Abstract
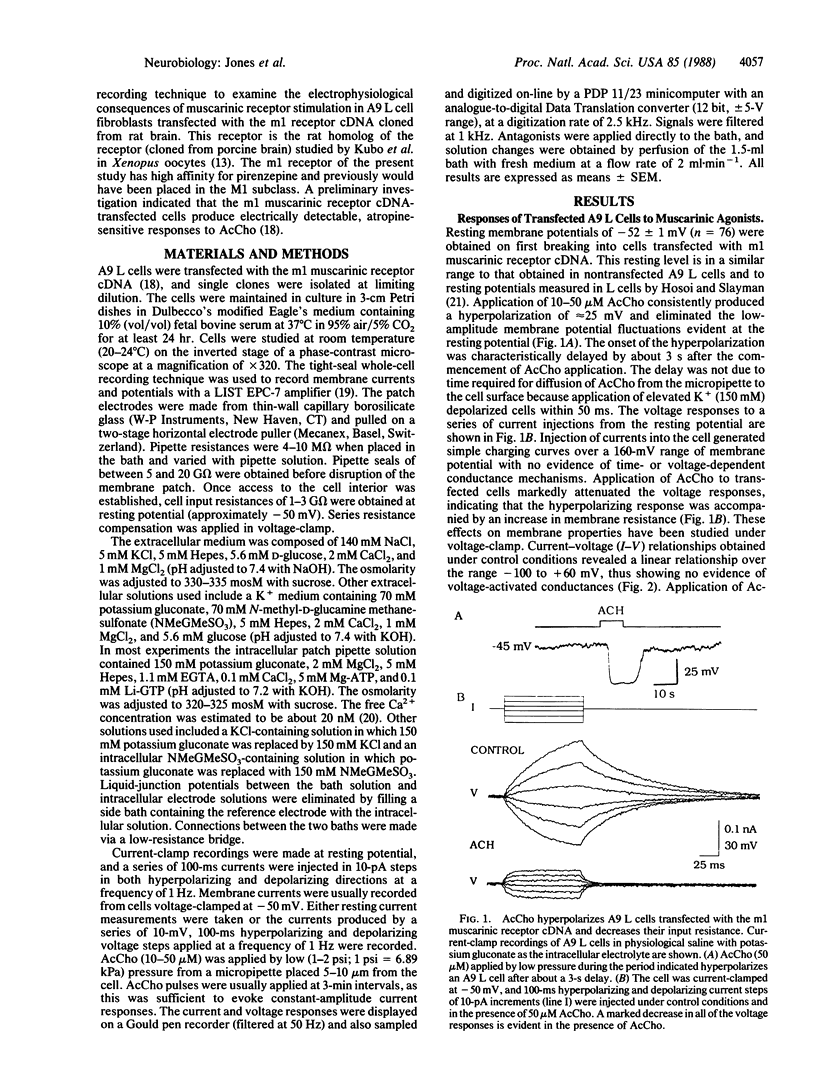
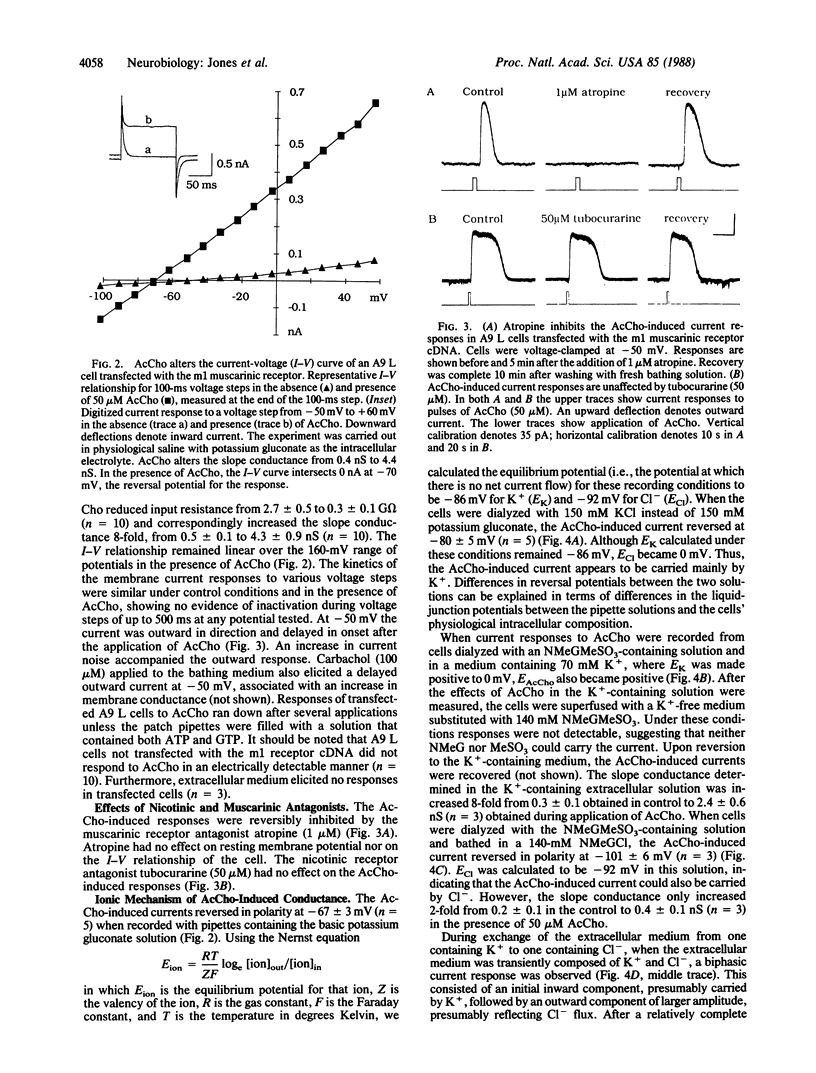
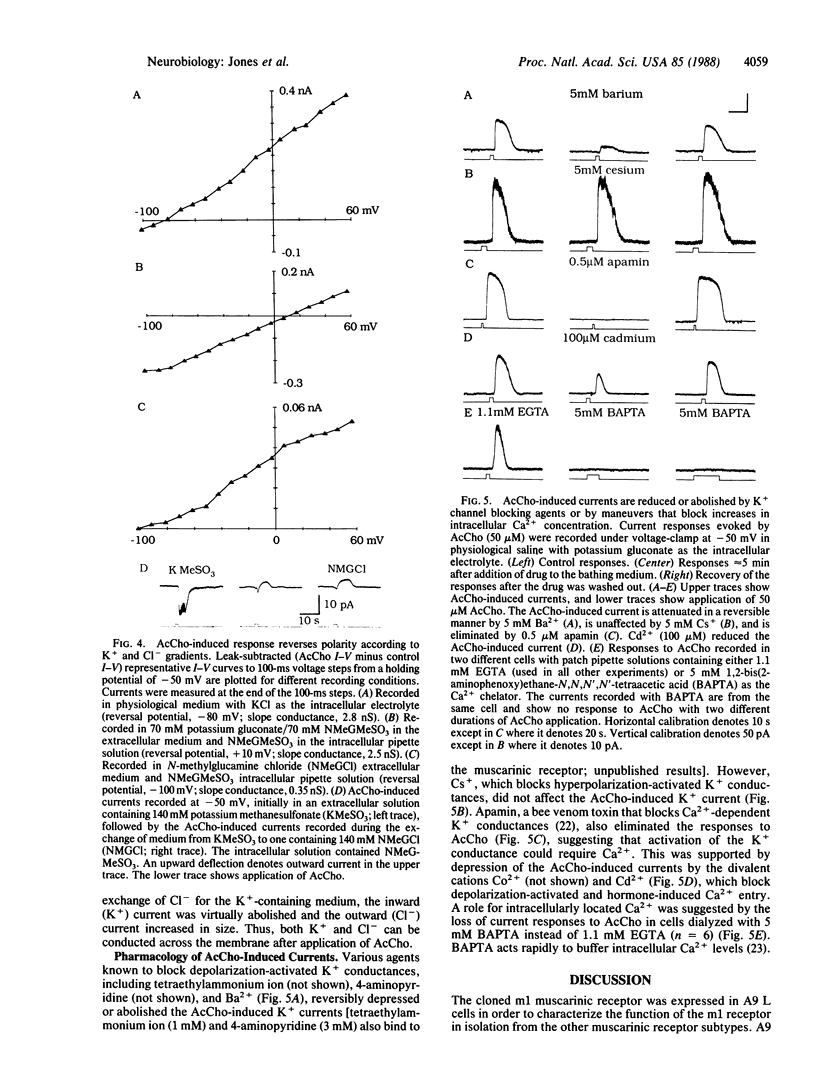
The electrophysiological properties of A9 L cells stably transfected with m1 muscarinic receptor cDNA were examined by using the whole-cell patch-clamp technique. In current-clamp recordings, acetylcholine (AcCho) elicited a hyperpolarization of all transfected cells studied but had no effect on nontransfected A9 L cells. In voltage-clamp recordings, AcCho elicited an outward current at -50 mV accompanied by an increase in conductance. The onset of the current response was consistently delayed by several seconds with respect to the onset of the application of AcCho and could not be accounted for by diffusion. The AcCho-induced currents were reversibly inhibited by the muscarinic receptor antagonist atropine (1 microM) but were unaffected by the nicotinic receptor antagonist tubocurarine (50 microM). Ion-substitution experiments replacing K+ with N-methyl-D-glucamine and Cl- with methanesulfonate indicated that the current was carried mainly by K+, although a minor part appeared to be carried by Cl-. The AcCho-induced current could be blocked by the K+ channel blocking agents tetraethylammonium ion, 4-aminopyridine, apamin, and Ba2+ but not by Cs+. The AcCho-induced current was inhibited when 5 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) was included in the patch pipette or when extracellular Cd2+ or Co2+ was applied, indicating a role for intracellular Ca2+ in the generation of the response. Thus, these results show that cloned m1 muscarinic receptors expressed in A9 L cells can activate a Ca2+-dependent K+ conductance, possibly via a second-messenger system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Masukawa L. M., Sjodin R. A., Livengood D. Uptake and release of 45Ca by Myxicola axoplasm. J Gen Physiol. 1981 Oct;78(4):413–429. doi: 10.1085/jgp.78.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshima H., Iio H., Anan M., Ishii H., Kobayashi S. Induction of muscarinic acetylcholine, serotonin and substance P receptors in Xenopus oocytes injected with mRNA prepared from the small intestine of rats. Brain Res. 1987 Apr;388(1):15–20. doi: 10.1016/0169-328x(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Buckley N. J., Jones S. V., Bonner T. I. Expression of a cloned muscarinic receptor in A9 L cells. Mol Pharmacol. 1987 Oct;32(4):450–455. [PubMed] [Google Scholar]
- Brann M. R., Collins R. M., Spiegel A. Localization of mRNAs encoding the alpha-subunits of signal-transducing G-proteins within rat brain and among peripheral tissues. FEBS Lett. 1987 Sep 28;222(1):191–198. doi: 10.1016/0014-5793(87)80218-1. [DOI] [PubMed] [Google Scholar]
- Brown D. Neuropharmacology. Acetylcholine and brain cells. 1986 Jan 30-Feb 5Nature. 319(6052):358–359. doi: 10.1038/319358a0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., North R. A. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. 1986 Jan 30-Feb 5Nature. 319(6052):405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Kubo T., Akiba I., Maeda A., Mishina M., Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987 Jun 18;327(6123):623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V., Morris A. P. The receptor-regulated calcium influx in mouse submandibular acinar cells is sodium dependent: a patch-clamp study. J Physiol. 1987 Mar;384:119–130. doi: 10.1113/jphysiol.1987.sp016446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hosoi S., Slayman C. L. Membrane voltage, resistance, and channel switching in isolated mouse fibroblasts (L cells): a patch-electrode analysis. J Physiol. 1985 Oct;367:267–290. doi: 10.1113/jphysiol.1985.sp015824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Lancaster B., Nicoll R. A. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987 Mar;7(3):733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Evans M. G., Tan Y. P., Trautmann A. Muscarinic response in rat lacrimal glands. J Exp Biol. 1986 Sep;124:15–32. doi: 10.1242/jeb.124.1.15. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. 1986 Jan 30-Feb 5Nature. 319(6052):402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Slow cholinergic excitation of guinea pig hippocampal neurons is mediated by two muscarinic receptor subtypes. Neurosci Lett. 1986 Jun 18;67(2):107–112. doi: 10.1016/0304-3940(86)90381-2. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- North R. A., Slack B. E., Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J Physiol. 1985 Nov;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukada T., Tanabe T., Takahashi H., Noda M., Haga K., Haga T., Ichiyama A., Kanagawa K., Hiranaga M., Matsuo Primary structure of the alpha-subunit of bovine adenylate cyclase-inhibiting G-protein deduced from the cDNA sequence. FEBS Lett. 1986 Mar 3;197(1-2):305–310. doi: 10.1016/0014-5793(86)80347-7. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Hisanaga Y., Hirono C. Induction of muscarinic cholinergic responsiveness in Xenopus oocytes by mRNA isolated from rat brain. Brain Res. 1985 Jul 15;338(2):346–350. doi: 10.1016/0006-8993(85)90166-0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Intracellular measurements of ion activities. Annu Rev Biophys Bioeng. 1983;12:91–116. doi: 10.1146/annurev.bb.12.060183.000515. [DOI] [PubMed] [Google Scholar]



