Abstract
• Background and Aims Rain-fed lowland rice commonly encounters stresses from fluctuating water regimes and nutrient deficiency. Roots have to acquire both oxygen and nutrients under adverse conditions while also acclimating to changes in soil-water regime. This study assessed responses of rice roots to low phosphorus supply in aerated and stagnant nutrient solution.
• Methods Rice (Oryza sativa ‘Amaroo’) was grown in aerated solution with high P (200 μm) for 14 d, then transferred to high or low (1·6 μm) P supply in aerated or stagnant solution for up to 8 d.
• Key Results After only 1 d in stagnant conditions, root radial oxygen loss (ROL) had decreased by 90 % in subapical zones, whereas near the tip ROL was maintained. After 4 d in stagnant conditions, maximum root length was 11 % less, and after 8 d, shoot growth was 25 % less, compared with plants in aerated solution. The plants in stagnant solution had up to 19 % more adventitious roots, 24 % greater root porosity and 26 % higher root/shoot ratio. Rice in low P supply had fewer tillers in both stagnant and aerated conditions. After 1–2 d in stagnant solution, relative P uptake declined, especially at low P supply. Aerated roots at low P supply maintained relative P uptake for 4 d, after which uptake decreased to the same levels as in stagnant solution.
• Conclusions Roots responded rapidly to oxygen deficiency with decreased ROL in subapical zones within 1–2 d, indicating induction of a barrier to ROL, and these changes in ROL occurred at least 2 d before any changes in root morphology, porosity or anatomy were evident. Relative P uptake also decreased under oxygen deficiency, showing that a sudden decline in root-zone oxygen adversely affects P nutrition of rice.
Keywords: Aerenchyma, phosphorus deficiency, phosphorus uptake, radial oxygen loss, rain-fed lowland rice, respiration, root morphology, root porosity, stagnant solution
INTRODUCTION
Rain-fed lowland rice in Asia commonly encounters stress conditions, most notably due to fluctuation in water regimes in the root zone, and nutrient deficiency. In the rain-fed lowlands, rice experiences intermittent anaerobic and aerobic conditions in the root zone (Zeigler and Puckridge, 1995). Deep root activity in aerobic soil is supported by oxygen uptake directly from the air-filled soil pores, whereas in anaerobic flooded soils oxygen supply is via internal diffusion in aerenchyma (Armstrong, 1979). Root acclimation to the changing water regimes may in turn affect plant morphological features, physiological function and nutrient uptake efficiency.
In waterlogged soil, roots acclimate by increasing the internal supply of oxygen to the root tip from the atmosphere, via formation of aerenchyma (Justin and Armstrong, 1987; Drew et al., 1994; Drew, 1997). This modification of the root cortex by aerenchyma formation enhances longitudinal oxygen diffusion, but might also decrease symplastic nutrient transport across the roots to the stele (Drew and Saker, 1986; Kronzucker et al., 1998). Furthermore, in order to minimize radial oxygen loss (ROL) from the main axes of adventitious roots, rice forms a barrier to gas diffusion near the root exterior (Colmer, 1998) believed to be related to increased suberization of the exodermis (Ranathunge et al., 2004). Although such a barrier might cause an inhibition of nutrient absorption by roots (Koncalova, 1990), direct studies of this are few, and the data available indicate that nutrient uptake might not be affected (Rubinigg et al., 2002). Furthermore, it has been proposed that the axial root with aerenchyma is inefficient in nutrient uptake, and that new fine lateral roots are induced for nutrient absorption (Kirk and Du, 1997; Kirk, 2003). The fine lateral roots comprise the bulk of the external surface, and the laterals are connected directly into the main water and solute transport vessels in the stele of the primary root (Matsuo and Hoshikawa, 1993). Moreover, roots of rice acclimate to growth in anaerobic condition by increasing the number of adventitious roots per plant, which presumably also contributes to waterlogging tolerance (Colmer, 2003b). Rice plants grown in aerobic soils have a different structure of roots; maximum root lengths are greater in aerobic than in anaerobic media, and plants in aerobic media have fewer adventitious roots and these have lower porosity and a much less pronounced barrier against ROL (Colmer, 2003b).
The intermittently waterlogged conditions that occur in soils of the rain-fed lowland rice ecosystem are likely to have adverse effects on phosphorus nutrition of rice on low P soils, due to changes in P availability with different water regimes (Huguenin-Elie et al., 2003; Seng et al., 2004) and possibly due to adverse effects of soil oxidation/reduction cycles on root function. The adaptations of plants for increased P acquisition include mycorrhizal symbioses, rhizosphere modification by secretion of organic acids (Gardner et al., 1983; Lipton et al., 1987; Lu et al., 1999) and proton release (Kirk and Du, 1997). Moreover, roots also increase surface area by root hair elongation and proliferation (Bates and Lynch, 1996; Ma et al., 2003). For rice in anaerobic root conditions, low P supply enhanced adventitious root elongation and lateral root development and elongation (Kirk and Du, 1997). Fan et al. (2003) reported that in maize roots, aerenchyma formation can be induced by low P status, and this in turn reduced respiration and P requirement in the roots. A similar decline in respiratory costs of root growth in rice with low P may enhance its adaptation to P-deficient flooded soils. However, the adaptation of rice roots in low P soils to the transitions from aerobic to anaerobic conditions and vice versa has not been studied. This study evaluated the early responses of morphology and physiology in rice to changes in oxygen and P supplies, and how these in turn affect P uptake and plant growth.
MATERIALS AND METHODS
Three experiments were conducted in a glasshouse at Murdoch University, Western Australia. During the two main experiments reported here, minimum/maximum temperatures and day lengths were 25/30 °C, 13 h and 35/42 °C, 12 h, respectively. Rice plants were harvested after 0 (i.e. initial), 1, 2, 4 and 8 d of treatments.
Plant culture
Seeds of rice (Oryza sativa L. ‘Amaroo’) were imbibed in deionized water for 48 h at room temperature. Germinated seeds were incubated on moist paper in the laboratory. After 3–7 d, seedlings were transferred onto a mesh screen on full-strength nutrient solution in a glasshouse for 2 weeks. Seedlings were transplanted to pots containing 12 L of aerated full-strength nutrient solution for a further 2 weeks before treatments were imposed (each pot held six seedlings, one plant was randomly taken for each harvest). Seven levels of P supply were tested in a preliminary experiment, to select 200 and 1·6 μm P as adequate (high) and deficient (low) levels for use in the experiments, respectively. The stagnant nutrient solution contained 0·1 % (w/v) agar to prevent convective movement in the pots (Wiengweera et al., 1997). Stagnant solution, however, does not fully simulate the sink for oxygen that exists in waterlogged soil. Reduced iron (Fe II) in flooded soil is a strong sink for ROL (Kirk, 2004). Thus, the role that ROL plays in the soil by diminishing toxicity of iron (Green and Etherington, 1977) and other soil toxins (Colmer, 2003a, and references therein) is absent in stagnant solution culture.
The composition of the full-strength nutrient solution (mol m−3) was: NO3−, 4·375; K+, 3·95; SO42−, 1·90; Ca2+, 1·50; NH4+, 0·625; Mg2+, 0·40; H2PO4−, 0·20; Na+, 0·20; H4SiO4, 0·10; and the micronutrients (mmol m−3) Cl, 50; B, 25; Mn, 2; Zn, 2; Ni, 1; Cu, 0·5; Mo, 0·5; Fe-EDTA, 50. The pH was adjusted to 6·5 using KOH or HCl. All chemicals used were of analytical grade. The nutrient solution was renewed every 7 d. Four treatments were used: high P level in aerated (A HP) or stagnant (S HP), and low P level in aerated (A LP) or stagnant (S LP) nutrient solutions.
Root and shoot growth measurements
An initial harvest was taken at the time treatments were imposed (day 0), and plants were then harvested after 1, 2, 4 and 8 d of treatment. The length of the longest individual root, numbers of root and numbers of tiller were recorded for each plant. Shoots and roots were excised and oven dried at 70 °C for 72 h, before root and shoot dry weights were determined.
Root porosity and aerenchyma measurements
At each harvest, the roots of one plant were cut into 50-mm segments for porosity measurement. Porosity (% gas spaces per unit tissue volume) of roots from each plant was evaluated by measuring root buoyancy before and after vacuum infiltration of the gas spaces in the roots with water (Raskin, 1983), using modified versions of the equations given by Thomson et al. (1990).
Autofluorescence of cells in root cross-sections
Fresh ‘thick’ adventitious roots (100–120 mm in length) were sampled. Roots were sectioned by hand at 10, 20 and 70 mm behind the apex, using a razor blade. Roots were sampled at 0 and 8 d. Root cross-sections were viewed under blue excitation U-MNB2 (excitation BP 460–490 nm, barrier filter IF 520, dichromatic mirror 500) using an Olympus BX51 photomicroscope and photographed with an Olympus DP70 camera. Autofluorescence can be attributed to walls impregnated with phenolic compound or lipid complexes and hence is an indicator of lignin, suberin and/or other phenolic compounds in cell walls (O'Brien and McCully, 1981; Yeung, 1998). The relative intensity of autofluorescence of cell walls in the layer of sclerenchymatous fibres on the outer side of the root cortex was scored on a three-point scale: 0, when intensity of autofluorescence of the walls in the sclerenchymatous layer was equal to that of the cell layers on either side of it; 1, when intensity of autofluorescence was greater than that in cell walls of cell layers on either side of it and the thickness of the walls was equal to the walls of cell layers on either side of it; 2, when the intensity of autofluorescence was greater than those in cell walls of the cell layers on either side of it and cell walls were thicker than those of cell layers on either side of it. Scores for autofluorescence intensity were made on three replicates (each replicate was a cross-section from an individual root) for cross-sections at 10, 20 and 70 mm behind the root tip.
ROL measurements
ROL was measured for two replicate plants (an adventitious root on an individual plant was one replicate) in both experiments, to provide a total of four replicates. Intact 101–127-mm adventitious roots were selected for ROL measurements in an O2-free root medium using cylindrical root-sleeving O2 electrodes (Armstrong and Wright, 1975) in a temperature-controlled room (30 °C) with a photosynthetically active radiation at shoot height of 150 µmol m−2 s−1. Root systems of intact plants were immersed in a transparent chamber (50 × 50 × 250 mm; breadth × width × height) of 0·1 % (w/v) agar solution with 5·0 mol m−3 KCl and 0·5 mol m−3 CaSO4. The shoots were in air and the stem base was held with wet cotton wool in a rubber lid sealed onto the top of the chamber. An intact adventitious root was selected and gently guided through a root-sleeving O2 electrode (height 5 mm, internal diameter 2·25 mm) with guides to keep the root located towards the centre of the cylindrical electrode. ROL measurements were taken at 10, 20, 30, 40, 50 and 70 mm behind the root tip. Root diameters at these positions along the roots were measured using a microscope with a calibrated eyepiece reticule.
Root oxygen consumption measurements
Roots in aerated solution culture at both high and low P supply were measured for oxygen consumption rates. Adventitious roots were separated into the 0–20-mm tip and 20–40-mm zone from the tip. The 20–40-mm zone contains expanded cells, whereas the 20-mm tip contains expanding and dividing cells, as well as some expanded cells. Locations further behind the tip were not used as lateral roots are present and/or the outer cell layers can have low permeability to oxygen; both situations would complicate interpretations of the data from these measurements. Each zone of root was cut into 5-mm segments for the measurements, which lasted at most 30 min; small root segments minimize the influence of boundary layers. The 5-mm root segments were placed in a sealed cuvette of known volume, which contained air-saturated treatment solutions (composition as used in the growth medium) with rapid mixing via a magnetic stir-bar. The rate of oxygen consumption was measured at 30 °C using the polarographic technique and equipment as described in Lambers and Steingrover (1978) and Lambers et al. (1993).
Phosphorus analyses
After root and shoot dry mass were determined, tissues were ground and ashed at 535 °C for 8 h. The ash was dissolved in 0·1 n HCl, and P was then determined using a colorimetric assay and a spectrophotometer (Murphy and Riley, 1962). Relative P uptake (total P uptake per unit root mass; mg P g−1 root dry weight) was calculated after subtracting the initial P content (at day 0).
Statistical analyses
Data on root and shoot growth, root porosity, ROL, root oxygen consumption rate and tissue P were analysed using linear models as general analyses of variance (ANOVA) to determine the main effects and interactions among treatments. Effects of treatments at each harvest time were analysed as separate experiments. Means were compared using least significant differences (l.s.d.) at P = 0·05.
RESULTS
Root and shoot growth
Overall, plants in stagnant solution had shorter roots than those in aerated solution (Table 1). After 4 d of treatments, the longest adventitious roots in aerated solution were 7 % longer at low P than at high P. By contrast, in stagnant solution the longest root at low P supply was 8 % shorter than at high P. The differences in adventitious root lengths were more clearly distinguished after 8 d of treatments (Table 1).
Table 1.
Lengths of longest roots of rice (‘Amaroo’) grown in aerated solution with high P (200 μm) and then transferred to aerated or stagnant nutrient solution at high and low (1·6 μm) P supply for 4 and 8 d
| Lengths of longest roots (cm) | ||||
|---|---|---|---|---|
| P level | Culture | Initial | 4 d | 8 d |
| Low P | Aerated | 34·8 ± 0·59 | 40·3 ± 2·17 | 45·2 ± 1·42 |
| Stagnant | 34·8 ± 0·59 | 32·7 ± 0·67 | 32·5 ± 3·87 | |
| High P | Aerated | 34·8 ± 0·59 | 37·4 ± 2·42 | 40·7 ± 4·87 |
| Stagnant | 34·8 ± 0·59 | 35·6 ± 1·50 | 37·1 ± 0·95 | |
| F-test | O × P* | O × P* | ||
Values are means of three replicates ± standard errors.l.s.d. for solution aeration and P interaction effects: 4 d, 3·04; 8 d, 2·62.
*Significant at P < 0·05 for O × P based on F-test for solution aeration and P interaction effects.
Stagnant culture for 8 d increased the number of adventitious roots per plant by 12–19 % (Table 2). However, P treatments did not influence the number of adventitious roots per plant in either stagnant or aerated solutions (Table 2). Tiller numbers were 20–33 % greater in plants in aerated solution compared with those in stagnant solution. Moreover, tiller numbers were decreased by low P supply under both root oxygen treatments. Likewise, the low oxygen treatment decreased shoot dry weight and low P also resulted in lower shoot dry weights (Table 3). The differential effect of stagnant conditions on root and shoot growth resulted in higher root/shoot ratio in stagnant than in aerated solution (Table 3).
Table 2.
Adventitious root and tiller numbers of rice (‘Amaroo’) grown in aerated solution at high P (200 μm) (initial condition) and then transferred to aerated or stagnant nutrient solution at low (1·6 μm) or high P (200 μm) supply for 8 d
| Condition | Adventitious root numbers (roots per plant) | Tiller numbers (tillers per plant) | |
|---|---|---|---|
| Initial | 55 ± 2·1 | 3·9 ± 0·3 | |
| Low P | Aerated | 142 ± 18·4 | 10·0 ± 0·9 |
| Stagnant | 161 ± 9·8 | 6·7 ± 0·6 | |
| High P | Aerated | 143 ± 24·0 | 12·0 ± 1·0 |
| Stagnant | 176 ± 7·5 | 8·0 ± 0·0 | |
| F-test | O* | O* and P* |
Values are means of three replicates ± standard errors. l.s.d. for solution aeration effects: adventitious root numbers, 22·52; tiller numbers, 0·71, and for P effects on tiller numbers, 0·71.
*Significant at P< 0·05, O and P based on F-test for solution aeration and P effects, respectively.
Table 3.
Root and shoot growth of rice (‘Amaroo’) in aerated solution at high P (200 μm) (initial condition) and then transferred to aerated or stagnant nutrient solution at low (1·6 μm) or high P (200 μm) supply for 8 d
| Condition | Root dry matter (g per plant) | Shoot dry matter (g per plant) | Relative growth rate (g g−1 d−1) | Root/shoot ratio | |
|---|---|---|---|---|---|
| Initial | 0·17 ± 0·01 | 0·65 ± 0·03 | - | 0·26 ± 0·010 | |
| Low P | Aerated | 0·83 ± 0·03 | 3·23 ± 0·12 | 0·159 ± 0·002 | 0·26 ± 0·001 |
| Stagnant | 0·77 ± 0·09 | 2·19 ± 0·27 | 0·137 ± 0·013 | 0·35 ± 0·002 | |
| High P | Aerated | 0·80 ± 0·09 | 3·24 ± 0·30 | 0·171 ± 0·011 | 0·25 ± 0·004 |
| Stagnant | 0·91 ± 0·04 | 2·66 ± 0·03 | 0·173 ± 0·004 | 0·34 ± 0·012 | |
| F-test | n.s. | O* | O* | O* |
Values are means of three replicates ± standard errors. l.s.d. for solution aeration effect on: shoot dry matter, 0·35; relative growth rate, 0·018; root/shoot ratio, 0·014.
*Significant at P < 0·05; O indicates F-test for solution aeration effects. n.s., no significant difference.
Porosity of adventitious roots
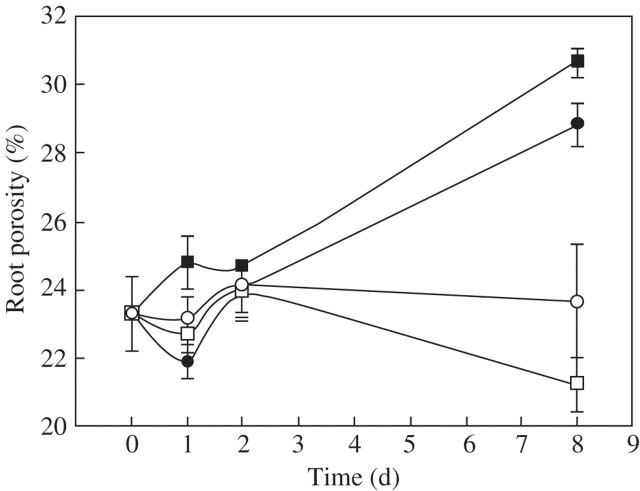
Porosity of the whole root system increased within 8 d after transfer from aerated to stagnant solution, from 23·6 to 28·8 % at low P supply and to 30·6 % at high P supply (Fig. 1). The porosity of roots in aerated solution at low P supply was maintained at 23·6 % and slightly decreased to 21·1 % at high P supply in aerated solution. Transverse sections were also taken to examine the percentage of root cross-sectional area occupied by aerenchyma. At 20 mm from the tip, aerenchyma formation in the roots of plants grown in stagnant solution was increased by 18 %. Rice roots grown in stagnant solution had two-fold greater aerenchyma formation at 70 mm than in aerated roots (data not shown). The observed increase in aerenchyma supported the trends of increased root porosity of rice in stagnant culture (Fig. 1).
Fig. 1.
Root porosity (% gas volume per unit root volume) of rice (‘Amaroo’) grown in aerated solution with high P and then transferred to aerated (open symbols) and stagnant (closed symbols) solutions at 1.6 μm P (circles) and 200 μm P (squares). Root porosity (%) was measured for the whole root system of each plant. Bars represent standard errors of three replicates.
Autofluorescence in walls of outer cell layers in roots
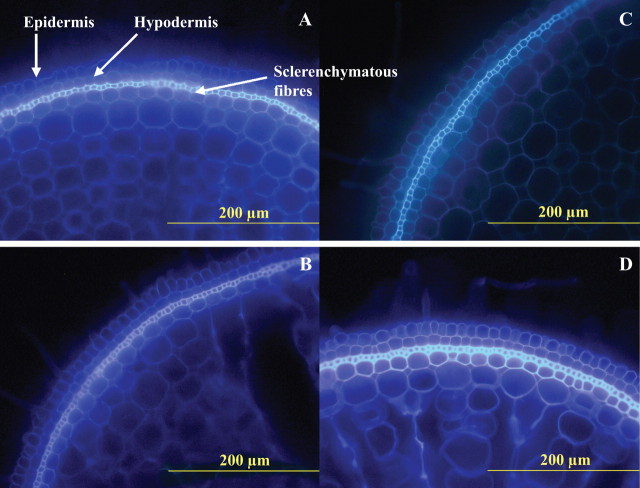
The relative intensity of autofluorescence indicates the secondary thickened walls or walls impregnated with phenolic compounds or lipid-complexes in the layer of fibre cells, as well as in the hypodermal layer and the layer immediately inside the fibre cells, in response to stagnant solution treatment for 8 d (Table 4). In aerated solution, the intensity of autofluorescence of cell walls in these outer cell layers remained unchanged during the 8 d of the experiment. The greatest increase in autofluorescence of cell walls in these outer cell layers for roots in stagnant compared with aerated solution was evident in root sections at 70 mm, rather than 20 mm, from the tip (Fig. 2).
Table 4.
Score for autofluorescence in the layer of sclerenchymatous fibre cells in rice roots when grown in aerated solution at high P (200 μm) and then transferred to aerated or stagnant nutrient solution at low (1·6 μm) or high P (200 μm) supply for 8 d
| Distance of sections from the root apex (mm) | Score of autofluorescence of sclerenchymatous fibre cells in rice roots* | |||
|---|---|---|---|---|
| Low P | High P | |||
| Aerated | Stagnant | Aerated | Stagnant | |
| 10 | 1·00 ± 0·00 | 2·00 ± 0·00 | 1·00 ± 0·00 | 1·67 ± 0·33 |
| 20 | 1·33 ± 0·33 | 1·50 ± 0·41 | 1·33 ± 0·33 | 1·67 ± 0·33 |
| 70 | 2·00 ± 0·00 | 2·00 ± 0·00 | 1·33 ± 0·33 | 2·00 ± 0·00 |
Values are means of three replicates ± standard errors.
*The relative intensity of autofluorescence of cell walls in the layer of sclerenchymatous fibres on the outer side of the root cortex was scored on a three-point scale: 0, when intensity of autofluorescence of the walls in the sclerenchymatous layers was equal to that of the cell layers on either side of it; 1, when intensity of autofluorescence was greater than that in cell walls of cell layers on either side of it and the thickness of the walls was equal to the walls of cell layers on either side of it; 2, when the intensity of autofluorescence was greater than those in cell walls of the cell layers on either side of it and had thicker walls than those of cell layers on either side of it.
Fig. 2.
Typical transverse sections of rice roots showing autofluorescence of the walls in the outer cell layers at 20 mm (A) and 70 mm (B) from the apex or in stagnant nutrient solution at 20 mm (C) and 70 mm (D). Plants were grown for 8 d after transfer to high P level (200 μm) in aerated nutrient solution. Increased autofluorescence in response to the stagnant treatment was evident for the walls of the sclerenchymatous fibres, as well as those in the hypodermal cell layer and the cells to the immediate interior of the fibre cells. Average scoring of sections in triplicate is given in Table 4.
Radial oxygen loss measurements
Only 1 d after transfer of aerated roots to stagnant solution, rates of ROL were unchanged at 10 mm behind the root tip, but had decreased markedly to low rates in the more basal positions measured at 20–70 mm behind the tip (Fig. 3). The ROL rates from the roots in stagnant solution were not affected by P treatment. For plants from aerated solution, those at high P supply had higher ROL rates (when transferred to the oxygen-free root medium for the ROL measurements) than the adventitious roots of plants grown in aerated solution at low P supply.
Fig. 3.
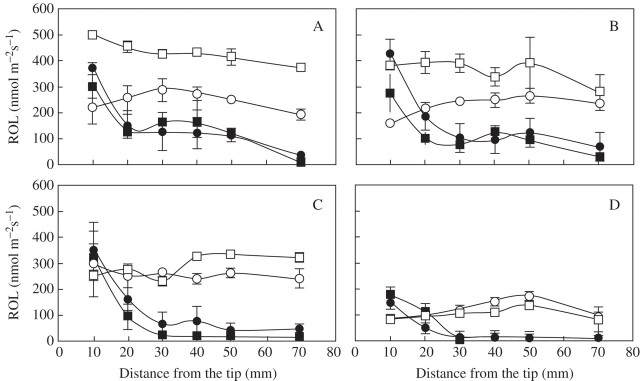
Rate of radial O2 loss (ROL) along adventitious roots of rice (‘Amaroo’) after transition to treatment solutions: aerated at 1.6 μm P (open circles) and 200 μm P (open squares); stagnant at 1·6 μm P (closed circles) and 200 μm P (closed squares). Rates of ROL were measured from one 101–127-mm adventitious root of each of four 28-d-old plants in each treatment, with the treatments imposed for 1 (A), 2 (B), 4 (C) or 8 (D) d. Bars represent standard errors of four replicates.
Two days after transfer, the ROL rates at 10 mm behind the tip of roots of plants grown in stagnant culture were higher than for roots of plants grown in aerated solution. During the first 2 d, low P supply decreased ROL rates for roots of plants in aerated culture compared with those at high P, but this difference was no longer present at later times. Furthermore, the ROL rates along the adventitious root axis for all plants gradually decreased with time, to be lowest after 8 d (Fig. 3). Although ROL rates from the roots on day 8 in aerated culture were decreased five-fold from the first day in treatments, these were still much higher than the ROL rates measured from roots of plants in stagnant solution, except at 10 mm behind the tip. The stagnant culture after 8 d decreased the ROL rates to almost zero along the basal positions measured at 30–70 mm behind the tip, whereas ROL near the tips was still relatively high compared with rates at earlier times (Fig. 3d).
Root oxygen consumption
Root oxygen consumption rates for root tissues of plants grown in aerated solution at low or high P supply for 4 d were measured for two zones of the roots (0–20 mm and 20–40 mm behind the tip). The 0–20-mm tips had 44–52 % higher oxygen consumption rates on a fresh weight basis than the 20–40-mm segments. Root oxygen consumption rates on a fresh mass basis in both tissue zones were not affected by P supply (Table 5).
Table 5.
O2 consumption rate of rice (‘Amaroo’) roots grown in aerated solution at high P (200 μm) and then transferred to aerated or stagnant nutrient solution at low (1·6 μm) or high P (200 μm) supply for 4 d
| O2 consumption rate (n mol O2 g−1 f. wt s−1) | ||
|---|---|---|
| Part of root | 1·6 μm P | 200 μm P |
| 0–20-mm tip | 4·67 ± 0·75 | 5·22 ± 0·47 |
| 20–40-mm zone | 2·61 ± 0·02 | 2·50 ± 0·30 |
Roots for each zone were cut into 5-mm segments and measured in the same solution as the growth medium. Values are means of four replicates ± standard errors. l.s.d. (P < 0·05) for effect of different root part, 0·97. f. wt, fresh weight.
Phosphorus uptake
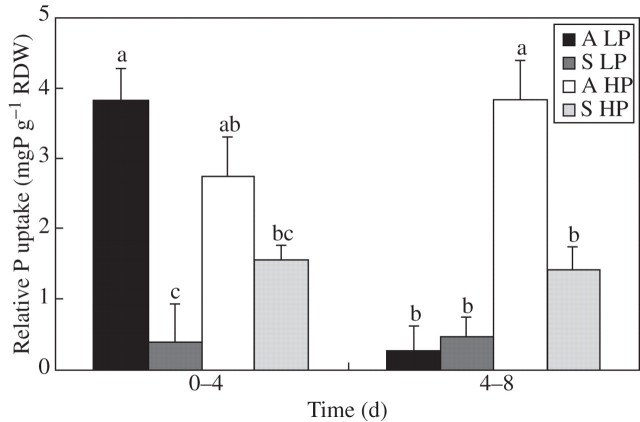
Two days after transition to stagnant culture, relative P uptake was decreased by 90 % at low P supply (Fig. 4). At 4 d, relative P uptake in stagnant plants declined also at high P compared with plants in aerated nutrient solution. Relative P uptake also declined in aerated solution at low P during the last 4 d in treatments.
Fig. 4.
Relative phosphorus uptake by rice (mg P g−1 relative dry weight) after transfer to treatment for 8 d: aerated at 1·6 μm P (A LP), and 200 μm P (A HP) and stagnant at 1·6 μm P (S LP) and 200 μm P (S HP) nutrient solution culture. Phosphorus uptake per unit rice root weight was measured after subtracting the initial (day 0) P content of the 28-d-old plants. Bars represent standard errors of three replicates.
Phosphorus content (i.e. milligrams per plant) of plants after 1 d did not differ among treatments (Table 6). Thereafter, P content for plants grown in stagnant solution at low P supply declined relative to aerated plants, so that after 8 d values were approx. 40 % lower than in plants in aerated solution. The low oxygen also affected the P content for plants at high P supply, by halving P content in stagnant culture. At 8 d, the low P treatment decreased the P accumulation in plants grown in both aerated or in stagnant solution culture by 37 % (Table 6).
Table 6.
Phosphorus content in whole rice plants grown in aerated solution at high P (200 μm) and then transferred to aerated or stagnant nutrient solution at low (1·6 μm) or high P (200 μm) supply for 1, 2, 4 and 8 d
| Time (day after transition) | Phosphorus content (mg per plant) | ||||
|---|---|---|---|---|---|
| Low P | High P | ||||
| Aerated | Stagnant | Aerated | Stagnant | F-test | |
| Initial | 0·62 ± 0·03 | 0·62 ± 0·03 | 0·62 ± 0·03 | 0·62 ± 0·03 | n.s. |
| 1 | 0·66 ± 0·07 | 0·66 ± 0·06 | 0·66 ± 0·09 | 0·62 ± 0·06 | n.s. |
| 2 | 0·86 ± 0·06 | 0·65 ± 0·05 | 0·81 ± 0·07 | 0·93 ± 0·01 | O × P* |
| 4 | 1·28 ± 0·08 | 0·69 ± 0·10 | 1·10 ± 0·12 | 0·89 ± 0·04 | O × P* |
| 8 | 1·36 ± 0·04 | 0·82 ± 0·11 | 2·16 ± 0·12 | 1·31 ± 0·06 | O* and P* |
Values are means of three replicates ± standard errors. l.s.d. for solution aeration and P interaction effects on phosphorus content in whole plants: 2 d after transition, 0·13; 4 d after transition, 0·28; l.s.d. for solution aeration effects on P content in whole plants at 8 d after transition, 0·19; and for P effects on phosphorus content in whole plants at 8 d after transition, 0·19.
*Significant at P < 0·05, O, P and O × P indicate F-test for solution aeration, P and solution aeration and P interaction effects. n.s., no significant difference.
DISCUSSION
Oxygen effect
The roots of plants grown in aerated solution had a porosity within the range found previously in 12 rice cultivars (20–26 %) grown in aerated solution culture for 35 d (Colmer, 2003b), and had high rates of ROL from basal zones when measured in an oxygen-free medium. Before transfer into stagnant solution, plants had been grown in aerated solution until early tillering (average 3·9 tillers per plant). After roots were exposed to stagnant culture for only 1 d, ROL declined markedly in basal zones. However, near the tips of the roots, ROL did not decline after transfer to stagnant solution, indicating that oxygen supply was not limiting to the distal portions of these roots. The decreasing ROL rates from basal zones of roots of rice, and many other plant species, have been interpreted in terms of a physical barrier to restrain radial oxygen diffusion from the root (Armstrong, 1979; Colmer, 1998, 2003a, b; McDonald et al., 2002). The barrier can enhance longitudinal oxygen transport via the aerenchyma to the root tip, by reducing losses to the rhizosphere. The rapid decline in ROL from basal zones preceded the increase in aerenchyma development for plants in stagnant culture. Root porosity was only slightly changed after 2 d in stagnant culture, but after 4–8 d more aerenchyma formation in roots was evident as an increase in the porosity (Fig. 1). This study is the first to demonstrate that the barrier to ROL can be induced in existing roots, an important addition to earlier work that showed induction of the barrier in roots that had formed in a stagnant medium (Colmer, 1998, 2003b).
Roots of rice contain a layer of sclerenchymatous fibre cells (with thickened secondary walls) on the outer side of the cortex that becomes the outermost layer when the two outer cell layers (epidermis and exodermis) are lost, at least in soil-grown roots (Clark and Harris, 1981). Cross-sections of roots taken immediately before treatments were imposed showed that autofluorescence of cell walls in this layer was evident at 70 mm from the apex, whereas at 10 and 20 mm the walls of the immature fibres exhibited very little autofluorescence (Table 4). Autofluorescence in the cell walls in this layer increased after 8 d in roots that had been in stagnant solution, suggesting impregnation of cell walls with phenolic compounds or lipid-complexes (e.g. lignin and/or suberin or other phenolic compounds). The walls of cells in the hypodermal layer (layer immediately exterior to the fibres) and those cells in the layer to the immediate interior of the fibres also showed increased autofluorescence, again indicating impregnation of materials also into these walls. Whether the barrier to the ROL relates directly to the intensity of autofluorescence in the layer of sclerenchymatous fibre cells, or to that for the adjacent cell layers, requires further work using more specific histochemical stains to assess anatomical changes in these layers within the same 2-d time frame after transfer to stagnant solution when ROL had already decreased.
Root acclimation to oxygen deficiency was reflected in changes in root morphology, but not until 4 d after transfer to stagnant solution. The morphological responses, which included decreases in root elongation and new adventitious root production and increased root porosity, occurred too late to explain changes in ROL on days 1–2; nevertheless, these changes might be significant for functioning of the root system in the longer term. The increased root porosity is essential for oxygen transport to tips of roots in anoxic media, especially as roots extend deeper into an oxygen-free medium (Armstrong, 1979). New adventitious roots help to offset the decreased root depth (restricted by the distance that internal oxygen can diffuse along roots; Armstrong, 1979) by adding to total root length and surface area for water and nutrient absorption. These new roots are therefore presumed to be of importance for longer term growth of rice in waterlogged soil. Even when these new roots form a barrier to ROL in basal parts of the main axis, water (Ranathunge et al., 2004) and nutrient (Rubinigg et al., 2002) uptake should still proceed near the root tips, and presumably even more so if lateral roots are formed (Kirk, 2003).
Phosphorus–oxygen interaction
After only 1 d at low P in aerated solution, ROL rates at all positions measured along roots were decreased (plants transferred into an oxygen-free root medium for measurements), but only temporarily for the first 2 d, after which the P effect disappeared (Fig. 3). The disappearance of the P effect on ROL rate may result from changes in sinks for oxygen in the root due to P deficiency. It is not expected to be due to the formation of a barrier to ROL, as the roots were aerated. Similarly, increased porosity in low P roots of aerated solution should have decreased the oxygen consumption in the cortex for respiration (Fan et al., 2003), as well as enhancing internal oxygen transport due to the decreased physical resistance to gas diffusion (Armstrong, 1979). Both the decreased resistance and the lower respiratory consumption of oxygen would be expected to increase oxygen supply to individual roots. However, on a whole-plant basis, low P also increased root/shoot ratio in aerated solution, which may have increased the overall requirement for oxygen in root respiration from day 4 onwards.
Oxygen deficiency depressed P uptake in the first 2–4 d and continued to do so for the last 4 d at both low and high P supply (Fig. 4). By contrast, in aerated solution P uptake remained higher until day 8 even at low P. One possible cause of the lower P uptake at low P in stagnant solution is the formation of a P depletion zone around the roots. Depletion of P in the rhizosphere is well known in soil (Kirk and Saleque, 1995) due to P uptake by the root and slow diffusion of P to the root surface. Stagnant solution culture is designed to minimize convective movement of solution. Wiengweera et al. (1997) also reported that stagnant solution can limit nutrient uptake by wheat due to the formation of depletion zones. Such depletion zones would be expected to develop more quickly at low P concentrations and this is consistent with the earlier decline in the P uptake at low P than at high P for plants in stagnant solution in the present study. A second possibility for declining P uptake is the formation of the barrier to ROL: the barrier to ROL has been suggested by several authors to decrease nutrient uptake (reviewed in Colmer, 2003a, although conversely other authors have suggested such effects might not occur, see Rubinigg et al., 2002). Moreover, Kirk (2003) argued that nutrient uptake by rice in an anoxic medium is mostly by fine lateral roots, and these normally remain permeable to oxygen, except under unusual rhizosphere conditions such as sulphide toxicity (Armstrong and Armstrong, 2005). Nevertheless, we consider that depletion zones adjacent to roots in stagnant agar probably were the major cause of decreased P uptake.
ROL in stagnant solution does not fully simulate processes affecting P uptake that occur in soil. In soil, ROL causes oxidation of ferrous iron, which acidifies the rhizosphere and increases the pool of plant-available P (Saleque and Kirk, 1995). Hence, in anoxic soils, ROL might increase solubilization of soil P in the rhizosphere. Stagnant solution contains no pool of P that can be solubilized by rhizosphere acidification.
Root morphological changes can be important adaptations to improve P uptake under low P supply in anoxic condition (Kirk and Du, 1997). In the present study, the morphological changes in roots under low P included reduced root elongation and increased number of adventitious roots, but these changes were observed at 4–8 d and hence followed the decrease in P uptake in stagnant solution. The morphological changes in roots will presumably be significant for the longer term adaption of rice to P acquisition in low P anoxic soil. Fine lateral roots on adventitious roots are considered to be the dominant surface for nutrient uptake (Kirk, 2003). Lorenzen et al. (2001) found the plants in low oxygen solution had reduced root length at low oxygen, which they suggested may have a negative effect on nutrient acquisition. However, increased numbers of adventitious roots under low P may offset the decrease in maximum root length under anoxic conditions, and hence increased numbers of adventitious roots should help to maintain or increase root surface area, which is important for P uptake under low P conditions (Wissawa, 2003).
Based on the results of Fan et al. (2003) with maize and bean, it was expected that low P in stagnant solution would increase root porosity. However, in the present study, P had no consistent effect on root porosity. Rice differs from dry land crops such as maize and bean in that it has high root porosity even under aerated conditions. Hence, the benefits of lower respiratory costs in roots has already been captured by aerated rice and may explain why low P failed to increase root porosity further in rice roots under stagnant solution. Moreover, Armstrong (1979) clearly showed that most benefit from increased root porosity is derived from the reduced resistance to internal oxygen diffusion, with the decreased demand for oxygen being a secondary benefit. The present study also examined root porosity over a short period of 8 d compared with 12–42 d as reported by Fan et al. (2003). Indeed, Lu et al. (1999) found no effect of low P on root porosity in three rice cultivars at 14 d and an increase in root porosity for two out of three cultivars at 28 d. Hence, it would appear that increased aerenchyma in rice roots under low P supply is a longer term acclimation of plants, or possibly a secondary response, to P deficiency.
In summary, only 1 d after transfer to stagnant conditions, rice roots had apparently formed a barrier to ROL, which decreased ROL by 90 % in subapical root zones, but not at the root tip. After 4 d in stagnant conditions, plants also showed increases in numbers of adventitious roots, root dry mass and root/shoot ratio. By contrast, maximum root length and shoot growth were decreased in stagnant conditions. Only 4 d after transition to stagnant conditions, relative P uptake declined, especially at low P supply. In aerated solutions, roots increased relative P uptake until 8 d. The effect of P deficiency was more severe on rice growth and relative P uptake of plants in stagnant than in aerated nutrient solution culture. In conclusion, roots responded rapidly to oxygen deficiency with decreased ROL in subapical root zones, at least 2 d before any change in root porosity or morphology was evident. Relative P uptake also decreased under oxygen deficiency, showing that a sudden decline in root-zone oxygen adversely affects P nutrition of rice.
Acknowledgments
We thank the Thailand Research Fund for a Royal Golden Jubilee PhD Scholarship, the McKnight Foundation for financial support, the School of Environmental Science, Murdoch University, and the School of Plant Biology, University of Western Australia, WA, Australia, for their help in establishing and maintaining the experiment, and Dr Al Imran Malik for technical assistance.
LITERATURE CITED
- Armstrong J and Armstrong W. (2005) Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Annals of Botany 96625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. (1979) In Woolhouse HW (Ed.). Advances in Botanical Research, Volume 7. Aeration in higher plantsAcademic Press pp. 225–332.
- Armstrong W and Wright EJ. (1975) Radial oxygen loss from roots: the theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum 3521–26. [Google Scholar]
- Bates TR and Lynch JP. (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell and Environment 19529–538. [Google Scholar]
- Clark LH and Harris WH. (1981) Observations on the root anatomy of rice (Oryza sativa L.). American Journal of Botany 68154–161. [Google Scholar]
- Colmer TD. (1998) The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany 491431–1436. [Google Scholar]
- Colmer TD. (2003a) Long-distance transport of gases in plants; a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 2617–36. [Google Scholar]
- Colmer TD. (2003b) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology 48223–250. [DOI] [PubMed] [Google Scholar]
- Drew MC and Saker LR. (1986) Ion transport to the xylem in aerenchymatous roots of Zea mays (L.). Journal of Experimental Botany 3722–33. [Google Scholar]
- Drew MC, Cobb DB, Johnson JR, Andrews D, Morgan PW, Jordan W, He CJ. (1994) Metabolic acclimation of root tips to oxygen deficiency. Annals of Botany 74281–286. [Google Scholar]
- Fan M, Zhu J, Richards C, Brown KM, Lynch JP. (2003) Physiological roles for aerenchyma in phosphorus-stressed root. Functional Plant Biology 30493–506. [DOI] [PubMed] [Google Scholar]
- Gardner W, Barber D, Parberry D. (1983) The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant and Soil 70107–124. [Google Scholar]
- Green MS and Etherington JR. (1977) Oxidation of ferrous iron by rice (Oryza sativa L.) roots: a mechanism of waterlogging tolerance? Journal of Experimental Botany 28678–690. [Google Scholar]
- Huguenin-Elie O, Kirk GJD, Frossard E. (2003) Phosphorus uptake by rice from soil that is flooded, drained or flooded then drained. European Journal of Soil Science 5477–90. [Google Scholar]
- Justin SHFW and Armstrong W. (1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106465–495. [Google Scholar]
- Kirk GJD. (2003) Rice root properties for internal aeration and efficient nutrient acquisition in submerged soil. New Phytologist 159185–194. [DOI] [PubMed] [Google Scholar]
- Kirk GJD. (2004) The biogeochemistry of submerged soil(John Wiley & Sons, Ltd, Chichester).
- Kirk GJD and Du LV. (1997) Changes in rice root architecture, porosity and oxygen and proton release under phosphorus deficiency. New Phytologist 135191–200. [Google Scholar]
- Kirk GJD and Saleque MA. (1995) Solubilization of phosphate by rice plants growing in reduced soil: prediction of the amount solubilized and the resultant increase in uptake. European Journal of Soil Science 46247–255. [Google Scholar]
- Koncalova H. (1990) Anatomical adaptations to waterlogging in roots of wetland graminoids: limitations and drawbacks. Aquatic Botany 38127–134. [Google Scholar]
- Kronzucker HJ, Kirk GJD, Siddiqi MY, Glass ADM. (1998) Effects of hypoxia on NH4+ fluxes in rice roots: kinetics and compartmental analysis. Plant Physiology 116581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H and Steingrover E. (1978) Efficiency of root respiration of a flood-tolerant and a flood-intolerant Senecio species as affected by low oxygen tension. Physiologia Plantarum 42179–184. [Google Scholar]
- Lambers H, van der Werf A, Bergkotte M. (1993) Respiration: the alternative pathway. In Hendry GAF and Grime JP (Eds.). Methods in comparative plant ecology(Chapman and Hall, London) pp. 140–144.
- Lipton D, Blanchar R, Blevins D. (1987) Citrate, malate and succinate concentration in exudates form P-deficient and P-stressed Medicago sativa L. seedlings. Plant Physiology 85315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen B, Brix H, Mendelssohn IA, McKee KL, Miao SL. (2001) Growth, biomass allocation and nutrient use efficiency in Cladium jamaicense and Typha domingensis as affected by phosphorus and oxygen availability. Aquatic Botany 70117–133. [Google Scholar]
- Lu Y, Wassmann R, Neue HU, Huang C. (1999) Impact of phosphorus supply on root exudation, aerenchyma formation and methane emission of rice plants. Biogeochemistry 47203–218. [Google Scholar]
- Lynch JP. (1995) Root architecture and plant productivity. Plant Physiology 1097–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Lauchli A, Epstein E. (1991) Vegetative growth of the common bean in response to phosphorus nutrition. Crop Science 31380–387. [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP. (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiology 1311381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Schortemeyer M. (2003) Aerenchyma formation and radial O2 loss along adventitious roots of wheat with only the apical root portion exposed to O2 deficiency. Plant, Cell and Environment 261713–1722. [Google Scholar]
- Marschner H. (1995) Mineral nutrition of higher plants 2nd edn (Academic Press, London).
- Matsuo T and Hoshikawa K. (1993) Science of the rice plant. I. Morphology(Food and Agriculture Policy Research Center, Tokyo).
- McDonald MP, Galwey NW, Colmer TD. (2002) Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant, Cell and Environment 25441–451. [Google Scholar]
- Morita S and Abe J. (1996) Development of root systems in wheat and rice. In Ito O, Johansen C, Adu-Gyamfi JJ, Katayama K, Kumar Rao JVDK, Rego TJ (Eds.). Dynamics of roots and nitrogen in cropping systems of the semi-arid tropics(Japan International Research Centre for Agricultural Sciences, Tsukuba, Japan) pp. 199–209.
- Murphy J and Riley JP. (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 2731–36. [Google Scholar]
- O'Brien TP and McCully ME. (1981) The study of plant structure: principles and selected methods.(Termarcarphi Private Limited, Melbourne).
- Ranathunge K, Kotula L, Steudle E, Lafitte R. (2004) Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. Journal of Experimental Botany 55433–447. [DOI] [PubMed] [Google Scholar]
- Raskin I. (1983) A method for measuring leaf volume, density, thickness and internal gas volume. Horticultural Science 18698–699. [Google Scholar]
- Robards AW, Clarkson DT, Sanderson J. (1979) Structure and permeability of epidermal/hypodermal layers of sand sedge (Carex arenaria, L.). Protoplasma 101331–347. [Google Scholar]
- Rubinigg M, Stulen I, Elzenga JT, Colmer TD. (2002) Spatial patterns of radial oxygen loss and nitrate net flux along adventitious roots of rice raised in aerated or stagnant solution. Functional Plant Biology 291475–1481. [DOI] [PubMed] [Google Scholar]
- Saleque MA and Kirk GJD. (1995) Root-induced solubilization of phosphate in the rhizosphere of lowland rice. New Phytologist 129325–336. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiology 116447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng V, Bell RW, Willett IR. (2004) Amelioration of growth reduction of lowland rice caused by a temporary loss of soil water saturation. Plant and Soil 2651–16. [Google Scholar]
- Thomson CJ, Armstrong W, Waters I, Greenway H. (1990) Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant, Cell and Environment 13395–403. [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell and Environment 231237–1245. [Google Scholar]
- Wiengweera A and Greenway H. (2004) Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. Journal of Experimental Botany 552121–2129. [DOI] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ. (1997) The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany 80115–123. [Google Scholar]
- Wissawa M. (2003) How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiology 1331947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EC. (1998) In Karcher SJ (Ed.). A beginner's guide to the study of plant structure. Proceedings of the 19th Workshop/Conference of the Association for Biology Laboratory Education (ABLE)Tested studies for laboratory teaching Vol. 9 pp. 125–142.
- Zhu J and Lynch JP. (2004) The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedings. Functional Plant Biology 31949–968. [DOI] [PubMed] [Google Scholar]
- Zeigler RS and Puckridge DW. (1995) Improving sustainable productivity in rice-based rainfed lowland systems of South and South-East Asia. Geo Journal 35307–324. [Google Scholar]