Abstract
• Background and Aims Habitats in mountains are often isolated. Plants growing in these sites face severe dispersal limitations, but also difficulties for recruitment. The focus was laid on the magnitude of genetic differences among populations but also on the size of potentially occurring clones.
• Methods RAPD fingerprints were obtained from 23 populations in southern Mongolia. Sampling covered the entire distribution range of Galitzkya macrocarpa; samples of G. potaninii represented only the Mongolian part of its mainly northern Chinese range.
• Key Results The Mongolian endemic G. macrocarpa showed moderately strong population differentiation (ΦST = 0·251), and limited evidence for isolation by distance. Local genetic diversity was not positively correlated to habitat size, and not reduced in peripheral populations. Clonal growth is possible, but most plants originate from sexual reproduction. In contrast, populations of G. potaninii were highly differentiated (ΦST = 0·550); and the most remote outposts had reduced genetic diversity. In these areas, isolation is expected to date back to glacial times.
• Conclusions Effects of natural fragmentation differ among species. Both are rare, but G. macrocarpa appears to be able to maintain genetic diversity over its range. Clonal growth is an option in its mixed reproduction strategy and allows survival under harsh conditions. In contrast, genetic structure in G. potaninii gives reason for concern, and further studies on population dynamics are needed.
Keywords: Conservation, Galitzkya macrocarpa, Galitzkya potaninii, clonal growth, fragmentation, endemics, genetic diversity, isolation, Mongolia, mountains
INTRODUCTION
Mountain habitats experience special climatic conditions that often differ tremendously from the surrounding lowlands and valleys. Steep topographic and therefore climatic gradients lead to heavily fragmented habitats characterized by barriers to migration and genetic exchange. Levels of natural fragmentation are thus generally high and several studies have demonstrated strong genetic effects and isolation by distance (Bauert et al., 1998; Schönswetter et al., 2002, 2004). Effects of habitat isolation should be especially pronounced where mountains rise from dry lowlands like the Central Asian Gobi. Several montane species of the Altay and Tien Shan mountains have relatively high moisture requirements and populations are widely isolated (Jäger, 2005). This renders genetic exchange — at least under current climatic conditions — difficult. So far only a few Central Asian mountain taxa have been studied in terms of genetic structure (Chen et al., 2005; Wesche et al., 2005c; Xia et al., 2005; Zhang et al., 2005). Thus, it is largely unknown whether natural fragmentation has strongly affected genetic exchange among populations, which would raise a need for subsequent studies on possible consequences for fitness parameters (Reed and Frankham, 2003; Frankham, 2005).
Genetic exchange relies on movement of pollen or seeds. However, climatic conditions in Central Asia are generally harsh and seedling establishment is exceedingly difficult (Lavrenko and Karamysheva, 1993; Gunin et al., 2003). Most dominant plant species are therefore perennial, and clonality is widespread (Li and Ge, 2001; Song et al., 2002; Setsuko et al., 2004). Patterns are similar in mountain areas where several species are known to survive unfavourable conditions by extended clonal growth over dozens or hundreds of years (Steinger et al., 1996; Escaravage et al., 1998; Keeler et al., 2002; Young et al., 2002; Yu et al., 2004; Wesche et al., 2005c). However, despite the fact that genetic diversity is expected to decrease with clonal growth being the dominant reproduction type (Honnay et al., 2006), recent studies on alpine plants found relatively high levels of clonal diversity (Li and Ge, 2001; Pluess and Stöcklin, 2004).
Here, is presented a study on the genetic structure of two closely related, long-lived Central Asian rock endemics: Galitzkya macrocarpa and G. potaninii (Brassicaceae). Both species are capable of clonal growth and occur in comparable habitats in south-western Mongolia and north-western China (Gubanov, 1996; Grubov, 2001). They have distinct distribution ranges with the range of G. macrocarpa being exclusively restricted to Mongolia. Populations are rare and known from few scattered locations in southern Mongolia (Gubanov, 1996; Grubov, 2001), where they are restricted to widely isolated mountain habitats (Wesche et al., 2005a). Whether isolation has caused genetic differentiation among populations was unknown. In the same region, an earlier study confirmed an almost complete reproductive collapse in stands of the clonal Juniperus sabina (Wesche et al., 2005c), so similar problems were expected for the species in the present study.
Patterns of random amplified DNA (RAPD) variation were examined for 23 Mongolian Galitzkya populations in order to answer the following questions: (a) How is genetic variation distributed among and within populations of G. macrocarpa and G. potaninii? Is there any evidence for restricted gene flow among populations; and is genetic similarity correlated with spatial distance? (b) How large is the genetic diversity of populations, and is there any correlation to habitat size of G. macrocarpa? (c) Is there evidence for extensive clonal growth in the fine-scale genetic structure of G. macrocarpa populations? (d) What are the implications for conservation of the Mongolian populations?
MATERIALS AND METHODS
Study species and study region
The genus Galitzkya (Brassicaceae) is restricted to Central and Middle Asia and comprises three species: Galitzkya spathulata (Steph. ex Willd.) V. Boczantzeva occurs from northern China to western Kazakhstan (Pavlov, 1961; Zhou et al., 2001) and is not included here for logistical reasons. Galitzkya macrocarpa (Iconn.-Galitz.) V. Boczantzeva is a true endemic of mountain ranges in southern Mongolia (Boczantzeva, 1979; Gubanov, 1996), while G. potaninii (Maxim.) V. Boczantzeva grows in mountains of south-west Mongolia, and in the Tien Shan and Quilian Shan in north-western China. So far neither species has been studied in terms of genetic structure, nor is there any information on ploidy levels.
Galitzkya macrocarpa and G. potaninii are suffruticose plants characterized by broad rosulate leaves. Individuals develop one main caudex which can form several branches (three to five) with increasing age. Plants are often successively buried in moving scree, resulting in the development of subterranean shoots. The number of laterally developed inflorescences ranges between 0 and 27 (mean of 5; R. Undrakh, unpbl. res.); these bear on average three to five silicles, each of which contains four to eight seeds. Both species have light seeds (mean weight: G. macrocarpa, 1·72 mg; G. potaninii, 1·05 mg) which are flat (mean surface area: G. macrocarpa, 5·69 mm2; G. potaninii, 5·19 mm2) with broad wings (mean width: G. macrocarpa, 1·66 mm; G. potaninii, 1·43 mm). Seeds are thus capable of dispersal by wind, but no detailed data are available. Plants are self-compatible, insect-pollinated (R. Undrakh, unpbl. res.), and seeds germinate readily without any apparent sign of dormancy.
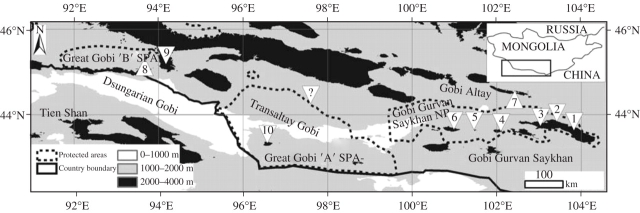
The study area comprises three protected areas in south-western Mongolia and covers some 80 000 km2 (Fig. 1). Here, G. potaninii occurs in the Dsungarian Gobi and Transaltay Gobi, while G. macrocarpa is restricted to the Gobi Altay region and the Transaltay Gobi where it was previously described from a single mountain range only (Edrengiyn Nuruu; Grubov, 2001). However, it was not possible to relocate that population, nor was it possible to find any of the two species in that mountain range. Both species avoid the relatively flat piedmont regions, which reach higher altitudes in central southern Mongolia; instead species prefer rock fissures or boulder areas. Galitzkya macrocarpa occurs between 1800 and 2600 m a.s.l., while G. potaninii covers the altitudinal range of 1500–2100 m a.s.l. in south-western Mongolia (Table 1). Both species grow on barely accessible rocks and boulders and are virtually unaffected by nomadic land use. Thus, the current levels of isolation and population fragmentation are not determined by human impact but by natural causes.
Fig. 1.
Position of sampling localities in southern Mongolia. Numbers of ranges refer to Table 1; the question mark indicates the site Edrengiyn Nuruu, where both species had previously been recorded (Grubov, 2001) but were not found in this study. Dotted lines demarcate the nature reserves (Great Gobi ‘A’ and ‘B’ Special Protected Areas, Gobi Gurvan Saykhan National Park) in the region.
Table 1.
List of sampled mountain ranges, populations, spatial extent of suitable moist mountain steppes, and number of specimens sampled (sample size is higher in population 1 due to the analysis of small-scale genetic structure)
| Range no. | Latitude/longitude (decimal degrees) | Elevation (m a.s.l.) | Habitat (km2) | Population no. | Sample size | No. used for population level analysis | |
|---|---|---|---|---|---|---|---|
| Galitzkya macrocarpa | |||||||
| 1 | Dund Saykhan | 43·620/103·820 | 2480 | 67·7 | 1 | 30 (+31) | 10 |
| Dund Saykhan | 43·630/103·810 | 2520 | 2 | 6 | 6 | ||
| Dund Saykhan | 43·630/103·777 | 2750 | 3 | 5 | 5 | ||
| 2 | Gegetiyn Am | 43·830/103·330 | 2070 | 60·5 | 4 | 10 | 10 |
| Gegetiyn Am | 43·800/103·190 | 2050 | 5 | 9 | 9 | ||
| Bayan Bor Nuruu | 43·792/103·134 | 2150 | 16·1 | 6 | 3 | ||
| 3 | Bayan Tsagaan | 43·794/102·515 | 2040 | 23·8 | 7 | 9 | 9 |
| Bayan Tsagaan | 43·717/103·101 | 2330 | 8 | 9 | 9 | ||
| Khavtsgaitiyn Am | 43·690/102·970 | 1950 | 9 | 1 | |||
| 4 | Sevrey Uul | 43·647/102·045 | 2080 | 7·2 | 10 | 10 | 10 |
| Sevrey Uul | 43·601/101·978 | 2300 | 11 | 6 | 6 | ||
| 5 | Gilbent Uul | 43·636/101·500 | 2050 | 2·7 | 12 | 5 | 5 |
| 6 | Nemegt Uul | 43·666/100·900 | 2600 | 28·3 | 13 | 2 | |
| Nemegt Uul | 43·700/100·910 | 2400 | 14 | 10 | 10 | ||
| Nemegt Uul | 43·622/100·434 | 2300 | 15 | 10 | 10 | ||
| 7 | Arts Bogd | 44·730/102·200 | 1800 | 78·6 | 16 | 3 | |
| Arts Bogd | 44·418/102·211 | 1830 | 17 | 9 | 9 | ||
| Arts Bogd | 44·483/102·561 | 2160 | 18 | 1 | |||
| Galitzkya potaninii | |||||||
| 8 | Gun Tamgijn Us | 45·250/93·667 | 1700 | No data | 19 | 10 | 10 |
| Takhin Tal | 45·561/93·613 | 1800 | 20 | 10 | 10 | ||
| 9 | Mongolian Altay | 46·423/94·221 | 2010 | 21 | 10 | 10 | |
| 10 | Atas Bogd | 43·321/96·645 | 2300 | 22 | 16 | 10 | |
| Atas Bogd | 43·390/96·411 | 1700 | 23 | 4 | |||
There are hardly any climate stations in the mountains but short-term measurements are available for the Dund Saykhan (east Gobi Gurvan Saykhan National Park; Fig. 1) and are expected to be fairly typical for the region. These suggest that mountains above 2300 m a.s.l. receive a total annual precipitation of 160–200 mm (Retzer, 2004). This renders them dry but nonetheless moister than the surrounding lowlands (mean annual precipitation <130 mm).
Data collection
Sample sizes differ among species because more populations of Galitzkya macrocarpa than of G. potaninii were found. Whereas the 18 populations of G. macrocarpa cover its entire distributional range, those of G. potaninii (five populations) represent only the northern part of that species’ range (Fig. 1). DNA samples are kept at our institute Halle; voucher specimens were deposited at the herbarium at the Martin-Luther-University Halle-Wittenberg (HAL). A population was defined as a group of plants separated from their closest conspecific by at least 1 km. The minimum and maximum distances between two populations were 1 and 275 km, respectively, for G. macrocarpa and 20 and 367 km, respectively, for G. potaninii.
The mountain steppes colonized by G. macrocarpa in the Gobi Gurvan Saykhan region have a mean inclination of 20° (Wesche et al., 2005b); suitable microhabitats are usually even steeper and several populations grew on vertical cliffs. Access is exceedingly difficult due to the heavily weathered rock. Whenever possible, at least nine plants per population (>1 m apart), which were always taken within a radius of 10 m, were sampled. In some cases, the terrain rendered sampling of nine plants impossible (Table 1). For the same reason, it was impossible to estimate population numbers. However, as G. macrocarpa is restricted to relatively moist mountain steppes (Gubanov, 1996), a vegetation map (von Wehrden et al., 2006) was used to estimate the extent of this habitat type in a given mountain range as a proxy for the potential population size (Table 1).
To assess the small-scale genetic structure of G. macrocarpa, all 67 shoots on a reasonably accessible site in the Dund Saykhan were mapped and sampled for genetic fingerprinting (range 1, population 1, Table 1; plot size 7 × 14 m, mean density 0·68 shoots m−2).
RAPD-PCR
Anonymous RAPD-, AFLP- and ISSR-markers are widely used in population genetics. All share the problem of dominance, and have been demonstrated to yield mostly similar results (Nybom and Bartish, 2000; Nybom, 2004). RAPDs were chosen as these have been employed previously for identifying clones in small-scale spatial studies (e.g. Steinger et al., 1996; Wesche et al., 2005c). As RAPDs formerly have been criticized in terms of reproducibility (Bachmann, 1994), reliability of data was ensured by repeating PCRs.
Tissues were stored in silica-gel directly after sampling. Genomic DNA was extracted from 25 mg of dried leaves with a standard kit (QIAGEN 2000; DNeasy Plant Mini Kit). Sixty primers were screened for readability and reproducibility (Random Primer Kits, Roth). This resulted in the selection of nine primers (D02, GGACCCAACC; D05, TGAGCGGACA; D07, TTGGCACGGG; D12, CACCGTATCC; D20, ACCCGGTCAC; N05, ACTGAACGCC; N09, TGCCGGCTTG; N12, CACAGACACC; N20, GGTGCTCCGT). DNA was amplified in 10-μL reaction volumes containing 8 ng DNA, 0·6 μmol L−1 primer (Roth), 0·2 mmol L−1 of each dNTP (Peqlab), 0·5 units Taq polymerase (Qbiogene), 1 μL buffer ×10 (Qbiogene) and 6·5 μL H2O. PCR was carried out in a thermocycler (Flexigene 384, Techne) that allowed for the simultaneous processing of all samples. The thermocycler was programmed for one cycle of 2 min at 94 °C followed by 36 cycles of 12 s at 94 °C, 45 s at 36 °C and 120 s at 72 °C with a final cycle of 7 min at 72 °C.
DNA fragments were separated by electrophoresis in 2% agarose gels with a TAE (Tris-acetate-EDTA) buffer system at 150 V for 150 min (equalling 10 cm distance) and stained with ethidium bromide. DNA bands were then visualized by UV light and documented using a video camera. Each sample was run in at least two independent RAPD-PCR amplification reactions. Gel pictures were analysed visually and digitalized with the help of the software RFLPSCAN PLUS Version 3.0 (Scanalytics); only bands in the range between 240 and 1500 bp were scored.
Data analysis
The nine primers used in the PCR yielded 150 polymorphic bands. RAPD data were coded into a simple matrix with ‘0’ for absent and ‘1’ for present bands. Only polymorphic bands were used as recommended by Nybom and Bartish (2000); these totalled 126 in G. macrocarpa and 68 in G. potaninii; 67 bands were shared among species (Table 2). It was possible to amplify DNA for 61 of the 67 mapped shoots of G. macrocarpa within population 1. The matrix for analysis of possible clonal structures contained 73 bands, of which 51 were polymorphic.
Table 2.
Summary of genetic information available for analysis
| G. macrocarpa | G. potaninii | Both species | |
|---|---|---|---|
| No. of samples | 138 (+31) | 50 | 188 |
| No. of polymorphic bands | 126 | 68 | 150 |
| No. of monomorphic bands | 9 | 14 | |
| No. of missing bands | 15 | 68 | |
| No. of shared bands | 67 | ||
| No. of private bands | 68 | 15 | |
| of these polymorphic | 68 | 11 | |
| of these monomorphic | 0 | 4 | |
| Bands with frequency <5% | |||
| of these private | 16 | 0 | |
| of these shared | 12 | 10 |
In dominant markers, application of standard measures of genetic diversity relies on several assumptions including presence of a Hardy–Weinberg equilibrium. For that reason, several approaches are usually combined to analyse dominant markers (e.g. Schönswetter et al., 2005). Sörensen similarity was chosen as an asymmetrical index that places strong weight on bands shared among individuals. Values were transformed to a distance measure by subtracting them from 1 (Legendre and Legendre, 1998). The distance matrix was used to calculate mean distance among individuals, as well as within and among populations. Sörensen distance was also used in ordination; principal co-ordinate analysis was performed on all samples on square-root transformed distances as these have metric properties (Legendre and Legendre, 1998). As ordinations confirmed clear differences among species, further analyses were performed separately for G. macrocarpa and G. potaninii.
A second approach was based on symmetric measures of genetic diversity. Data of all populations with a sample size ≥5 (13 of G. macrocarpa, four of G. potaninii) were subjected to a hierarchical analysis of molecular variance (AMOVA) (Excoffier et al., 1992) with three levels for variance partitioning: among mountain regions, among populations within mountain regions, and within populations (Table 1). In parallel to F-statistics, Φ-statistics were calculated to assess the genetic differentiation among populations. Significances were tested by a permutation procedure with 9999 runs. Measures for intra-population diversity used in this study include the percentage of polymorphic loci, the percentage of polymorphic bands among those present in a given population, the number of private bands (those restricted to the given population or mountain range, respectively), mean Sörensen distance among plants in a population, and average gene diversity over all loci (Schneider et al., 2000).
In populations 1 and 22 more than ten specimens were sampled, so statistics were compared on the population level for populations with subsample size n ≥ 5 with those with n ≥ 9, and again for those with n ≥ 9, but with a maximum of ten specimens included. Except for the number of polymorphic sites, no statistic was affected by changing sample size, confirming that RAPD-based assessments are relatively insensitive to sampling intensity (Nybom and Bartish, 2000). Thus, figures based on those 13 populations with at least five samples available are reported (Table 1). In populations 1 and 22, ten samples were randomly chosen from the larger data set for analysis at the population level.
For G. macrocarpa, isolation by distance was tested with Mantel tests. Pairwise ΦST-values were used as symmetrical measures of genetic distances, which were tested against a matrix of spatial distances in kilometres. Mean Sörensen distances among populations were also tested against the same geographic distances. In all Mantel tests, significances were tested with 9999 permutations.
Asymmetric analyses were performed with PC-ORD 4·32 (McCune and Mefford, 1999) and CANOCO 4.5 (ter Braak and Smilauer, 2002); genetic analysis was done with Arlequin ver. 2.000 (Schneider et al., 2000). Simple bivariate correlations were calculated with SPSS 12.0 (SPSS, 2003).
RESULTS
Differences between Galitzkya macrocarpa and G. potaninii
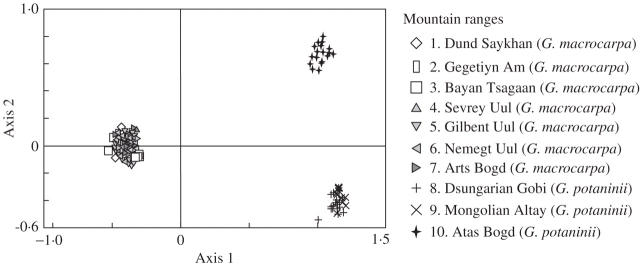
Among the 188 bands obtained, 68 were only found in G. macrocarpa, while G. potaninii had 15 private bands (Table 2). No single band was sufficient to characterize samples of G. macrocarpa as all 68 private bands were polymorphic. Of the 15 private bands of G. potaninii, four were monomorphic and characterized that species. Principal co-ordinate analysis of all 23 populations sampled showed that species were clearly and unequivocally differentiated along ordination axis 1 (Fig. 2), which captured 43·7 % of the total variance. The much less important axis 2 (7·9 % explained variance) differentiated populations of G. potaninii of the Dsungarian Gobi (lower right corner) from those in the Transaltay Gobi. In comparison, samples of G. macrocarpa formed a closed group in the left-hand part of the ordination diagram.
Fig. 2.
Principal co-ordinate analysis of RAPD data for G. macrocarpa and G. potaninii (based on square-root transformed Sörensen similarity; explained variance axis 1 = 43·7 %; axis 2 = 7·9 %; axis 3 = 3·4 %).
Inter-population structure
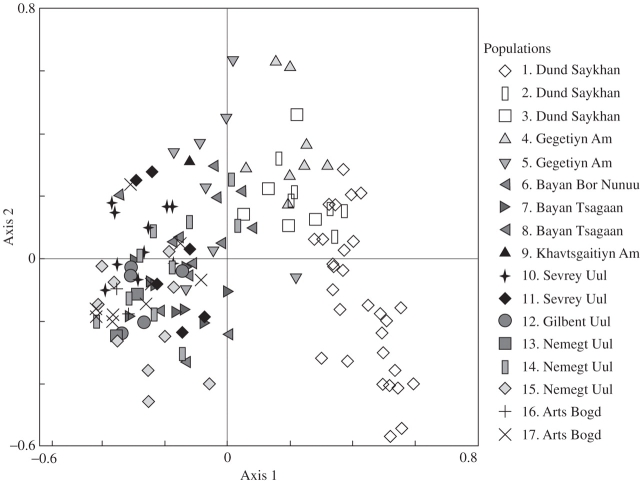
Except for one sample (in population 4), all others in this data set, and in that for G. potaninii, had distinct phenotypes, and specimens were thus considered genetic individuals. The ordination revealed relatively weak genetic differentiation between G. macrocarpa populations and mountain ranges (Fig. 3). The first three axes together explained only 14·7 % of the variance, indicating the absence of any simple genetic structure. Although samples within populations generally clustered together, populations — and even different mountain ranges — showed clear overlaps in the ordination diagram. This pattern was supported by the AMOVA (Table 3A) that found 74·9 % of the total genetic variation within populations, and 16·2 % among populations. This left 8·9 % for differences between mountain ranges.
Fig. 3.
Principal co-ordinate analysis of RAPD data for G. macrocarpa (all 138 available samples; PCA based on square-root transformed Sörensen Similarity; explained variance axis 1 = 8·6 %; axis 2 = 6·1 %; axis 3 = 5·2 %).
Table 3.
AMOVA table for (A) populations of Galitzkya macrocarpa in the South Gobi, which were nested within the main mountain ranges (13 populations, n ≥ 5 and ≤ 10): ΦSC = 0·178; ΦST = 0·251; ΦCT = 0·088; and (B) G. potaninii populations, which were nested within the regions Dsungarian Gobi and Transaltay Gobi (only those four populations with n = 10): ΦSC = 0·181; ΦST = 0·550; ΦCT = 0·451
| Source of variation | d.f. | Sum of squares | Variance components | % variation |
|---|---|---|---|---|
| (A) G. macrocarpa | ||||
| Among regions | 6 | 257·700 | 1·106*** | 8·9 |
| Among populations within regions | 6 | 156·272 | 2·029*** | 16·2 |
| Within populations | 95 | 889·944 | 9·368*** | 74·9 |
| Total | 1303·917 | 12·504 | ||
| (B) G. potaninii | ||||
| Among regions | 2 | 159·175 | 5·067 | 45·1 |
| Among populations within regions | 1 | 16·250 | 1·120*** | 10·0 |
| Within populations | 36 | 181·900 | 5·053*** | 44·9 |
| Total | 39 | 357·325 | 11·240 | |
***P < 0·001.
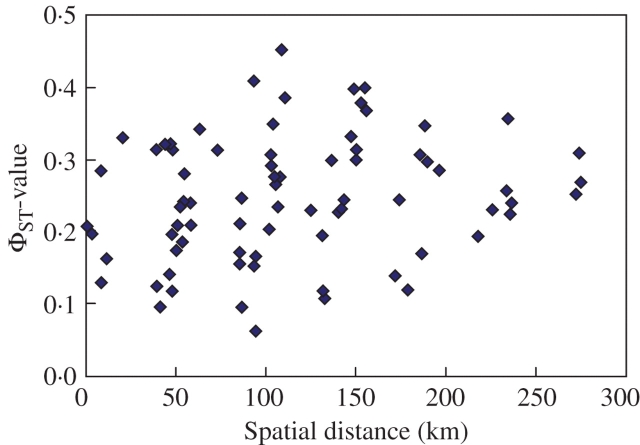
Differences among groups and among ranges, as well as values for Φ-statistics, were highly significant. The overall ΦST-value of 0·251 gives evidence for spatial isolation. This is confirmed by pair-wise ΦST-values, which tended to be lower within mountain ranges than among them (Fig. 4). The results of the Mantel tests indicated limited isolation by distance, as the pair-wise ΦST-values among populations and their geographical distances showed a weak and not significant correlation (standardized Mantel statistic RM = 0·213, P = 0·100; 9999 permutations). The respective correlation for mean Sörensen distance between populations was higher than that for the ΦST-values and significant (standardized Mantel statistic RM = 0·345, P = 0·008, 9999 runs).
Fig. 4.
Scatterplot indicating the relationship between geographic and genetic distance among populations.
A separate PCoA for G. potaninii yielded a similar picture as the general ordination (Fig. 2), so the data are not shown. The AMOVA also indicated strong differentiation in G. potaninii, and suggested a moderate degree of variability within the populations (44·9 %, Table 3B), while 45·1 % of the variation was accounted for by the different regions. The ΦST-value of 0·550 indicates strong genetic differentiation among populations. This differentiation was highly significant, as were differences among regions.
Intra-population diversity
Values for average gene diversity were <0·18 and those of mean Sörensen distance within populations were <0·22 for the 13 populations of G. macrocarpa (Table 4). Values within the more extensively sampled population 1 were similar, when several subsets were compared. The number of private alleles was low for most populations. However, when data were pooled on the level of mountain ranges, five out of seven mountain ranges were characterized as having exclusive alleles: Dund Saykhan (five), Gegetiyn Am/Bayan Bor Nuruu (two), Bayaan Tsagaan (three), Gilbent Uul (one) and Nemegt Uul (two). Genetic diversity within populations was not correlated with the size of the suitable habitat (for average gene diversity, Pearson’s r = –0·01, n.s.; for mean Sörensen distance within populations, r = 0·03, n.s.).
Table 4.
Genetic diversity within populations of G. macrocarpa and G. potaninii
| Population no. | Range no. | n | No. of bands present | No. of bands polymorphic | % polymorphic | Private bands | Gene diversity | Sörensen distance |
|---|---|---|---|---|---|---|---|---|
| Galitzkya macrocarpa | ||||||||
| 1 | 1 | 10 | 76 | 42 | 55 | 1 | 0·134 | 0·163 |
| 2 | 1 | 6 | 71 | 37 | 52 | 0 | 0·146 | 0·184 |
| 3 | 1 | 5 | 71 | 33 | 46 | 1 | 0·141 | 0·170 |
| 4 | 2 | 10 | 73 | 40 | 55 | 0 | 0·116 | 0·143 |
| 5 | 2 | 9 | 79 | 57 | 72 | 1 | 0·172 | 0·222 |
| 7 | 3 | 9 | 75 | 49 | 65 | 1 | 0·153 | 0·191 |
| 8 | 3 | 9 | 86 | 57 | 66 | 1 | 0·165 | 0·205 |
| 10 | 4 | 10 | 84 | 62 | 74 | 1 | 0·170 | 0·215 |
| 11 | 4 | 6 | 64 | 36 | 56 | 0 | 0·129 | 0·182 |
| 12 | 5 | 5 | 78 | 44 | 56 | 1 | 0·165 | 0·190 |
| 14 | 6 | 10 | 82 | 57 | 70 | 1 | 0·158 | 0·203 |
| 15 | 6 | 10 | 76 | 53 | 70 | 0 | 0·155 | 0·199 |
| 17 | 7 | 9 | 84 | 45 | 54 | 0 | 0·127 | 0·142 |
| r | 0·374 | 0·541 | 0·491 | 0·022 | 0·017 | 0·164 | ||
| Galitzkya potaninii | ||||||||
| 19 | 8 | 10 | 41 | 21 | 51 | 3 | 0·111 | 0·124 |
| 20 | 10 | 10 | 43 | 23 | 53 | 3 | 0·138 | 0·152 |
| 21 | 9 | 10 | 41 | 20 | 49 | 2 | 0·106 | 0·117 |
| 22 | 10 | 10 | 47 | 42 | 89 | 14 | 0·240 | 0·336 |
Figures refer to symmetrical measures of molecular diversity (average gene diversity over all loci, see Schneider et al., 2000), and the mean of the asymmetrical Sörensen distance among samples of a given population (only those with n ≥ 5).
Percentages of polymorphic bands were calculated with respect to the number of bands present in a given population.
For G. macrocarpa, Pearson correlations of diversity measures and specimen number are given.
Genetic diversity within populations of G. potaninii was lower in the Dsungarian Gobi than in the Transaltay Gobi (Table 4). Strong isolation among regions colonized by G. potaninii was also indicated by the higher numbers of private bands at the population level (Table 4). Moreover, 20 bands were restricted to populations found in the Dsungarian Gobi and 14 were restricted to samples from the Transaltay Gobi.
Spatial extent of clones in G. macrocarpa
Non-sexual regeneration occurs in G. macrocarpa (Fig. 5). Shoots tended to grow together and there was evidence of clumping. The 51 polymorphic bands constituted 52 different phenotypes, five of which occurred twice, and two, three times. Thus, most shoots originated from sexual reproduction. Shoots with identical phenotypes — though rare overall — always grew next to each other. The maximum distance covered by one clone was 2 m. The importance of sexual reproduction was also suggested by the intense flowering of the species, and also ramets within clones were usually flowering (Fig. 5).
Fig. 5.
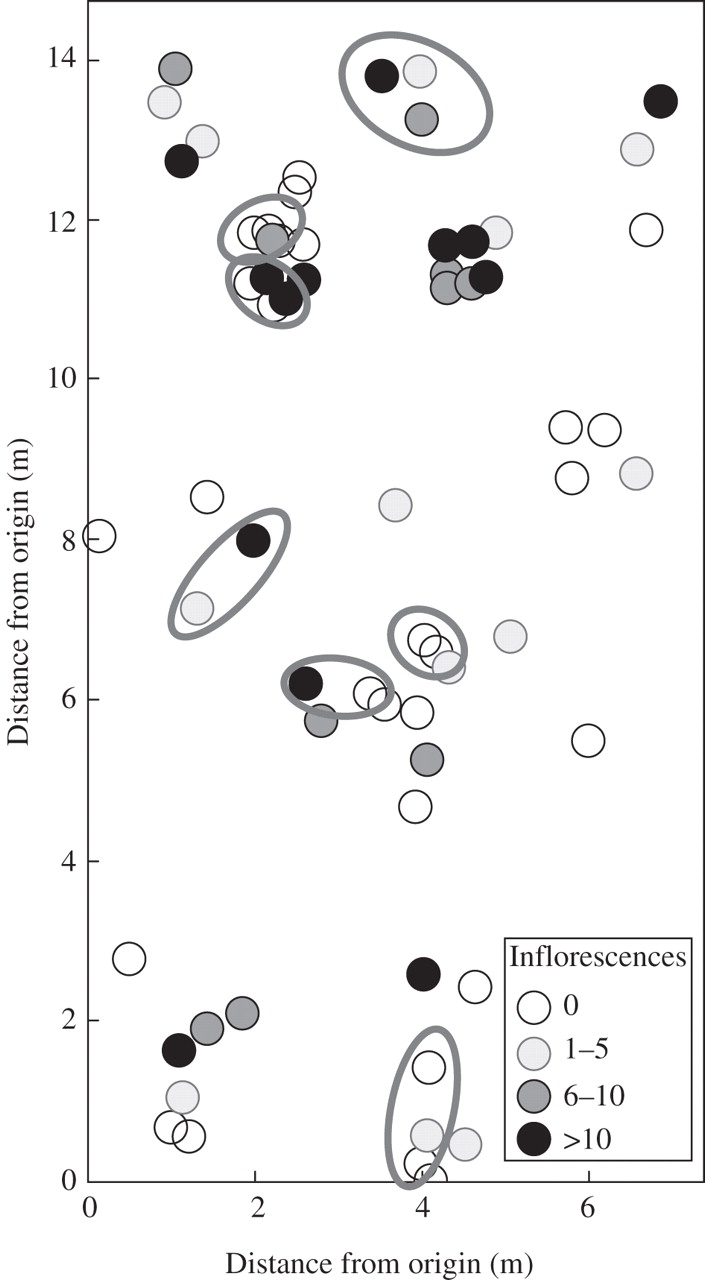
Small-scale distribution of shoots of G. macrocarpa. Dots indicate individual shoots, shading corresponds to its number of inflorescences. Outlined areas include shoots with an identical marker phenotype.
DISCUSSION
Genetic structure
Our analyses of genetic structure revealed clear differentiation among populations, but levels differed tremendously among the two Galitzkya species. The ΦST-value of 0·251 for G. macrocarpa is not unexpectedly high. Meta-analysis of RAPD-based estimates of FST/ΦST-values (Nybom and Bartish, 2000; Nybom, 2004) demonstrated that ΦST-values are significantly related to live-form, with long-lived perennials showing the lowest figures (mean 0·25), and species with a mixed-breeding system being characterized by intermediate levels (means 0·25–0·4; Nybom and Bartish, 2000; Nybom, 2004). Endemics do not differ from more widespread species in this respect. Compared with other alpine perennials, pairwise ΦST-values among G. macrocarpa populations are also intermediate (Fig. 4), as values between 0·1 and 0·5 are widely reported (e.g. Gugerli et al., 1999; Stehlik et al., 2001; Schönswetter et al., 2002, 2004; Young et al., 2002). In comparison, the overall ΦST-value of 0·550 for G. potaninii appears relatively high. In alpine plants, similarly high ΦST-values were usually interpreted as evidence of prolonged isolation, assumed to date back to the last glacial period (Schönswetter et al., 2002, 2004; Reisch et al., 2003). Pronounced differentiation in Dsungarian populations of G. potaninii may also be related to founder effects, and subsequent severe isolation. Another option is that populations in the Dsungarian Gobi and in the Transaltay Gobi originated from different refugia, which can not be assessed without data from the Chinese part of the range, but seems less likely with respect to the regional topography. Thus, there are good reasons to suspect that G. potaninii populations in the Dsungarian Gobi have been isolated for extended periods of time.
Comparatively low levels of population differentiation imply that in G. macrocarpa, isolation may be less severe or has occurred much more recently (Max et al., 1999). This is indicated by the overlap of populations in the PCoA (Fig. 3) and by the results of the AMOVA analysis (Table 3). Most of the genetic variance is kept within populations (>70 %), a pattern often described for mountain plants (Gugerli et al., 1999; Cotrim et al., 2003; Pluess and Stöcklin, 2004), for endemic plants from Tibet (Chen et al., 2005) and from Central Asian deserts (Li and Ge, 2001; Ge et al., 2003; Sheng et al., 2005). However, available data does not allow a generalization of such a pattern, as higher levels of population differentiation have also been reported, both from Tibet (Chen et al., 2005; Xia et al., 2005) and from Central Asia (Ge et al., 2003, 2005).
Variation among populations and regions was nonetheless significant in G. macrocarpa, so there clearly is an impact of spatial isolation, albeit less severe than that documented for G. potaninii. Correspondingly, only part of the Mantel tests of spatial and genetic differences gave significant values for the standardized Mantel statistic, suggesting that much of the variation among populations of G. macrocarpa is not related to spatial distance. Because Galitzkya species are insect-pollinated, long-distance seed dispersal, rather than pollen flow, is likely to be responsible for gene flow among mountain ranges (Pluess and Stöcklin, 2004). Available data for the similarly sized Brassicaceae Biscutella laevigata, which also has winged seeds and grows on exposed rocks in Europe, suggests a limited dispersal capacity with <1·5 % of the seeds flying >100 m (Tackenberg, 2001; Tackenberg et al., 2003). However, as micro-scale wind patterns are of outmost importance for seed uplift, and weather observations suggest that upgoing winds can be very strong in the region studied (>8 on the Beaufort scale), there is at least a potential for effective uplift of seeds and, consequently, occasional long-distance dispersal.
Within population diversity
RAPDs are dominant markers and estimates of genetic diversity are relatively crude. However, values of average gene diversity obtained for the study species were closely related to those calculated based on simple multivariate similarity (Sörensen distance vs. average gene diversity, r = 0·921). This supports the notion that relative comparisons within datasets should be possible (Nybom and Bartish, 2000; Nybom, 2004). In G. macrocarpa, genetic diversity of populations was not positively correlated to habitat size, and figures did not differ much between mountains. In all mountain ranges, the potential habitat size was above 7 km2, which provides ample space for a relatively small plant like this, but results of the AMOVA indicate that there is significant genetic variation among populations within a given mountain range, so habitats are probably not continuously colonized. Thus, the real populations of G. macrocarpa may be much smaller than that suggested by the estimates derived from the vegetation map (scale 1 : 250 000; von Wehrden et al., 2006).
Figures for intra-population genetic diversity are nonetheless relatively low when compared with studies on other fragmented plant species performed by the present working group with the same methodology (Dittbrenner et al., 2005; Hensen and Oberprieler, 2005; Hensen et al., 2005) and, also, though this has to be treated with caution because of differing marker systems, compared with other alpine plants (Schönswetter et al., 2002, 2004; Cotrim et al., 2003). It cannot be ruled out that the low genetic diversity is related to random losses of alleles in small populations. However, genetic variability was not further reduced in peripheral populations, and there was only limited evidence for isolation by distance. Thus, it is suspected that current patterns are more easily explained by effects on the entire species. Pronounced range contractions and possible bottlenecks are plausible with respect to the strong climatic changes that these dry mountain ranges experienced in the Quaternary (Gunin et al., 1999).
Galitzkya potaninii differs also in this respect. Low levels of genetic diversity were found in the Dsungarian Gobi, where habitats are relatively small and populations are widely isolated from the main range in the north-western Chinese uplands (Fig. 1). However, levels were higher in the population from the Atas Bogd, which is geographically less isolated from the remainder of its range. Thus, in the peripheral populations in the Dsungarian Gobi, isolation has apparently prevented genetic exchange resulting in overall lower levels of genetic diversity than in the less isolated populations. Similar results have been obtained for montane plants in North America (Godt et al., 1996).
Clonal growth in G. macrocarpa
Identical specimens were hardly found in the overall sample of 13 G. macrocarpa populations, and the small-scale mapping revealed that 61 ramets on 98 m2 represent 52 genets (Fig. 5). The few clones reached a maximum extension of up to 2 m, which should translate to an age of several decades, or even centuries. However, sexual reproduction is clearly the more important mode of recruitment in the life cycle of G. macrocarpa and suggests that establishment of sexual propagules, and thus also gene flow, is possible. Studies on genetic structure have demonstrated higher importance of clonal growth in a number of Central Asian desert species (Li and Ge, 2001; Su et al., 2003; Xu et al., 2003), and the number of clonal plants is thought to increase with increasing aridity of the sites (Song et al., 2002). Clonal growth in alpine plants has been described for several growth forms including perennial grasses (Steinger et al., 1996; Linhart and Gehring, 2003), perennial herbs (Diggle et al., 1998; Jones and Gliddon, 1999), and shrubby species (Escaravage et al., 1998; Young et al., 2002). In some alpine plants such as Saxifraga cernua (Bauert et al., 1998) extensive clonal growth correlates with reduced genetic diversity. In the region studied, the prostrate Juniperus sabina forms clones totalled up to 100 m in diameter while sexual reproduction had practically ceased (Wesche et al., 2005c).
It is concluded that sexual reproduction is apparently effective in G. macrocarpa, gene flow seems to be present and there is no direct evidence for loss of genetic diversity due to clonal growth. In these respects, G. macrocarpa compares well with the clonal alpine species Geum reptans that maintains local clonal and genetic diversity, and shows only moderated isolation by distance among populations (Pluess and Stöcklin, 2004). A similarly mixed strategy of sexual and asexual reproduction was found in Lloydia serotina (Jones and Gliddon, 1999), Carex scopulorum (Linhart and Gehring, 2003) and Rutidosis leiolepis (Young et al., 2002). If site conditions become unfavourable for seed production or seedling establishment, extended periods of time can be survived by vegetative growth while at the same time guarding against potential risks associated with exclusively clonal growth (Honnay and Bossyut, 2005). Thus, G. macrocarpa appears to be well adapted to an essentially harsh environment where pronounced intra-annual variability renders opportunities for sexual regeneration rare events.
Recommendations for species’ conservation
Galitzkya macrocarpa is completely restricted to Mongolia. Its overall distribution range is approx. 30 000–40 000 km2, though the actual potential habitats cover well below 1000 km2 (Table 1). It clearly is a rare species, but no evidence of shrinking populations was found (Wesche et al., 2005a). Thus, at present the species cannot be regarded as vulnerable according to standard criteria (IUCN, 2001).
Current levels of fragmentation could still threaten long-term survival. Estimates of gene flow based on ΦST-values (Wright, 1931) are crude but still widely used (Wang, 2004). In the case of G. macrocarpa, gene flow is estimated at 0·75 exchanged individuals per generation (ΦST = 0·251). This is less than the minimum of one migrant per generation — a rule-of-thumb value that is thought to be sufficient to maintain genetic exchange. On the other hand, a ΦST value of 0·251 is not an unusual figure compared with other species of perennial herbs, and similar levels of population differentiation have also been described for naturally fragmented montane species (Pluess and Stöcklin, 2004; Chen et al., 2005). In the present case, fragmentation is largely controlled by the current levels of aridity. Less drought-tolerant vegetation types were more widespread in the southern Mongolian mountains some 4000–2000 years bp (Gunin et al., 1999; Jäger, 2005), and current levels of fragmentation in G. macrocarpa should have been reached later than that.
With respect to its overall limited distribution, Mongolian populations of G. potaninii certainly have importance for conservation of that species. The present estimate for the ΦST-value of 0·550 corresponds to only 0·20 exchanged individuals per population, a very low figure that indicates ‘severe fragmentation’ (IUCN, 2001). Further data on distribution and population structure, especially in the Chinese part of its range, are thus urgently needed to assess if the species has not already become endangered.
At present, there is no immediate need for conservation action in G. macrocarpa, but levels of fragmentation and genetic diversity imply that some monitoring is also required. As possible threats may be related to climate change, rather than land use, they may be beyond conservation action in situ. Because the greater part of genetic variability is captured within populations, sampling for conservation ex situ should concentrate on representing a high number of individuals rather than representing all populations; a strategy which has already been proposed for other Central Asian endemics (Young et al., 2002; Ge et al., 2003).
Acknowledgments
Invaluable technical assistance was provided by B. Müller, who was aided by A. Fothe, C. Kilian and K. Ronnenberg. H. von Wehrden, V. Clausnitzer, R. Tungalag and members of the Gobi Gurvan Saykhan Research Project helped during fieldwork, which would not have been possible without financial support by the German Science Foundation (DFG), Bundesministerium für wirtschaftliche Zusammenarbeit und Entwickung (BMZ), the Schimper-Foundation and the Gesellschaft für technische Zusammenarbeit (gtz). The administration of the Gobi Gurvan Saykhan National Park gave institutional support, as did R. Samyaa and Ts. Jamsran at the National University of Mongolia, Ulaan Baatar. E. Jäger assisted in compiling the data on distributional ranges; D. McCluskey kindly checked our English. Data presentation and analysis benefited from the comments of two anonymous referees and the handling editor. This is contribution no. 266 in the series ‘Results of the Mongolian–German Biological Expedition since 1962’.
LITERATURE CITED
- Bachmann K. (1994) Tansley Review No. 63. Molecular markers in plant ecology. New Phytologist 126403–418. [DOI] [PubMed] [Google Scholar]
- Bauert MR, Kälin M, Baltisberger M, Edwards PJ. (1998) No genetic variation detected within isolated relict populations of Saxifraga cernua in the Alps using RAPD markers. Molecular Ecology 71519–1527. [Google Scholar]
- Boczantzeva V. (1979) The new genus Galitzkya V. Boczantzeva (Cruciferae). Botanicheskiy Jurnal 641440–1442. [Google Scholar]
- ter Braak CJF and Smilauer P. (2002) Canoco 4·5 reference manual.(Ceske Budejovice: Biometris, Wageningen).
- Chen S, Xia T, Chen S, Zhou Y. (2005) RAPD profiling in detecting genetic variation in endemic Coelonema (Brassicaceae) of Qinghai-Tibet Plateau of China. Biochemical Genetics 43189–201. [DOI] [PubMed] [Google Scholar]
- Cotrim HC, Chase MW, Pais MS. (2003) Silene rothmaleri P.Silva (Caryophyllaceae), a rare, fragmented but genetically diverse species. Biodiversity and Conservation 121083–1098. [Google Scholar]
- Diggle PK, Lower S, Ranker TA. (1998) Clonal diversity in alpine populations of Polygonum viviparum (Polygonaceae). Israel Journal of Plant Sciences 159606–615. [Google Scholar]
- Dittbrenner A, Hensen I, Wesche K. (2005) Genetic structure and RAPD diversity of the rapidly declining Angelica palustris (Apiaceae) in Eastern Germany in relation to population size and seed production. Plant Species Biology 20191–200. [Google Scholar]
- Escaravage N, Questiau S, Pornon A, Doche B, Taberlet P. (1998) Clonal diversity in a Rhododendron ferrugineum L. (Ericaceae) population inferred from AFLP markers. Molecular Ecology 7975–982. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankham RD. (2005) Genetics and extinction. Biological Conservation 126131–140. [Google Scholar]
- Ge X-Y, Yu Y, Zhao N-X, Chen H-S, Qi W-Q. (2003) Genetic variation in the endangered Inner Mongolia endemic shrub Tetraena mongolica Maxim (Zygophyllaceae). Biological Conservation 111427–434. [Google Scholar]
- Ge X-Y, Yu Y, Yuan Y-M, Huang H-W, Yan C. (2005) Genetic diversity and geographic differentiation in endangered Ammopiptanthus (Leguminosae) populations in desert regions of northwest China as revealed by ISSR analysis. Annals of Botany 95843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt MJW, Johnson BR, Hamrick JL. (1996) Genetic diversity and population size in four rare southern Appalachian plant species. Conservation Biology 10796–805. [Google Scholar]
- Grubov VI. (2001) Key to the vascular plants of Mongolia(Science Publishers, Plymouth, UK) Vols I and II..
- Gubanov IA. (1996) Conspectus of the flora of Outer Mongolia (vascular plants).(Valang Publishers, Moscow) [in Russian].
- Gugerli F, Eichenberger K, Schneller JJ. (1999) Promiscuity in populations of the cushion plant Saxifraga oppositifolia in the Swiss Alps as inferred from random amplified polymorphic DNA (RAPD). Molecular Ecology 8453–461. [Google Scholar]
- Gunin PD, Vostokova EA, Dorofeyuk NI. (1999) Vegetation dynamics of Mongolia.(Kluwer Academic Publishers, Dordrecht).
- Gunin PD, Slemnev NN, Tsoog S. (2003) Seed regeneration of dominant plants in ecosystems of the desert zone of Mongolia: dynamics of undergrowth populations. Botaniceskij Zurnal 881–17. [Google Scholar]
- Hensen I and Oberprieler C. (2005) Effects of population size on genetic diversity and seed production in the rare Dictamnus albus (Rutaceae) in Central Germany. Conservation Genetics 663–73. [Google Scholar]
- Hensen I, Oberprieler C, Wesche K. (2005) Genetic structure, population size, and seed production of Pulsatilla vulgaris Mill. (Ranunculaceae) in Central Germany. Flora 2003–14. [Google Scholar]
- Honnay O and Bossyut B. (2005) Prolonged clonal growth: escape route or route to extinction? Oikos 108427–432. [Google Scholar]
- Honnay O, Jacquemyn H, Roldan-Ruiz I, Hermy M. (2006) Consequences of prolonged clonal growth on local and regional genetic structure and fruiting success of the forest perennial Maianthemum bifolium. Oikos 11221–30. [Google Scholar]
- IUCN red list categories and criteria IUCN. (2001) Version 3·1. Gland: IUCN.
- Jäger EJ. (2005) The occurrence of forest plants in the desert mountains of Mongolia and their bearing on the history of the climate. Erforschung biologischer Ressourcen der Mongolei 9237–245. [Google Scholar]
- Jones B and Gliddon C. (1999) Reproductive biology and genetic structure in Lloydia serotina. Plant Ecology 141151–161. [Google Scholar]
- Keeler KH, Williams CF, Vescio LS. (2002) Clone size of Andropogon gerardii Vitman (Big Bluesteem) at Konza Prairie, Kansas. American Midland Naturalist 147295–304. [Google Scholar]
- Lavrenko EM and Karamysheva ZV. (1993) Steppes of the former Soviet Union and Mongolia. In Coupland RT (Ed.). Natural grasslands. Ecosystems of the world 8b.(Elsevier, Amsterdam).
- Legendre P and Legendre L. (1998) Numerical ecology.(Elsevier, Amsterdam).
- Li A and Ge S. (2001) Genetic variation and clonal diversity of Psammochloa villosa (Poaceae) detected by ISSR markers. Annals of Botany 87585–590. [Google Scholar]
- Linhart YB and Gehring JL. (2003) Genetic variability and its ecological implications in the clonal plant Carex scopulorum Holm. in Colorado tundra. Arctic, Antarctic and Alpine Research 35429–433. [Google Scholar]
- McCune B and Mefford MJ. (1999) PC-ORD. Multivariate analysis of ecological data.(MjM Software, Gleneden Beach, OR).
- Max K, Mouchaty SK, Schwaegerle KE. (1999) Allozyme and morphological variation in two subspecies of Dryas octopetala (Rosaceae) in Alaska. American Journal of Botany 861637–1644. [PubMed] [Google Scholar]
- Nybom H. (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Molecular Ecology 131143–1155. [DOI] [PubMed] [Google Scholar]
- Nybom H and Bartish IV. (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspectives in Plant Ecology, Evolution and Systematics 393–114. [Google Scholar]
- Pavlov NV. (1961) Flora Kazachstana.(Izdat. Akad. Nauk Kazachsk. SSR, Alma-Ata) [in Russian].
- Pluess AR and Stöcklin J. (2004) Population genetic diversity of the clonal plant Geum reptans (Rosaceae) in the Swiss Alps. American Journal of Botany 912013–2021. [DOI] [PubMed] [Google Scholar]
- Reed DH and Frankham R. (2003) Correlation between fitness and genetic diversity. Conservation Biology 17230–237. [Google Scholar]
- Reisch C, Poschlod P, Wingender R. (2003) Genetic variation of Saxifraga paniculata Mill. (Saxifragaceae): molecular evidence for glacial relict endemism in central Europe. Biological Journal of the Linnean Society 8011–21. [Google Scholar]
- Retzer V. (2004) Carrying capacity and forage competition between livestock and a small mammal, the Mongolian Pika (Ochotona pallasi) in a non-equilibrium ecosystem, South-Gobi, Mongolia.(Görich & Weiershäuser Verlag, Marburg).
- Schneider S, Roessli D, Excoffier L. (2000) Arlequin ver. 2·000: a software for population genetics data analysis.(Genetics and Biometry Laboratory, University of Geneva, Geneva, Switzerland).
- Schönswetter P, Tribsch A, Barfuss M, Niklfeld H. (2002) Several Pleistocene refugia detected in the high alpine plant Phyteuma globulariifolium Sternb. & Hoppe (Campanulaceae) in the European Alps. Molecular Ecology 112637–2647. [DOI] [PubMed] [Google Scholar]
- Schönswetter P, Tribsch A, Stehlik I, Niklfeld H. (2004) Glacial history of high alpine Ranunculus glacialis (Ranunculaceae) in the European Alps in a comparative phylogeographical context. Biological Journal of the Linnean Society 81183–195. [Google Scholar]
- Schönswetter P, Stehlik I, Holderegger R, Tribsch A. (2005) Molecular evidence for glacial refugia of mountain plants in the European Alps. Molecular Ecology 143547–3555. [DOI] [PubMed] [Google Scholar]
- Setsuko S, Ishida K, Tomaru N. (2004) Size distribution and genetic structure in relation to clonal growth within a population of Magnolia tomentosa Thunb. (Magnoliaceae). Molecular Ecology 132645–2653. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Zheng W-H, Pei K-Q, Ma K-P. (2005) Genetic variation within and among populations of a dominant desert tree Haloxylon ammodendron (Amaranthaceae) in China. Annals of Botany 96245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Dong M, Jiang G. (2002) Importance of clonal plants and plant species diversity in the Northeast China Transect. Ecological Research 17705–716. [Google Scholar]
- SPSS. (2003) SPSS for Windows 12·0G.(SPSS Inc, Chicago, IL).
- Stehlik I, Schneller JJ, Bachmann K. (2001) Resistance or emigration: response of the high-alpine plant Eritrichium nanum (L.) Gaudin to the ice age within the Central Alps. Molecular Ecology 10357–370. [DOI] [PubMed] [Google Scholar]
- Steinger T, Körner C, Schmid B. (1996) Long-term persistence in a changing climate: DNA analysis suggests very old stages of clones of alpine Carex curvula. Oecologia 10594–99. [DOI] [PubMed] [Google Scholar]
- Su H, Qu L-J, He K, Zhang Z, Wang J, Chen Z, Gu H. (2003) The Great Wall of China: a physical barrier to gene flow? Heredity 90212–219. [DOI] [PubMed] [Google Scholar]
- Tackenberg O. (2001) Methoden zur Bewertung gradueller Unterschiede des Ausbreitungspotentials von Pflanzenarten.(J. Cramer, Berlin).
- Tackenberg O, Poschlod P, Bonn S. (2003) Assessment of wind dispersal potential in plant species. Ecological Monographs 73191–205. [Google Scholar]
- Wang J. (2004) Application of the one-migrant-per-generation rule to conservation and management. Conservation Biology 18332–343. [Google Scholar]
- von Wehrden H, Wesche K, Reudenbach C, Miehe G. (2006) Mapping of large-scale vegetation pattern in southern Mongolian semi-deserts—an application of LANDSAT 7 data. Erdkunde 60261–272. [Google Scholar]
- Wesche K, Jäger EJ, von Wehrden H, Undrakh R. (2005a) Status and distribution of four endemic vascular plants in the Gobi Altay. Mongolian Journal of Biological Sciences 33–11. [Google Scholar]
- Wesche K, Miehe S, Miehe G. (2005b) Plant communities of the Gobi Gurvan Sayhan National Park (South Gobi Aimag, Mongolia). Candollea 60149–205. [Google Scholar]
- Wesche K, Ronnenberg K, Hensen I. (2005c) Lack of sexual reproduction in dry mountain steppe populations of the clonal shrub Juniperus sabina L. in southern Mongolia. Journal of Arid Environments 63390–405. [Google Scholar]
- Wright S. (1931) Evolution in Mendelian populations. Genetics 1697–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Chen S, Chen S, Ge X. (2005) Genetic variation within and among populations of Rhodiola alsia (Crassulaceae) native to the Tibetan Plateau as detected by ISSR markers. Biochemical Genetics 4387–101. [DOI] [PubMed] [Google Scholar]
- Xu L, Wang Y-L, Wang X-M, Zhang LJ, Yue M, Gu F-X, et al. (2003) Genetic structure of Reaumuria soongorica population in Fukang desert, Xinjang and its relationship with ecological factors. Acta Botanica Sinica 45 pp. 787–794. [Google Scholar]
- Young AG, Hill JH, Murray BG, Peakall R. (2002) Breeding system, genetic diversity and clonal structure in the sub-alpine forb Rutidosis leiolepis F.Muell. (Asteraceae). Biological Conservation 10671–78. [Google Scholar]
- Yu F, Dong M, Krusi B. (2004) Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytologist 162697–704. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chiang TY, Miehe G, Liu JQ, Abbott RJ. (2005) Phylogeography of the Qinghai-Tibetan Plateau endemic Juniperus przewalskii (Cupressaceae) inferred from chloroplast DNA sequence variation. Molecular Ecology 143513–3524. [DOI] [PubMed] [Google Scholar]
- Zhou T, Lu L, Yang G, Al-Shehbaz IA. (2001) Brassicaceae. In Commitee FoCE (Ed.). Flora of China.(Science Press, Brassicaceae through Saxifragaceae. Beijing) Vol. 8.