Abstract
• Background and Aims In clonal plants, internode connections allow translocation of photosynthates, water, nutrients and other substances among ramets. Clonal plants form large systems that are likely to experience small-scale spatial heterogeneity. Physiological and morphological responses of Fragaria vesca to small-scale heterogeneity in soil quality were investigated, together with how such heterogeneity influences the placement of ramets. As a result of their own activities plants may modify the suitability of their habitats over time. However, most experiments on habitat selection by clonal plants have not generally considered time as an important variable. In the present study, how the foraging behaviour of clonal plants may change over time was also investigated.
• Methods In a complex of environments with different heterogeneity, plant performance was determined in terms of biomass, ramet production and photosynthetic activity. To identify habitat selection, the number of ramets produced and patch where they rooted were monitored.
• Key Results Parent ramets in heterogeneous environments showed significantly higher maximum and effective quantum yields of photosystem II than parents in homogeneous environments. Parents in heterogeneous environments also showed significantly higher investment in photosynthetic biomass and stolon/total biomass, produced longer stolons, and had higher mean leaf size than parents in homogeneous environments. Total biomass and number of offspring ramets were similar in both environments. However, plants in homogeneous environments showed random allocation of offspring ramets to surrounding patches, whereas plants in heterogeneous environments showed preferential allocation of offspring to higher-quality patches.
• Conclusions The results suggest that F. vesca employs physiological and morphological strategies to enable efficient resource foraging in heterogeneous environments and demonstrate the benefits of physiological integration in terms of photosynthetic efficiency. The findings indicate that short-term responses cannot be directly extrapolated to the longer term principally because preferential colonization of high-quality patches means that these patches eventually show reduced quality. This highlights the importance of considering the time factor in experiments examining responses of clonal plants to heterogeneity.
Keywords: Chlorophyll fluorescence, foraging behaviour, Fragaria vesca, habitat selection, physiological integration, source–sink hypothesis
INTRODUCTION
Clonal plants dominate many natural communities (Kliměs et al., 1997). Clonal growth allows plants to form large systems consisting of a variable number of ramets located at some distance from each other, and remaining connected by stolon or rhizome internodes for a variable period of time. This kind of reproduction implies that clonal plants are likely to experience small-scale spatial heterogeneity. In many ecosystems, such small-scale heterogeneity can often be observed over distances at scales of <50 cm (Salzman, 1985; Lechowick and Bell, 1991; Caldwell and Pearcy, 1994), and is relevant for many ecological plant processes (Hartgering and Bazzaz, 1984; Price and Marshall, 1999; Day et al., 2003).
A large number of studies (e.g. Hartnett and Bazzaz, 1983; Slade and Hutchings, 1987; Stuefer, 1997; Alpert, 1999; Saitoh et al., 2002) have shown that connections among ramets of clonal plants by stolon or rhizome internodes allow transport of photosynthates, water and nutrients from established ramets (parent ramets) to developing ramets (offspring ramets). This physiological integration generally confers a net benefit to the newly established offspring ramets, especially under unfavourable conditions, because they can import essential resources from established ramets growing under more favourable conditions. In this way, physiological integration may buffer the clonal plants against local effects resulting from spatial and temporal changes in the quality of a habitat (Hartnett and Bazzaz, 1983; Hutchings and Bradbury, 1986; Wijesinghe and Handel, 1994). Physiological integration may also enable clonal plants to rapidly colonize and exploit higher stress patches that would otherwise be unexploitable by independent ramets or plants (Hartnett and Bazzaz, 1983; Silander, 1985; Saitoh et al., 2002).
It has been demonstrated that plants have a wide variety of specific receptors enabling environmental information, such as light fluxes, water pulses and the presence of nutrients, to be acquired. These receptors may influence plant growth through signal-transduction pathways (Caldwell and Pearcy, 1994). Several authors (Schellner et al., 1982; Bülow-Olsen et al., 1984; Eriksson, 1986; Kleijn and van Groenendael, 1999; Wijesinghe and Hutchings, 1999) have pointed out that these mechanisms for acquiring information about the environment might allow clonal plants to perceive small-scale patterns in habitat quality, and to establish new ramets in the best patches.
In many studies on habitat selection by clonal plants, it has been stated that species may develop morphological and physiological plasticity as a strategy for foraging more efficiently for the resources they need (Wijesinghe and Hutchings, 1996, 1999; Welham et al., 2002; Day et al., 2003). However, most of these studies have only addressed morphological responses to resource heterogeneity, in spite of the importance of physiological processes such as photosynthesis for overall plant performance and thus for understanding the ecology of clonal plants, such as the ways in which they colonize space and overcome establishment risks.
Likewise, most previous experiments investigating the responses of clonal plants to environmental heterogeneity have used simple heterogeneity models with only two types of patch (Salzman and Parker, 1985; Slade and Hutchings, 1987; Stuefer et al., 1996; Price and Marshall, 1999). In natural environments, clonal plants often have to cope with more complex habitats, where homogeneous and heterogeneous environments may differ both in the total amount of resources and in the spatial segregation of these. However, little research has been done under these more realistic scenarios of heterogeneity.
In addition, previous experiments dealing with the physiological or morphological strategies of clonal plants for resource foraging have not generally considered time as an important variable. Most experiments have investigated short-term responses, but these cannot necessarily be extrapolated to the longer term. Notably, as a result of their own activities plants may modify the suitability of their habitats. If plants selectively locate their shoots in nutrient-rich, high-quality patches, these may be deprived of nutrients and thus converted into low-quality patches. Therefore, small-scale patterns of habitat quality can be expected to change over time, with corresponding implications for plant responses. The duration of the experiment may thus affect conclusions about the relative benefits of different habitat characteristics (Fransen and de Kroon, 2001; Fransen et al., 2001).
In the present study, an 11-month experiment was performed to investigate whether small-scale spatial heterogeneity of the habitat affects the pattern of placement of new ramets by the clonal species Fragaria vesca. The physiological and morphological responses exhibited by plants to cope with habitats of different complexity, and the consequences for plant performance in terms of biomass, new ramet production and photosynthetic activity, as estimated by chlorophyll fluorescence parameters were investigated. As far as is known, only two previous studies (Hartnett and Bazzaz, 1983; Roiloa and Retuerto, 2005) have examined the influence of physiological integration on the photosynthetic rates of clonal plants.
Specifically the following hypotheses were tested. Our first hypothesis stated that plants growing in homogenous high-quality habitats will root their new ramets randomly in the different patches, whereas plants growing in heterogeneous habitats will preferentially root their new ramets in high-quality patches. Significant differences in number of ramets among patches in the heterogeneous habitat and a random distribution of ramets among the patches of the homogeneous habitat are predicted. Our second hypothesis was that in heterogeneous habitats, low-quality patches will eventually be occupied by ramets, after conditions in the high-quality patches have deteriorated as a consequence of ramet growth. Thus, initial differences in number of ramets among patches in the heterogeneous habitat will disappear over time. Finally, assuming that physiological integration allows the redistribution of resources among interconnected ramets according to source–sink relationships (Pitelka and Ashmun, 1985; Salzman and Parker, 1985; Marshall, 1990), we hypothesized a greater integration of clones in heterogeneous habitats, with ramets acting as sources or sinks for assimilates, depending on the quality of the habitats on which they grew. Since it is widely considered that photosynthetic rates in a given plant location may be regulated, at least in part, by the net requirement for photosynthate export from that location (Sweet and Wareing, 1966; Neales and Incoll, 1968; Meyer and Whitlow, 1992), it is predicted that photosynthetic rates of ramets are driven, in part, by the steepness of the source–sink gradient, and thus, ramets in high quality patches of heterogeneous habitats will show enhanced photosynthetic activity with respect to ramets in homogeneous habitats. It is also predicted that as a consequence of the greater integration, heterogeneous habitats with patches of contrasting quality, could maintain similar biomass production to homogeneous high-quality habitats.
MATERIALS AND METHODS
Species and propagation
Fragaria vesca L., wild strawberry, is a perennial, winter-green herb of the Rosaceae family, with above-ground ramets connected by stolons. Vegetative spread allows F. vesca to form fairly large and long-lived clonal systems that are likely to experience significant environmental heterogeneity. All plant material used in these experiments came from 20 plants collected at a field site located 10 km south of Santiago de Compostela (north-west Spain) and propagated vegetatively for 2 years in an open-end greenhouse at the University of Santiago de Compostela before the experiment began. The plants might or might not differ in genotype. To avoid possible confounding effects of genotypes, the plants were assigned randomly to the treatments.
Experimental design
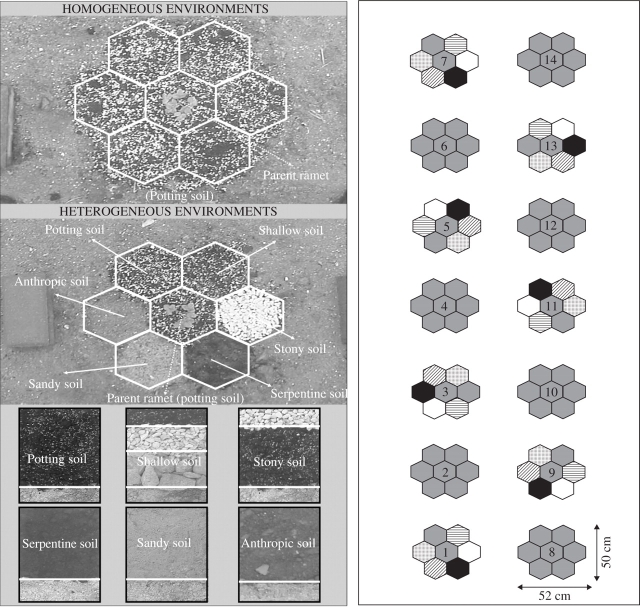
In early 2002, two types of experimental environment were constructed under natural light conditions at the field station of the University of Santiago de Compostela. Both types of environment, which will be referred to as homogenous and heterogeneous, were built into the soil of the field station, using a steel frame. This frame consisted of seven hexagonal compartments each with area 260 cm2, arranged as six compartments around a central compartment (Fig. 1). One patch type was assigned to each compartment, in all cases with substrate depth 20 cm (thus substrate volume 5·2 L). The central compartment, both in homogeneous and heterogeneous environments, was filled with a 3 : 1 mixture of potting soil (Pindstrup Plus-Orange Mosebrug, Sotopalacios, Burgos, Spain) and perlite (Europerlita S.A., Barcelona, Spain). This substrate, with all main nutrients and trace elements added, may be regarded as fertile soil, and was that used previously for vegetative propagation of the plants used in the experiments, providing optimal growth conditions for F. vesca. All patches in the homogeneous environments were made up of this same mixture of potting soil and perlite. The six patch types assigned to the marginal compartments in the heterogeneous environments were: sandy soil (washed beach sand), stony soil (a 2-cm layer of 10–15 mm gravel above potting soil), serpentine soil (natural soil formed from parent materials with unusually high Mg and Fe content), shallow soil (a 2-cm layer of potting soil above gravel and pebbles), an anthropic soil (that of the field station) and a mixture of potting soil and perlite (like that of the central compartment). An overall assessment of the differences in total resource availability between homogeneous and heterogeneous habitats, and between the different patches of the heterogeneous habitats is given in Table 1. Although the total resource availability differed between the homogeneous and heterogeneous environments, this did not affect the contrast of our two first hypotheses. However, it was not possible to test our third hypothesis unless either the total resource amount was held constant or, as we predicted, the growth was better in the heterogeneous treatment despite having lower total resource amount.
Fig. 1.
Experimental layout and schematic representation of the two treatments: heterogeneity (homogenous versus heterogeneous environments) and patch type. Each homogenous and heterogeneous environment was replicated seven times, in the layout shown to the right.
Table 1.
Mineral concentrations in homogeneous and heterogeneous environments and in the different patches of the heterogeneous environments (mg L−1)
| Homogeneous | Heterogeneous | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Total | Potting | Shallow | Stony | Serpentine | Sandy | Anthropic | |
| N | 76·9 | 40·5 | 76·9 | 7·7 | 57·7 | 0·96 | 5·8 | 57·7 |
| P | 134·6 | 57·4 | 134·6 | 13·5 | 115·4 | 0·76 | 0·96 | 1·9 |
| K | 230·8 | 100 | 230·8 | 19·2 | 192·3 | 19·2 | 7·7 | 0·2 |
| Mg | 5·8 | 4279 | 5·8 | 0·38 | 3·8 | 29000 | 903·8 | 38·5 |
| Fe | 13·5 | 26005 | 13·5 | 1·3 | 11·5 | 136000 | – | 46000 |
| Ni | – | 157·5 | – | – | – | 1038·5 | – | 63·7 |
| Cr | – | 733·5 | – | – | – | 5000 | – | 134·6 |
nd, Not determined.
The position of the different quality cells in the marginal compartments of the heterogeneous treatment was rotated to avoid possible confounding effects of orientation. Once the environments had been prepared the frame was removed, to allow root interactions. Each type of environment was replicated seven times. The 14 replicates were laid out in a two-row arrangement, with equal spacing between them, on a flat, fully exposed and homogeneous plot of the field station (Fig. 1). The homogeneous environmental conditions of the plot allow possible confounding factors to be ruled out. As can be seen from Fig. 1, the two types of environment were interspersed regularly, as recommended by Hurlbert (1984).
In early March 2002, 14 plants with no offspring ramets were selected for size uniformity from the plant stock. Each of these plants, which will be referred to as parent ramets, was randomly assigned to one of the environment types and rooted in the centre of the central compartment, giving each patch an a priori equal chance of being colonized. The plants were allowed to grow for 11 months (March 2002 to January 2003). Substrates were watered daily to field capacity throughout the experiment.
Measurements
Chlorophyll fluorescence
Measurements of chlorophyll fluorescence parameters were initiated when all patches of heterogeneous and homogeneous environments had been colonized by rooted ramets. Every week during the last 3 months of the experiment, chlorophyll fluorescence parameters were measured with a portable pulse-amplitude-modulated fluorometer (MINI-PAM photosynthesis yield analyser; Walz GmbH, Effeltrich, Germany) by the saturation pulse method (see Schreiber et al., 1998). Measuring light and saturating light pulses (>4000 μmol photons m−2 s−1, 0·8 s pulse length) were applied through optical fibres at an angle of 60° and 12 mm distance from the leaf (Halogen Lamp 8V/20W, Bellaphot; Osram, Munich, Germany).
The chlorophyll fluorescence parameters recorded were as follows. The maximum quantum yield of photosystem II (PSII) was assessed by the ratio Fv/Fm = (Fm − F0)/Fm (see Bolhàr-Nordenkampf et al., 1989), where F0 and Fm are defined as minimal and maximal fluorescence yields of a dark-adapted sample, with all PSII reaction centres fully open (i.e. all primary acceptors oxidized). This parameter was measured just before dawn to ensure a dark-adaptation time sufficient for all PSII reaction centres to open. The maximum quantum yield of PSII is an estimate of the efficiency of excitation energy capture by open PSII reaction centres (Butler and Kitajima, 1975) and is correlated with the amount of carbon gained per unit of light absorbed (Bolhàr-Nordenkampf and Öquist, 1993). The effective quantum yield of PSII was calculated as ΦPSII = 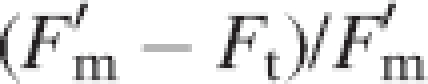 (see Genty et al., 1989), where
(see Genty et al., 1989), where 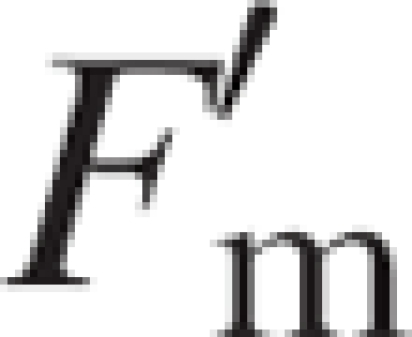 is defined as the maximal fluorescence yield reached in a pulse of saturating light with an illuminated sample and Ft is the fluorescence yield of the leaf at a given photosynthetic photon flux density. This parameter, which measures the proportion of the light absorbed by chlorophyll that is used in photochemistry (Maxwell and Johnson, 2000), was measured on seven dates, under the ambient light conditions prevailing at midday [photosynthetic photon flux density = 1071·4 ± 23·2 μmol m−2 s−1 (mean ± s.e.), n = 98]. Previous studies have demonstrated that ΦPSII can be used to predict CO2 assimilation rates precisely and quickly (Genty et al., 1989; Demmig-Adams et al., 1990; Edwards and Baker, 1993; Andrews et al., 1995). Both fluorescence parameters were measured in three fully developed leaves per parent and offspring ramet, and the resulting mean values (n = 3) were used in the analyses.
is defined as the maximal fluorescence yield reached in a pulse of saturating light with an illuminated sample and Ft is the fluorescence yield of the leaf at a given photosynthetic photon flux density. This parameter, which measures the proportion of the light absorbed by chlorophyll that is used in photochemistry (Maxwell and Johnson, 2000), was measured on seven dates, under the ambient light conditions prevailing at midday [photosynthetic photon flux density = 1071·4 ± 23·2 μmol m−2 s−1 (mean ± s.e.), n = 98]. Previous studies have demonstrated that ΦPSII can be used to predict CO2 assimilation rates precisely and quickly (Genty et al., 1989; Demmig-Adams et al., 1990; Edwards and Baker, 1993; Andrews et al., 1995). Both fluorescence parameters were measured in three fully developed leaves per parent and offspring ramet, and the resulting mean values (n = 3) were used in the analyses.
Growth
At the end of the experiment, the plant material in each patch of the different environments was harvested separately, dried to constant mass at 60 °C, and weighed to determine root, leaf, stem and stolon biomass. The biomass of the stolons that they produced was included in the biomass of each ramet. The length of stolons (internode length), number of disconnected ramets, leaf areas and biomass allocation ratios (root/total biomass, aboveground/total biomass and stolon/total biomass) were also calculated. The number of disconnected ramets was calculated for all the patches at the end of the experiment. During the experiment, the production of new ramets was monitored (number of ramets and patch where they rooted) on 19 dates to identify possible preferences for colonization of particular patches.
Data analysis
The experimental design comprised two factors: ‘heterogeneity’ (with two levels, homogeneous and heterogeneous) and ‘patch type’ (six levels corresponding to six patches in the homogeneous and heterogeneous environments). Prior to analyses, all variables were examined for normality and homoscedasticity. When significant violations were found, non-parametric statistics were used. Differences between parent ramets at the end of the experiment, in biomass, leaf area, biomass allocation ratios, ramet production and stolon lengths, were analysed by one-way analyses of variance (ANOVA) with ‘heterogeneity’ as factor. Differences between parent ramets in fluorescence parameters related to photosynthesis were compared by repeated-measures analyses of variance (ANOVAR), with heterogeneity as the between-subject factor. Variations among offspring ramets in biomass, leaf area, biomass allocation ratios, ramet production and stolon lengths were investigated by a profile analysis, a form of repeated measures ANOVA (Quinn and Keough, 2002), with ‘heterogeneity’ as the main between-subject effect and ‘patch type’ as the within subject effect. Due to the difficulties in achieving an adequate number of replicates (since it took a long time to colonize some patches), the differences between offspring ramets in maximum quantum yield were only evaluated for the last 5 weeks of the experiment, using profile analysis with ‘heterogeneity’ as the between-subject effect and ‘patch type’ and ‘time’ as within-subject factors. For the analysis of differences between offspring ramets in effective quantum yield, a sufficient number of replicates was only obtained on a single date; thus, the significance of the differences was determined by a profile analysis, such as indicated above for the analyses of offspring biomass. To test the hypothesis that ‘heterogeneity’ induced variation in ramet placement changes over time we used a profile analysis with ‘heterogeneity’ as the between-subject factor and with ‘patch type’ and ‘time’ as the within-subject factors. Differences in the number of ramets established in the different patches were analysed, separately for homogeneous and heterogeneous environments, by Kruskal–Wallis tests, with one for each of the dates on which ramet establishment was monitored. Finally, Kruskal–Wallis tests were also performed to determine whether the number of disconnected ramets differed as a function of ‘heterogeneity’ or ‘patch type’.
The level of significance accepted was set at P < 0·05. All statistical tests were performed with SPSS 11.0.
RESULTS
Photosynthetic efficiencies
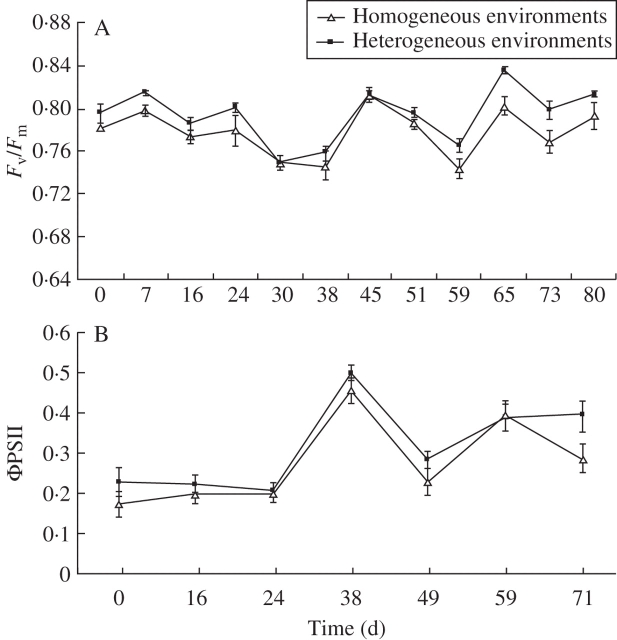
It was found that the maximum and effective quantum yields of PSII in parent ramets growing under heterogeneous environments were significantly higher than in parent ramets growing under homogeneous environments (ANOVAR: F1,12 = 5·29, P = 0·040 for Fv/Fm, and F1,12 = 7·49, P = 0·018 for ΦPSII; Fig. 2). Considering the photosynthetic performances of offspring ramets, no significant effect of heterogeneity or patch type was detected (profile analysis: P always >0·099 for Fv/Fm, and P always >0·091 for ΦPSII).
Fig. 2.
Mean values (± s.e.) of (A) photosynthetic efficiency in parent ramets growing in heterogeneous and homogeneous environments, as estimated by the maximum quantum yield of PSII (Fv/Fm) (ANOVAR indicated significant between-subjects effects: F1,12 = 5·29, P < 0·05), and (B) the effective quantum yield of PSII (ΦPSII). ANOVAR indicated significant between-subjects effects: F1,12 = 7·50, P < 0·05.
Growth
Parent ramets growing in heterogeneous environments showed significantly higher investment in photosynthetic biomass (above-ground/total biomass) (ANOVA: F1,12 = 5·61; P = 0·036), mean leaf area (ANOVA: F1,12 = 10·52; P = 0·007) and stolon/total biomass (ANOVA: F1,12 = 12·70; P = 0·004) than parent ramets growing in homogeneous environments (Table 2). We found similar significant effects for stolon length (ANOVA: F1,12 = 4·99; P = 0·045; Table 2). No significant effects of heterogeneity on the rest of variables examined were detected.
Table 2.
Mean (± s.e.) and ANOVA P-values for above-ground : total biomass ratio, mean leaf area (mm2), stolon : total biomass ratio and stolon length (cm) of parent ramets growing in homogeneous or heterogeneous environments
| Homogeneous | Heterogeneous | P-values | |
|---|---|---|---|
| Above-ground : total biomass | 0·582 ± 0·051 | 0·746 ± 0·057 | 0·036 |
| Mean leaf area (mm2) | 1089·28 ± 176·27 | 2014·02 ± 265·35 | 0·007 |
| Stolon : total biomass | 0·031 ± 0·007 | 0·113 ± 0·015 | 0·004 |
| Stolon length (cm) | 12·67 ± 4·16 | 22·41 ± 1·30 | 0·045 |
In all cases ANOVA indicated significant differences between homogeneous and heterogeneous environments (d.f. 1,12).
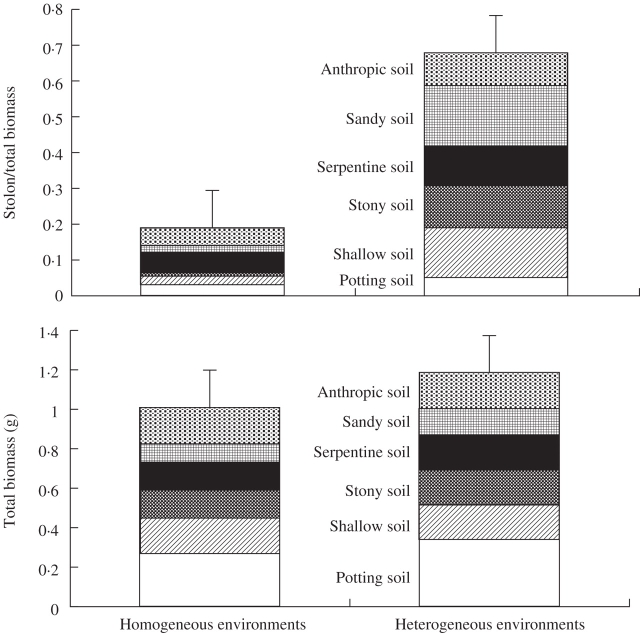
Offspring ramets growing in heterogeneous environments showed a significantly higher investment in stolon biomass (profile analysis: between-subject effect F1,12 = 13·161, P = 0·003, for stolon/total biomass; Fig. 3) than those growing in homogeneous environments. However, these differences were not significant for stolon length (profile analysis: between-subject effect F1,12 = 2·373, P = 0·149). The total biomass of offspring ramets produced in homogeneous and heterogeneous environments did not differ significantly (profile analysis: between-subject effect F1,12 = 0·223, P = 0·646; Fig. 3). No significant effects were detected on the rest of the growth variables studied.
Fig. 3.
Mean values (± s.e.) for stolon : total biomass ratio and total biomass (g) of offspring ramets growing in homogeneous and heterogeneous environments. Profile analysis indicated significant differences between the two types of environment for stolon : total biomass ratio (between subject effect: F1,12 = 13·161, P = 0·003), but not for total biomass (between subject effect: F1,12 = 0·223, P = 0·646).
No significant effect of heterogeneity or patch type was found on the number of disconnected ramets (P-values for Kruskal–Wallis tests always >0·094).
At the end of the experiment, there were no offspring ramets in the central patch of any of the environments. Some stolons were long enough to leave the experimental areas. These stolons were left to grow outside but they were not included in the analyses. In any case, we did not detect differences between homogeneous and heterogeneous environments in the biomass outside the area (F1,82 = 0·075, P = 0·785).
Habitat selection
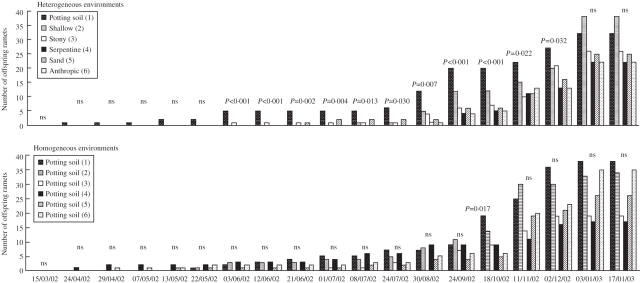
The significant interaction term between ‘heterogeneity’, ‘patch type’ and ‘time’ indicates that ramet placement in the patches differed between homogeneous and heterogeneous environments over time (profile analysis: F80,960 = 1·556, P = 0·002). The results show that in heterogeneous environments the parent ramets did not establish new ramets in any of the patches until April 2002 (Fig. 4). Between April and May, parent ramets only established new ramets in the highest-quality patches, the potting soil patches. Between June and December ramets additionally colonized other types of patches. Kruskal–Wallis tests for each of the 11 data sets obtained in that period detected significant effects of patch type on the number of ramets established in the heterogeneous environments, with more ramets in the potting soil patches (Fig. 4). Parent ramets did not establish offspring ramets in all six surrounding patches until the end of August. Kruskal–Wallis tests for the two data sets obtained in January did not detect any significant effects of patch type on the mean number of ramets established in the different patches of the heterogeneous environments (see Fig. 4).
Fig. 4.
Time-course of the colonization of patches in homogeneous and heterogeneous environments by ramets. For each date, columns show the total number of offspring ramets (n = 7 replicates) established in the different patches making up the heterogeneous and homogeneous environments. P-values for Kruskal–Wallis tests are shown (ns = non-significant differences). Profile analysis: F80,960 = 1·556, P = 0·002, for the heterogeneity × patch type × time interaction effect.
The process of colonization of new patches was faster in homogeneous than in heterogeneous environments (Fig. 4). At the start of July, parent ramets had already established at least one ramet in each of the patches that made up the homogeneous environments, about 2 months earlier than in the heterogeneous environments. Although Fig. 4 seems to suggest a possible effect of orientation on the number of ramets established in the patches of the homogeneous environments (four contiguous patches showed more ramets), Kruskal–Wallis tests for the relevant data sets (i.e. homogenous environments, on dates on which ramets had been established) did not detect any significant effect of patch position on the number of ramets established, with the single exception of October. To confirm the results of the Kruskal–Wallis test, circular statistics were used to test the uniformity of distribution of ramets around a circle in the homogeneous environments. The Rayleigh test for grouped data (grouping interval 60°) (Batschelet, 1981; Zar, 1984) applied to the mean number of ramets in the different patch positions (six positions, n = 7 replicates) confirmed that the ramets were uniformly distributed around the circle in the homogeneous environments (P always >0·2).
The total number of ramets established on the different dates on which new ramets were present did not differ significantly between homogeneous and heterogeneous environments, except on July 24 (Kruskal–Wallis test: χ2 = 4·442, d.f. 1, P = 0·035).
DISCUSSION
Regarding our first hypothesis of a preferential location of ramets in high-quality habitats, the results of this study confirm our predictions. It was found that in heterogeneous environments, F. vesca first rooted new ramets in the highest-quality patches (Fig. 4), whereas in homogeneous environments ramets were rooted randomly on the different patches (Fig. 4). These results did not reveal the mechanism for ramet location, whether active foraging or delay (or even suppression) of root formation. Some authors have suggested a mechanistic explanation for the selective placement of ramets. Wijesinghe and Hutchings (1999) observed that plants of the stoloniferous Glechoma hederacea initially extend some ramets into both favourable and unfavourable patches, apparently in order to gauge local habitat quality. In line with this, Schellner et al. (1982) and Eriksson (1986) have suggested that stolon tips of clonal plants might sense areas with high nutrient concentrations, water availability or light flux, increasing the probability of placing ramets in favourable sites. Moreover, Salzman (1985) and Oborny (1994) have interpreted that the preferential colonization of certain habitats is the result of a facultative reduction by plants of resource allocation for growth in unfavourable habitats. Soil type-dependent differences in air humidity may be a plausible mechanism to explain the variation in initial ramet placement. Organic potting compost inherently holds more water, thereby increasing the humidity experienced by plants close to the soil surface. High humidity, in turn, may stimulate root formation.
Our second hypothesis was that in the heterogeneous environments, initial among-patch differences in the number of ramets will disappear over time. Our results confirm this prediction. From June to December significantly higher numbers of rooted ramets were consistently observed in the potting soil patches of the heterogeneous environments. This pattern was no longer observed at the end of the experiment, in January, when no significant effects of patch type on number of ramets rooted in the heterogeneous environments were detected. The accumulation of biomass in the different patches can be expected to have followed a similar trend. At the end of the experiment, no significant effect of patch type on the total biomass of offspring ramets was observed. A plausible explanation is that as a result of the preferential allocation of ramets to the high-quality patches in the heterogeneous environments, these patches become low-quality patches by overcrowding. At their maximum density the ramets cast shade or, having achieved optimal ramet spacing, allocate to spreading new ramets elsewhere. Mechanisms that tend to minimize competition among parts of the same clone have been described. Gruntman and Novoplansky (2004) have suggested that the clonal system may recognize itself and avoid competition by starting growth in other patches. Although previous studies have shown that the potential benefits of selective root placement in heterogeneous soils may be reduced by patch depletion (van Vuuren et al., 1996; Frasen et al., 1998; Hodge et al., 1999), nutrient depletion is discarded as an explanation because at the end of the experiment the roots did not occupy the whole soil volume. In fact, a considerable volume of soil (5200 mL per patch) was used. This volume of soil is clearly enough for maintaining F. vesca growth during the 11 months of the experiment. Whatever the explanation, the results of the present study support the model of habitat selection proposed by Fretwell (1972) for animal populations. This simple model assumes that individuals can move into habitats without restrictions (‘ideal free distribution’) and that the suitability of habitats is not constant but affected by the density of the individuals, so that overcrowding reduces suitability. The model predicts that when density is high in good habitats, good and poor habitats will have equal suitability. Given that plants can change the quality of patches, with corresponding implications for plant responses, the results of the present study, as well as the results of others (Fransen and de Kroon, 2001; Fransen et al., 2001), point to the importance of considering time as a key factor when investigating the foraging behaviour of clonal plants.
Our third hypothesis was that parent ramets will send resources to offspring ramets in poor-quality environments and that, in line with this, photosynthetic efficiency will be higher in parent ramets growing in heterogeneous environments than in parent ramets growing in homogeneous environments. The results of the present study show that the maximum and effective quantum yields of PSII in parent ramets growing in heterogeneous environments were significantly higher than in parent ramets growing in homogeneous environments. It can be argued that these differences, although statistically significant, are quantitatively quite small. However, they may accumulate throughout development to contribute substantially to differences in carbon gain and to produce large differences in fitness, as Thomson et al. (2003) have suggested. These results, along with the maintenance of the photochemical activity of offspring ramets growing in the low quality patches of the heterogeneous environments, support our hypothesis of greater integration of clones in heterogeneous environments, and are in line with results of Alpert et al. (2003), showing that a higher potential for resource sharing is selected for in more heterogeneous habitats. It is suggested that the observed enhancement in photosynthetic efficiency allowed parent ramets in heterogeneous environments to attend to the increased photosynthate demand from ‘dependent’ offspring, especially those growing in low-quality patches. The present data are also consistent with the findings of Hartnett and Bazzaz (1983), who reported an enhancement of photosynthetic rates in illuminated ramets connected to shaded ramets of Solidago canadensis, and with the findings of Roiloa and Retuerto (2005), indicating an enhancement of photosynthetic activity in parent ramets of F. vesca supporting offspring ramets growing under water-stress conditions. Day et al. (2003) have suggested that physiological responses to heterogeneity, such as increased rates of uptake of nutrients, may explain why some species produce more biomass in heterogeneous environments than in homogenous environments, as reported by some previous authors (Birch and Hutchings, 1994; Einsmann et al., 1999). Other kinds of physiological response to heterogeneity, such as the increased photosynthetic efficiencies reported in our study, may be also important for explaining the surprising result that plants in heterogeneous but mostly lower-quality habitats produce as much biomass as plants in uniformly high-quality homogeneous environments.
Changes in CO2 assimilation measured by gas exchange correlate significantly with fluorescence-based indices of the effective quantum yield of PSII photochemistry (Genty et al., 1989; Demmig-Adams et al., 1990; Edwards and Baker, 1993; Andrews et al., 1995). Gas exchange and fluorescence-based indices measure different components of the photosynthetic process associated with CO2 fixation (carbon reactions) and with light-harvesting and electron transport (light reactions), respectively. The electrons transferred along the photosystems provide energy and substrates for carboxylation, but a certain proportion may also be consumed in processes that do not result in carbon fixation, mainly photorespiration (Krall and Edwards, 1992; Björkman and Demmig-Adams, 1995). The present study provides evidence of how the light reactions of the photosynthetic process are affected by physiological integration, and strongly suggest that CO2 fixation may also be affected.
In addition to greater photosynthetic efficiencies, parent ramets growing in heterogeneous environments also exhibited morphological responses to heterogeneity. Parent ramets in heterogeneous environments showed a significantly higher investment in photosynthetic biomass (above-ground/total biomass) and stolon/total biomass, produced longer stolons, and had higher mean leaf size than parent ramets in homogeneous environments. Morphological plasticity in response to heterogeneity may reflect the placement of structures to ensure efficient foraging for resources (Hutchings and de Kroon, 1994; Alpert and Stuefer, 1997; Cain, 1994; Einsmann et al., 1999), and can therefore be considered an adaptive response to heterogeneity (Jain, 1979). Several authors have reported that ramets growing under unfavourable conditions generated longer internodes, but in smaller numbers, than ramets established in favourable areas; they interpreted this response as a strategy for escape from unfavourable conditions (Hartnett and Bazzaz, 1983; Sutherland and Stillman, 1988; Welham et al., 2002; Macek and Lepš, 2003). A higher investment was also observed in stolon biomass in offspring ramets growing under heterogeneous conditions. However, there were no differences in stolon length. This should mean thicker stolons, which could be an indication for more transport capacity. For the offspring ramets, the escape theory does not seem to work.
In terms of total biomass and production of new ramets, the putatively different foraging strategies to exploit heterogeneous environments (preferential allocation of ramets to high-quality patches) and homogeneous environments (random allocation of ramets to patches) produced similar results.
In conclusion, the present study confirms that F. vesca shows physiological and morphological responses enabling more efficient foraging for resources in heterogeneous environments. Perhaps the most interesting result of the study is that it demonstrates the benefits of physiological integration in terms of feedback regulation of photosynthesis. This is an important aspect of physiological integration that deserves to receive more attention, since it may be important for explaining the overall better performance of clonal plants in heterogeneous environments. This study shows that the greater integration in heterogeneous environments allows maintaining similar biomass in the patchy treatment (despite having lower total resource amount) and in the homogeneous environment.
The present study also highlights the importance of time in experiments examining responses of clonal plants to heterogeneity. Most previous experiments of this type have tended to be short, and have not considered that clonal plants by their own expansion may themselves reduce heterogeneity among microsites. The present study indicates that short-term responses cannot be directly extrapolated to the long term.
Acknowledgments
P. Alpert and two anonymous referees made helpful comments on an earlier version of this paper. S.R.R. was supported by a grant from the Department of Education and Universities (Autonomous Government of Galicia).
LITERATURE CITED
- Alpert P. (1999) Effects of clonal integration on plant plasticity in Fragaria chiloensis. Plant Ecology 14199–106. [Google Scholar]
- Alpert P and Stuefer JF. (1997) Division of labour in clonal plants. In de Kroon H and van Groenendael J (Eds.). The ecology and evolution of clonal plants.(Backhuys Publishers, Leiden) pp. 137–154.
- Alpert P, Holzapfel C, Slominski C. (2003) Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. Journal of Ecology 9127–35. [Google Scholar]
- Andrews JR, Fryer MJ, Baker NR. (1995) Characterization of chilling effects on photosynthetic performance of maize crops during early season growth using chlorophyll fluorescence. Journal of Experimental Botany 461195–1203. [Google Scholar]
- Batschelet E. (1981) Circular statistics in biology.(Academic Press, London).
- Birch CPD and Hutchings MJ. (1994) Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. Journal of Ecology 82653–664. [Google Scholar]
- Björkman O and Demmig-Adams B. (1995) Regulation of photosynthetic light energy capture, conversion and dissipation in leaves of higher plants. In Schulze ED and Caldwell MM (Eds.). Ecophysiology of photosynthesis(Springer, Berlin) pp. 17–48.
- Bolhàr-Nordenkampf HR and Öquist G. (1993) Chlorophyll fluorescence: a tool in photosynthesis research. In Hall DO, Scurlock JMO, Bolhár-Nordenkampf HR, Leegood RC, Long SP (Eds.). Photosynthesis and production in a changing environment.(Chapman and Hall, London) pp. 193–206.
- Bolhàr-Nordenkampf HR, Long SP, Baker NR, Öquist G, Scheiber U, Lechner EG. (1989) Chorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Functional Ecology 3497–514. [Google Scholar]
- Bülow-Olsen A, Sackville Hamilton NR, Hutchings MJ. (1984) A study of growth form in genets of Trifolium repens L. as affected by intra- and interplant contacts. Oecologia 61383–387. [DOI] [PubMed] [Google Scholar]
- Butler W and Kitajima M. (1975) Fluorescence quenching in photosystem II of chloroplasts. Biochimica et Biophysica Acta 376116–125. [DOI] [PubMed] [Google Scholar]
- Caldwell MM and Pearcy RW. (1994) Exploitation of environmental heterogeneity by plants. Ecophysiological processes above and belowground.(Academic Press, San Diego).
- Cain ML. (1994) Consequences of foraging in clonal plant species. Ecology 75933–944. [Google Scholar]
- Day KJ, John EA, Hutchings MJ. (2003) The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Functional Ecology 17454–463. [Google Scholar]
- Demmig-Adams B, Máguas C, Adams WW III, Meyer A, Kilian E, Lange OL. (1990) Effect of high light on the efficiency of photochemical energy conversion in a variety of lichen species with green and blue-green phycobionts. Planta 180400–409. [DOI] [PubMed] [Google Scholar]
- Einsmann JC, Jones RH, Pu M, Mitchell RJ. (1999) Nutrient foraging traits in 10 co-occurring plant species of contrasting life forms. Journal of Ecology 87609–619. [Google Scholar]
- Edwards GE and Baker NR. (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynthesis Research 3789–102. [DOI] [PubMed] [Google Scholar]
- Eriksson O. (1986) Mobility and space capture in the stoloniferous plant Potentilla anserine. Oikos 4682–87. [Google Scholar]
- Fransen B and de Kroon H. (2001) Long-term disadvantages of selective root placement: root proliferation and shoot biomass of two perennial grass species in a 2-year experiment. Journal of Ecology 89711–722. [Google Scholar]
- Fransen B, de Kroon H, Berendse F. (1998) Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 115351–358. [DOI] [PubMed] [Google Scholar]
- Fransen B, de Kroon H, Berendse F. (2001) Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology 822534–2546. [Google Scholar]
- Fretwell SD. (1972) Populations in a seasonal environment.(Princeton University Press, Princeton, NJ).
- Genty BE, Briantais JM, Baker NR. (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 99087–92. [Google Scholar]
- Gruntman M and Novoplansky A. (2004) Physiologically mediated self/non-self discrimination in roots. Proceeding of the National Academy of Sciences of the USA 1013863–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgering AP and Bazzaz FA. (1984) Seedling-scale environmental heterogeneity influences individual fitness and population structure. Ecology 65198–206. [Google Scholar]
- Hartnett DC and Bazzaz FA. (1983) Physiological integration among intraclonal ramets in Solidago canadensis. Ecology 64779–788. [Google Scholar]
- Hodge A, Robinson D, Griffiths BS, Fitter AH. (1999) Nitrogen capture by plants grown in N-rich organic patches of contrasting size and strength. Journal of Experimental Botany 501243–1252. [Google Scholar]
- Hurlbert SH. (1984) Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54187–211. [Google Scholar]
- Hutchings MJ and Bradbury IK. (1986) Ecological perspectives on clonal perennial herbs. BioScience 36178–182. [Google Scholar]
- Hutchings MJ and de Kroon H. (1994) Foraging in plants: the role of morphological plasticity in resources acquisition. Advances in Ecological Research 25159–238. [Google Scholar]
- Jain SK. (1979) Adaptive strategies: polymorphism, plasticity and homeostasis. In Solbrig OT, Jain S, Johnson GB, Raven PH (Eds.). Topics in plant population biology.(Columbia University Press, New York, NY) pp. 160–187.
- Kleijn D and van Groenendael JM. (1999) The exploitation of heterogeneity by a clonal plant in habitats with contrasting productivity levels. Journal of Ecology 87873–884. [Google Scholar]
- Kliměs L, Kliměsova J, Hendriks R, van Groenendael J. (1997) Clonal plant architecture: a comparative analysis of form and function. In de Kroon H and van Groenendael J (Eds.). The ecology and evolution of clonal plants.(Backhuys Publishers, Leiden) pp. 1–29.
- Krall JP and Edwards GE. (1992) Relationship between photosystem II activity and CO2 fixation in leaves. Physiologia Plantarum 86180–187. [Google Scholar]
- Lechowicz MJ and Bell G. (1991) The ecology and genetics of fitness in forest plants. II. Microspatial heterogeneity of the edaphic environment. Journal of Ecology 79687–696. [Google Scholar]
- Macek P and Lepš J. (2003) The effect of environmental heterogeneity on clonal behaviour of Prunella vulgaris L. Plant Ecology 16831–43. [Google Scholar]
- Marshall C. (1990) Source–sink relations of interconnected ramets. In Groenendael J and de Kroon H (Eds.). Clonal growth in plants: regulation and function.(SPB Academic, The Hague) pp. 23–41.
- Maxwell K and Johnson GN. (2000) Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 51659–668. [DOI] [PubMed] [Google Scholar]
- Meyer GA and Whitlow TH. (1992) Effects of leaf and sap feeding insects on photosynthetic rates of goldenrod. Oecologia 92480–489. [DOI] [PubMed] [Google Scholar]
- Neales TF and Incoll LD. (1968) The control of leaf photosynthesis rate by the level of assimilate concentration in the leaf: a review of the hypothesis. Botanical Review 34107–125. [Google Scholar]
- Oborny B. (1994) Growth rules in clonal plants and environmental predictability—a simulation study. Journal of Ecology 82341–351. [Google Scholar]
- Pitelka LF and Ashmun JW. (1985) Physiology and integration of ramets in clonal plants. In Jackson JBC, Buss LW, Cook RE (Eds.). Population biology and evolution of clonal organisms.(Yale University Press, New Haven, CT) pp. 339–437.
- Price EAC and Marshall C. (1999) Clonal plants and environmental heterogeneity. Plant Ecology 1413–7. [Google Scholar]
- Quinn GP and Keough MJ. (2002) Experimental design and data analysis for biologists.(Cambridge University Press, Cambridge).
- Roiloa SR and Retuerto R. (2005) Presence of developing ramets of Fragaria vesca L. increases photochemical efficiency in parent ramets. International Journal of Plant Sciences 166795–803. [Google Scholar]
- Saitoh T, Seiwa K, Nishiwaki A. (2002) Importance of physiological integration of dwarf bamboo to persistence in forest understorey: a field experiment. Journal of Ecology 9078–85. [Google Scholar]
- Salzman AG. (1985) Habitat selection in a clonal plant. Science 228603–604. [DOI] [PubMed] [Google Scholar]
- Salzman AG and Parker MA. (1985) Neighbours ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia 65273–277. [DOI] [PubMed] [Google Scholar]
- Schellner RA, Newell SJ, Solbrig OT. (1982) Studies on the population biology of the genus Viola. IV. Spatial pattern of ramets and seedlings in three stoloniferous species. Journal of Ecology 70273–290. [Google Scholar]
- Schreiber U, Bilger W, Hormann H, Neubauer C. (1998) Chlorophyll fluorescence as a diagnostic tool: basics and some aspects of practical relevance. In Raghavendra AS (Ed.). Photosynthesis: a comprehensive treatise.(Cambridge University Press, Cambridge) pp. 320–336.
- Silander JA Jr. (1985) Microevolution in clonal plants. In Jackson JBC, Buss LW, Cook RE (Eds.). Population biology and evolution of clonal organisms.(Yale University Press, New Haven, CT) pp. 107–152.
- Slade AJ and Hutchings MJ. (1987) An analysis of the costs and benefits of physiological integration between ramets in the clonal perennial herb Glechoma hederacea. Oecologia 73425–431. [DOI] [PubMed] [Google Scholar]
- Stuefer JF. (1997) Division of labour in clonal plants? PhD Thesis, University of Utrecht, The Netherlands.
- Stuefer JF, de Kroon H, During HJ. (1996) Exploitation of environmental heterogeneity by spatial division of labour in a clonal plant. Functional Ecology 10328–334. [Google Scholar]
- Sutherland WJ and Stillman RA. (1988) The foraging tactics of plants. Oikos 52239–244. [Google Scholar]
- Sweet GB and Wareing PF. (1966) Role of plant growth in regulating photosynthesis. Nature 21077–79. [Google Scholar]
- Thompson VP, Cunninham SA, Ball MC, Nicotra AB. (2003) Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia 134167–175. [DOI] [PubMed] [Google Scholar]
- van Vuuren MMI, Robinson D, Griffiths BS. (1996) Nutrient inflow and root proliferation during the exploitation of a temporally and spatially discrete source of nitrogen in soil. Plant and Soil 178185–192. [Google Scholar]
- Welham CVJ, Turkington R, Sayre C. (2002) Morphological plasticity of white clover (Trifolium repens L.) in response to spatial and temporal resource heterogeneity. Oecologia 130231–238. [DOI] [PubMed] [Google Scholar]
- Wijesinghe DK and Handel SN. (1994) Advantages of clonal growth in heterogeneous habitats: an experiment with Potentilla simplex. Journal of Ecology 82495–502. [Google Scholar]
- Wijesinghe DK and Hutchings MJ. (1996) Consequences of patchy distribution of light for the growth of the clonal herb Glechoma hederacea. Oikos 77137–145. [Google Scholar]
- Wijesinghe DK and Hutchings MJ. (1999) The effects of environmental heterogeneity on the performance of Glechoma hederacea: the interactions between patch contrast and patch scale. Journal of Ecology 87860–872. [Google Scholar]
- Zar JH. (1984) Biostatistical analysis.(Prentice-Hall, London).