Abstract
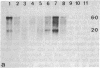
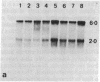
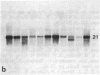
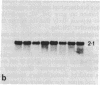
Screening of cDNA libraries prepared from the frontal cortex of an Alzheimer disease patient and from fetal human brain has led to isolation of the cDNA for a core protein of the paired helical filament of Alzheimer disease. The partial amino acid sequence of this core protein was used to design synthetic oligonucleotide probes. The cDNA encodes a protein of 352 amino acids that contains a characteristic amino acid repeat in its carboxyl-terminal half. This protein is highly homologous to the sequence of the mouse microtubule-associated protein tau and thus constitutes the human equivalent of mouse tau. RNA blot analysis indicates the presence of two major transcripts, 6 and 2 kilobases lon g, with a wide distribution in normal human brain. Tau protein mRNAs were found in normal amounts in the frontal cortex from patients with Alzheimer disease. The proof that at least part of tau protein forms a component of the paired helical filament core opens the way to understanding the mode of formation of paired helical filaments and thus, ultimately, the pathogenesis of Alzheimer disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudier J., Cole R. D. Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987 Dec 25;262(36):17577–17583. [PubMed] [Google Scholar]
- Baudier J., Lee S. H., Cole R. D. Separation of the different microtubule-associated tau protein species from bovine brain and their mode II phosphorylation by Ca2+/phospholipid-dependent protein kinase C. J Biol Chem. 1987 Dec 25;262(36):17584–17590. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion J. P., Cheetham M. E., Robinson P. A., Couck A. M., Anderton B. H. Isolation of cDNAs coding for epitopes shared by microtubule-associated proteins and neurofibrillary tangle in Alzheimer's disease. FEBS Lett. 1987 Dec 21;226(1):28–32. doi: 10.1016/0014-5793(87)80544-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977 Oct 25;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Caput D., Kirschner M. W. Studies on the expression of the microtubule-associated protein, tau, during mouse brain development, with newly isolated complementary DNA probes. J Cell Biol. 1984 Mar;98(3):1090–1097. doi: 10.1083/jcb.98.3.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Feinstein S. C., Shooter E. M., Kirschner M. W. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985 Nov;101(5 Pt 1):1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Kirschner M. W. Tau protein function in living cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Scherson T., Giveon D., Behar L., Littauer U. Z. Modulation of mRNA for microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4892–4896. doi: 10.1073/pnas.79.16.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goedert M. Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer's disease. EMBO J. 1987 Dec 1;6(12):3627–3632. doi: 10.1002/j.1460-2075.1987.tb02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Miyata Y., Sakai H., Miyoshi T., Ogawara H., Akiyama T. Protein kinase C phosphorylates tau and induces its functional alterations. FEBS Lett. 1987 Jun 15;217(2):237–241. doi: 10.1016/0014-5793(87)80670-1. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Duffy L. K., Dowling M. M., Abraham C., McCluskey A., Selkoe D. J. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. C., Brion J. P., Calvert R., Chin T. K., Eagles P. A., Downes M. J., Flament-Durand J., Haugh M., Kahn J., Probst A. Alzheimer's paired helical filaments share epitopes with neurofilament side arms. EMBO J. 1986 Feb;5(2):269–276. doi: 10.1002/j.1460-2075.1986.tb04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve R. L., Harris P., Kosik K. S., Kurnit D. M., Donlon T. A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986 Dec;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Nukina N., Kosik K. S., Selkoe D. J. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci U S A. 1987 May;84(10):3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. L. New perspectives on Alzheimer's disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Gaskin F., Fu S. M. Neurofibrillary tangles in senile dementia of the Alzheimer type share an antigenic determinant with intermediate filaments of the vimentin class. Am J Pathol. 1983 Dec;113(3):373–381. [PMC free article] [PubMed] [Google Scholar]