Abstract
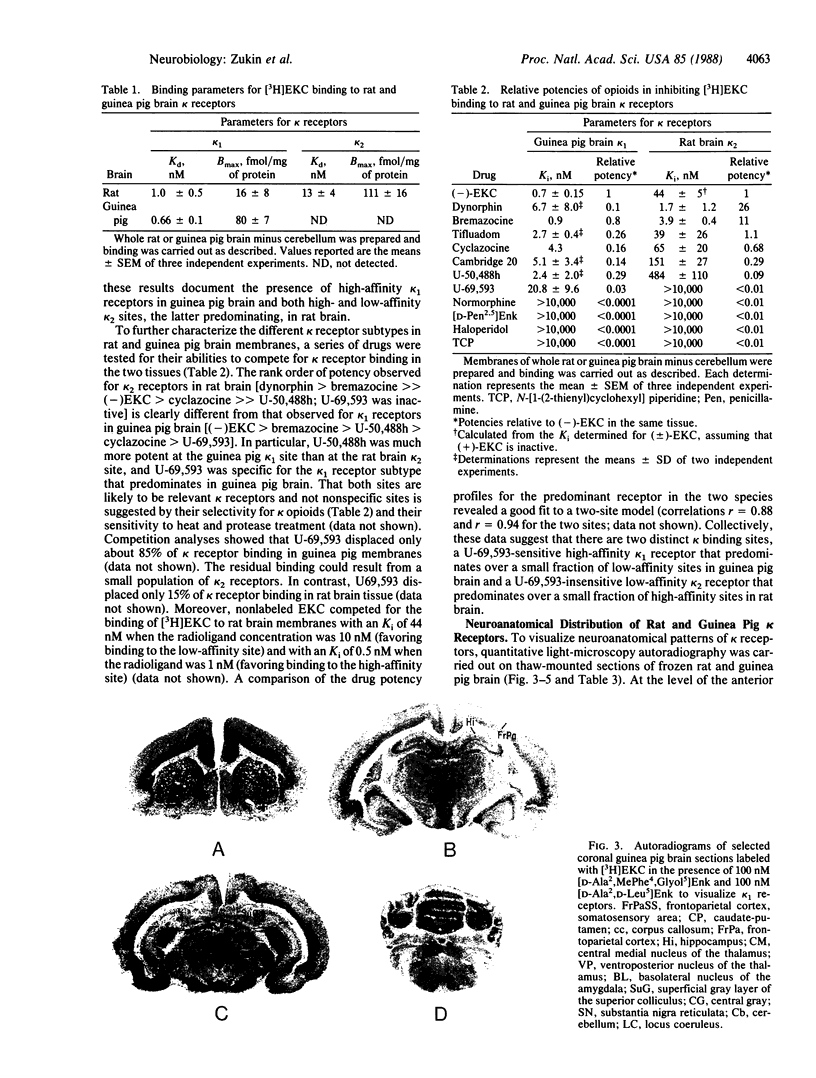
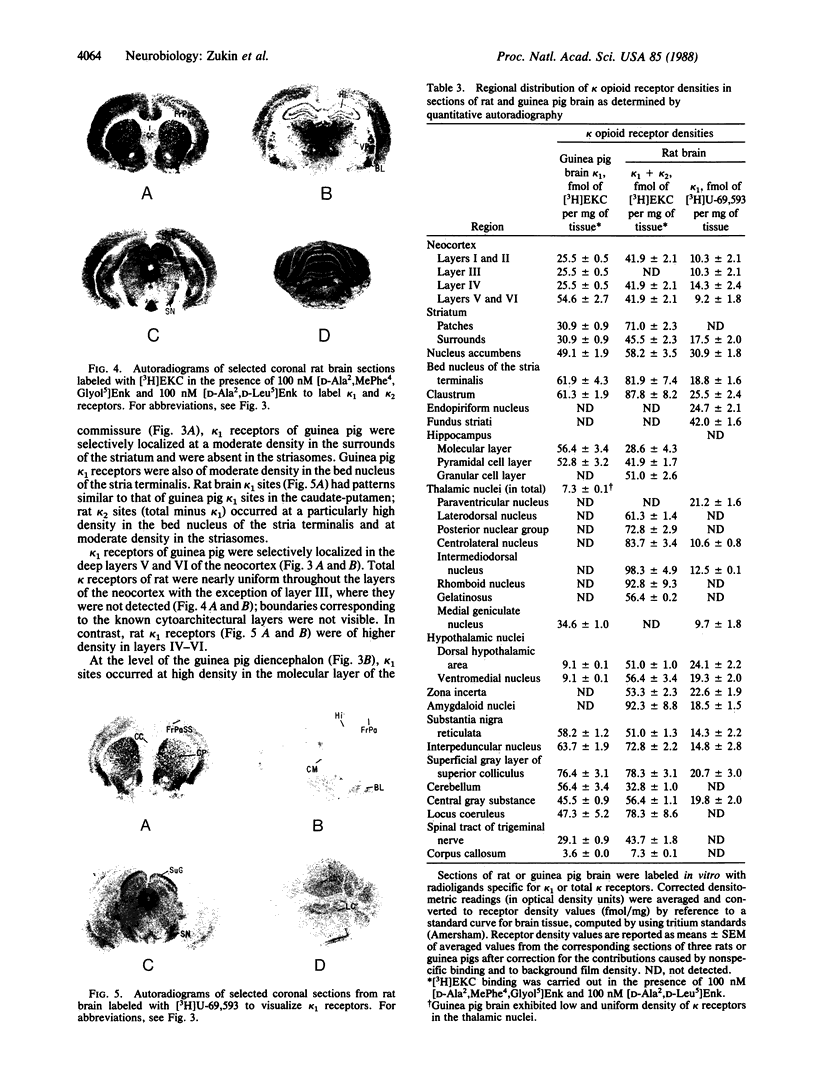
kappa opioid receptors (kappa receptors) have been characterized in homogenates of guinea pig and rat brain under in vitro binding conditions. kappa receptors were labeled by using the tritiated prototypic kappa opioid ethylketocyclazocine under conditions in which mu and delta opioid binding was suppressed. In the case of guinea pig brain membranes, a single population of high-affinity kappa opioid receptor sites (kappa sites; Kd = 0.66 nM, Bmax = 80 fmol/mg of protein) was observed. In contrast, in the case of rat brain, two populations of kappa sites were observed--high-affinity sites at low density (Kd = 1.0 nM, Bmax = 16 fmol/mg of protein) and low-affinity sites at high density (Kd = 13 nM, Bmax = 111 fmol/mg of protein). To test the hypothesis that the high- and low-affinity kappa sites represent two distinct kappa receptor subtypes, a series of opioids were tested for their abilities to compete for binding to the two sites. U-69,593 and Cambridge 20 selectively displaced the high-affinity kappa site in both guinea pig and rat tissue, but were inactive at the rat-brain low-affinity site. Other kappa opioid drugs, including U-50,488, ethylketocyclazocine, bremazocine, cyclazocine, and dynormphin (1-17), competed for binding to both sites, but with different rank orders of potency. Quantitative light microscopy in vitro autoradiography was used to visualize the neuroanatomical pattern of kappa receptors in rat and guinea pig brain. The distribution patterns of the two kappa receptor subtypes of rat brain were clearly different. The pattern of rat high-affinity kappa sites paralleled that of guinea pig in the caudate-putamen, mid-brain, central gray substance of cerebrum, and substantia nigra; interspecies differences were apparent throughout most of the rest of the brain. Collectively, these data provide direct evidence for the presence of two kappa receptor subtypes; the U-69,593-sensitive, high-affinity kappa 1 site predominates in guinea pig brain, and the U-69,593-insensitive, low-affinity kappa 2 site predominates in rat brain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Castanas E., Bourhim N., Giraud P., Boudouresque F., Cantau P., Oliver C. Interaction of opiates with opioid binding sites in the bovine adrenal medulla: II. Interaction with kappa sites. J Neurochem. 1985 Sep;45(3):688–699. doi: 10.1111/j.1471-4159.1985.tb04047.x. [DOI] [PubMed] [Google Scholar]
- Cherubini E., North R. A. Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1860–1863. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow T., Zukin R. S. Solubilization and preliminary characterization of mu and kappa opiate receptor subtypes from rat brain. Mol Pharmacol. 1983 Sep;24(2):203–212. [PubMed] [Google Scholar]
- Demoliou-Mason C. D., Barnard E. A. Solubilization in high yield of opioid receptors retaining high-affinity delta, mu and kappa binding sites. FEBS Lett. 1984 May 21;170(2):378–382. doi: 10.1016/0014-5793(84)81348-4. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H. Kappa opiate receptors localized by autoradiography to deep layers of cerebral cortex: relation to sedative effects. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5703–5707. doi: 10.1073/pnas.79.18.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y., Hiller J. M., Simon E. J. Solubilization and characterization of mu, delta, and kappa opioid binding sites from guinea pig brain: physical separation of kappa receptors. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4217–4221. doi: 10.1073/pnas.81.13.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S., Kim H. S., Wood P. L. Effects of kappa opiate agonists on neurochemical and neuroendocrine indices: evidence for kappa receptor subtypes. Life Sci. 1986 Aug 18;39(7):637–644. doi: 10.1016/0024-3205(86)90045-7. [DOI] [PubMed] [Google Scholar]
- James I. F., Chavkin C., Goldstein A. Preparation of brain membranes containing a single type of opioid receptor highly selective for dynorphin. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7570–7574. doi: 10.1073/pnas.79.23.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James I. F., Goldstein A. Site-directed alkylation of multiple opioid receptors. I. Binding selectivity. Mol Pharmacol. 1984 May;25(3):337–342. [PubMed] [Google Scholar]
- Kosterlitz H. W., Paterson S. J., Robson L. E. Characterization of the kappa-subtype of the opiate receptor in the guinea-pig brain. Br J Pharmacol. 1981 Aug;73(4):939–949. doi: 10.1111/j.1476-5381.1981.tb08749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Morris B. J., Herz A. Autoradiographic localization in rat brain of kappa opiate binding sites labelled by [3H]bremazocine. Neuroscience. 1986 Nov;19(3):839–846. doi: 10.1016/0306-4522(86)90302-7. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Tempel A., Zukin R. S. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaysse P. J., Gardner E. L., Zukin R. S. Modulation of rat brain opioid receptors by cannabinoids. J Pharmacol Exp Ther. 1987 May;241(2):534–539. [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Dynorphin and neoendorphin peptides decrease dorsal root ganglion neuron calcium-dependent action potential duration. J Pharmacol Exp Ther. 1985 Jul;234(1):49–56. [PubMed] [Google Scholar]
- Zukin R. S., Maneckjee R. Solubilization and characterization of opiate receptors. Methods Enzymol. 1986;124:172–190. doi: 10.1016/0076-6879(86)24015-x. [DOI] [PubMed] [Google Scholar]