Abstract
• Background and Aims Reactive oxygen species are frequently produced when plants are exposed to abiotic stresses. Among the detoxication systems, two enzymes, ascorbate peroxidase and glutathione reductase (GR) play key roles. GR has also a central role in keeping the reduced glutathione pool during stress thus allowing the adjustments on the cellular redox reactions. The aim of this work was to study the variations in cytosolic and dual-targeted GR gene expression in the leaves of cowpea plants submitted to progressive drought, rapid desiccation and application of exogenous abscisic acid (ABA).
• Methods Two cowpea (Vigna unguiculata) cultivars, one drought-resistant (‘EPACE-1’), the other drought-sensitive (‘1183’) were submitted to progressive drought stress by withholding irrigation. Cut-off leaves were air-dried or treated with exogenous ABA. Two GR cDNAs, one cytosolic, the other dual-targeted to chloroplasts and mitochondria were isolated by PCR and cloned in plasmid vectors. Reverse-transcription PCR was used to study the variations in GR gene expression.
• Key Results Two new cDNAs encoding a putative dual-targeted and a cytosolic GR were cloned and sequenced from leaves of V. unguiculata. Drought stress induced an up-regulation of the expression of the cytosolic GR gene directly related to the intensity of the stress in both cultivars. The expression of dual-targeted GR was up-regulated by the drought treatment in the susceptible cultivar only. Under a fast desiccation, the ‘1183’ cultivar responded later than the ‘EPACE-1’, although in ‘EPACE-1’ it was the cytosolic isoform which responded and in ‘1183’ the dual-targeted one. Exogenous ABA enhanced significantly the activity and expression levels of GR in both cultivars after treatment for 24 h.
• Conclusions These results demonstrate a noticeable activation in both cultivars of the antioxidant metabolism under a progressive water stress, which involves both GR genes in the case of the susceptible cultivar. Under a fast desiccation, the susceptible cultivar responded later than the resistant one, suggesting a weaker capacity of response versus the resistant one. Exogenous ABA probably acts on GR gene expression via a mediated signal transduction pathway.
Keywords: Reactive oxygen species, drought tolerance, glutathione reductase, abscisic acid, gene expression, Vigna unguiculata
INTRODUCTION
Environmental stresses such as drought lead to enhanced generation of reactive oxygen species (ROS), which will cause the breakdown of membrane lipids (Monteiro de Paula et al., 1993) and DNA damage (Monk et al., 1989; Halliwell and Gutteridge, 1999), and eventually lead to cellular death (Girotti, 2001). ROS in plants are removed by a variety of antioxidant enzymes and/or lipid-soluble and water-soluble scavenging molecules (Foyer et al., 1994; Allen, 1995; Chaudière and Ferrari-Iliou, 1999; Lascano et al., 2003), the antioxidant enzymes being the most efficient mechanisms against oxidative stress (Halliwell and Gutteridge, 1999). Apart from catalase, various peroxidases and peroxiredoxins (Dietz, 2003), four enzymes are involved in the ascorbate-glutathione cycle, a pathway that allows the scavenging of superoxide radicals and H2O2 (Asada, 1999): ascorbate peroxidase (APX), dehydroascorbate reductase, monodehydroascorbate reductase and glutathione reductase (GR). Most of the ascorbate-glutathione cycle enzymes are located in the stroma of chloroplasts, the cytosol, mitochondria and peroxisomes (Jiménez et al., 1998).
APX and GR which are, respectively, the first and last enzyme in this cycle, are responsible for H2O2 detoxification in green leaves (Foyer and Harbinson, 1994). APX is considered to be a key antioxidant enzyme in plants (Orvar and Ellis, 1997) and GR has a central role in maintaining the reduced glutathione (GSH) pool during stress (Pastori et al., 2000). In the case of GR, two cDNAs types have been isolated from plants, one type encoding the cytosolic isoforms (cGR) (Lee et al., 1998; Stevens et al., 2000) and the other type encoding GR proteins dual-targeted to both chloroplasts and mitochondria (dtGR) (Mullineaux et al., 1996; Rudhe et al., 2002; Chew et al., 2003).
Moreover, it is well known that the amount of the plant hormone abscisic acid (ABA) increases as a result of water stress, thus playing important roles as a stress signal, not only under water limitation but in plant adaptation to numerous stress conditions (Suhita et al., 2004). During water stress, ABA has also been shown to cause an increased production of 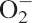 radicals and H2O2, enhancing the activities of antioxidant enzymes such as GR (Jiang and Zhang, 2001).
radicals and H2O2, enhancing the activities of antioxidant enzymes such as GR (Jiang and Zhang, 2001).
Drought resistance often depends on the ability of the plant to develop adaptative strategies under stress conditions. To better understand these mechanisms, comparative studies were carried out using a previously developed plant system consisting of two cowpea (Vigna unguiculata) cultivars, ‘EPACE-1’ and ‘1183’, differing in their tolerance to drought. In fact, several studies, undertaken both at the whole-plant and at the cellular level, have established that ‘EPACE-1’ is tolerant to drought whereas ‘1183’ is more susceptible (Roy-Macauley et al., 1992; El-Maarouf et al., 1999; Cruz de Carvalho et al., 2001; Matos et al., 2001; Diop et al., 2004).
Comparative studies using a previously developed plant system focused on the characterization of APX genes and their response to water deficit in two cowpea cultivars (d'Arcy-Lameta et al., 2006). GR like APX is part of the glutathione cycle but is also involved in keeping glutathione under the reduced GSH form, thus allowing numerous redox reactions in the cell. Hence, the fate of the GR isoforms under water deficit can be questioned.
The aim of the present work was to study GR (EC 261·6·4·2) activity in leaves of cowpea cultivars, which differ in their degree of drought tolerance, when submitted to progressive drought stress, air desiccation and ABA treatment. To better understand the contribution of GR in withstanding a highly oxidative environment, the cDNAs corresponding to cGR and dtGR isoforms were isolated and characterized and the gene expression level of both GR isoforms measured for both cultivars in the same conditions as those used in GR activity studies.
MATERIALS AND METHODS
Plant culture and sample treatments
Two Vigna unguiculata (L.) Walp (Vu) cultivars, one tolerant to drought (‘EPACE-1’) and the other susceptible (‘1183’) were grown in greenhouse conditions as described by d'Arcy-Lameta et al. (2006). Progressive drought stress was applied by withholding irrigation on 5-week-old plants. Leaf water potentials were measured daily at 0010 h (after 4 h light) with a pressure bomb (PMS instrument, Corvallis, USA) (Scholander et al., 1964). The second and the third well-developed leaves from the top were harvested when plants reached leaf water potentials of Ψw= −1·0 ± 0·1 MPa (S1 plants)—mild water stress; Ψw= −1·5 ± 0·2 MPa (S2 plants)—moderate stress; and Ψw= −2·0 ± 0·2 MPa (S3 plants)—severe stress. Leaves from control plants (Ψw= −0·5 ± 0·1 MPa) were harvested at the beginning of the drought treatment. Desiccation and ABA experiments were performed on well-watered plants from which the 2nd and 3rd leaves were cut off under water. Detached leaves were left to dry (desiccation treatment) at 24 °C under dim light for 30 min, 2 h or 5 h, or the petioles of cut-off leaves were placed in contact with 100 μm aqueous solution of mixed ABA isomers (Sigma, St Quentin Fallavier, France) (ABA treatment) under ambient conditions for 30 min, 2 h and 24 h or placed in water (control treatment). All the leaves sampled were frozen in liquid nitrogen and kept at −80 °C until needed.
Glutathione reductase activity
One hundred milligrams of frozen leaf material was homogenized in a ground glass homogenizer in an ice bath, with 1 mL extraction buffer (0·15 m Hepes, pH 8·0 containing 1·0 mm EDTA and 0·1 % Triton X-100), according to Foyer et al. (1995). The homogenates were transferred to Eppendorf® tubes then centrifuged at 13 000 g for 5 min. The supernatants were assayed for GR activity by following the decrease in absorbance at 340 nm (extinction coefficient 6·2 mm−1 cm−1) upon the addition of oxidized glutathione to a final concentration of 1·0 mm.
The reaction medium (1 mL) contained 50 mm Hepes buffer (pH 8·0), 1·0 mm EDTA, 0·1 mm NADPH, 1·0 mm GSSG and extract, according to Foyer et al. (1995). Corrections were made for GSSG-independent NADPH oxidation. Protein contents were determined according to Bradford (1976), with bovine serum albumin as a standard. All chemical products were purchased from Sigma France.
Total RNA and mRNA isolation
Frozen leaf material was ground with a mortar and pestle in liquid nitrogen and about 100 mg fresh material were used for total RNA extraction, using RNEasy Plant Minikit (Qiagen, France) according to the manufacturer's instructions. Messenger RNAs were obtained from total RNAs using Oligotex mRNA purification System (Qiagen). Total and mRNAs were quantified with a Nanodrop ND-1000 spectrophotometer (Starlab, USA) at 260 nm.
Reverse transcription–PCR amplification (RT-PCR) and cloning of GR cDNA fragments
GR protein sequences collected from GenBank™ (http://www.ncbi.nlm.nih.gov) were aligned using the CLUSTALW program (Thompson et al., 1994). Oligonucleotides were designed from consensus regions and used as primers in RT-PCR amplification of cowpea GR cDNA fragments.
The primers for dual-targeted GR were: 5′-TGT GTA ATA CGA GGA TGT GTG CCG-3′ (forward) and 5′-ACT TGG GTG AAT GCC TAC AGT GGC-3′ (reverse) and for cytosolic GR were: 5′-GGA GCA TCT TAT GGA GGT GAA C-3′ (forward) and 5′-CAG TTT TTT CTT GTC GCC CAG-3′ (reverse).
RT-PCR was performed on ‘1183’ drought-stressed (S2) leaf total RNAs. The RT-PCR reaction contained 100 ng total RNA, 15 pmol each oligo-nucleotide, dNTP mix (10 mm each), 1 unit of a mix of reverse transcriptase and Taq Pol enzymes (one-step reverse transcription–PCR system; Qiagen) and the manufacturer's buffer in a total volume of 25 μL. RT-PCR was performed in a thermal cycler (Mastercycler Gradient, Eppendorf AG, Germany), with a first step (50 °C for 30 min, 95 °C for 15 min) followed by 30 cycles (denaturation step at 95 °C for 30 s, annealing at 60·3 °C, 50 s for cytosolic GR and 63 °C 50 s for the dual-targeted, extension 72 °C, 1 min 10 s). After the last cycle, a final extension was carried out for 7 min at 72 °C. Amplified GR cDNA fragments were visualized after separation on 1 % (w/v) agarose gels, using a UV light transilluminator (Snapshot, Syngene) and purified (Wizard PCR Prep, Promega, France).
Cloning was performed using the pGEM-T easy vector plasmid system I (Promega) following the manufacturer's instructions and used to transform Escherichia coli strain GT869 competent cells by heat shock. After selection of the recombinant bacteria, plasmid DNA was isolated using Wizard® Plus SV minipreps DNA purification system kit (Promega). Sequencing was performed on both strands by Genoscreen (Lille, France).
The nucleotide sequence data are registered in Genbank under the following accession numbers: dtGR, DQ267474; cGR, DQ267475.
GR expression analysis
GR expression analysis was carried out by RT-PCR reactions, using the one-step RT-PCR kit (Qiagen). Total RNA extracts from control, water-deficit, desiccation and ABA conditions were used as templates. Specific primers were used to amplify cDNA fragments for dual-targeted (1200 bp) and cytosolic GR (1000 bp) with the same program (cf. §2·4, except 35 cycles instead of 30). To evaluate the amount of template RNA in each RT-PCR reaction, fragments of cowpea S19 gene coding for a ribosomal protein (drought response studies) and cowpea 18S gene encoding a ribosomal RNA (desiccation and ABA treatment studies) with near constitutive expression, were PCR amplified. Amplification products were visualized on 1·2 % agarose (w/v) gels using a UV light transilluminator (Snapshot, Syngene). Densitometric evaluation of DNA bands were performed with the Imager 1D/2D software (Appligene, Strasbourg, France). Relative transcript levels were calculated with reference to the controls taken as 1 (100 %).
Rapid amplification of 5′- and 3′-cDNA ends (RACE)
PCR amplification of the 5′- and 3′-regions of cDNAs for both GR genes was achieved by the use of the 5′/3′-RACE Kit (Roche Diagnostics, Meylan, France), ‘1183’ purified poly (A)+ mRNA as a template and following the manufacturer's instructions. Since amplifications were not in the plateau phase, semi-quantitative estimations of transcript levels were acceptable.
RESULTS
Total soluble GR activity
Due to the loss of membrane integrity induced by drought stress, measurements of enzymatic activity in the different cell compartments are very imprecise (Pham-Thi and Vieira da Silva, 1975; Ferrari-Iliou et al., 1994) hence, the GR enzymatic activity was referred to as ‘total soluble GR activity’. The GR-specific activities were measured both in the soluble leaf fraction of control and of drought-stressed plants. GR activity was 16 % higher in control ‘EPACE-1’ leaves than in control ‘1183’ leaves (Fig. 1, top). The total soluble GR activity was found constant for ‘EPACE-1’ in S1, S2 and S3 leaves, although slightly lower than in the control. For ‘1183’ the beginning of the drought stress induced a 23 % decrease in GR activity (in S1 as well as in S2) and then the activity increased in S3 (57 % more than in S2).
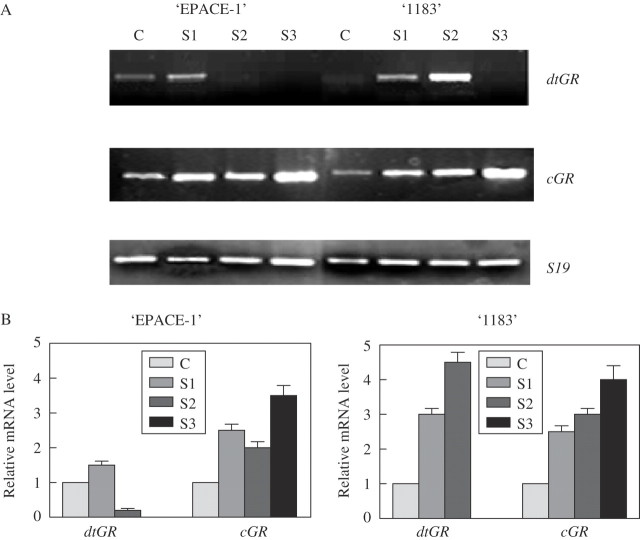
Fig. 1.
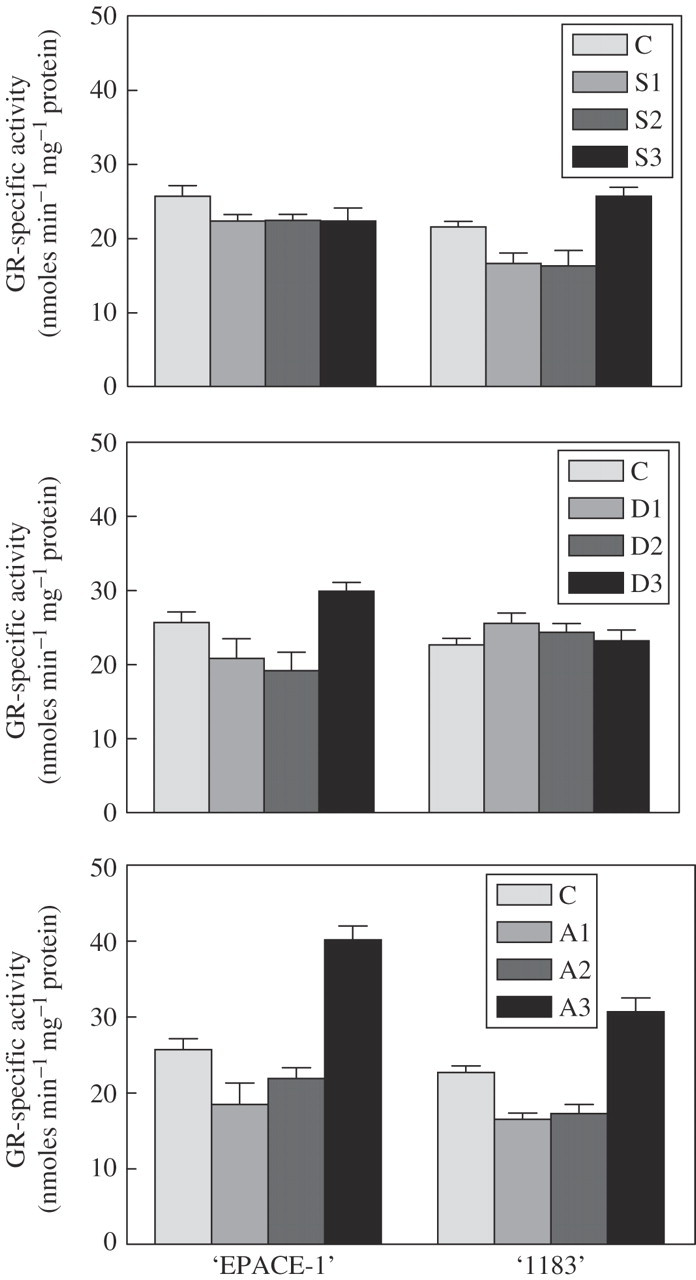
GR-specific activity in cowpea (Vigna unguiculata) ‘EPACE-1’ and ‘1183’ leaves. GR-specific activity was measured in control and drought-stressed plants (top), under a desiccation experiment (middle) and after ABA-treatment (bottom). Values are means ± s.d. of three to five independent experiments. GR activity was assayed by following the oxidation of NADPH (decrease in absorbance at 340 nm) and expressed in nmol mg−1 protein min−1. Control plants (C), Ψw = −0·5 ± 0·1 MPa; S, droughted plants: S1, Ψw = −1·0 ± 0·1 MPa; S2, Ψw = −1·5 ± 0·2 MPa; S3, Ψw = −2·0 ± 0·2 Mpa; D, desiccation treatment for 0·5 h (D1), 2 h (D2), 5 h (D3); A, ABA treatment for 0·5 h (A1), 2 h (A2), 24 h (A3).
In the desiccation experiments (Fig. 1, middle), the GR activity in ‘EPACE-1’ leaves decreased up to 19 % after 30 min (D1), 25 % after 2 h air-drying (D2) but then increased by 36 % after 5 h (D3). For ‘1183’ GR activity was nearly constant throughout the experiment (Fig. 1, middle).
The effect of ABA on GR activity of detached leaves was detectable after a 24 h-immersion of leaf petioles in 100 μm ABA, both in ‘EPACE-1’ and ‘1183’ cultivars, the higher increase in activity being found for ‘EPACE-1’ leaves (56 % and 42 % more than in controls, for ‘EPACE-1’ and ‘1183’, respectively) (Fig. 1, bottom).
Isolation of GR isoform cDNAs from cowpea
For cowpea dtGR, consensus regions were identified by aligning GR amino acid sequences of soybean and pea (accession numbers L11632 and X60373, respectively). A final 1997-bp cDNA was obtained for dtGR, its open reading frame (ORF) starting with an ATG codon at 64 and interrupted by a stop codon at position 1617 (GenBank accession number DQ267474). A 63-bp 5′-UTR and a 377-bp 3′-UTR flanked the ORF. The deduced protein had 544 amino acids residues with a calculated molecular weight of 58·5 kDa (www.infobiogen.fr/services/analyseq/cgi-bin/mwcalc_out.pl). At the amino acid level, the putative protein shared 87 % and 80·3 % identity with soybean (Tang and Webb, 1994) and pea (Creissen et al., 1992) dual-targeted GR, respectively. A putative targeting signal peptide of 52 amino acids residues was detected in the N-terminal domain of the dual isoform (www.cbs.dtu.dk/services/ChloroP/) (Fig. 2), its cleavage leading to a putative mature polypeptide of 52·7 kDa.
Fig. 2.

Alignment of cytosolic GR (cGR) amino acid sequences from Vigna unguiculata (Vu) and Pisum sativum (Ps, Genebank accession no. X98274) and of dual-targeted GR (dtGR) from Vigna unguiculata and Glycine max (Gm, no. L11632) amino acid sequences. The alignment was obtained using the program Clustalw (Thompson et al., 1994). *, identity; ‡, strongly similar; †, weakly similar; ↓, cleavage site for dual-targeted isoform (the targeting signal peptide is in italics); a, conserved arginine residues for NADPH binding; b, glutathione binding residue; the redox-active disulfite bridge is underlined (from Stevens et al., 1997).
For cowpea cGR, amino acid sequences from pea (X98274), Arabidopsis thaliana (P48641) and rice (D85751) were aligned and consensus regions identified. A final 1734-bp cDNA fragment was amplified for cGR, its ORF starting with an ATG codon at position 58 and ended by a stop codon at position 1558 (GenBank accession number DQ267475). A 57-bp 5′-UTR and a 175-bp 3′-UTR flanked the ORF. The deduced protein had 500 amino acids residues with a calculated molecular weight of 53·6 kDa, and shared 89·2 %, 80·2 % and 74·6 % identity with pea (Stevens et al., 1997), Arabidopsis (Sato et al., 2000) and rice (Kaminaka et al., 1998) cytosolic GR, respectively (Fig. 2).
The redox-active disulfide bridge, the glutathione-binding residues and the conserved arginine residues for NADP binding were referenced as in Stevens et al. (1997) (Fig. 2). The hydrophobicity profiles of the cGR and of the dtGR (without its signal peptide) from Vigna leaves were found very similar, the spectra being mainly negative (data not shown) thus confirming their solubility, respectively, in the cytosol and in the stroma of chloroplast or matrix of mitochondria.
Expression of the GR gene in response to progressive drought stress
As shown in Fig. 3, the transcript level was relatively higher for the cGR isoform than for the dual one for both cultivars. The drought treatment induced an up-regulation of cGR transcripts which was directly related to the intensity of the water deficit. This expression pattern of the cGR isoform was similar for both tolerant and susceptible cultivars, although slightly more pronounced in the case of the susceptible ‘1183’. Regarding the dual targeted GR isoform, significant differences were found between the two cultivars. The tolerant ‘EPACE-1’ responded to the beginning of the drought treatment with a slight increase in dtGR expression but, as the intensity of the drought treatment increased, there was a down-regulation on dtGR expression and no transcripts were detected in S2 and S3 leaves. For ‘1183’, the expression was highly dependent on the intensity of the drought treatment, reaching a peak in S2. The dual isoform was not detected in S3.
Fig. 3.
Effect of progressive drought on mRNA level of dtGR, cGR and S19 in ‘EPACE-1’ and ‘1183’ Vigna unguiculata leaves. Control plants (C), Ψw = −0·5 ± 0·1 MPa; S, droughted plants: S1, Ψw = −1·0 ± 0·1 MPa; S2, Ψw = −1·5 ± 0·2 MPa; S3, Ψw = −2·0 ± 0·2 MPa. (A) Gel analysis of GR isoenzyme transcripts; RT-PCR was carried out on 100 ng total RNA with 35 cycles, results were visualized using a UV light transilluminator. (B) Relative mRNA level. The mRNA levels were quantified with the Imager 1D/2D software and normalized to the respective S19 gene. Relative transcript levels were calculated with reference to the controls taken as 1 (100 %). Results are means ± s.d. of three independent experiments.
Expression of the GR gene under desiccation treatment
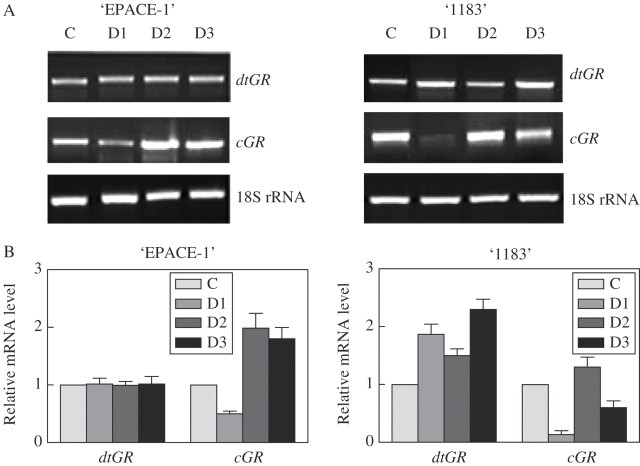
In the desiccation experiments (Fig. 4), differences were found between cultivars. Regarding ‘EPACE-1’, the dtGR isoform did not respond to the desiccation treatment, whereas cGR isoform expression was increased after 2 h and 5 h drying in the air. In the case of ‘1183’, both the cGR and the dtGR isoforms were up-regulated by air drying, the first after being treated for 2 h and 5 h, the second after 30 min and 5 h but with a down-regulation after 2 h of treatment.
Fig. 4.
Effect of desiccation (D) on mRNA level of dtGR and cGR and 18S rRNA in ‘EPACE-1’ and ‘1183’ Vigna unguiculata leaves. Desiccated leaves were air-dried at 24 °C under dim light for 0·5 h (D1), 2 h (D2) or 5 h (D3). Leaves from control plants (C) were taken prior to treatments. (A) Gel analysis of GR isoenzyme transcripts; RT-PCR was carried out on 100 ng total RNA with 35 cycles; results were visualized using a UV light transilluminator. (B) Relative mRNA level. The mRNA levels were quantified with the Imager 1D/2D software and normalized to the respective S19 gene. Relative transcript levels were calculated with reference to the controls taken as 1 (100 %). Results are means ± s.d. of three independent experiments.
Expression of the GR gene under ABA treatment
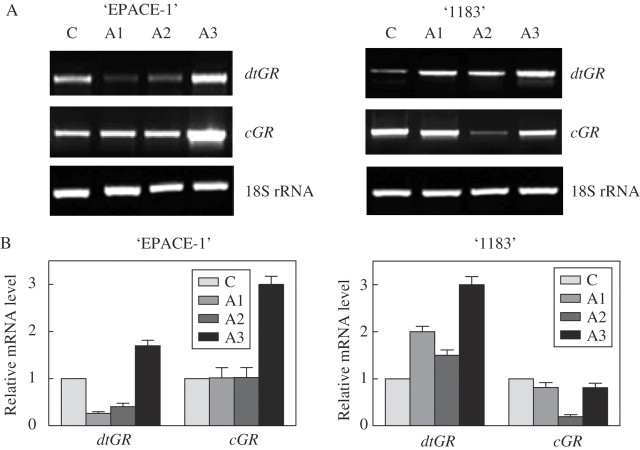
An up-regulation by ABA of both cGR and dtGR from ‘EPACE-1’ and dtGR from ‘1183’ was noticed after treatment for 24 h (Fig. 5), which correlated well with the data on enzymatic activity (Fig. 1). Cytosolic GR from ‘1183’ did not give a clear response but rather showed a down-regulation after treatment for 2 h.
Fig. 5.
Effect of exogenous abscisic acid (ABA) on mRNA level of dtGR and cGR in ‘EPACE-1’ and ‘1183’ Vigna unguiculata leaves. Exogenous ABA (100 μm) was applied by immersing petioles of excised leaves at 24 °C under dim light for 30 min (A1), 2 h (A2) and 24 h (A3). (A) Gel analysis of GR isoenzyme transcripts. RT-PCR was carried out on 100 ng total RNA with 35 cycles; results were visualized using a UV light transilluminator. (B) Relative mRNA level. The mRNA levels were quantified with the Imager 1D/2D software and normalized to the respective S19 gene. Relative transcript levels were calculated with reference to the controls taken as 1 (100 %). Results are means ± s.d. of three independent experiments.
DISCUSSION
In response to progressive drought-stress variations on GR activity were not significant in ‘EPACE-1’, whereas GR activity increased by 57 % in ‘1183’ for the lower water potential, hence the most severe water stress (S3, Fig. 1, top). A similar response was observed for APX activity in ‘EPACE-1’, whereas for ‘1183’ an important increase was observed at S2 level (d'Arcy-Lameta et al., 2006). The effects of water stress reported in the literature on GR and APX activities were contradictory and depended both on the degree of tolerance of the plant and on the kind of water stress. For example, table-top desiccation of leaves for 5 h acted on ‘EPACE-1’ but not in ‘1183’ cultivar and the results obtained were different from those found during progressive drought stress (Fig. 1, top and middle).
Since the measure of total soluble GR activity gave only an overall view of the role of this enzyme when facing stress conditions, the cDNAs corresponding to the GR isoforms were cloned and sequenced. The experiments revealed two GR isoforms, one cytosolic (cGR), and the other known to be dual-targeted to both mitochondria and chloroplasts (dtGR).
Most chloroplast and mitochondrial precursor proteins are targeted specifically to either chloroplasts or mitochondria. Although GR activity has been detected in mitochondria as well as in peroxisomes, no gene corresponding to these organelles has been isolated. Moreover, it was recently reported for Arabidopsis thaliana and pea that a single gene encoded a GR protein targeted to both chloroplastic and mitochondrial compartments (Creissen et al., 1995; Rudhe et al., 2002; Chew et al., 2003), the import of the protein being dependent on the N-terminal targeting signal as the mature protein alone was not imported into chloroplasts or mitochondria (Chew et al., 2003). A 52-amino acid sequence was found for the signal peptide in cowpea leaves (Fig. 2) and soybean (Tang and Webb, 1994) versus 60 amino acids for pea (Chew et al., 2003).
Under progressive drought stress and for both cultivars, cGR expression was directly related to the intensity of the drought stress with a maximal transcript level for the lowest leaf water potential (S3; Fig. 3). This could translate an active antioxidant-protective mechanism acting in drought-stressed susceptible and tolerant cowpea plants. Regarding the dual isoform, the pattern of transcript accumulation was different between the tolerant and the susceptible cultivars. In the tolerant cultivar, the progression of the drought treatment down-regulated dtGR expression, whereas in the susceptible cultivar it highly stimulated dtGR expression, at least until moderate water stress was reached. However, dtGR transcripts were not found in S3 (severe water stressed) leaves of either cultivar. It is interesting to note that a highly similar pattern of gene expression was observed between ‘EPACE-1’ GR (Fig. 3) and APX (d'Arcy-Lameta et al., 2006) under progressive drought, for the cytosolic isoforms as well as for the chloroplastic ones. Under progressive drought stress the level of transcripts was relatively higher for cytosolic GR than for the dual isoform (Fig. 3), this being in accordance with the results reported on pea GR (Stevens et al., 1997) and cowpea APX (d'Arcy-Lameta et al., 2006). As mentioned by Foyer and Noctor (2003), induced antioxidant genes encode cytosolic rather than chloroplastic antioxidative proteins even if the major stress should be located in the chloroplast. As drought stress induced an up-regulation of the expression of cGR in either cultivars, ‘EPACE-1’ (or ‘1183’) cytosolic GR cDNA could be used to transform drought-susceptible plants in order to improve their capacity to withstand oxidative stress.
The lack of water in the case of progressive drought, strongly affected the expression of the cytosolic isoform in both cultivars, and the higher level of transcripts was found for the most severe water stress. This might be a general feature since it was also found in several other plants and under different stresses: progressive drought stress in pea (Stevens et al., 1997), paraquat treatment, ozone fumigation and cold stress in Brassica campestris (Lee et al., 1998), high light stress in Arabidopsis (Karpinski et al., 1997) and drought and ABA treatment in rice (Kaminaka et al., 1998). Stevens et al. (1997) argued that plastidial and mitochondrial GR might be non-inducible since the organelles are regularly exposed to oxidative stress due to ROS production by photosynthesis and respiration, having hence acquired a housekeeping function. This might be true for the tolerant cowpea cultivar ‘EPACE-1’, but dtGR expression in the susceptible ‘1183’ also responded to the drought treatment. Lee et al. (1998) stated that the cytosol was the first cellular compartment concerned in the case of paraquat or ozone treatment after apoplastic and plasmalemma defences were breached. The same was seen for water, cold or high-light stress (Karpinski et al., 1997; Stevens et al., 1997). This could explain the rapid expression of the cytosolic GR mRNAs facing a stress and the greater stability of the enzyme in the chloroplast versus the cytosol as suggested by Foyer et al. (1995).
Contrary to progressive drought on whole plants, a fast desiccation of cut-off leaves induced a rise of leaf GR specific activity in the resistant cultivar, ‘EPACE-1’, only and after treatment for 5 h (Fig. 1, middle) in accordance with the increase of the expression level of cGR after air drying for 2 h and 5 h (Fig. 4). This shows a rapid response of the cytosolic ascorbate/glutathione cycle, suggesting a rapid increase of cytosolic ROS from the beginning of the drying period thus enhancing the cytosolic GR transcription level in ‘EPACE-1’ leaves. However, it is interesting to notice that ‘1183’ leaves did react to the air dryness by increasing the level of the dual-targeted GR isoform after treatment for 5 h. A possible explanation could be that under a desiccation treatment which leads to a fast water loss (as opposed to a progressive water stress), the susceptible cultivar did not adapt its metabolism in totality. The same phenomenon has been described in the case of lipid metabolism in cowpea drought-stressed plants (El-Maarouf et al., 2001).
Water stress is known to act on cellular leaf functions (Pham-Thi and Vieira da Silva, 1975) and to induce oxidative stress and ABA accumulation in plant cells (Jiang and Zhang, 2001, 2002; Foyer and Noctor, 2003). Guan et al. (2000) suggested that ROS might be involved in the ABA signal transduction pathway leading to the activation of one catalase (Cat1) in maize. These authors argued that ABA dependent and ABA independent pathways interact and converge to activate stress genes. Reactive oxygen signalling is likely to be a modulator of ABA response under stress in addition to sugar sensing (Verslues and Zhu, 2005). In ‘EPACE-1’, the expression level of both isoforms highly increased after ABA-treatment of cut-off leaves for 24 h but regarding ‘1183’ this was true only for dtGR. The stimulation of the GR cytosolic isoform expression by ABA was also described on rice by Kaminaka et al. (1998) after immersion of seedlings roots for 24 h in 1 mm ABA. The authors argued that gene expression of the cytosolic GR was regulated under environmental stresses via an ABA-mediated signal transduction pathway. Recent evidence suggests that ABA is involved in the cellular signalling process as a secondary messenger that induces antioxidant defences under water stress (Jiang and Zhang, 2002). In fact, ABA treatment of leaves of maize seedlings increased the levels of  and H2O2, which lead to the activation of antioxidant enzymes such as superoxide dismutase, catalase, APX and GR (Jiang and Zhang, 2001). The antioxidant effect, however, depends on the concentration of ABA used: in cowpea detached leaves a 100 μm ABA solution was used which should be a low enough concentration to induce an antioxidative defence response against oxidative damage. A higher concentration of ABA (1 mm) induces an excessive generation of ROS leading to oxidative damage in plant cells (Jiang and Zhang, 2001). Nevertheless, as mentioned by Park et al. (2003), the upstream steps of the ABA-induced ROS generation pathway remain largely unknown.
and H2O2, which lead to the activation of antioxidant enzymes such as superoxide dismutase, catalase, APX and GR (Jiang and Zhang, 2001). The antioxidant effect, however, depends on the concentration of ABA used: in cowpea detached leaves a 100 μm ABA solution was used which should be a low enough concentration to induce an antioxidative defence response against oxidative damage. A higher concentration of ABA (1 mm) induces an excessive generation of ROS leading to oxidative damage in plant cells (Jiang and Zhang, 2001). Nevertheless, as mentioned by Park et al. (2003), the upstream steps of the ABA-induced ROS generation pathway remain largely unknown.
Dual-targeted GR seems to act on ROS scavenging in the susceptible cultivar, ‘1183’, in the case of progressive drought stress, fast desiccation or ABA treatment but only in the case of ABA treatment for the tolerant cultivar ‘EPACE-1’. This could suggest that in the tolerant cultivar there are other protective mechanisms operating which are more implicated in organelle ROS scavenging (Foyer et al., 1994; Iturbe-Ormaetxe et al., 1998).
Furthermore, as previously suggested (Pastori et al., 2000), it must be taken into consideration that the increase in GR activity and gene expression is not uniquely required for the scavenging of ROS, but is also implicated in the maintenance of high concentrations of GSH for the adjustments on the cellular redox reactions.
Supplementary Material
LITERATURE CITED
- Allen RD. (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiology 1071049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Arcy-Lameta A, Ferrari-Iliou R, Contour-Ansel D, Pham-Thi AT, Zuily-Fodil Y. (2006) Isolation and characterization of four ascorbate peroxidase cDNA responsive to water deficit in cowpea leaves. Annals of Botany 97133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. (1999) The water-water cycle in chloroplast: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50601–639. [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Analytical Biochemistry 72248–254. [DOI] [PubMed] [Google Scholar]
- Chaudière J and Ferrari-Iliou R. (1999) Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chemistry Toxicology 37949–962. [DOI] [PubMed] [Google Scholar]
- Chew O, Whelan J, Miller AH. (2003) Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defences in plants. Journal of Biological Chemistry 27846869–46877. [DOI] [PubMed] [Google Scholar]
- Creissen G, Edwards EA, Enard C, Wellburn A, Mullineaux P. (1992) Molecular characterization of glutathione reductase cDNAs from pea (Pisum sativum L.). The Plant Journal 2129–131. [PubMed] [Google Scholar]
- Creissen G, Reynolds H, Xue Y, Mullineaux P. (1995) Simultaneous targeting of pea glutathione reductase and of a bacterial fusion protein to chloroplasts and mitochondria in transgenic tobacco. The Plant Journal 8167–175. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho MH, d'Arcy-Lameta A, Roy-Macauley H, Gareil M, El-Maarouf H, Pham-Thi AT, Zuily-Fodil Y. (2001) Aspartic protease in leaves of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata L. Walp): enzymatic activity, gene expression and relation to drought susceptibility. FEBS Letters 492242–246. [DOI] [PubMed] [Google Scholar]
- Dietz KJ. (2003) Plant peroxiredoxins. Annual Review of Plant Biology 5493–107. [DOI] [PubMed] [Google Scholar]
- Diop NN, Kidric M, Repellin A, Gareil M, d'Arcy-Lameta A, Pham-Thi AT, Zuily-Fodil Y. (2004) A multicystatin is induced by drought-stress in cowpea (Vigna unguiculata (L.) Walp) leaves. FEBS letters 577545–550. [DOI] [PubMed] [Google Scholar]
- El-Maarouf H, Zuily-Fodil Y, Gareil M, d'Arcy-Lameta A, Pham-Thi AT. (1999) Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp differing in drought tolerance. Plant Molecular Biology 391257–1265. [DOI] [PubMed] [Google Scholar]
- El-Maarouf H, d'Arcy-Lameta A, Gareil M, Zuily-Fodil Y, Pham-Thi AT. (2001) Cloning and expression under drought of cDNAs coding for two PI-PCLs in cowpea leaves. Plant Physiology Biochemistry 39167–172. [Google Scholar]
- Ferrari-Iliou R, d'Arcy-Lameta A, Pham Thi AT, Zuily-Fodil Y, Mazliak P. (1994) Effect of drought on photodynamic peroxidation of leaf total lipophilic extracts. Phytochemistry 371237–1243. [Google Scholar]
- Foyer CH and Harbinson J. (1994) Oxygen metabolism and the regulation of photosynthetic electron transport. In Foyer CH and Mullineaux PM (Eds.). Causes of photo-oxidative stress and amelioration of defense systems in plants(CRC Press, Boca Raton, FL) pp. 1–42.
- Foyer CH and Noctor G. (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum 119355–364. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. (1994) Photoxidative stress in plants. Physiologia Plantarum 92696–717. [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L. (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiology 1091047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti AW. (2001) Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects and cytoprotective mechanisms. Journal of Photochemistry and Photobiology 63103–113. [DOI] [PubMed] [Google Scholar]
- Guan LM, Zhao J, Scandalios JG. (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signalling molecule for the response. The Plant Journal 2287–95. [DOI] [PubMed] [Google Scholar]
- Halliwell B and Gutteridge JMC. (1999) Free radicals in biology and medicine 3rd edn (Oxford University Press, New York, NY).
- Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M. (1998) Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiology 116173–181. [Google Scholar]
- Jiang M and Zhang J. (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiology 421265–1273. [DOI] [PubMed] [Google Scholar]
- Jiang M and Zhang J. (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimental Botany 532401–2410. [DOI] [PubMed] [Google Scholar]
- Jiménez A, Hernandez JA, Ros Barcelo A, Sandalio LM, del Rio LA, Sevilla F. (1998) Mitochondrial and peroxisomal ascorbate peroxidase of pea leaves. Physiologia Plantarum 104687–692. [Google Scholar]
- Kaminaka H, Morita S, Nakajima M, Masumura T, Tanaka K. (1998) Gene cloning and expression of cytosolic glutathione reductase in rice (Oriza sativa L.). Plant Cell Physiology 391269–1280. [DOI] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM. (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. The Plant Cell 9627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano HR, Melchiorre MN, Luna CM, Trippi VS. (2003) Effect of photoxidative stress induced by paraquat in two wheat cultivars with differential tolerance to water stress. Plant Science 164841–848. [Google Scholar]
- Lee H, Jo J, Son D. (1998) Molecular cloning and characterization of the gene encoding glutathione reductase in Brassica campestris. Biochimica Biophysica Acta 1395309–314. [DOI] [PubMed] [Google Scholar]
- Matos AR, d'Arcy-Lameta A, França M, Pêtres S, Edelman L, Kader JC, et al. (2001) A novel patatin-like gene stimulated by drought stress encodes a galactolipid acyl hydrolase. FEBS Letters 491188–192. [DOI] [PubMed] [Google Scholar]
- Monk LS, Fagerstedt KV, Crawford RMM. (1989) Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiologia Plantarum 76456–459. [Google Scholar]
- Monteiro de Paula F, Pham-Thi AT, Zuily-fodil Y, Ferrari-Iliou R, Vieira da Silva J, Mazliak P. (1993) Effects of water stress on the biosynthesis and degradation of polyunsaturated lipid molecular species in leaves of Vigna unguiculata. Plant Physiology Biochemistry 31707–715. [Google Scholar]
- Mullineaux PM, Enard C, Hellens R, Creissen G. (1996) Characterization of a glutathione reductase gene and its genetic locus from pea (Pisum sativum L.). Planta 200186–194. [DOI] [PubMed] [Google Scholar]
- Orvar BL and Ellis BE. (1997) Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. The Plant Journal 111297–1305. [Google Scholar]
- Park KY, Jung JY, Park J, Hwang JU, Kim YW, Hwang I, Lee Y. (2003) A role for phosphatidylinositol 3-phosphate in abscisic acid-induced reactive oxygen species generation in guard cells. Plant Physiology 13292–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori G, Foyer CH, Mullineaux P. (2000) Low temperature-induced changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. Journal Experimental Botany 51107–113. [PubMed] [Google Scholar]
- Pham-Thi AT and Vieira da Silva J. (1975) Action d'un traitement osmotique sur l'ultrastructure de feuilles de cotonniers (Gossypium hirsutum L. et G. anomalum Waw et Peyr.). Comptes rendus de l'Académie des Sciences , Paris 2802857–2860. [Google Scholar]
- Roy-Macauley H, Zuily-Fodil Y, Kidric M, Pham-Thi AT, Vieira da Silva J. (1992) Effect of drought stress on proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. Physiologia Plantarum 8590–96. [Google Scholar]
- Rudhe C, Chew O, Whelan J, Glaser E. (2002) A novel in vitro system for simultaneous import of precursor proteins into mitochondria and chloroplasts. The Plant Journal 30213–220. [DOI] [PubMed] [Google Scholar]
- Sato S, Nakamura Y, Kaneko T, Katoh T, Asamizu E, Tabata S. (2000) Structural analysis of Arabidopsis thaliana chromosome 3. I. Sequence features of the regions of 4,504,864 bp covered by sixty P1 and TAC clones. DNA Research 7131–135. [DOI] [PubMed] [Google Scholar]
- Scholander F, Hammel H, Hemmingsten E, Bradstreet E. (1964) Hydrostatic pressure and osmotic potential in leaves of mangrove and some other plants. Proceedings of the National Academy of Sciences of the USA 52119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG, Creissen GP, Mullineaux PM. (1997) Cloning and characterization of a cytosolic glutathione reductase cDNA from pea (Pisum sativum L.) and its expression in response to stress. Plant Molecular Biology 35641–654. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Creissen GP, Mullineaux PM. (2000) Characterization of pea cytosolic glutathione reductase expressed in transgenic tobacco. Planta 211537–545. [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology 1341536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X and Webb MA. (1994) Soybean root nodule cDNA encoding glutathione reductase. Plant Physiology 1041081–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighing, positions-specific gap penalties and weight matrix choice. Nucleic Acids Research 224673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE and Zhu JK. (2005) Before and beyond ABA: upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochemical Society Transactions 33375–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.