Abstract
• Background and Aims Several families of tropical plants have thermogenic flowers that show a 2-d protogynous sequence. Most are pollinated by large beetles that remain for the entire period in the flowers, where they compete for mates and feed. Active beetles require high body temperatures that they can achieve endogenously at great energy expense or attain passively and cheaply in a warm environment. Floral heating is therefore hypothesized to be a direct energy reward to endothermic beetles, in addition to its accepted role in enhancing scent production.
• Methods This study measures the pattern of floral heat production (as temperature in 20 flowers and respiration rates in five flowers) in Victoria amazonica at field sites in Guyana and correlates floral temperatures with body temperatures necessary for activity in visiting Cyclocephala hardyi beetles.
• Key Results Thermogenesis occurred in a bimodal pattern, with peaks associated with the arrival and departure of beetles near sunset. Peak CO2 production rates averaged 2·9 µmol s−1, equivalent to a heat production of 1·4 W. Heat was generated mainly in the floral chamber on the first evening and by the stamen complex on the second. Mean chamber temperature remained between 29·3 and 34·7 °C during the first night, when ambient temperature was 23·5–25·2 °C. Beetles actively competed for mates and consumed stylar processes in the floral chamber, where their mean thoracic temperature was 33·2 °C. At the lower ambient temperatures outside of the flower, beetles capable of sustained flight had a similar mean temperature of 32·0 °C.
• Conclusions Floral heating is not only associated with attraction, but continues throughout the night when beetles are active inside the flower and increases again when they leave. Floral chamber temperatures similar to activity temperatures of actively endothermic beetles imply that thermogenesis is an energy reward.
Keywords: Pollination biology, thermogenesis, respiration, beetle, endothermy, Nymphaeaceae, Guyana, Victoria amazonica, Cyclocephala hardyi
INTRODUCTION
Thermogenesis occurs in several diverse families of primitive seed plants, including arum lilies (Araceae), lotus (Nelumbonaceae) and water lilies (Nymphaeaceae), among others (Thien et al., 2000). These plants are all insect-pollinated and typically have protogynous flowers or inflorescences in which the female parts are receptive to pollination first and the male parts shed pollen sometime later. Insects attracted to flowers in the female phase often remain in them for about 1 d until the male phase. Intense thermogenesis is characteristic of inflorescences that are pollinated chiefly by beetles (Bernhardt, 2000), and this association, originating in the Cretaceous (Gottsberger, 1988), is not uncommon today. In the neotropics, it is estimated that 900 species of thermogenic plants are pollinated by the scarab beetle genus Cyclocephala alone (Schatz, 1990). The role of thermogenesis is almost always considered to augment scent production but, in many cases, it persists well past the period of attraction and throughout the period of insect residence. Some flowers even physiologically regulate their temperature to achieve a warm, stable internal environment in the face of changing environmental temperatures (Seymour and Schultze-Motel, 1997; Seymour, 2001). Floral temperatures only a few degrees above ambient have recently been demonstrated to be a direct energy reward to Cyclocephala beetle visitors to an arum lily Philodendron solimoesence (Seymour et al., 2003b).
Pollination of the Amazon water lily Victoria amazonica has been known to be associated with Cyclocephala beetles since Robert Schomburgk's description in 1837 (Prance and Arias, 1975). In a detailed, year-long study of V. amazonica in Brazil, Prance and Arias (1975) recorded the pattern of anthesis, including floral temperatures, the role of beetles as pollen vectors and many other aspects of floral biology. However, there are no observations of beetle activities within the flowers, and the thermal requirements of the beetles for activity remain unknown. Because the ‘heat reward’ hypothesis has been tested only once in the Araceae, it is desirable to test it in Victoria, a member of the Nymphaeaceae. This study therefore evaluates the temperatures maintained within the flowers in relation to the preferred activity temperatures of the beetle visitors.
The source and intensity of floral heating are also unknown in V. amazonica. Knoch (1899) determined that the warmest parts of the flower were the stylar processes and inner staminodes, but Lamprecht et al. (2002a) concluded that the stamens of Victoria cruziana were the major heat source. The present study resolves this ambiguity and provides the first field measurements of floral respiration throughout anthesis. These measurements are important, because common measurements of temperature elevation do not necessarily represent the true rate of heat production, as temperature is influenced by evaporative, convective and radiant heat loss, which are complex (Seymour, 2001).
MATERIALS AND METHODS
Flowers
Victoria amazonica was studied during 5–17 October 2005, in ponds adjacent to the Rupununi River, near Karanambu Ranch, Guyana.
Temperatures in the floral chamber and ambient air were logged throughout the 2-d sequence of 15 flowers. Loggers (1·7 cm iButtons; Dallas Semiconductor) were taped to a string in pairs. One logger was placed in the floral chamber through a cut at the top of unopened buds and the other was held above the flower on the string tied to a pole pushed into the mud.
Open flow respirometry was carried out on five flowers in the field. Each was isolated in a floating respirometry hood fashioned from a floating expanded polystyrene base (30 × 30 cm and 6 cm thick) and a 2·3-L clear polyethylene hood that permitted light to reach the flower, but shaded it from direct sunlight. Thermocouples were placed inside and outside of the hood and into the floral chamber. Air was pumped through the hood at about 360 mL min−1 into a 100-mL bottle with wet filter paper to humidify the gas, into a mass flowmeter (Toptrak; www.sierrainstruments.com), calibrated with a bubble flowmeter (Gilibrator; www.sensidyne.com), and finally into a CO2 analyser (280; David Bishop, Warwickshire, UK), calibrated with a precision gas mixer. Gas from the hood was compared with ambient air in an identical separate channel. Data were logged (Squirrel; www.grantdataloggers.com) at 2-min intervals for 46 h, to include the first and second nights of thermogenesis. Rate of CO2 production (MCO2) was calculated every 30 min according to Seymour and Blaylock (1999). After the measurements were finished, the flowers were cut, dissected into the major parts and weighed.
Respirometry on parts of other flowers employed the same equipment but with 100–200 mL flow-through chambers. First- and second-day flowers were cut in the evening and taken to camp with the cut pedicel in water. The flowers were quickly cut into sections as described in the results. Each section, representing one-quarter or one-half of the flower was placed in the chamber. Equilibrium was complete by 5 min, and the sample was replaced with another. The parts were then weighed.
Beetles
Cyclocephala hardyi were observed flying from second-day to first-day flowers in the evening. They disappeared into the flowers immediately on landing, but were observed within the floral chamber with an optical borescope (www.olympusindustrial.com).
Thoracic temperatures of two groups of beetles were measured between 2130 and 2300 h, during their most active period. One group of beetles was removed from the flower by cutting a window in the floral chamber and shaking them onto a wooden platform in the boat. The other group was taken to camp and encouraged to fly within a large mosquito net over a hammock. They were placed on the hammock and allowed to crawl up to a raised fold, which often stimulated flight. If flight duration was sufficient to show that they could gain height, they were measured immediately upon landing. Temperatures were taken within 10 s with a thermocouple inside a 25-ga hypodermic needle and a thermometer (model 52; www.fluke.com).
RESULTS
Flowers
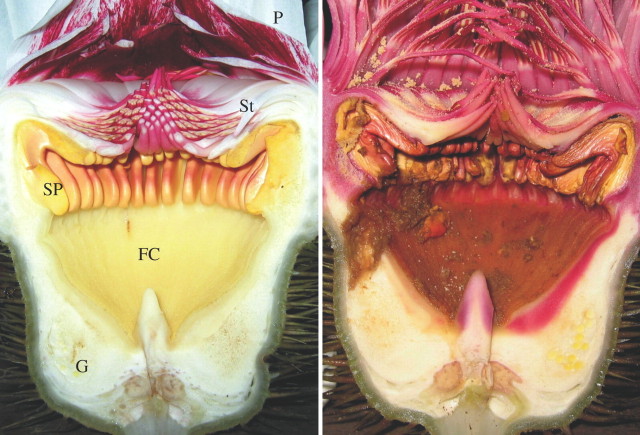
Victoria amazonica flowers progress through a 2-d sequence. This description uses anatomical definitions according to Schneider (1980). The sepals began to loosen during the first day and began to open quickly after 1800 h. At this time, the stamens, including the outer staminodia (infertile stamens), middle fertile stamens and inner staminodia (termed paracarpels by earlier authors) were erect and formed an approx. 12-mm tunnel into the floral chamber (Fig. 1). The petals remained expanded, erect and white all night and the next morning. Inside the floral chamber, stylar processes (termed carpellary appendages or food bodies by earlier authors) encircled the upper rim. By the evening of the second day, the petals had turned variegated white–pink and relaxed to water level, revealing the outer pink staminodia. At this time, the stamens and inner staminodia closed the floral chamber, but pointed upward at the centre, and the anthers on the under sides of the fertile stamens were covered with pollen (Fig. 1). Beetles had consumed parts of the stylar processes and some had burrowed into the floor of the chamber. In general, this sequence was as described by Prance and Arias (1975) except for minor differences in petal movements and colouring.
Fig. 1.
Sagittal sections through Victoria amazonica flowers on the first evening (left; female phase) and the second evening (right; male phase). Petals (P), stamens and staminodes (St), stylar processes (SP), floral chamber (FC) and gynoecium (G) are indicated. The spot at the back of the floral chamber in the left photograph is the point of thermocouple entry. Note the pollen released from the stamens in the male phase and the beetle damage, including consumption of stylar processes and a burrow into the gynoecial tissue.
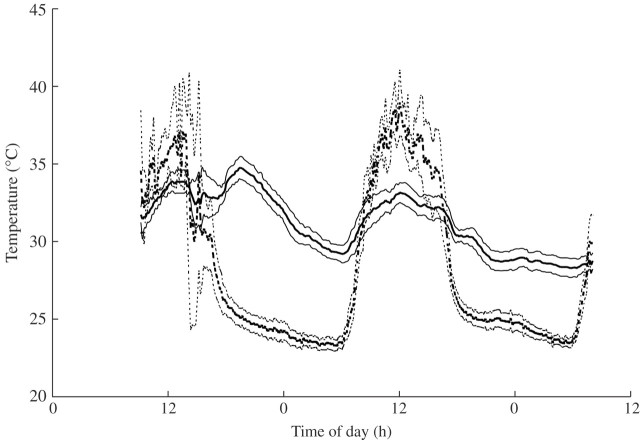
Mean floral chamber temperature peaked at 34·7 °C at 1940 h on the first day and gradually fell during the night to a mean low of 29·3 °C at 0620 h (Fig. 2). Meanwhile mean ambient air temperatures decreased from 25·2 to 23·5 °C. Thus the temperature difference decreased from 9·5 to 5·8 °C throughout the night. The grand mean floral chamber temperature was 31·7 °C between sunset at 1844 h and sunrise at 0641 h, while the mean air temperature was 24·1 °C. On the second night, mean floral temperature was lower at the same times of day, decreasing from 30·2 °C at 1940 h to 28·3 °C at 0620 h. Because mean air temperature decreased from 25·1 to 23·9 °C during this period, the temperature difference decreased slightly, from 5·1 to 4·4 °C. During the day, temperatures in unhooded flowers rose in response to rising ambient temperature, but remained considerably below it during the hottest part of the day, due to evaporative cooling. In contrast, high humidity caused floral chamber temperatures in hooded flowers to be similar to inside hood temperatures.
Fig. 2.
Temperatures in the floral chamber (continuous lines) and ambient air (dashed lines) of 15 Victoria amazonica flowers throughout the 2-d sequence of flowering. Data are means and 95 % confidence intervals. Air temperatures are influenced by solar radiation and precipitation.
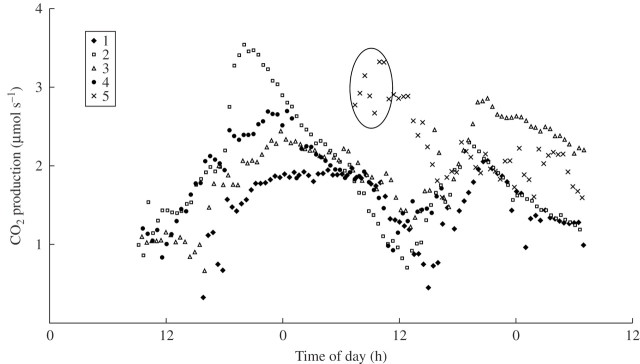
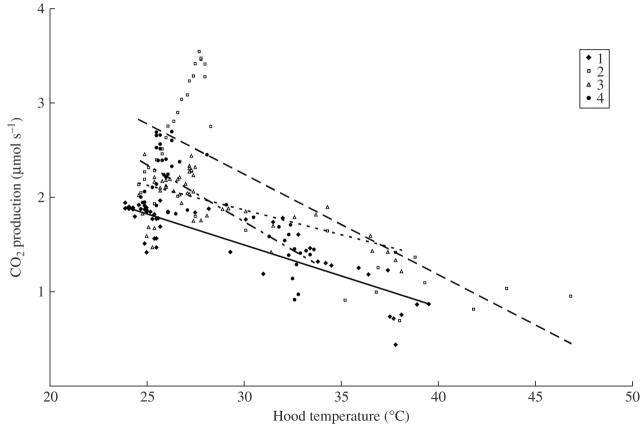
Respiration (MCO2) showed a bimodal pattern of higher rates during the nights than during the days (Fig. 3). In general, rates were somewhat higher on the first night compared with the second, but the maximum rates could occur on either night. The timing of the peak was also variable between flowers, occurring between 2000 h and 0020 h. The lowest rates occurred between 1100 h and 1450 h, during the hottest part of the second day (Fig. 2). MCO2 was negatively correlated with hood temperature during the 24-h period of beetle residence (Fig. 4).
Fig. 3.
Rates of respiration of five Victoria amazonica flowers in the field. Data within the oval were taken during a 4-h period of artificial cooling of ambient temperature around flower 5 (see text).
Fig. 4.
Relationship between respiration rate and ambient (inside hood) temperature throughout the 24-h period of beetle residence (from 1800 h on the first day). Linear regressions are significant (R2 = 0·41–0·75; P < 0·05). Symbols as in Fig. 3.
An attempt was made to influence the pattern of warming by artificially stabilizing the air temperature at about 23 °C around flower 5. Ice water was dribbled as necessary on a cloth wrapped around the hood between 0615 h and 1030 h on the morning of the second day. The results were that respiration remained high during the 4 h of treatment and the subsequent decline in rate was delayed in comparison to the other flowers (Fig. 3). Unfortunately, the respiration rate before 0615 h is not known, because of technical problems.
The flowers used for respirometry were dissected into five parts: (1) the ovary consisting of the walls of the floral chamber, without peduncle; (2) the stylar processes consisting of yellow, spongy, C-shaped tissue excised from the inside of the floral chamber; (3) the stamens including all of the staminodia and stamens and the tissue at their base; (4) the petals consisting of all free petals; and (5) the sepals consisting of four large and four small spiny sepals. These parts made up the entire flower. The masses of these tissues in the first-day flower are summarized in Table 1.
Table 1.
Mass of seven first-day flowers and rates of CO2 production (MCO2) from parts of two first-day and two second-day flowers
| Ovary | Stylar processes | Stamens | Petals | Sepals | Total | ||
|---|---|---|---|---|---|---|---|
| Wet mass (g) | Mean | 90·0 | 11·9 | 59·1 | 78·2 | 55·6 | 282·1 |
| 95 % CI | 22·8 | 3·8 | 10·0 | 9·4 | 14·2 | 19·1 | |
| % | 28 | 4 | 19 | 29 | 20 | 100 | |
| MCO2 (μmol s−1) | |||||||
| First-day | Mean | 0·23 | 2·53 | 0·80 | 0·23 | – | 3·79 |
| 95 % CI | 0·09 | 0·29 | 0·28 | 0·02 | – | 0·69 | |
| % | 6 | 67 | 21 | 6 | 100 | ||
| Second-day | Mean | 0·16 | 0·07 | 1·23 | – | – | 1·46 |
| 95 % CI | – | 0·04 | 0·45 | – | – | 0·33 | |
| % | 11 | 5 | 84 | – | – | 100 | |
| MCO2 (pmol s−1 g−1) | |||||||
| First-day | Mean | 2·0 | 162·2 | 11·8 | 3·4 | ||
| 95 % CI | 0·3 | 37·0 | 4·6 | 1·1 | |||
| Second-day | Mean | – | 6·7 | 18·7 | – | ||
| 95 % CI | – | 1·0 | 2·7 | – | |||
Data refer to whole flowers or mass-specific tissues.
Total values for respiration data do not include negligible contributions from sepals or second-day petals.
Most floral respiration was due to the stylar processes on the first evening, but their contribution declined to very low values on the second evening (Table 1). This decrease was due to a decrease in mass-specific respiration, not to a significant loss of tissue by feeding beetles. Meanwhile respiration by the stamens increased about 50 % from the first to the second evenings. Total respiration of all floral parts (Table 1) was consistent with total values in intact field flowers (Fig. 3), indicating that measuring quickly after cutting did not reduce metabolic intensity. The mean mass of five field respirometry flowers was 282 g (± 23 CI) and mean maximum MCO2 was 2·9 μmol s−1 (± 0·5 CI). This gives a mean mass-specific respiration rate for the whole flower of 10·3 pmol s−1 g−1.
Beetles
The beetles were of two species. The larger species was identified as Cyclocephala hardyi Endrödi by examining the male paramere (Prance and Arias, 1975). The body mass of 17 beetles was 0·84 g (± 0·04 CI), length 22·6 mm (± 0·7) and width 10·9 mm (± 0·4). The smaller species was probably C. verticalis Burmeister because it was within the size range and time of year reported by Prance and Arias (1975). The body mass of six beetles was 0·25 g (± 0·02 CI), length 15·1 mm (± 0·6) and width 7·7 mm (± 0·7). Cyclocephala hardyi was much more common in the flowers, so all measurements refer to it.
Beetles flew directly from second-day to first-day flowers when they opened between about 1800 h and 1830 h. Beetles were observed eating the stylar processes. Males were seen mounting females in the floral chamber, his clasping claws on the forelegs hooked on her elytra. On one occasion, two males were seen competing for access to a female within the floral chamber. One was successful in attaching to the elytra with his forelegs, and he used his back legs to kick away the other male, which attempted to dislodge him from alternate sides.
Mean thoracic temperature was 33·2 °C (± 0·05 CI) in 22 C. hardyi removed from the floral chambers of three first-day flowers between 2100 and 2230 h. There was no significant difference between 15 males and seven females (t-test; P = 0·97). Ambient temperature was 24·9 °C during this period. Thoracic temperatures of 12 captive beetles averaged 32·0 °C (± 0·08 CI) after short, but sustainable flight under a mosquito net. This was slightly, but significantly, lower than the temperatures of beetles taken immediately from the floral chamber (t-test; P = 0·02). Ambient temperature was 25·8–27·2 °C during the period of testing, and temperatures of non-endothermic beetles would have been similar (Heinrich, 1993).
DISCUSSION
Flowers
Thermogenesis in V. amazonica is dynamic. It shifts from the stylar processes that produce 67 % of the heat on the first evening to the stamens that produce 84 % of the heat on the second evening (Table 1). The shift is due mainly to a large decrease in heat produced by stylar process and to a much smaller increase in heat produced by the stamens. These observations clarify the ambiguity in earlier studies (Knoch, 1899; Lamprecht et al., 2002a).
On a mass-specific basis, the stylar processes of V. amazonica are the most metabolically active. Assuming 473 J mmol−1 (Wieser, 1986) and that carbohydrate is the energy source (Knoch, 1899), they produce 77 mW g−1 on the first evening. Next powerful are the stamens at 9 mW g−1 on the second evening. The mean mass-specific respiration rate of 162 pmol s−1 g−1 is lower than those from several species of arum lilies that range between 360 and 890 pmol s−1 g−1 (Seymour et al., 2003a). Considering respiration of the entire flower, however, gives an indication of a substantial total heat production. The mean maximum respiration rate was 2·9 μmol s−1, which corresponds to a heat production of 1·4 W to attain a temperature about 10 °C higher than the air. This compares with about 1 W for the sacred lotus Nelumbo nucifera to maintain a 20 °C elevation (Seymour and Schultze-Motel, 1998), and 0·6 W in skunk cabbage Symplocarpus foetidus maintaining a 33 °C elevation (Seymour, 2004). This demonstrates the futility of assessing thermogenesis by measuring only temperature elevation.
Some thermogenic flowers are thermoregulatory, i.e. they increase heat production when the ambient temperature decreases, which tends to stabilize floral temperature. Differing degrees of this ability have been demonstrated in four species of Araceae (Nagy et al., 1972; Knutson, 1974; Seymour and Schultze-Motel, 1999) and Nelumbonaceae (Seymour and Schultze-Motel, 1996), but it has never been demonstrated in any Nymphaeaceae, although thermogenesis is not uncommon (Ervik and Knudsen, 2003). Data from V. amazonica at first lead to the conclusion that heat production is not regulated in relation to ambient temperature. Floral chamber temperature (Fig. 2) and respiration rate (Fig. 3) both fall along with ambient temperature during the last half of the first night. Clearly, heating is related to light cycle and has a bimodal pattern, with major bouts of heating on the first and second evenings, a pattern similar to that in species of non-thermoregulating, protogynous Philodendron subg. Philodendron (Gibernau and Barabé, 2000).
Nevertheless, there is preliminary evidence that V. amazonica has some thermoregulatory capacity. There was an overall decrease in respiration at higher ambient temperatures in all flowers (Fig. 4); however, this pattern may not be causal, but simply correlate with nocturnal heating. Similar nocturnal heating occurs in Nelumbo nucifera, but it has been shown experimentally that heating is related to ambient temperature, not time of day or light intensity (Seymour and Schultze-Motel, 1998). When the hood over a flower was artificially cooled 5 or 4 h after sunrise, its respiration remained high and the floral chamber temperature increased from 27·6 to 30·7 °C when all other flowers were steeply decreasing (Fig. 3). It is unfortunate that this experiment could not be repeated, and that respiration before the cooling period was unknown. Therefore a conclusion that Victoria is thermoregulatory must be tentative until further testing in controlled temperature enclosures becomes possible.
Beetles
Plants have evolved many elaborate mechanisms to encourage visits by animals that carry pollen efficiently between individuals. These mechanisms usually involve two aspects—attraction and reward. The cues for attraction are appearance and scent, and the rewards are usually offered as food energy in the form of nectar, pollen or other flower parts. While heat production is almost always considered to enhance the scent, its role as a direct energy reward has received little attention. It is clear that heating in V. amazonica is involved with both attraction and reward. Attraction is implied by the coincidence of major heating, a powerful, fruity scent and arrival of beetles on the first evening (Prance and Arias, 1975; Kite et al., 1991; Lamprecht et al., 2002b). Heat reward is implied by the coincidence of temperatures of the floral chamber and the bodies of active beetles. The case of V. amazonica and C. hardyi appears similar to that of the epiphytic arum lily Philodendron solimoescence and its pollinator C. colasi in French Guiana (Seymour et al., 2003b). In both, beetles are attracted to the inflorescence in the evening and remain in the floral chamber for approx. 24 h. Although the floral chamber temperature is only a few degrees warmer than the outside air during the night, the metabolic rates of active beetles in the floral chamber are reduced to as low as a fifth of that required outside. Metabolic rates of active C. hardyi were not measured, but it was shown that the beetles attain body temperatures of about 31 °C (flight temperature) in the floral chamber without having to generate their own excess heat. In this case, the mean temperature elevation in the floral chamber of 7·6 °C throughout the night would probably have saved more energy than estimated for C. colasi. Because Cyclocephala beetles spend most of their adult lives inside warm flowers, these savings represent a substantial component of their energy budgets.
As in the case of Philodendron, in which beetles consume the sterile male florets that are the major heat-producing organs, C. hardyi beetles consume the starchy stylar processes that are the main source of heat during the first night. Sometimes almost all of the stylar processes are consumed if there are many beetles (up to 47) present (Prance and Arias, 1975). This can be viewed as an energy trade-off; reduced heating by the flower may be compensated to some extent by consumption of more food. In the present case, flowers with access to beetles cooled about −5·2 °C between 2000 h and 0500 h on the first night (Fig. 2), while the five hooded flowers either increased temperature (+ 1·1 °C) or decreased less (−1·0 to −3·4 °C).
The role of elevated temperatures during the second night may also be associated with beetle activity, but no definite statements can be made. The fact that the major source of heat shifts from the floral chamber to the stamens indicates that warming on the second night is not simply a repeat of the first night. Warming in the stamens might attract beetles away from the floral chamber and encourage more activity within their loose structure (Fig. 1), leading to greater adhesion of pollen. Heating would certainly prepare them for their departure flight by providing an appropriate flight temperature. However, the role of continued high rates of heat production for hours after the beetles leave is unknown. Heating might be associated with continued production of pollen, but without beetles to carry it away, the only purpose for this would be self-pollination, which is possible in V. amazonica (Prance and Arias, 1975).
This preliminary study suggests future lines of inquiry concerning the role of thermogenesis in Victoria. In particular, it would be interesting to test for relationships between numbers of beetle visitors and the pattern of heating. Consumption of stylar processes by large numbers of beetles might decrease the total rate of heating by the flower, by destroying thermogenic tissue. However, this loss might be compensated by increased heat production of the remaining tissue, if the flower tissue is truly thermoregulatory. Another possibility is that heat production by numerous beetles could offset decreased floral heat production, such that floral temperature is maintained. The present experiments do not approach this question because no beetles were present in the respirometer. Careful measurements of flowers with different numbers of introduced beetles would be necessary. It would also be interesting to investigate the role of floral heating and beetle visitation on the seed set of Victoria by untangling the effects of high temperature and beetle numbers. Greater fertilization might be expected if warm beetles moved around more inside the flower, or if more beetles were present. Such questions could be approached by independently manipulating beetle number and floral temperature with a temperature-controlled enclosure. Finally, more experiments involving artificially manipulated ambient temperature are necessary to determine the extent of temperature regulation in Victoria. Thermoregulatory flowers increase respiration rate with decreasing tissue temperature.
Acknowledgments
This research was supported by the Australian Research Council and the Adelaide Botanic Garden. We thank Stephen Forbes and David Forwood (ABG) for organizing of the expedition. Prime Minister Samuel Hinds, Inge Nathoo and John Caesar facilitated our work in Guyana and the Environmental Protection Agency helpfully issued our permit (280905 BR 039). At Karanambu, we appreciate the assistance of Kaslyn Holder, Ashley Holland, Jose George, Peter Copley and Gilbert Dashorst in the field, and Diane, Melanie and Edward McTurk for warm hospitality.
LITERATURE CITED
- Bernhardt P. (2000) Convergent evolution and adaptive radiation of beetle-pollinated angiosperms. Plant Systematics and Evolution 222293–320. [Google Scholar]
- Ervik F and Knudsen JT. (2003) Water lilies and scarabs: faithful partners for 100 million years? Biological Journal of the Linnean Society 80539–543. [Google Scholar]
- Gibernau M and Barabé D. (2000) Thermogenesis in three Philodendron species (Araceae) of French Guiana. Canadian Journal of Botany 78685–689. [Google Scholar]
- Gottsberger G. (1988) The reproductive biology of primitive angiosperms. Taxon 37630–643. [Google Scholar]
- Heinrich B. (1993) The hot-blooded insects.(Harvard University Press, Cambridge, MA).
- Kite G, Reynolds T, Prance GT. (1991) Potential pollinator-attracting chemicals from Victoria (Nymphaeaceae). Biochemical Systematics and Ecology 19535–539. [Google Scholar]
- Knoch E. (1899) Untersuchungen über die Morphologie, Biologie und Physiologie der Blüte von Victoria regia. Bibliotheca Botanica 91–60. [Google Scholar]
- Knutson RM. (1974) Heat production and temperature regulation in eastern skunk cabbage. Science 186746–747. [DOI] [PubMed] [Google Scholar]
- Lamprecht I, Schmolz E, Blanco L, Romero CM. (2002a) Energy metabolism of the thermogenic tropical water lily, Victoria cruziana. Thermochimica Acta 394191–204. [Google Scholar]
- Lamprecht I, Schmolz E, Blanco L, Romero CM. (2002b) Flower ovens: thermal investigations on heat producing plants. Thermochimica Acta 391107–118. [Google Scholar]
- Nagy KA, Odell DK, Seymour RS. (1972) Temperature regulation by the inflorescence of Philodendron. Science 1781195–1197. [DOI] [PubMed] [Google Scholar]
- Prance GT and Arias JR. (1975) A study of the floral biology of Victoria amazonica (Poepp.) Sowerby (Nymphaeaceae). Acta Amazonica 5109–139. [Google Scholar]
- Schatz GE. (1990) Some aspects of pollination biology in central American forests. In Bawa KS and Hadley M (Eds.). Reproductive ecology of tropical forest plants(UNESCO, Paris) Vol. 7 pp. 69–84. [Google Scholar]
- Schneider P. (1980) Contribution to flight physiology in beetles. 4. Body temperature, flight behaviour and wing beat frequency. Zoologischer Anzeiger 2051–19. [Google Scholar]
- Seymour RS. (2001) Biophysics and physiology of temperature regulation in thermogenic flowers. Bioscience Reports 21223–236. [DOI] [PubMed] [Google Scholar]
- Seymour RS. (2004) Dynamics and precision of thermoregulatory responses of eastern skunk cabbage Symplocarpus foetidus. Plant, Cell and Environment 271014–1022. [Google Scholar]
- Seymour RS and Blaylock AJ. (1999) Switching off the heater: influence of ambient temperature on thermoregulation by eastern skunk cabbage Symplocarpus foetidus. Journal of Experimental Botany 501525–1532. [Google Scholar]
- Seymour RS and Schultze-Motel P. (1996) Thermoregulating lotus flowers. Nature 383305. [Google Scholar]
- Seymour RS and Schultze-Motel P. (1997) Heat-producing flowers. Endeavour 21125–129. [Google Scholar]
- Seymour RS and Schultze-Motel P. (1998) Physiological temperature regulation by flowers of the sacred lotus. Philosophical Transactions of the Royal Society of London B 353935–943. [Google Scholar]
- Seymour RS and Schultze-Motel P. (1999) Respiration, temperature regulation and energetics of thermogenic inflorescences of the dragon lily Dracunculus vulgaris (Araceae). Proceedings of the Royal Society of London Series B Biological Sciences 2661975–1983. [Google Scholar]
- Seymour RS, Gibernau M, Ito K. (2003a) Thermogenesis and respiration of inflorescences of the dead horse arum Helicodiceros muscivorus, a pseudo-thermoregulatory aroid associated with fly pollination. Functional Ecology 17886–894. [Google Scholar]
- Seymour RS, White CR, Gibernau M. (2003b) Heat reward for insect pollinators. Nature 426243–244. [DOI] [PubMed] [Google Scholar]
- Thien LB, Azuma H, Kawano S. (2000) New perspectives in the pollination biology of basal angiosperms. International Journal of Plant Sciences 161S225–S235. [Google Scholar]
- Wieser W. (1986) Bioenergetik. Energietransformationen bei Organismen.(Georg Thieme, Stuttgart).