Abstract
• Background and Aims Determining the mode of action of allelochemicals is one of the challenging aspects in allelopathic studies. Recently, allelochemicals have been proposed to cause oxidative stress in target tissue and induce an antioxidant mechanism. α-Pinene, one of the common monoterpenoids emitted from several aromatic plants including forest trees, is known for its growth-inhibitory activity. However, its mechanism of action remains unexplored. The aim of the present study was to determine the inhibitory effect of α-pinene on root growth and generation of reactive oxygen species, as indicators of oxidative stress and changes in activities of antioxidant enzymes.
• Methods Effects of α-pinene on early root growth were studied in five test species, Cassia occidentalis, Amaranthus viridis, Triticum aestivum, Pisum sativum and Cicer arietinum. Electrolyte leakage, lipid peroxidation, hydrogen peroxide generation, proline accumulation, and activities of the enzymes superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), catalase (CAT) and glutathione reductase (GR) were studied in roots of C. occidentalis.
• Key Results α-Pinene inhibited the radicle growth of all the test species. Exposure of C. occidentalis roots to α-pinene enhanced solute leakage, and increased levels of malondialdehyde, proline and hydrogen peroxide, indicating lipid peroxidation and induction of oxidative stress. Activities of the antioxidant enzymes SOD, CAT, GPX, APX and GR were significantly elevated, thereby indicating the enhanced generation of reactive oxygen species (ROS) upon α-pinene exposure. Increased levels of scavenging enzymes indicates their induction as a secondary defence mechanism in response to α-pinene.
• Conclusions It is concluded that α-pinene inhibits early root growth and causes oxidative damage in root tissue through enhanced generation of ROS, as indicated by increased lipid peroxidation, disruption of membrane integrity and elevated antioxidant enzyme levels.
Keywords: α-Pinene, radicle growth, lipid peroxidation, hydrogen peroxide, proline content, electrolyte leakage, membrane integrity, antioxidant enzymes, oxidative damage, Cassia occidentalis
INTRODUCTION
Plants synthesize an array of chemical compounds that are involved in a variety of plant–plant, plant–microbe and plant–herbivore interactions. These exhibit a great structural and functional diversity and are produced within plants as a result of secondary metabolism (Hadacek, 2002). Although initially regarded as being functionless, these compounds are now known to play an important role in plant defence mechanisms (Berenbaum, 1995), to provide reproductive fitness (Facchini, 1999) and to impart allelopathic properties (Seigler, 1996); they thus serve as an excellent source of lead compounds for the development of new herbicides (Duke et al., 2000). Among the different classes of natural plant products, monoterpenes—the components of volatile essential oils from a number of plant species—are involved in a variety of ecological interactions and also serve as pollinator attractants and as protectants (Langenheim, 1994; Harrewijn et al., 2001). In addition, they are potent germination inhibitors and suppress early root growth (Muller and Muller, 1964; Abrahim et al., 2000; Romagni et al., 2000; Singh et al., 2002, 2006; Zunino and Zygadlo, 2004; Nishida et al., 2005). However, the exact mode of inhibitory action of monoterpenes remains unknown. Recently, Nishida et al. (2005) reported that five volatile monoterpenoids, namely eucalyptol, α- and β- pinene, camphene and camphor (which are present in the foliage, soil and airspace around Salvia leucophylla), when used in their purified form (0·1–10 mm) inhibited cell proliferation in roots of Brassica campestris by interfering with organelle and nuclear DNA synthesis within the meristem cells. Monoterpenes such as 1,8-cineole, thymol, geraniol and camphor (21, 2·0, 1·9 and 7·4 mg mL−1, respectively, in the headspace) have been reported to inhibit maize root growth and induce oxidative stress by production of malondialdehyde (Zunino and Zygadlo, 2004). Production of reactive oxygen species (ROS), and related oxidative stress in general, has been proposed as one of the major mechanisms of action of the phytotoxins (Weir et al., 2004).
α-Pinene is one of the major components of volatiles released by a wide range of species throughout the world, including those in the tropics (Keller and Lerdau, 1999; Geron et al., 2001, 2002), Mediterranean species (Llusià and Peñuelas, 2000) and northern coniferous forest trees (Geron et al., 2000). Plants emitting high levels include Eucalyptus sp., Pinus sp. and Quercus sp., although thousands of other species also emit monoterpenes, e.g. Salvia species. α-Pinene inhibits seed germination and primary root growth in maize (Abrahim et al., 2000) and disrupts energy metabolism by acting as an uncoupler of oxidative phosphorylation and inhibiting the electron transport chain (Abrahim et al., 2003). However, not much is known about its exact mode of action, although an increase in malondialdehyde levels in maize roots has been reported (Scrivanti et al., 2003). It is likely that exposure to α-pinene induces oxidative stress in the target tissue and results in the observed inhibitory action. However, details regarding the level and extent of oxidative stress and the induction of anti-oxidative enzyme mechanisms due to α-pinene exposure are lacking. A study was therefore performed to investigate the impact of α-pinene on (1) the germination and early radicle growth of five plant species; (b) the induction of oxidative stress, measured in terms of lipid peroxidation, membrane integrity, hydrogen peroxide generation and proline content; and (c) the levels of induction of antioxidant enzyme mechanisms (in terms of activities of superoxide dismutase, catalase, ascorbate peroxidase, guaiacol peroxidase and glutathione reductase) in the roots.
MATERIALS AND METHODS
Materials
(–)-α-Pinene of technical grade (purity 98 %) was used in the experiments (Lancaster Synthesis Ltd., Morecambe, UK). Seeds of chickpea (Cicer arietinum L. ‘GL-470’, wheat (Triticum aestivum L. ‘HD-2329’), and pea (Pisum sativum L. ‘AP-1’) were purchased from Punjab Agricultural University, Ludhiana, India, whilst seeds of coffee weed (Cassia occidentalis L.) and green amaranth (Amaranthus viridis L.) were collected locally from wild stands. All chemicals used in the enzymatic studies were of technical grade and procured from either Sisco Research Laboratory Pvt. Ltd., India, Sigma Co., St. Louis, USA, Merck Ltd., India or Loba-Chemie Pvt. Ltd., India.
Growth studies
Growth studies were conducted with different concentrations of α-pinene, namely 1, 2·5, 5 and 10 mm (= 0·136–1·36 mg mL−1) under laboratory conditions. Solutions of α-pinene were prepared by dissolving the requisite amount of α-pinene in Tween-80 and making up to final volume with distilled water. The final concentration of Tween-80 in the α-pinene solutions was 100 mg L−1 (0·1 %). Tween-80 was used because of its surface activity to dissolve α-pinene in water, and the same amount was added to the distilled water that served as the control. The concentrations of α-pinene used in the study are environmentally relevant and comparable to those reported by previous workers under natural conditions (Wilt et al., 1993; White, 1994; Amaral and Knowles, 1998). Amaral and Knowles (1998) reported that α-pinene concentrations in the range of 5–10 mg g−1 soil dry weight are ecologically relevant to those found in natural forest soils, whilst White (1994) and Wilt et al. (1993) reported that concentrations of the monoterpenes under natural conditions ranged from 3–5 mg g−1 soil dry weight.
Seeds of all test plants were imbibed in distilled water for 6 h except C. occidentalis, which were imbibed for 24 h. After imbibition, 15 seeds of each test species (30 for A. viridis) were placed in 15-cm diameter Petri dishes lined with two layers of Whatman No. 1 filter paper moistened with 8 mL of α-pinene solution or distilled water (control). The Petri dishes were then sealed with cellotape and Parafilm®. Each treatment was replicated five times. All Petri dishes were placed in a growth chamber maintained at 30/16 °C day/night temperature (± 2 °C) and 16/8 h light/dark, with a photon flux density of approximately 150 μmol photons m−2 s−1 and relative humidity of 78 ± 2 %. After a week, the number of seeds that germinated was counted and their root length was measured. The entire experiment was repeated. For further studies involving analysis and enzymatic assays, only the roots of C. occidentalis were chosen, as this species was affected the most in the growth studies. These were excised and frozen at –80 °C prior to enzyme extraction and other analysis.
Effect on membrane integrity
Loss of membrane integrity (an indicator of cellular damage) was studied in terms of ion (electrolyte) leakage from the roots of C. occidentalis by measuring conductivity of the bathing medium containing α-pinene, as per the method of Duke and Kenyon (1993). Root tissue (100 mg) collected from 10-d-old seedlings of C. occidentalis (grown under controlled conditions as described above) was dipped in 5 mL of 1 mm MES buffer (2-[N-morpholino]ethanesulfonic acid sodium salt, pH 6·5) containing 2 % sucrose (w/v) and α-pinene (1, 2·5, 5·0 and 10·0 mm) dissolved in Tween-80. A parallel control containing all the materials except α-pinene was also maintained. The conductivity of the bathing medium was measured with a conductivity meter (ECOSCAN CON5; Eutech Instruments Pte. Ltd., Singapore) at regular intervals in the dark (0, 1, 2, 4, 8, 12, 16, 18 and 20 h) followed by exposure to light for a further 10 h (measurements at 22, 24, 26, 28 and 30 h). The root samples were then boiled for 15 min in order to measure the maximum electrolyte leakage. For each treatment there were five replicates, and the experiment was repeated.
Determination of lipid peroxidation
Lipid peroxidation was measured in terms of malondialdehyde content (MDA) as per the method of Heath and Packer (1968). Roots (100 mg) were extracted with trichloroacetic acid (TCA, 0·1 %, w/v) and centrifuged at 10 000 g for 10 min. MDA level was used as an index of lipid peroxidation and was expressed as nmol g−1 fresh weight (f. wt). One mL of the supernatant was added to 4 mL of 0·5 % thiobarbituric acid (TBA, made in 20 % TCA). The mixture was incubated at 95 °C for 30 min followed by quick cooling over ice, and then centrifuged at 10 000 g for 10 min. The absorbance of the supernatant was determined at 532 nm and corrected for non-specific absorbance at 600 nm. MDA amount was determined using the extinction coefficient of 155 mm−1cm−1 and expressed as nmol g−1 f. wt.
Hydrogen peroxide (H2O2) content
H2O2 content was determined using the method given by Velikova et al. (2000). Root tissue (100 mg) was extracted with 5 mL of 0·1 % TCA and centrifuged at 12000 g for 15 min. Then 0·5 mL of supernatant was mixed with 0·5 mL of 10 mM phosphate buffer (pH 7·0) and 1 mL of 1 m potassium iodide and the absorbance was determined at 390 nm. The amount of H2O2 read using the extinction coefficient 0·28 μm−1 cm−1 and expressed as nmol g−1 f. wt.
Determination of free proline content
Proline content was measured following the method described by Bates et al. (1973). Dried and powdered root tissue (100 mg) was digested in 3 mL of 3 % sulfosalicylic acid for 30 min at 100 °C followed by centrifugation at 2000 g for 5 min at 25 °C. To 0·2 mL of the extract was added 0·4 mL of distilled water and 2 mL of the reagent mixture (consisting of 30 mL glacial acetic acid, 20 mL distilled water and 0·5 g Ninhydrin). The samples were boiled for 1 h, cooled and extracted with 6 mL of toluene. The absorbance of the toluene phase was determined at 520 nm and proline content was calculated from a standard curve and expressed as mg g−1 f. wt.
Preparation of enzyme extract
Enzyme extract was prepared by homogenizing 200 mg of frozen root tissue (from each treatment or control) in 10 mL of sodium phosphate buffer (0·1 m, pH 7·0). The homogenate was filtered through a triple layer of cheese-cloth and centrifuged at 15 000 g at 4 °C. The supernatant was collected, stored at 4 °C and used as the enzyme extract for analysis of superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), ascorbate peroxidase (APX; EC 1.11.1.11), guaiacol peroxidase (GPX; EC 1.11.1.7) and glutathione reductase (GR; EC 1.6.4.2). Total protein content was determined using a 0·5-mL aliquot of the enzyme extract as described by Lowry et al. (1951) using bovine serum albumin as standard.
Activities of antioxidant enzymes
Superoxide dismutase (SOD) was assayed following the method of Beauchamp and Fridovich (1971) by measuring its ability to inhibit photochemical reduction of nitro blue tetrazolium chloride (NBT). The reaction mixture (4 mL) contained 63 μm NBT, 13 mm methionine, 0·1 mm EDTA (ethylene diamine tetraacetic acid), 13 μm riboflavin, 0·05 m sodium carbonate and 0·5 mL enzyme extract (0·5 mL distilled water in the case of the control). Test-tubes were kept under two 15 W fluorescent lamps for 20 min and then transferred to the dark for 20 min. The absorbance was determined at 560 nm and activity was expressed as enzyme units mg−1 protein. One unit of the enzyme activity was defined as the enzyme required for 50 % inhibition of the reduction of NBT in comparison with the tubes lacking the enzyme.
Catalase (CAT) activity was measured as per the method of Cakmak and Marschner (1992). The reaction mixture (2 mL) consisted of 25 mm phosphate buffer (pH 7·0), 10 mm H2O2 and 0·2 mL of enzyme extract. The activity was determined by measuring the rate of disappearance of H2O2 for 1 min at 240 nm, and calculated using an extinction coefficient of 39·4 mm−1 cm−1 and expressed as enzyme units g−1 f. wt. One enzyme unit was defined as the amount of enzyme required to oxidize 1 μm of H2O2 min−1.
Glutathione reductase (GR) activity was determined spectrophotometrically by monitoring GSSG (glutathione oxidized)-dependent oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm as per the method of Foyer and Halliwell (1976). The reaction mixture (2 mL) contained 25 mm phosphate buffer (pH 7·0), 0·1 mm EDTA, 0·5 mm GSSG, 0·12 mm NADPH and 0·2 mL enzyme extract. Absorbance at 340 nm was read immediately after addition of the enzyme extract at time zero and after 5 min. The enzyme activity was measured in terms of NADPH left unoxidized using an extinction coefficient 6·224 mm−1 cm−1, and expressed as enzyme units g−1 f. wt. One enzyme unit was defined as the amount of enzyme required to oxidize 1μm of NADPH min−1.
Ascorbate reductase (APX) was assayed as per the method of Nakano and Asada (1981). The reaction mixture (2 mL) consisted of 25 mm phosphate buffer (pH 7·0), 0·1 mm EDTA, 0·25 mm ascorbate, 1·0 mm H2O2 and 0·2 mL enzyme extract. The enzyme activity was determined using an extinction coefficient of 2·8 mm−1 cm−1 by measuring the decrease in absorbance at 290 nm for 1 min. It was expressed as enzyme units g−1 f. wt. One enzyme unit was defined as the amount of enzyme required to oxidize 1 μm of ascorbate min−1.
Guaiacol peroxidase (GPX) activity was measured using the method of Egley et al. (1983). The reaction mixture (2 mL) consisted of 25 mm phosphate buffer (pH 7·0), 0·05 % guaiacol, 1·0 mm H2O2, 0·1 mm EDTA and 0·2 mL of the enzyme extract. Increase in absorbance was measured at 470 nm due to oxidation of guaiacol. The enzyme activity was calculated using an extinction coefficient of 26·6 mm−1 cm−1 and expressed as enzyme units g−1 f. wt. One enzyme unit was the amount of enzyme that catalyses oxidation of 1μm guaiacol min−1.
Statistical analysis
For each treatment five replicates were maintained in a completely randomized manner and repeated. Five tissue sample replicates were used for enzymatic assay and all other analyses. Data is presented as mean ± s.e. and was analysed by one-way analysis of variation followed by separation of treatment means from the control at P < 0·01 and 0·05 by post hoc application of Dunnett's test.
RESULTS AND DISCUSSION
The results indicate that α-pinene inhibited seed germination of C. occidentalis, A. viridis and T. aestivum, and the effect was species- as well as concentration-dependent. However, there was no effect on seed germination of P. sativum and C. arietinum (Table 1). At higher concentrations of α-pinene (5 or 10 mm), the germination of T. aestivum was completely suppressed (Table 1), whilst the germination of A. viridis and C. occidentalis was significantly reduced (P < 0·01). Regardless of the effect on seed germination, α-pinene inhibited radicle elongation in all the plant species tested (Table 2). The inhibitory effect on radicle length was statistically significant (P < 0·01), except in C. occidentalis and P. sativum where the reduction was insignificant at 1·0 mm α-pinene concentration. At 5·0 mm concentration, radicle growth was reduced in the range of 49–66 % in A. viridis, C. arietinum and P. sativum, whereas in C. occidentalis nearly 89 % reduction was observed (Table 2). Reduction in germination and radicle elongation in maize caused by α-pinene has been reported previously by Abrahim et al. (2000) and Scrivanti et al. (2003). Several studies have shown that volatile monoterpenes are potent inhibitors of seed germination and root elongation. For example, Vaughn and Spencer (1993) reported that several monoterpenes, particularly oxygenated ones, when used as a headspace volatile (in the concentration range 20–350 μm) decreased germination of four weed species (Abutilon theophrasti, Lolium multiflorum, Amaranthus retroflexus and Digitaria sanguinalis). Romagni et al. (2000) observed that cineoles (both 1,4- and 1,8-) at concentrations ranging from 10–1000 μg g−1 in sand inhibited germination and radicle elongation of barnyard grass (Echnichloa crus-galli) and sicklepod (Cassia obtusifolia). Singh et al. (2006) reported that citronellal at concentrations ranging from 5–100 μg g−1 in sand severely affected the germination and early growth of six weeds, namely billy goat weed (Ageratum conyzoides), common lambsquarters (Chenopodium album), ragweed parthenium (Parthenium hysterophorus), prickly malvastrum (Malvastrum coromandelianum), coffee weed (Cassia occidentalis) and littleseed canarygrass (Phalaris minor). The concentrations of monoterpenes used by these workers are similar to the range observed under natural environmental conditions (see below). Although the exact mechanism for the observed inhibitory activity of terpenes remains largely unknown, some studies have shown that volatile terpenes, namely 1,8-cineole (at 25 μg g−1 in sand; Romagni et al., 2000) and citronellal (at 2500 μm, ≈ 25 μg g−1 in sand; Singh et al., 2006) inhibit mitotic activity in growing root tips of onion. α-Pinene (at 1000 μm concentration) inhibits mitotic activity in root tip cells of onion (H. P. Singh et al., unpubl. data). Nishida et al. (2005) have reported that volatile monoterpenes such as α- and β-pinene, eucalyptol and camphor in their purified form inhibited root growth of Brassica campestris by inhibiting cell proliferation in root apical meristems, and decreased the mitotic index at concentrations ranging from 200–1250 μm.
Table 1.
Effect of α-pinene on the germination percentage of test species measured after 7 d
| Plant species | |||||
|---|---|---|---|---|---|
| Concentration (mm) | C. occidentalis | A. viridis | P. sativum | C. arietinum | T. aestivum |
| 0 | 100 ± 0 | 95·5 ± 3·85 | 100 | 100 | 100 |
| 1 | 100 ± 0ns | 93·3 ± 0 ns | 100ns | 100ns | 100ns |
| 2·5 | 77·8 ± 4·87** | 91·1 ± 3·85 ns | 100ns | 100ns | 100ns |
| 5 | 55·6 ± 12·73** | 86·7 ± 0** | 100ns | 100ns | 0** |
| 10 | 50·0 ± 8·33** | 84·5 ± 3·85** | 100ns | 100ns | 0** |
Values are means ± s.e. (n =5).
*, ** indicate significant difference from controls at P < 0·05 and P < 0·01, respectively, after applying Dunnett's test; ns: non-significant.
Table 2.
Effect of α-pinene on radicle growth (cm) of test species measured after 7 d
| Plant species | |||||
|---|---|---|---|---|---|
| Concentration (mm) | C. occidentalis | A. viridis | P. sativum | C. arietinum | T. aestivum |
| 0 | 3·77 ± 0·28 | 4·68 ± 0·35 | 5·83 ± 0·09 | 10·46 ± 0·05 | 12·42 ± 0·22 |
| 1 | 3·55 ± 0·02ns | 4·03 ± 0·19* | 5·23 ± 0·35ns | 9·29 ± 0·26** | 10·31 ± 0·75** |
| 2·5 | 2·74 ± 0·04** | 3·12 ± 0·02** | 3·78 ± 0·17** | 8·20 ± 0·23** | 5·52 ± 0·39** |
| 5 | 0·61 ± 0·05** | 2·37 ± 0·44** | 1·98 ± 0·14** | 4·37 ± 0·29** | 0** |
| 10 | 0·41 ± 0·12** | 1·26 ± 0·10** | 1·29 ± 0·07** | 3·40 ± 0·35** | 0** |
Values are means ± s.e. (n = 5).
*, ** indicate significant difference from controls at P < 0·05 and P < 0·01, respectively, after applying Dunnett's test; ns: non-significant.
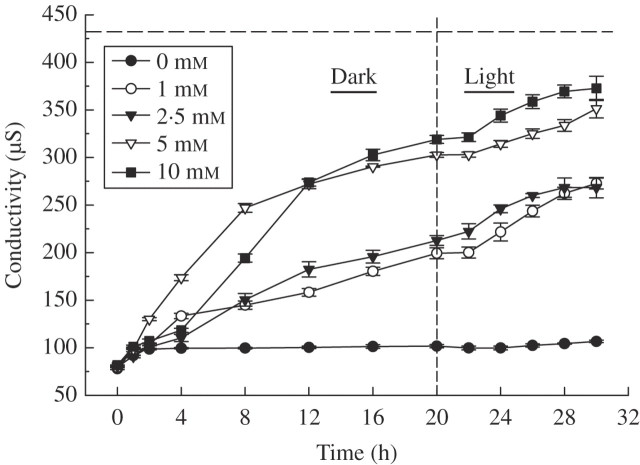
Exposure to α-pinene caused significant excessive ion leakage from C. occidentalis roots, as measured by increased conductivity of the bathing medium (MES buffer). This indicates that α-pinene disrupts membrane permeability resulting in solute leakage, and the effect was concentration dependent (Fig. 1). Solute leakage increased steadily with time up to 20 h in the dark and then for another 10 h in the light. After 30 h, the conductivity of the bathing medium containing 5·0 or 10 mm α-pinene was 80–86 % of the maximum (approx. 432·05 μS) as obtained upon boiling the tissue (Fig. 1). The ion leakage was irrespective of light or dark conditions and such a response indicates occurrence of enhanced respiration and oxidative stress (Dayan et al., 2000). There was, however, not much change in electrolyte leakage in either the 1·0 or 2·5 mm α-pinene treatment (Fig. 1). Increased conductivity levels indicate that α-pinene caused stress resulting in disruption of membrane integrity. Membrane disruption by monoterpenoids, as one of the mechanisms for their fungicidal and bactericidal activity resulting in cell death, has previously been suggested (Harrewijn et al., 2001). A decrease in membrane permeability could be due to peroxidation of polyunsaturated fatty acids in the biomembranes resulting in the formation of several byproducts, including malondialdehyde (MDA; Kappus, 1985; Maness et al., 1999). In order to explore this, the amount of MDA as an indicator of lipid peroxidation was measured (see below). Enhanced lipid peroxidation and electrolyte leakage resulting in loss of membrane integrity are among the key factors that determine cellular injury.
Fig. 1.
Effect of α-pinene on electrolyte leakage (measured as conductivity) of C. occidentalis roots determined one week after treatment. The dashed horizontal line indicates maximum ion leakage (432·05 μS) achieved after boiling samples; the vertical line at 20 h indicates the point of transition from dark to light conditions. Bars indicate s.e. (n = 5).
In general, various types of environmental stresses (including abiotic, xenobiotic and herbicidal) mediate their impact through oxidative stress caused by generation of reactive oxygen species, ROS (Smirnoff, 1995; 1998; Blokhina et al., 2003). ROS, such as singlet oxygen (1O2), superoxide radicles ( ), hydroxyl radicles (OH·) and hydrogen peroxide (H2O2), are highly reactive and toxic molecules that can cause oxidative damage to membranes, DNA, proteins, photosynthetic pigments and lipids (Apel and Hirt, 2004). Recently, ROS generation and related oxidative stress has been proposed as one of the modes of action of plant growth inhibition by allelochemicals (Weir et al., 2004). However, very little is known about the action of allelochemicals/phytotoxins in inducing ROS-mediated oxidative damage. Bais et al. (2003) reported that (–)-catechin, a putative phytotoxin, inhibits plant growth due to a severe oxidative burst in root tips, resulting in cell death. To explore whether α-pinene induces a similar response, various non-enzymatic indicators (such as membrane leakage, lipid peroxidation, proline content and hydrogen peroxide) and enzymatic mechanisms linked with oxidative stress were assessed in roots of C. occidentalis.
), hydroxyl radicles (OH·) and hydrogen peroxide (H2O2), are highly reactive and toxic molecules that can cause oxidative damage to membranes, DNA, proteins, photosynthetic pigments and lipids (Apel and Hirt, 2004). Recently, ROS generation and related oxidative stress has been proposed as one of the modes of action of plant growth inhibition by allelochemicals (Weir et al., 2004). However, very little is known about the action of allelochemicals/phytotoxins in inducing ROS-mediated oxidative damage. Bais et al. (2003) reported that (–)-catechin, a putative phytotoxin, inhibits plant growth due to a severe oxidative burst in root tips, resulting in cell death. To explore whether α-pinene induces a similar response, various non-enzymatic indicators (such as membrane leakage, lipid peroxidation, proline content and hydrogen peroxide) and enzymatic mechanisms linked with oxidative stress were assessed in roots of C. occidentalis.
Levels of MDA (the main thiobarbituric acid-reactive species, or TBARS) increased in roots of C. occidentalis upon exposure to α-pinene. An increase in the levels of TBARS is an indicator of lipid peroxidation and membrane damage. The increase was significant (P < 0·01) at 2·5 mm or higher concentrations (Fig. 2A). Heath and Packer (1968) observed that environmental stress caused enhanced MDA levels in the target tissue due to ROS generation and resulted in lipid peroxidation. The results in the current study match previous work that has shown that monoterpenes, including α-pinene, enhance lipid peroxidation (Scrivanti et al., 2003; Zunino and Zygadlo, 2004). Increased lipid peroxidation therefore indicates that α-pinene exposure results in oxidative stress due to generation of ROS species and causes a loss of cell integrity. Under a variety of abiotic stresses, plants accumulate higher levels of proline (Upadhyaya et al., 1989) and this indicates induction of oxidative damage. Treatment with α-pinene significantly (P < 0·01, except at 1 mm) increased the amount of endogenous proline in root tissue of C. occidentalis (Fig. 2B). Proline content increased by nearly 1·3-fold at 2·5 mm α-pinene concentration compared with the control, whereas at 5·0 mm concentration the increase was nearly 1·9-fold (Fig. 2B). Proline acts as an electron acceptor and prevents damage to membranes (Ain-Lhout et al., 2001). It also provides protection against ROS-induced disruption of photosystems (Hare et al., 1998).
Fig. 2.
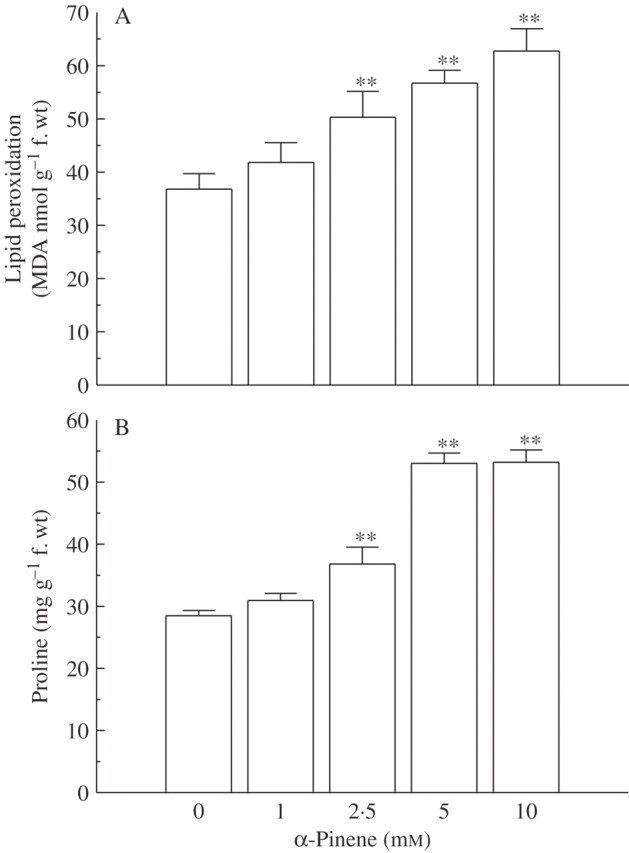
Effect of α-pinene on (A) lipid peroxidation and (B) proline accumulation in C. occidentalis roots (n = 5) determined a week after treatment. Bars indicate s.e.; ** indicates significance from control at P < 0·01 applying Dunnett's test.
To avoid the cellular damage due to ROS generation, plants produce a number of antioxidant enzymes that are induced and provide secondary protection against oxidative stress (Mittler, 2002; Apel and Hirt, 2004; Mittler et al., 2004). Increased ROS generation due to α-pinene is also indicated by enhanced activities of scavenging enzymes such as SOD, CAT, APX and GPX. Activity of SOD in C. occidentalis roots increased significantly in response to α-pinene compared with controls (P < 0·01; Fig. 3A). The increase was concentration-dependent and ranged from nearly 2·5-fold over controls at 1·0 mm to a nearly 23-fold increase at 5 mm α-pinene. However, there was a decrease in SOD activity at 10 mm α-pinene compared with the 5 mm concentration (Fig. 3A). SOD is the major scavenger of superoxide (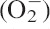 ) to form H2O2 and O2, and plays an important role in defence activity against the cellular damage caused by environmental stress (Meloni et al., 2003). Increased levels of SOD activity indicate an induction of oxidative stress caused by excessive generation of
) to form H2O2 and O2, and plays an important role in defence activity against the cellular damage caused by environmental stress (Meloni et al., 2003). Increased levels of SOD activity indicate an induction of oxidative stress caused by excessive generation of 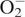 , presumably resulting from α-pinene exposure. An increase in SOD activity in response to phenolic allelochemicals contained in root exudates of cucumber (Cucumis sativus) has also been reported (Yu et al., 2003).
, presumably resulting from α-pinene exposure. An increase in SOD activity in response to phenolic allelochemicals contained in root exudates of cucumber (Cucumis sativus) has also been reported (Yu et al., 2003).
Fig. 3.
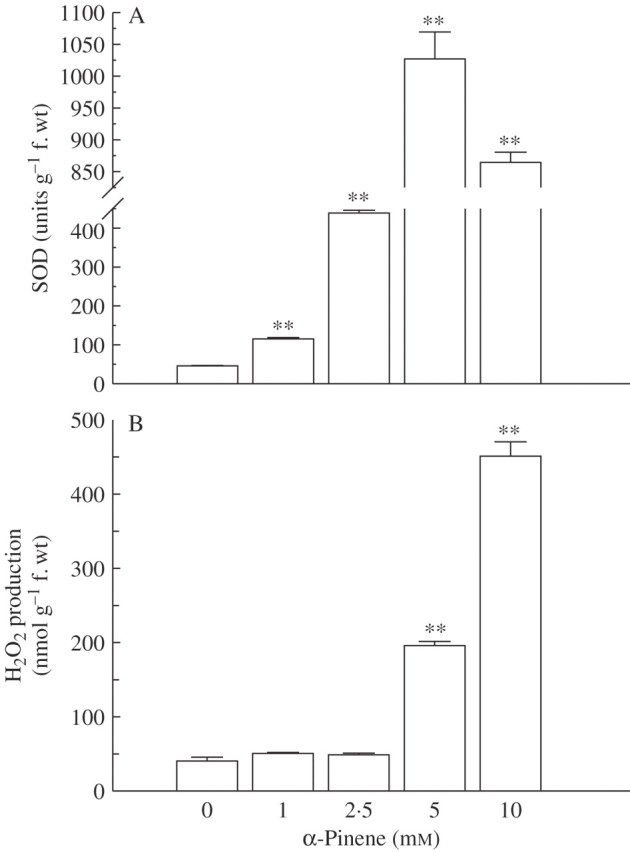
Effect of α-pinene on (A) activity of superoxide dismutase (SOD) and (B) hydrogen peroxide production in roots of C. occidentalis determined one week after treatment. Bars indicate s.e. (n = 5); ** indicates significant difference from the control at P < 0·01, after applying Dunnett's test.
The content of H2O2 also increased in response to α-pinene treatment compared with the control, and the increase was significant (P < 0·01) at 5 mm or higher concentration (Fig 3B). At 5·0 mm concentration, there was a nearly 4·5-fold increase in H2O2 amount, whereas the increase was nearly 11-fold at 10 mm concentration. Increased levels of H2O2 further enhance lipid peroxidation and oxidative stress levels in the target tissues. In the chloroplasts, H2O2 interferes with the activities of SH-group-containing enzymes such as fructose-1,6-biphosphatase and inhibits photosynthetic activity (Takeda et al., 1995). Among different ROS produced in response to environmental stresses, H2O2 acts as a major signalling molecule and serves as an effective mode of defence (Foyer et al., 1997). H2O2 is further reduced to H2O by CAT in the peroxisomes, by APX in the chloroplasts and cytosol, and by GPX in the cell wall (Blokhina et al., 2003), and APX is the most important peroxidase detoxifying H2O2 to water (Noctor and Foyer, 1998).
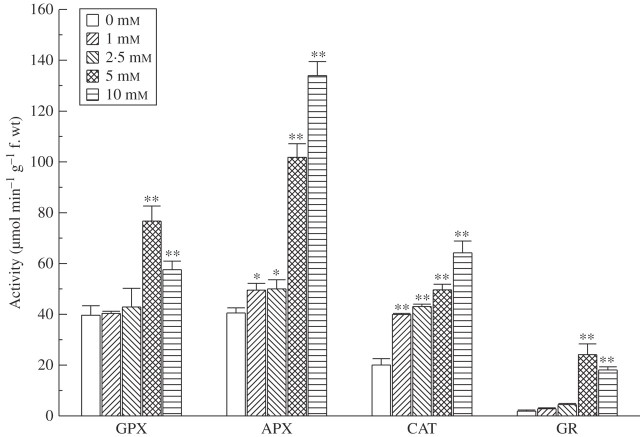
Activity of CAT increased steadily with α-pinene concentration and it nearly doubled in response to 1 mm α-pinene (Fig. 4). At 5·0 mm α-pinene concentration, the increase was nearly 2·5-fold and it further increased to nearly 3·2-fold at 10 mm. Likewise, APX activity also increased significantly in response to α-pinene exposure: it showed an increase of nearly 25 % (significant at P < 0·05) over the control at 1 and 2·5 mm α-pinene. In response to 5·0 and 10·0 mm α-pinene, it increased by nearly 2·5 and 3·3-fold (significant at P < 0·01; Fig. 4). In contrast, there was not much increase in the activity of GPX at the lower concentrations (1 and 2·5 mm) compared with the control. However, at higher concentrations GPX activity showed a significant (P < 0·01) increase that was nearly double at 5·0 mm α-pinene compared with the control.
Fig. 4.
Effect of α-pinene on activities of GPX, APX, CAT and GR in roots of C. occidentalis estimated one week after treatment. Bars indicate s.e. (n = 5); *, ** indicate significant difference from the control at P < 0·05 and P < 0·01, respectively, after applying Dunnett's test.
A similar trend was observed with the GR assay as with SOD, APX, GPX and CAT. GR, a flavoenzyme in the ascorbate–glutathione cycle, uses NADPH as an electron donor and converts oxidized glutathione (GSSG) to the reduced form, GSH (Noctor and Foyer, 1998) and provides protection against oxidative damage (Aono et al., 1995). Activity of GR increased steadily upon exposure to α-pinene and this increase was statistically significant at P < 0·01, except at 1 mm concentration. Activity increased by nearly 2·4 times at 2·5 mm α-pinene compared with the control, whereas at 5 mm α-pinene exposure the increase was nearly 13-fold over the control (Fig. 4). Increased activity of GR indicates that α-pinene induces oxidative stress since GR is involved in providing protection from oxidative damage against abiotic stresses (Aono et al., 1995).
A number of reports indicate that oxidative stress induces an increase in the responses of enzymatic systems linked to ROS-scavenging process (Apel and Hirt, 2004; Jones and Smirnoff, 2005). The results obtained in the present study showed that among the antioxidant enzymes assayed, activities of CAT, GPX and APX showed an increase in the range of 2- to 3·2-fold upon α-pinene exposure compared with controls. The activity of GR increased by nearly 13-fold over the control. APX and GR are the main components of the ascorbate–glutathione cycle that provides one of the main defences against oxidative damage in plants (Smirnoff, 1996; Becana et al., 2000). In fact, GR is a key enzyme in providing protection against a variety of environmental and abiotic stresses (Aono et al., 1995; Dalton, 1995; Romero-Puertas et al., 2006). Activity of GR and hence GSH production is generally elevated in plants upon exposure to xenobiotics and various environmental stresses (Foyer et al., 1991). GSH is an essential thiol-containing molecule providing defence against oxidative damage induced by abiotic stresses. It is used by glutathione-S-transferases to detoxify xenobiotics (Edwards et al., 2000).
The results obtained in the present study indicate that α-pinene inhibits root growth and induces oxidative stress. However, under natural field conditions the effect depends upon its availability in the soil. Although a lot of information is available regarding foliar release of volatile monoterpenes into the atmosphere from forests and vegetation, information about their release from the roots and underground plant parts is largely unknown. Roots synthesize and accumulate a variety of volatile monoterpenes and sesquiterpenes that enter the rhizosphere soil upon release (Wichtmann and Stahl-Biskup, 1987; Ji et al., 1993; Kovacevic et al., 2002). Monoterpenes enter into the soil medium by several mechanisms, such as by leaching from litter (Angelini et al., 2003) or foliar parts (Weidenhamer et al., 1994), by root exudation (Janson, 1993; Napierala-Filipiak et al., 2002), by rhizo-deposition of volatilized monoterpenes (Muller, 1970), and also by direct release. Among these, leaching from litter is the main source of monoterpenes in the soil (Wood, 1996). Many plant roots also release volatile monoterpenes into the soil; for example, roots of ponderosa pine (Pinus ponderosa) release monoterpenes including α-pinene (Latta et al., 2000), 1,8-cineole is released from roots of Arabidopsis thaliana (Steeghs et al., 2004) and sesquiterpene (E)-β-caryophyllene is released from roots of corn (Rasmann et al., 2005). However, little is known about the amount and persistence of these monoterpenes in the soil.
The concentrations of monoterpenes used in the present study (1–10 mm or 0·136–1·36 mg mL−1) are ecologically relevant in view of the reported levels of monoterpenes under natural conditions. For example, Paavolainen et al. (1998) observed that the concentration of monoterpenes was very high in the soil micro-air (1·00–2·06 mg g−1 soil) of Picea abies forests and α-pinene accounted for nearly 38 % of it. These workers ascribed these high levels to their release from plant roots. The concentration of monoterpenes is much higher in the senescent litter layer (White, 1994), which may contain concentrations in the range of nearly 3–5 mg g−1 (Wilt et al., 1993; White, 1994); these are released through leaching and decomposition into the soil system. Forest litter is the main source of release of monoterpenes into the soil in such forests and, as a result, the superficial layers of forest soil have a very high concentration of these compounds (White, 1994).
In conclusion, the present study shows that α-pinene (at ecologically relevant concentrations) inhibited the germination and radicle growth of the test species examined. Exposure to α-pinene induced oxidative stress through the enhanced generation of ROS, which was accompanied by membrane damage, enhanced lipid peroxidation levels, proline accumulation and by activation of antioxidant enzyme systems. Increased levels of scavenging enzymes indicate their induction as a secondary defence mechanism in response to α-pinene. However, the genetic mechanisms that are involved bringing about such responses to α-pinene remain unknown.
Acknowledgments
Financial support from the Department of Science and Technology (DST), New Delhi to H.P. Singh in the form of a Fast Track Project, and from the Council of Scientific and Industrial Research (CSIR), New Delhi to S. Kaur and K. Arora, are gratefully acknowledged.
LITERATURE CITED
- Abrahim D, Braguini WL, Kelmer-Bracht AM, Ishii-Iwamoto EL. (2000) Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. Journal of Chemical Ecology 26611–624. [Google Scholar]
- Abrahim D, Francischini AC, Pergo EM, Kelmer-Bracht AM, Ishii-Iwamoto EL. (2003) Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiology and Biochemistry 41985–991. [Google Scholar]
- Ain-Lhout F, Zunzunegui FA, Diaz Barradas MC, Tirado R, Clavijio A, Garcia Novo F. (2001) Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant and Soil 230175–183. [Google Scholar]
- Amaral JA and Knowles R. (1998) Inhibition of methane consumption in forest soils by monoterpenes. Journal of Chemical Ecology 24723–734. [Google Scholar]
- Angelini LG, Carpanese G, Cioni PL, Morelli I, Macchia M, Flamni G. (2003) Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. Journal of Agricultural and Food Chemistry 516158–6164. [DOI] [PubMed] [Google Scholar]
- Aono M, Saji H, Fujiyama K, Sugita M, Kondo N, Tanaka K. (1995) Decrease in activity of glutathione reductase enhances paraquat sensitivity in transgenic Nicotina tabacum. Plant Physiology 107645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K and Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55373–399. [DOI] [PubMed] [Google Scholar]
- Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 3011377–1380. [DOI] [PubMed] [Google Scholar]
- Bates LS, Walderen RD, Taere ID. (1973) Rapid determination of free proline for water stress studies. Plant and Soil 39205–207. [Google Scholar]
- Beauchamp C and Fridovich I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry 44276–286. [DOI] [PubMed] [Google Scholar]
- Becana M, Dalton DA, Moran JF, Iturbe-Ormaetxe I, Matamoros MA, Rubio MC. (2000) Reactive oxygen species and antioxidants in legume nodules. Physiologia Plantarum 109372–381. [Google Scholar]
- Berenbaum MR. (1995) The chemistry of defence: the theory and practice. Proceedings of the National Academy of Sciences, USA 922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 91179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I and Marschner H. (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiology 981222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA. (1995) Antioxidant defences of plants and fungi. In Ahmad S (Ed.). Oxidative stress and antioxidant defences in biology.(Chapman and Hall, New York) pp. 398–455.
- Dayan FE, Romagni JG, Duke SO. (2000) Investigating the mode of action of natural phytotoxins. Journal of Chemical Ecology 202079–2093. [Google Scholar]
- Duke SO and Kenyon WH. (1993) Peroxidizing activity determined by cellular leakage. In Böger P and Sandmann G (Eds.). Target assays for modern herbicides and related phytotoxic compounds.(CRC Press, Boca Raton, FL) pp. 61–66.
- Duke SO, Dayan FE, Romagni JG, Rimando AM. (2000) Natural products as sources of herbicides: current status and future trends. Weed Research (Oxford) 4099–111. [Google Scholar]
- Edwards R, Dixon DP, Walbot V. (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in death. Trends in Plant Science 5193–198. [DOI] [PubMed] [Google Scholar]
- Egley GH, Paul RN, Vaughn KC, Duke SO. (1983) Role of peroxidase in the development of water impermeable seed coats in Sida spinosa L. Planta 157224–232. [DOI] [PubMed] [Google Scholar]
- Facchini PJ. (1999) Plant secondary metabolism out of evolutionary abyss. Trends in Plant Science 4381–418. [DOI] [PubMed] [Google Scholar]
- Foyer CH and Halliwell B. (1976) Presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 13321–25. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Edwards EA, Mullineaux PM. (1991) The role of ascorbate in plants, interactions with photosynthesis and regulatory significance. In Pell E and Steffen K (Eds.). Active oxygen/oxidative stress and plant metabolism: Proceedings of the 6th Annual Penn State Symposium in Plant Physiology.(American Society of Plant Physiologists, Rockville, MD) pp. 131–144.
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiologia Plantarum 100241–254. [Google Scholar]
- Geron C, Ramussen R, Arnts RR, Guenther A. (2000) A review and synthesis of monoterpene speciation from forests in the United States. Atmospheric Environment 341761–1781. [Google Scholar]
- Geron C, Harley P, Guenther A. (2001) Isoprene emission capacity for US tree species. Atmospheric Environment 353341–3352. [Google Scholar]
- Geron C, Guenther A, Greenberg J, Loescher HW, Clark D, Baker B. (2002) Biogenic volatile organic compound emissions from a lowland tropical wet forest in Costa Rica. Atmospheric Environment 363793–3802. [Google Scholar]
- Hadacek F. (2002) Secondary metabolites as plant traits: current assessment and future perspectives. Critical Reviews in Plant Sciences 21273–322. [Google Scholar]
- Hare PD, Cress WA, van Staden J. (1998) Dissecting the roles of osmolyte accumulation during stress. Plant and Cell Environment 21535–553. [Google Scholar]
- Harrewijn P, van Oosten AM, Piron PM. (2001) Natural terpenoids as messengers: A multidisciplinary study of their production, biological functions and practical applications(Kluwer, Dordrecht).
- Heath RL and Packer L. (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125189–198. [DOI] [PubMed] [Google Scholar]
- Janson RW. (1993) Monoterpene emission from Scots pine and Norwegian spruce. Journal of Geophysical Research—Atmospheres 982839–2850. [Google Scholar]
- Ji L, Xu ZL, Pan JG. (1993) GC-MS analysis of the essential oil from the root of Ligusticum brachylobum Franch. Zhongguo Zhong Yao Za Zhi 18294–295. [PubMed] [Google Scholar]
- Jones MA and Smirnoff N. (2005) Reactive oxygen species in plant development and pathogen defence. In Smirnoff N (Ed.). Antioxidants and reactive oxygen species in plants.(Blackwell Publishing, Oxford).
- Kappus H. (1985) Lipid peroxidation: mechanisms, analysis, enzymology and biological relevance. In Sies H (Ed.). Oxidative stress.(Academic Press, Inc., New York) pp. 273–310.
- Keller M and Lerdau M. (1999) Isoprene emission from tropical forest canopy leaves. Global Biochemical Cycles 1319–29. [Google Scholar]
- Kovacevic N, Pavlovic M, Menkovic N, Tzakou O, Couladis M. (2002) Composition of the essential oil from roots and rhizomes of Valeriana pancicii Halácsy & Bald. Flavour and Fragrance Journal 17355–357. [Google Scholar]
- Langenheim JH. (1994) Higher plant terpenoids: a phytocentric overview of their ecological roles. Journal of Chemical Ecology 201223–1280. [DOI] [PubMed] [Google Scholar]
- Latta RG, Linhart YB, Lundquist L, Snyder MA. (2000) Patterns of monoterpenes variation within individual trees in ponderosa pine. Journal of Chemical Ecology 261341–1357. [Google Scholar]
- Llusià J and Peñuelas J. (2000) Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. American Journal of Botany 87133–140. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NT, Farr AL, Randall RJ. (1951) Protein measurement with the folin-phenol reagent. Journal of Biological Chemistry 193265–275. [PubMed] [Google Scholar]
- Maness P-C, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. (1999) Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Applied and Environmental Microbiology 654094–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni DA, Oliva MA, Martinez CA, Cambraia J. (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environmental and Experimental Botany 4969–76. [Google Scholar]
- Mittler R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7405–410. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. (2004) Reactive oxygen gene network of plants. Trends in Plant Science 9490–498. [DOI] [PubMed] [Google Scholar]
- Muller CH. (1970) Phytotoxins as plant habitat variables. Recent Advances in Phytochemistry 3106–121. [Google Scholar]
- Muller WH and Muller CH. (1964) Volatile growth inhibitors produced by Salvia species. Bulletin of the Torrey Botanical Club 91327–330. [Google Scholar]
- Nakano Y and Asada K. (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22867–880. [Google Scholar]
- Napierala-Filipiak A, Werner A, Mardarowicz M, Gawdzik J. (2002) Concentrations of terpenes in mycorrhizal roots of Scots pine (Pinus sylvestris L.) seedlings grown in vitro. Acta Physiologia Plantarum 24137–143. [Google Scholar]
- Nishida N, Tamotsu S, Nagata N, Saito C, Sakai A. (2005) Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. Journal of Chemical Ecology 311187–1203. [DOI] [PubMed] [Google Scholar]
- Noctor G and Foyer CH. (1998) Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49249–279. [DOI] [PubMed] [Google Scholar]
- Paavolainen L, Kitunen V, Smolander A. (1998) Inhibition of nitrification in forest soils by monoterpenes. Plant and Soil 205147–154. [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ. (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434732–737. [DOI] [PubMed] [Google Scholar]
- Romagni JG, Allen SN, Dayan FE. (2000) Allelopathic effects of volatile cineoles on two weedy plant species. Journal of Chemical Ecology 26303–313. [Google Scholar]
- Romero-Puertas MC, Corpas FJ, Sandalio LM, Leterrier M, Rodríguez-Serrano M, del Río LA, Palma JM. (2006) Glutathione reductase from pea leaves: response to abiotic stress and characterization of the peroxisomal isozyme. New Phytologist 17043–52. [DOI] [PubMed] [Google Scholar]
- Scrivanti LR, Zunino M, Zygadlo M. (2003) Tagetes minuta and Schinus areira essential oils as allelopathic agents. Biochemical, Systematics and Ecology 31563–572. [Google Scholar]
- Seigler DS. (1996) Chemistry and mechanism of allelopathic interactions. Agronomy Journal 88876–885. [Google Scholar]
- Singh HP, Batish DR, Kaur S, Ramezani H, Kohli RK. (2002) Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Annals of Applied Biology 141111–116. [Google Scholar]
- Singh HP, Batish DR, Kaur S, Kohli RK, Arora K. (2006) Phytotoxicity of the volatile monoterpene citronellal against some weeds. Zeitschrift für Naturforschung, C 61334–340. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. (1995) Antioxidant systems and plant response to environment. In Smirnoff N (Ed.). Environment and plant metabolism: flexibility and acclimation.(BIOS Scientific Publishers, Oxford) pp. 217–243.
- Smirnoff N. (1996) The function and metabolism of ascorbic acid in plants. Annals of Botany 78661–669. [Google Scholar]
- Smirnoff N. (1998) Plant resistance to environmental stress. Current Opinion in Biotechnology 9214–219. [DOI] [PubMed] [Google Scholar]
- Steeghs M, Bais HP, de Gouw J, Goldan P, Kuster W, Northway M, Fall R, Vivanco JM. (2004) Proton-transfer-reaction mass spectrometry (PTR-MS) as a new tool for real time analysis of root-secreted volatile organic compounds (VOCs) in Arabidopsis thaliana. Plant Physiology 13547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Yokota A, Shigeoka S. (1995) Resistance of photosynthesis to hydrogen peroxide in algae. Plant and Cell Physiology 361089–1095. [Google Scholar]
- Upadhyaya A, Davis TD, Walser RH, Galbraith AB, Sankhla N. (1989) Uniconazole-induced alleviation of low-temperature damage in relation to antioxidant activity. HortScience 24955–957. [Google Scholar]
- Vaughn SF and Spencer GF. (1993) Volatile monoterpenes as potential parent structures for new herbicides. Weed Science 41114–119. [Google Scholar]
- Velikova V, Yordanov I, Edreva A. (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Science 15159–66. [Google Scholar]
- Weidenhamer J, Menelaou MA, Macias FA, Fischer NH, Richardson DR, Williamson GB. (1994) Allelopathic potential of menthofuran monoterpenes from Calamintha ashei. Journal of Chemical Ecology 203345–3359. [DOI] [PubMed] [Google Scholar]
- Weir TL, Park S-W, Vivanco JM. (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Current Opinion in Plant Biology 7472–479. [DOI] [PubMed] [Google Scholar]
- White CS. (1994) Monoterpenes: their effects on ecosystem nutrient cycling. Journal of Chemical Ecology 201381–1406. [DOI] [PubMed] [Google Scholar]
- Wichtmann EM and Stahl-Biskup E. (1987) Composition of the essential oils from caraway herb and root. Flavour and Fragrance Journal 283–89. [Google Scholar]
- Wilt FM, Miller GC, Everett RL, Hackett M. (1993) Monoterpene concentrations in fresh, senescent, and decaying foliage of single-leaf pinyon (Pinus monophylla Torr. & Frem: Pinaceae) from the western Great Basin. Journal of Chemical Ecology 19185–194. [DOI] [PubMed] [Google Scholar]
- Wood SE. (1996) Loss of foliar monoterpenes from Umbellularia californica leaf litter and their influence on nitrification potential in soil beneath the trees. PhD dissertation, University of California, Santa Cruz.
- Yu JQ, Ye SF, Zhang MF, Hu WH. (2003) Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus), and allelochemicals on photosynthesis and antioxidant enzymes in cucumber. Biochemical, Systematics and Ecology 31129–139. [Google Scholar]
- Zunino MP and Zygadlo JA. (2004) Effect of monoterpenes on lipid oxidation in maize. Planta 219303–309. [DOI] [PubMed] [Google Scholar]