membranes (Amersham Biosciences, Piscataway, NJ, USA) using 10× SSC (1× SSC is 0·15 m NaCl and 0·015 m sodium citrate). The RNA was fixed by baking at 80 °C for 30 min. Pre-hybridization was performed at 65 °C for 15 min in a solution of NaH2PO4 (pH = 7·2), 1 mm EDTA and 7 % SDS (w/v). 32P-labelled cDNAs of the 3′-UTR of each PIP gene were added to the solution. Hybridization was performed overnight, followed by three consecutive 5 min washings, first with 4× SSC and 0·1 % SDS (w/v) at 65 °C, secondly with 0·4× SSC and 0·1 % SDS (w/v) at 65 °C, and finally with 0·4× SSC and 0·1 % SDS (w/v) at room temperature. Detection of the labelled probe was performed by phosphorimaging. Quantification of gene expression was performed by dividing the intensity value of each band by the intensity of the corresponding rRNA stained with ethidium bromide (Martínez and Chrispeels, 2003). All RNA samples were taken 2 h after the lights were turned on in order to avoid oscillations of aquaporin expression during the day (Lopez et al., 2003). Blots were repeated three times with three different sets of plants.
Preparation of microsomes and immunodetection
The microsome purification, SDS–PAGE, transfer of proteins to nitrocellulose membranes (blotting) and blocking were all carried out as described by Aroca et al. (2005). The blots were incubated in Tris-buffered saline buffer (TBS) with 0·05 % Tween-20 in the presence of the antibody at an appropriate dilution. The dilution and incubation of the antibody for PIP2 were carried out as described by Aroca et al. (2005). Antibody against PIP1 from Arabidopsis thaliana (Kammerloher et al., 1994) was incubated at a 1 : 1000 dilution overnight at 4 °C and the secondary antibody (mouse anti-chicken IgG coupled to horseradish peroxidase; Sigma) at a dilution of 1 : 10 000 for 1 h at room temperature. The signal was developed using a chemiluminescent substrate (West-Pico, Super Signal; Pierce, Rockford, IL, USA).
The immunoblots were repeated three times with different samples. To quantify the inmunoblot signal, the intensity of each band or bands (30 or 60 kDa) was measured using Adobe PhotoShop 5·5 (Adobe Systems, Mountain View, CA, USA), corrected for the background and normalized against the intensity of the corresponding whole Coomassie brillant blue line (Aroca et al., 2005). Samples for microsome purification were taken 2 h after the lights were turned on in order to avoid fluctuation of aquaporin expression during the day (Lopez et al., 2003).
Abscisic acid quantification
Quantification of ABA in leaf and root extracts was carried out using a solid-phase radioimmunoassay based on a monoclonal antibody (DBPA1) raised against free (S)-ABA as described previously (Vernieri et al., 1991; Aroca et al., 2003).
RESULTS
Water relations parameters
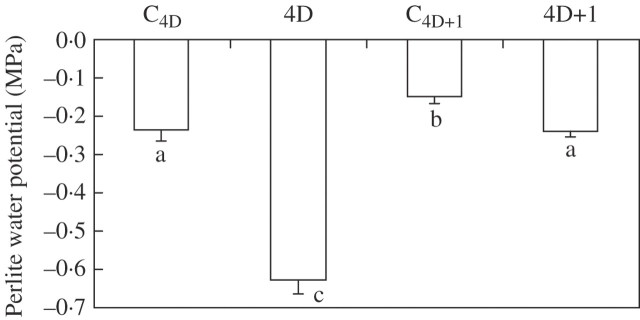
In order to determine the degree of water deficit experienced by plant roots, the Perlite water potential was measured. A 4 d period without watering reduced the Perlite water potential from −0·23 ± 0·03 to −0·63 ± 0·04 MPa (Fig. 1). Twenty-four hours after re-watering the pots, the Perlite water potential increased up to the previous control values (–0·24 ± 0·02 MPa), although on this day control pots had a slight higher water potential (Fig. 1).
Fig. 1.
Perlite water potential of common bean plants subjected to drought for 4 d (4D) and 1 d after re-watering (4D + 1), and the corresponding control plants (C4D and C4D+1). Bars represent the mean ± s.e. (n = 10). Bars with the same letter indicate that there are no significant differences (P > 0.05) among them after ANOVA and Fisher l.s.d. tests.
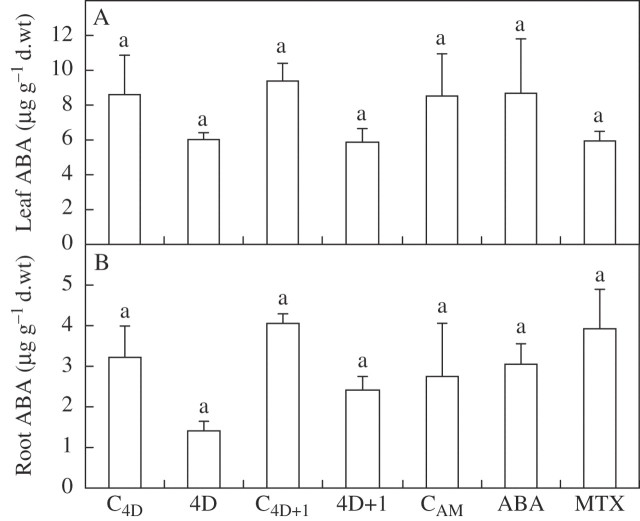
Neither water deficit nor ABA or MTX treatments caused a significant (P > 0·05) change in any of the leaf water status parameters measured, i.e. leaf relative water content, leaf osmotic potential and leaf osmotic potential at full turgor (data not shown). At the same time, no treatment caused any significant change to ABA levels either in the leaves or in the roots (Fig. 2A, B).
Fig. 2.
Leaf (A) and root (B) endogenous ABA content of common bean plants subjected to drought for 4 d (4D) and 1 d after re-watering (4D + 1), and the corresponding control plants (C4D and C4D+1), or of plants leaf sprayed 24 h earlier with 100 μm ABA or 200 μm MTX, and unsprayed control plants (CAM). Bars represent the mean ± s.e. (n = 4). Bars with the same letter indicate that there are no significant differences (P > 0.05) among them after ANOVA and Fisher l.s.d. tests.
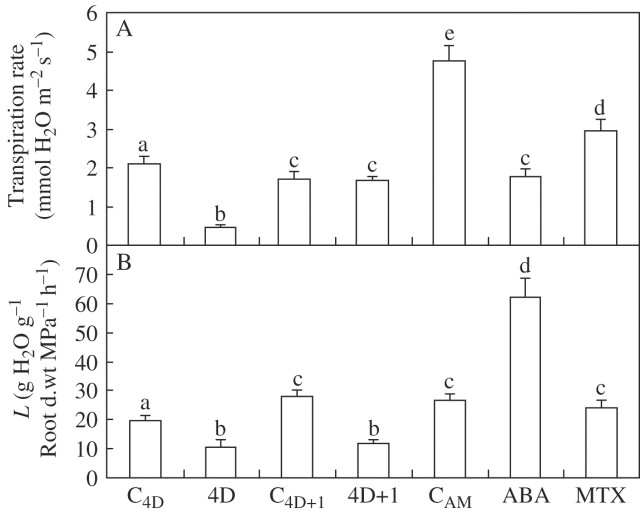
The transpiration rate decreased significantly (P < 0·05) after 4 d of withholding water to 22 % with respect to the corresponding control plants (Fig. 3A). One day after re-watering, plants exposed to water deficit recovered their control transpiration rates (Fig. 3A). Control plants on day 5 (C4D+1) had a lower transpiration rate than control plants on day 4 (Fig. 3A).
Fig. 3.
Leaf transpiration rate (A) and osmotic root hydraulic conductance (L; B) of common bean plants subjected to drought for 4 d (4D) and 1 d after re-watering (4D + 1), and the corresponding control plants (C4D and C4D+1), or of plants leaf sprayed 24 h earlier with 100 μm ABA or 200 μm MTX, and unsprayed control plants (CAM). Bars represent the mean ± s.e. (n = 20). Bars with the same letter indicate that there are no significant differences (P > 0.05) among them after ANOVA and Fisher l.s.d. tests.
In another set of experiments, young bean plants were sprayed with ABA (100 μm) or MTX (200 μm). Both ABA and MTX sprayed on the leaves reduced the transpiration rate, the effect being more pronounced in the ABA-treated plants (Fig. 3A). It was noted that control plants from this experiment had a higher leaf transpiration rate than those of the drought experiment (Fig. 3A). This difference could be due to the different ages of the plants (i.e. the plants from drought experiment were 5 d older than plants from the ABA experiment).
Root hydraulic conductance (L) was measured in plants exuding under atmospheric pressure, i.e. as a result of the pressure created by the osmotic gradient between the perlite solution and the point of exudation (Steudle, 2000). Droughted plants had lower L values than control plants (19·8 ± 1·7 and 10·6 ± 2·7 g H2O g−1 root dry weight MPa−1 h−1 for control and droughted plants, respectively). However, plants subjected to water deficit did not recover control L values after re-watering (Fig. 3B). Also, control plants on day 5 (C4D+1) had a higher L than control plants on day 4 (Fig. 3B). Plants treated with ABA but not those treated with MTX had an elevated L (Fig. 3B). The L values found in plants sprayed with 100 μm ABA were similar to those found previously in P. vulgaris plants exposed hydroponically to 100 μm ABA (Aroca, 2006).
Cloning of new P. vulgaris PIP genes
Using degenerate primers for the conserved amino acids of PIP proteins and a preparation of P. vulgaris root cDNA as a template, three different partial sequences were obtained that showed sequence identity with PIP sequences after a BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST). The full-length sequences of the three cDNAs were obtained using the RACE technique. The alignment of the predicted amino acid sequences of the three cDNAs with AtPIP1;1 (accession no. X75881) and AtPIP2;3 (accession no. D13254) was done by the T-COFFEE method (http://www.ch.embnet.org/software/TCoffee.html). As shown in Fig. 4, two of the genes corresponded to the PIP1 subgroup, and were called PvPIP1;1 (also identified earlier by Campos et al., 1997; accession no. U97023) and PvPIP1;2 (accession no. AY995196). The other cDNA corresponded to proteins in the PIP2 subgroup, and was called PvPIP2;1 (accession no. AY995195). The three proteins each have six putative transmembrane domains (Fig. 4) as predicted by the algorithm of Tusnády and Simon (2001) (http://www.enzim.hu/hmmtop). Amino acid identity correlation between PvPIP1;1 and PvPIP1;2, PvPIP1;1 and PvPIP2;1, and PvPIP1;2 and PvPIP2;1 was 83·4, 70·6 and 70·0 %, respectively. Amino acid identity correlation between PvPIP1;1 and AtPIP1;1, PvPIP1;2 and AtPIP1;1, and PvPIP2;1 and AtPIP2;3 was 84·1, 85·0 and 77·5 %, respectively. Based on this homology, it is concluded that the P. vulgaris genes cloned are putative aquaporins.
Fig. 4.

Alignment of the deduced amino acid sequence of P. vulgaris cDNAs PvPIP1;1 (accession no. U97023), PvPIP1;2 (accession no. AY995196) and PvPIP2;1 (accession no. AY995195), and comparison with AtPIP1;1 (accession no. X75881) and AtPIP2;3 (accession no. D13254). Two conserved NPA motifs are in bold. Putative membrane-spanning regions are in bold and italics. Putative phosphorylation sites at Ser117 and Ser280 are in italics, underlined and in bold. Regions recognized by antibodies are underlined. Asterisks indicate conserved sites in all the genes aligned. : and . indicate degree of amino acid similarity among protein sequences.
Expression of PIP genes under various conditions
The expression of PIP genes was analysed by determining the abundance of mRNA using northern blots, with specific probes for each gene corresponding to the 3′-UTRs. The 3′-UTR is specific for each gene and was used to ensure the specificity of the signal (Jang et al., 2004; Alexandersson et al., 2005; Aroca et al., 2005; Zhu et al., 2005). In fact, the identity among the three probes used in the present study was <1 % (data not shown). However, a possible cross-reaction with other PIP genes of P. vulgaris still cannot be excluded. It is assumed that studying only three PIP genes from a gene family composed most probably of 10–15 genes (Chaumont et al., 2001; Johanson et al., 2001; Sakurai et al., 2005) can draw a restricted picture. However, it can be a starting point for analysis of how PIP genes are regulated by drought, ABA and a decline in transpiration rate.
In leaves, only expression of the PvPIP2;1 gene was detected (Fig. 5A). Moreover, such expression was only detected in plants that had a reduced transpiration rate (i.e. plants droughted for 4 d, and plants treated with ABA or MTX).
Fig. 5.
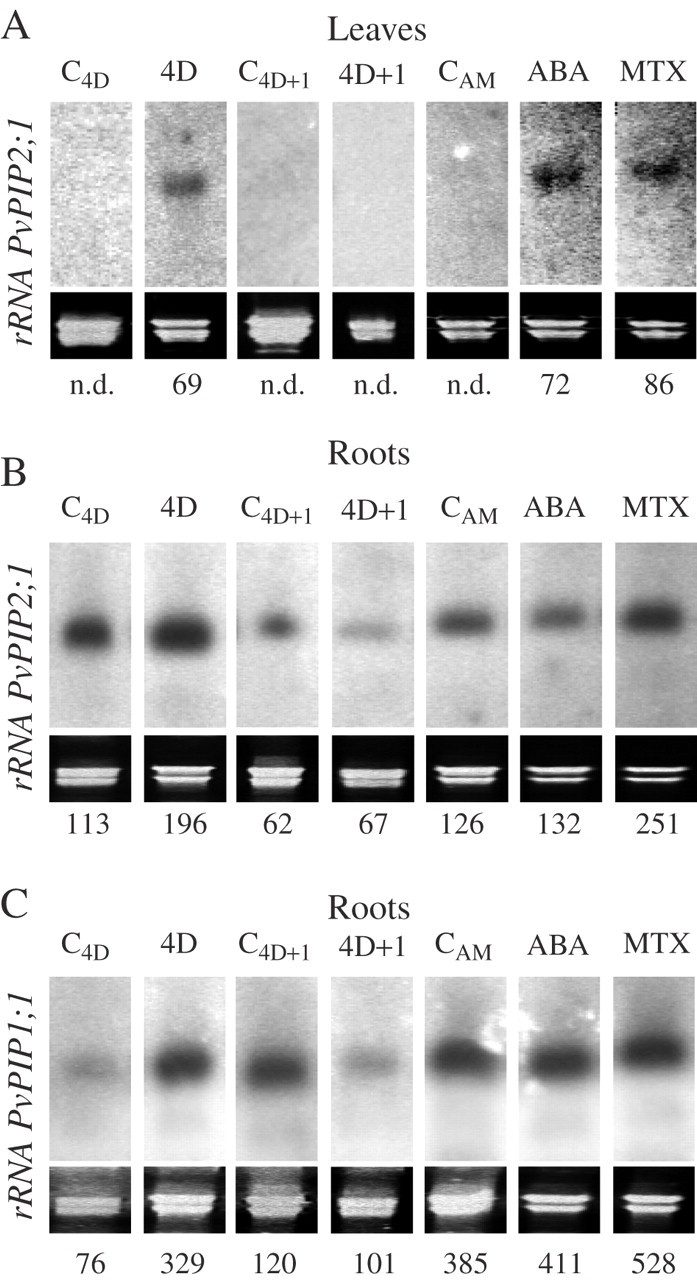
Northern blots of the PvPIP2;1 gene in leaves (A) and roots (B) and the PvPIP1;1 gene in roots (C) of common bean plants subjected to drought for 4 d (4D) and 1 d after re-watering (4D + 1), and the corresponding control plants (C4D and C4D+1), or of plant leaves sprayed 24 h earlier with 100 μm ABA or 200 μm MTX, and unsprayed control plants (CAM). The signal from the PvPIP1;2 gene was the same as that for PvPIP1;1 (data not shown). Blots were repeated three times with different sets of plants; representative blots are shown. Quantification of the gene expression was performed by dividing the intensity value of each band by the intensity of the corresponding rRNA stained with ethidium bromide. n.d., not detected.
The three genes analysed had increased mRNA levels in root tissues after 4 d of drought; these were reduced 1 d after re-watering (Fig. 5B, C). The expression of the PvPIP1;2 gene was identical to that of PvPIP1;1 (data not shown). C4D+1 plants showed more PvPIP1;1 and PvPIP1;2 expression in their roots than did C4D plants (Fig. 5C; data not shown for PvPIP1;2). In the ABA and MTX experiment, MTX treatment caused increases in expression of PvPIP1;1 (99 %) and PvPIP2;1 (37 %), while ABA had essentially no effect (Fig. 5B and C).
PIP protein abundance
To ascertain the levels of the PIP proteins, antibodies against the N-terminal region of AtPIP1;1 (Kammerloher et al., 1994) and the C-terminal region of AtPIP2;3 (Daniels et al., 1994) were used. Given the degree of sequence identity shown in Fig. 4, it is reasonable to expect that these antibodies would recognize the corresponding P. vulgaris PIP proteins. It is likely that each antiserum recognized multiple PIP1 and PIP2 proteins, and that several PIP proteins present in the cells were not recognized (Alexandersson et al., 2005). At the same time, knowing the limitation of using heterologous antibodies, only striking results were highlighted.
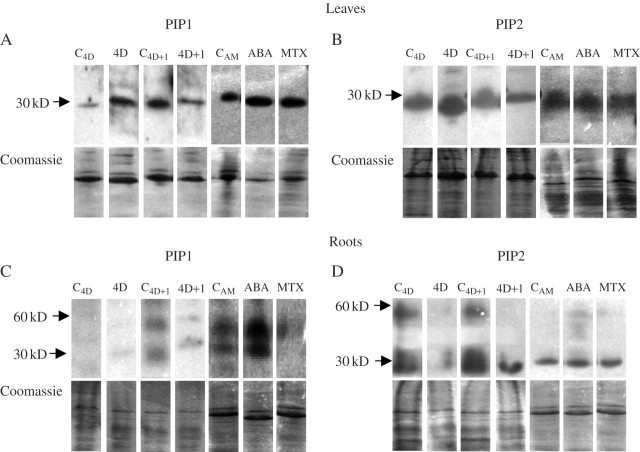
The amounts of PIP1 and PIP2 protein recognized by the antibodies used were increased by drought treatment in leaves, and decreased again after re-watering (Fig. 6A and B). At the same time, leaves of C4D+1 plants showed a higher amount of PIP1 protein than C4D plants (Fig. 6A). Also, ABA and MTX treatments increased the amounts of PIP1 protein in the leaves (Fig. 6A). No other changes of PIP protein abundance at leaf level were observed (Fig. 6A and B).
Fig. 6.
Western blots using antibodies against PIP1 (A and C) and PIP2 (B and D) proteins in leaves (A and B) and in roots (C and D) of common bean plants subjected to drought for 4 d (4D) and 1 d after re-watering (4D + 1), and the corresponding control plants (C4D and C4D+1), leaves sprayed 24 h earlier with 100 μm ABA or 200 μm MTX, and unsprayed control plants (CAM). Blots were repeated three times with different sets of plants; representative blots are shown. The corresponding Coomassie-stained gel shows the protein loaded in each lane.
In roots, the blots showed two bands at around 60 and 30 kDa, respectively, in almost all treatments. PIP1 proteins recognized by the antibody were almost only detected on ABA-treated plants, although a slight signal could be seen on C4D+1 and CAM plants (Fig. 6C). Drought treatment caused a strong reduction of PIP2 protein in roots, and this was only partially recovered after re-watering (Fig. 6D). No other changes in PIP protein abundance at the root level were observed (Fig. 6C and D).
DISCUSSION
Neither water deficit nor ABA or MTX treatments caused a significant (P > 0·05) change in leaf or root ABA contents (Fig. 2), with values of the same order of magnitude as have been found previously in P. vulgaris tissues (Vernieri et al., 2001; Wakrim et al., 2005). Zhang et al. (1997) found that after 24 h of feeding maize leaves with ABA, only 8 % remain unmodified. At the same time, these authors calculated that the daily increase of leaf ABA during a dry period was only about 5 %, mainly by ABA synthesized by leaves suffering water deficit. During the present drought experiment, bean leaves did not suffer any water deficit, and therefore it is not strange that leaf and root ABA levels did not change. Also, Trejo and Davies (1991) found that bean plants closed their stomata after 3 d of water deficit without any change in leaf ABA contents or leaf water status. At the same time, Wakrim et al. (2005) did not find any increase in leaf ABA contents in bean plants even after 20 d of drought treatment. Although no change in endogenous ABA contents caused by drought was seen here, it is possible that ABA could act during drought treatment by changing its internal localization, moving from symplastic to apoplastic zones (Wilkinson and Davies, 2002).
Drought is one of the most common stressful conditions that plants experience in their environment, and most frequently it has a negative effect on hydraulic conductance of the roots (Vandeleur et al., 2005). It is commonly accepted that under transpiring conditions water comes from the soil to the root xylem following mainly the apoplastic path governed by a hydrostatic pressure gradient; however, when transpiration is restricted by stressful conditions such as drought, more of the water follows the cell-to-cell path, flowing across membranes of living cells (see Steudle, 2000). The drought treatment imposed in the experiments described here could be defined as a moderate stress since no effect on leaf water status was observed (data not shown). Nevertheless, significant effects on leaf transpiration rate and root hydraulic conductance (L) were detected (Fig. 3). Here, L was measured under an osmotic gradient using detached root systems exuding under atmospheric pressure, and presumably this water followed mainly the cell-to-cell path (crossing membranes of cells). Drought reduced L significantly, at a time when the transpiration rate fell almost to zero (Fig. 3). Thus, droughted plants decreased the water permeability of their root membrane cells to avoid a possible loss of water from the roots to the soil, as has been proposed earlier (Steudle, 2000). However, after re-watering, plants continued to have very low L values, while the transpiration rate recovered (Fig. 2). Such behaviour indicates that water should be following an apoplastic path as has been reported before for other plant species subjected to drought (Ionenko et al., 2003; Siemens and Zwiazek, 2004). At the same time, no consistent relationship between transpiration rate and L measured under an osmotic pressure gradient was observed, in accordance with the results of others who measured L under hydrostatic pressure conditions (Henzler et al., 1999; Huxman et al., 1999; Wan and Zwiazek, 2001). For example, a parallel decrease of the transpiration rate with an increase of L was found for control plants of the drought experiment (Fig. 3). Therefore, it is plausible to assume that an increase in the cell-to-cell path was taking place.
The decrease of L caused by drought was correlated with a strong decline of the abundance of the PIP2 proteins recognized by the antibody and measured by immunoblotting (Fig. 6D). Similar results correlating PIP2 protein abundance decline and lower L values were observed in Arabidopsis plants subjected to saline stress (Boursiac et al., 2005). However, mRNA expression of the PvPIP2;1 gene increased in root tissues as a result of drought (Fig. 5B). PIP proteins form a large family that is subdivided into PIP1 and PIP2 proteins (Luu and Maurel, 2005). Arabidopsis thaliana has five PIP1 and eight PIP2 proteins (Johanson et al., 2001), whereas Zea mays has six PIP1 and seven PIP2 proteins (Chaumont et al., 2001); recently, three PIP1 and eight PIP2 proteins were found in rice (Sakurai et al., 2005). Therefore, it is possible that other PIP2 genes were downregulated during drought and PIP2 protein abundance also decreased.
On the other hand, ABA treatment raised both L and the abundance of PIP1 root protein recognized by the antibody (Figs 3B and 6C). In several studies it has been observed that PIP2 proteins have more water channel activity than PIP1 proteins when they are expressed in heterologous systems (Fetter et al., 2004; Temmei et al., 2005). However, it has also been demonstrated that PIP1 proteins upregulate the activity of PIP2 proteins (Fetter et al., 2004; Temmei et al., 2005). Also, a correlation between root PIP1 protein abundance and L has been seen before in maize (Lopez et al., 2003; Aroca et al., 2005). However, to our knowledge, this is the first time where such a correlation has been seen in ABA-treated plants. However, it is not known which PIP1 genes were recognized by the antibody used and, as in the case of PIP2 proteins, specific antibodies for each P. vulgaris PIP gene are needed to get a complete picture.
In roots, the blots showed two bands at around 60 and 30 kDa, respectively, in almost all treatments, as is usually common in PIP blots (Alexandersson et al., 2005; Boursiac et al., 2005). Thus, it is possible that PIP proteins present in P. vulgaris root extracts form stronger dimers than PIP proteins of leaf extracts. Other authors found similar results comparing different extracts or antibodies used. Thus, Fraysse et al. (2005) using different PIP antibodies in spinach extracts found that some of them recognized two bands (at around 56 and 28 kDa), and others only recognized one band at 28 kDa. Also, Henzler et al. (1999), using the same PIP1 antibody that has been used here, found two band in protein extracts from Lotus japonicus roots, but only one in Arabidopsis roots.
Most commonly, drought stress induces a downregulation of PIP gene expression in roots (Smart et al., 2001; Jang et al., 2004; Porcel et al., 2006). However, here the expression of the three PIP genes examined increased after 4 d of water deprivation (Fig. 5B and C). However, the PIP genes cloned here decreased their expression in roots the day after re-watering (4D + 1 plants), and the expression was barely changed either by ABA or by MTX treatment (Fig. 5B, C). Thus, it is plausible that the root upregulation of the PIP gene expression shown here could be caused by a direct effect of the low water content of the substrate and the inherent fall of the soil water potential. In fact, Hill et al. (2004) have proposed that aquaporins could function as osmosensors in plant membranes.
In the leaves, only PvPIP2;1 mRNA was detected (Fig. 5A). It is known that some PIP genes are tissue specific (Aroca et al., 2005; Sakurai et al., 2005) and, since the PIP genes cloned here came from root RNA, it is not surprising that two of them were not detected in leaf tissues. Moreover, expression was only detected in leaves of plants that had a reduced transpiration rate (4D, ABA and MTX plants). At the same time, the abundance of PIP1 protein recognized by the antibody also increased in the same groups of plants (Fig. 4A). Morillon and Chrispeels (2001) found that a decrease in the transpiration rate caused an increase of membrane water permeability of isolated leaf protoplasts. It is plausible that such a rise of water permeability was mediated by aquaporins.
In summary, drought treatment caused a strong decline of L that did not recover after re-watering, indicating some increase in the water flowing by the apoplastic path. At the same time, the decline of L was correlated with a strong decline of PIP2 protein abundance in the roots. Also, the rise of L caused by ABA was accompanied by a rise of PIP1 protein abundance in the roots. The increase of the drought-induced expression of the three genes examined in the roots was not correlated with any of the drought factors studied and results from the low water potential of the soil. Finally, in the leaves, the increase of PvPIP2;1 expression and PIP1 protein abundance induced by drought was correlated with a reduction in the transpiration rate.
Supplementary Material
Acknowledgments
We are grateful to Drs I. Martínez and M. J. Martín for their help during the experiments and their valuable comments. We are also indebted to Dr A. R. Schäffner (Ludwig-Maximilians Universität) for kindly providing antibody against AtPIP1;1. The authors also thank the two anonymous referees for their constructive comments. R.A. was supported by a postdoctoral fellowship from the Ministerio de Educación y Ciencia (Spain).
LITERATURE CITED
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G. (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, et al. (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Molecular Biology 59469–484. [DOI] [PubMed] [Google Scholar]
- Aroca R. (2006) Exogenous catalase and ascorbate modify the effects of abscisic acid (ABA) on root hydraulic properties in Phaseolus vulgaris L. plants. Journal of Plant Growth Regulation 2510–17. [Google Scholar]
- Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Díaz M, Tognoni F, Pardossi A. (2003) Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Science 165671–679. [Google Scholar]
- Aroca R, Amodeo G, Fernádez-Illescas S, Herman EH, Chaumont F, Chrispeels MJ. (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiology 137341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé RM, Sylvia DM, Park S, Buttery BR, Sexton AM, Moore JL, Cho K. (2004) Partitioning mycorrhizal influence on water relations of Phaseolus vulgaris into soil and plant components. Canadian Journal of Botany 82503–514. [Google Scholar]
- Boursiac Y, Chen S, Luu DT, Sorieul M, van den Dries N, Maurel C. (2005) Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiology 139790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA. (2004) Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. Journal of Experimental Botany 552331–2341. [DOI] [PubMed] [Google Scholar]
- Broughton WJ, Hernández G, Blair M, Beebe S, Gepts P, Vanderleyden J. (2003) Beans (Phaseolus spp.)—model food legumes. Plant and Soil 25255–128. [Google Scholar]
- Campos F, Solórzano RM, Garciarrubio A, Covarrubias A. (1997) A Phaseolus vulgaris cDNA encoding a putative aquaporin (Accession No. U97023) (PGR 97-124). Plant Physiology 115113.9306694 [Google Scholar]
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiology 1251206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ. (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is homolog of the tonoplast water channel protein TIP. Plant Physiology 1061325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ and Zhang JH. (1991) Root signals and the regulation of growth and development of plants in drying soils. Annual Review of Plant Physiology and Plant Molecular Biology 4255–76. [Google Scholar]
- Downs RJ and Helmers H. (1975) Environment and the experimental control of plant growth(Academic Press, London).
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraysse LC, Wells B, McCann M, Kjellbom P. (2005) Specific plasma membrane aquaporins of the PIP1 subfamily are expressed in sieve elements and guard cells. Biology of the Cell 97519–534. [DOI] [PubMed] [Google Scholar]
- Henzler T, Waterhouse RN, Smith AJ, Carvajal M, Cooke DT, Schäffner AR, et al. (1999) Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 21050–60. [DOI] [PubMed] [Google Scholar]
- Hill AE, Schachar-Hill B, Shachar-Hill Y. (2004) What are aquaporins for? Journal of Membrane Biology 1971–32. [DOI] [PubMed] [Google Scholar]
- Hose E, Steudle E, Hartung W. (2000) Abscisic acid and hydraulic conductivity of maize roots: a study using cell- and root-pressure probes. Planta 211874–882. [DOI] [PubMed] [Google Scholar]
- Huxman KA, Smith SD, Neuman DS. (1999) Root hydraulic conductivity of Larrea tridentate and Helianthus annus under elevated CO2. Plant, Cell and Environment 22325–330. [Google Scholar]
- Ionenko IF, Anisimov AV, Romanov AV. (2003) Effect of water stress and mercuric chloride on the translational diffusion of water in maize seedlings roots. Russian Journal of Plant Physiology 5079–83. [Google Scholar]
- Irigoyen JJ, Pérez de Juan J, Sánchez-Díaz M. (1996) Drought enhances chilling tolerance in a chilling-sensitive maize (Zea mays) variety. New Phytologist 13453–59. [Google Scholar]
- Jang JK, Kim DG, Kim YO, Kim JS, Kang HS. (2004) An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology 54713–725. [DOI] [PubMed] [Google Scholar]
- Javot H and Maurel C. (2002) The role of aquaporins in root water uptake. Annals of Botany 90301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD, Holobrada M, Hartung W. (1997a) Growth of Zea mays L. plants with their seminal roots only. Effects on plant development, xylem transport, mineral nutrition and the flow and distribution of abscisic acid (ABA) as a possible shoot to root signal. Journal of Experimental Botany 481229–1239. [Google Scholar]
- Jeschke WD, Peuke AD, Pate JS, Hartung W. (1997b) Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. Journal of Experimental Botany 481737–1747. [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, et al. (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiology 1261358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerloher W, Fisher U, Piechottka GP, Schäffner AR. (1994) Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant Journal 6187–199. [DOI] [PubMed] [Google Scholar]
- Klein M, Geisler M, Suh SJ, Kolukisaoglu HU, Azevedo L, Plaza S, et al. (2004) Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increase drought susceptibility. Plant Journal 39219–236. [DOI] [PubMed] [Google Scholar]
- Lian H-L, Yu X, Ye Q, Ding X-S, Kitagawa Y, Kwak S-S, et al. (2004) The role of aquaporin RWC3 in drought avoidance in rice. Plant and Cell Physiology 45481–489. [DOI] [PubMed] [Google Scholar]
- Liu F, Andersen MN, Jacobsen S-E, Jensen CR. (2005) Stomatal control and water use efficiency of soybean (Glycine max L. Merr) during progressive soil drying. Environmental and Experimental Botany 5433–40. [Google Scholar]
- Lopez F, Bousser A, Sissoëff I, Gaspar M, Lachaise B, Hoarau J, Mahé A. (2003) Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant and Cell Physiology 441384–1395. [DOI] [PubMed] [Google Scholar]
- Luu DT and Maurel C. (2005) Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant, Cell and Environment 2885–96. [Google Scholar]
- Martínez IM and Chrispeels MJ. (2003) Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular process. Plant Cell 15561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology 1302101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC and Schroeder JI. (2004) Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiology 135702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon R and Chrispeels MJ. (2001) The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proceedings of the National Academy of Sciences of the USA 9814138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM. (2006) PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Molecular Biology 60389–404. [DOI] [PubMed] [Google Scholar]
- Pospíšilová J. (2003) Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biologia Plantarum 46491–506. [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant and Cell Physiology 461568–1577. [DOI] [PubMed] [Google Scholar]
- Saliendra NZ and Meinzer FC. (1992) Genotypic, developmental and drought-induced differences in root hydraulic conductance of contrasting sugarcane cultivars. Journal of Experimental Botany 431209–1217. [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to functions in plants. Plant Cell 14869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens JA and Zwiazek JJ. (2004) Changes in water flow properties of solutions culture-grown trembling aspen (Populus tremuloides) seedlings under different intensities of water-deficit stress. Physiologia Plantarum 12144–49. [DOI] [PubMed] [Google Scholar]
- Smart LB, Moskai WA, Cameron KD, Bennet AB. (2001) MIP genes are down-regulated under drought stress in Nicotiana glauca. Plant and Cell Physiology 42686–693. [DOI] [PubMed] [Google Scholar]
- Steudle E. (2000) Water uptake by roots: effects of water deficit. Journal of Experimental Botany 511531–1542. [DOI] [PubMed] [Google Scholar]
- Suga S, Komatsu S, Maeshima M. (2002) Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant and Cell Physiology 431229–1237. [DOI] [PubMed] [Google Scholar]
- Temmei Y, Uchida S, Hoshino D, Kanzawa N, Kuwahara M, Sasaki S, Tsuchiya T. (2005) Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Letters 5794417–4422. [DOI] [PubMed] [Google Scholar]
- Trejo CL and Davies WJ. (1991) Drought-induced closure of Phaseolus vulgaris L. stomata precedes leaf water deficit and any increase in xylem ABA concentration. Journal of Experimental Botany 421507–1515. [Google Scholar]
- Tusnády GE and Simon I. (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17849–850. [DOI] [PubMed] [Google Scholar]
- Vandeleur R, Niemitz C, Tilbrook J, Tyerman SD. (2005) Roles of aquaporins in root responses to irrigation. Plant and Soil 274141–161. [Google Scholar]
- Vernieri P, Pardossi A, Tognoni F. (1991) Influence of chilling and drought on water relations and abscisic acid accumulation in bean. Australian Journal of Plant Physiology 1825–35. [Google Scholar]
- Vernieri P, Lenzi A, Figaro M, Tognoni F, Pardossi A. (2001) How the roots contribute to the ability of Phaseolus vulgaris L. to cope with chilling-induced water stress. Journal of Experimental Botany 522199–2206. [DOI] [PubMed] [Google Scholar]
- Wakrim R, Wahbi S, Tahi H, Aganchich B, Serraj R. (2005) Comparative effects of partial root drying (PRD) and regulated deficit irrigation (RDI) on water relations and water use efficiency in common bean (Phaseolus vulgaris L.). Agriculture, Ecosystems and Environment 106275–287. [Google Scholar]
- Wan X and Zwiazek JJ. (2001) Root water flow and leaf stomatal conductance in aspen (Populus tremuloides) seedlings treated with abscisic acid. Planta 213741–717. [DOI] [PubMed] [Google Scholar]
- Weig A, Deswarte C, Chrispeels MJ. (1997) The major intrinsic proteins family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiology 1141347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S and Davies WJ. (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant, Cell and Environment 25195–210. [DOI] [PubMed] [Google Scholar]
- Yu Q, Hu Y, Li J, Wu Q, Lin Z. (2005) Sense and antisense expression of plasma membrane aquaporin from Brassica napus in tobacco and its effects on plant drought resistance. Plant Science 169647–656. [Google Scholar]
- Zhang J, Jia W, Zhang D-P. (1997) Re-export and metabolism of xylem-delivered ABA in attached maize leaves under different transpirational fluxes and xylem ABA concentrations. Journal of Experimental Botany 481557–1564. [Google Scholar]
- Zhu C, Schraut D, Hartung W, Schäffner AR. (2005) Differential responses of maize MIP genes to salt stress and ABA. Journal of Experimental Botany 562971–2981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.