Abstract
Background
Controversy still surrounds the question whether yeasts found in the gut are causally related to disease, constitute a health hazard, or require treatment.
Methods
The authors present the state of knowledge in this area on the basis of a selective review of articles retrieved by a PubMed search from 2005 onward. The therapeutic recommendations follow the current national and international guidelines.
Results
Yeasts, mainly Candida species, are present in the gut of about 70% of healthy adults. Mucocutaneous Candida infections are due either to impaired host defenses or to altered gene expression in formerly commensal strains. The expression of virulence factors enables yeasts to form biofilms, destroy tissues, and escape the immunological attacks of the host. Yeast infections of the intestinal mucosa are of uncertain clinical significance, and their possible connection to irritable bowel syndrome, while plausible, remains unproved. Yeast colonization can trigger allergic reactions. Mucosal yeast infections are treated with topically active polyene antimycotic drugs. The adjuvant administration of probiotics is justified on the basis of positive results from controlled clinical trials.
Conclusion
The eradication of intestinal yeasts is advised only for certain clearly defined indications.
Keywords: gastrointestinal mycosis, candidiasis, yeast infection, pathogenesis, treatment
Three decades ago, on the basis of case reports, the American physician C. O. Truss (e1– e3) proposed the hypothesis that an unhealthy lifestyle and increased intake of drugs, modified foods, and pollutants could lead to overgrowth of Candida species in the intestine. This would generally reduce the defenses of the host organism and trigger a multiorgan Candida-associated complex of symptoms (“Candida hypersensitivity syndrome”). Since then, this topic has been repeatedly and vigorously discussed by experts and laymen. In 1996, C. Seebacher even claimed that “mycophobia” was spreading. Many of the publications supporting Truss’s hypothesis are not to be taken seriously. Abnormal conditions and severe diseases have often been uncritically linked to the mere detection of fungi in the gut, leading to the initiation of antimycotic therapy. This provoked justifiably critical publications during the 1990s, although some of these were exaggeratedly polemical (for example, e4). An objective scientific discussion on intestinal Candida colonization, with evaluation according to criteria of environmental medicine, was started in 2004, under the management of the Robert Koch Institute (1). Nevertheless, some questions remain open.
Since then, microbiological, molecular biological, and experimental clinical studies have succeeded in clarifying additional facts, so that it appears to be appropriate to re-evaluate the clinical importance of yeasts in the gut.
Methods
The report of the Commission on Methods and Quality Assurance in Environmental Medicine (Kommission “Methoden und Qualitätssicherung in der Umweltmedizin”) on the Pathogenetic Significance of Candida Colonization of the Intestine appeared in 2004 (1). In order to identify later relevant literature, the database PubMed was searched and evaluated from 2005. The following search terms (MeSH) were used as selection criteria in the preparation of the present review article: “Candida/pathogenicity,” “fungi/pathogenicity/virulence factors,” “Candida/clinical trials, humans,” “mycoses/microbiology/drug therapy AND gastrointestinal tract OR urogenital system.” Publications on molds were not considered. Therapeutic statements are in accordance with national and international guidelines.
Intestinal microflora and fungi
Already at birth, microbial colonization starts in the gastrointestinal tract, which has previously been sterile. Intestinal microflora—now also known as microbiota—is established in successive stages and consists of numerous types of microorganism. More than 99% of this microbiota consists of bacterial and archaeal species (2). In addition, Candida yeasts are detectable in 96% of neonates by the end of the first month of life (e5). The constitutional development process is complete after 3 to 5 years and each individual then has an individual microflora compatible with his/her immune system. The special anatomical and physiological features of the individual compartments of the mouth, stomach and intestine offer disparate ecological niches and they are colonized with site-specific microbe communities (2).
Table 1 contains groups of important microorganisms detected by culture. The concentration ranges given for the individual counts of life microbes indicate the great individual variability in the microflora of adults. The findings also show that fungi are detectable in all gastrointestinal sections of about 70% of healthy adults (1). Most of these are members of the Candida genus. Normally 101 to 103 fungal cells per g stool are found, which is much lower than the corresponding values for bacteria – 1011 to 1012 bacteria per g stool. Fungi of other genera are occasionally detected in stool. Although these may be pathogens of the respiratory tract or skin, they are thought to be only transient in the digestive tract. Examples include Aspergillus, Mucor, Cryptococcus, Rhodotorula, and Trichosporon.
Table 1. Information on microorganism groups and counts in different sections of the gastrointestinal tract.
| Microorganisms | Stomach | Jejunum | Ileum | Colon |
| Aerobic and facultatively anaerobic microorganism groups | ||||
| Enterobacteria | 0–102 | 0–103 | 102–106 | 104–1010 |
| Enterococci | 0–103 | 0–104 | 102–106 | 105–1010 |
| Staphylococci | 0–102 | 0–103 | 102–105 | 104–107 |
| Lactobacilli | 0–103 | 0–104 | 102–105 | 106–1010 |
| Fungi | 0–102 | 0–102 | 102–103 | 102–106 |
| Anaerobic microorganism groups | ||||
| Bacteroides spp. | rare | 0–102 | 103–107 | 1010–1012 |
| Bifidobacteria | rare | 0–103 | 103–105 | 108–1012 |
| Anaerobic streptococci | rare | 0–103 | 102–104 | 108–1011 |
| Clostridia | rare | rare | 102–104 | 106–1011 |
| Eubacteria | rare | rare | rare | 109–1012 |
(Essential microorganism groups compiled as in [2].
Figures in colony forming units (CFU) per mL or per g intestinal content.
Even though Candida species are relatively often detected in stool, it is unclear whether these fungi are physiological and (useful) intestinal symbionts. As long as the site-specific microbial communities are intact and the innate immune system is functioning, Candida species behave like commensal members of the gastrointestinal microflora (3, 4, 5). They are then guests which cause no damage. For example, oral and esophageal candidiasis is only manifest when CD4+ Th1 lymphocytes deficiency and reduced formation of proinflammatory cytokines (IL-12, INF-gamma) prevent effective defense against fungi (6, e6). Candida infections may arise as a consequence of
Janus-headed Candida
Candida spp. are yeasts which are normally present as individual cells and which predominantly replicate asexually by budding. The expression “yeast fungus” is pleonastic, as yeasts belong to the kingdom of the fungi. Candida albicans is a diploid polymorphic yeast with eight pairs of chromosomes. It can also replicate under anaerobic conditions, as found in the human colon (e8). Almost 200 Candida species are known, although few are important for man. The most important of these are C. albicans, C. glabrata, C. krusei, C. dubliniensis, C. tropicalis, C. parapsilosis, C. guilliermondii, and C. lusitaniae (3, 4, e10). One reason that this list is short is that about two thirds of Candida species are incapable of growing at 37oC (e10). Molecular genetic studies have shown that there is much greater variety in individual Candida flora (e11).
Candida yeasts are classified as opportunistic pathogens, meaning that they are pathogens only under specific conditions (4, e7). Overgrowth of yeasts is normally inhibited by both specific (immune system) and non-specific defense systems (intestinal flora, peristalsis, intestinal enzymes, defensins, and others).
Changes in the qualitative or quantitative composition of the bacterial flora in the gastrointestinal tract—for example, after administration of antibiotics (9)—or a deficiency in specific parameters of the host’s immune system evidently enhance the virulence of opportunistic Candida strains through gene regulation mechanisms (10, e7, e12). Their damaging activities can manifest at two different levels:
Superficial infections of the skin, mucus membranes, or epithelia (skin or mucosal mycoses, Candida vaginoses)
Invasive penetration into deeper tissue layers, distribution in blood and dissemination in various organs (invasive or systemic mycoses or candidoses).
Pathogenicity factors of Candida strains
Pathogenic yeasts bear genes coding for specific pathogenicity factors and for other properties important for the infection process (10, 11, e7). These primarily include adhesion factors, which mediate binding of yeasts to cell surfaces, such as epithelium or endothelium, and aggression factors. The latter destroy tissues and include secreted aspartate proteinases, phospholipases, and lipases. Aggression factors are partially responsible for the invasiveness of fungi (e5). Strongly virulent Candida strains are capable of expressing several pathogenicity factors simultaneously (e10, e13– e15). Moreover, pathogenic yeasts have developed so-called escape mechanisms, to avoid the attacks of the immune system (6).
The first step in infection is interaction with the host cells by adhesion. C. albicans can express various adhesins, which bind to extracellular matrix proteins of mucosal or endothelial cells (12, e16). The close association with cell surfaces stimulates the formation of biofilms (e5). Biofilms are three dimensional consortia of microorganisms, which adhere to a surface and are enclosed by extracellular polymeric substances. Biofilm formation is directed by the emission and reception of signal molecules between the participating microorganisms (“quorum sensing”) (e8, e14). One of the quorum sensing molecules used by Candida species is the oxylipin farnesol (e8, e17). The biofilm surrounds the yeast cells like a protective cocoon and largely keeps out attacks from the environment, including the immune system. In addition, resistance to antimycotics is enhanced by changes in metabolic reactions in the yeast cells within the biofilm (13). For example, so-called efflux pumps in the cell membrane are activated; these extrude absorbed antimycotics from yeast cells (e5, e14). Another example is the reduction in the synthesis of the cell membrane component ergosterol, which makes the yeast cells less sensitive to antimycotics by a factor of 30 to 2000 (13).
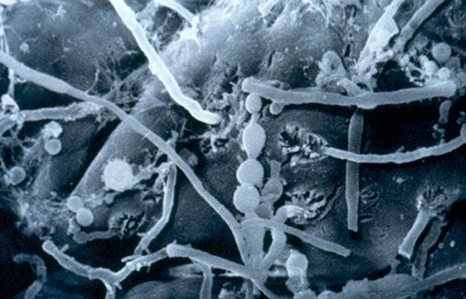
Ten different secreted aspartate proteinases (SAPs) are known for C. albicans. Depending on the situation, different tissue-specific SAPs are induced and these are important for the invasion of tissues and organs (4, 10, e18). Moreover, these Candida proteinases inactivate various host defense factors, such as immunoglobulins, complement factors and serum protease inhibitors, thus reducing opsonizing and microbicidal activity in blood. The term “phenotypic switching” means the rapid change in cell surface structure. This has been observed in some Candida strains and makes it more difficult for the immune system to recognize these yeast cells. It also seems to be associated with biofilm formation (14, e19– e21). During the reversible change from the roundish yeast cell shape to the protracted hyphae form (figure), the incorporation of beta-glucan molecules into the external wall layers is blocked (5). As a consequence, the pattern recognition receptors (toll-like receptors) of the innate immune system can no longer recognize the fungi or initiate an immune reaction, as they are “designed” for the recognition of the beta-glucan of the Candida cell wall (15, e5, e9, e22). The transition from the yeast to the hyphae form—and back—is controlled by various factors, including the synthesis of prostaglandins (PGE2) and leukotrienes (LTB4) (15, 16, e23– e26), or by contact with bacterial peptidoglycans (17). Any use of antibiotics leads to the release of peptidoglycans from the cell wall of intestinal bacteria, possibly thus increasing the formation of hyphae from C. albicans and enhancing its invasive potency (9).
Figure.
Candida albicans on human small intestine mucosa (biopsy material, scanning electron microscope image, 3000-fold magnification). Center of image: spherical budding cells; to the right and left of these: thread-like, aggressive fungal hyphae (taken by Ms A Lorenz, with kind permission of Ardeypharm GmbH)
Some Candida strains avoid the attacks of the immune system by concealing themselves in host cells. They can survive unharmed in epithelial cells (e27) or in non-activated macrophages (6, e28) and even replicate there.
As a result of transcriptional flexibility (11, e13), Candida species are extremely adaptable to the environmental conditions produced by the host, such as pH, partial CO2 pressure, amino acid availability, and iron deficiency (8, 9, 18). The yeast genome can be slightly modified by repeated point mutations (“microevolution”) and this can help the yeast to overcome the initial protective measures initiated by the host after the first contact (11). Moreover, chromosomal rearrangements can lead to the deletions of the chromosome sections mediating sensitivity to antimycotics. Recombination can also lead to the duplication of genes for efflux pumps, thus furthering the elimination of toxic substances.
Clinical significance of Candida infections
In contrast to other medically important fungi, such as Histoplasma capsulatum, Cryptococcus neoformans or Aspergillus fumigatus, human pathogenic Candida species are rarely found in environmental samples (4). In particular, Candida albicans is always associated with man or with warm blooded animals. Candida infections are contact infections. The pathogen reservoir is nevertheless not thought to be fungal spores, cells, or mycelium fragments taken up through the respiratory tract, but the Candida cells colonizing human mucosal surfaces, vaginal epithelium or skin—usually regarded as harmless commensals (7).
It is generally accepted that invasive candidoses are a real threat in hospitals. C. albicans is the yeast species most frequently isolated from clinical material and is involved in more than 50% of mucocutaneous and systemic yeast infections. On the other hand, there has recently been a clear increase in the proportion of non-albicans candidoses. Thus, infections with C. glabrata and C. krusei have increased more since 1990 than those with C. albicans (e29, e30). In the USA, for example, 11.5% of an approximate 80 000 blood infections per year are caused by Candida species. This is accompanied by a mortality rate of more than 30% (e31).
In many industrial countries, Candida mycoses are in fourth place among nosocomial infections in intensive care units (e31). Up to 20% of infections of medical implants, such as central venous catheters, bladder catheters or artificial heart valves, are due to infections with Candida albicans (e32). The predilection for forming biofilms on catheters and implants makes treatment with antimycotics enormously more difficult.
Table 2 lists predisposing factors for fungal infections, corresponding to the practical criteria of “very young, very old or very ill.” Aside from oral soor, esophagitis and nappy rash, it remains unclear to what extent intestinal fungi are responsible for specific gastrointestinal diseases. Thus, Candida-associated infectious diarrhea has been frequently described in neonates, undernourished children, older patients, the severely or chronically ill, in intensive care units, or after long term antibiotic treatment (e33– e40). Nevertheless, these cases are rather rare in comparison to bacterial or viral intestinal infections.
Table 2. Causes and predisposing factors for fungal infections.
| Causes | Factors (examples) |
| Physiological states with high susceptibility to fungi | Premature babies |
| Neonates | |
| Elderly | |
| Persons during phases of hormonal adjustment (e.g. pregnancy, menopause) | |
| Diseases, pathological conditions, operations | Hormonal diseases (e.g. diabetes mellitus, adrenal dysfunction) |
| Immune deficiencies | |
| Infections | |
| Chronic inflammatory bowel diseases | |
| Hematological oncological diseases | |
| Malignant tumors | |
| Visceral operations | |
| Organ transplantation | |
| Alcoholism | |
| Bedridden patients | |
| Therapeutic measures | Antibiotics |
| Corticosteroids | |
| Immunosuppressives | |
| Intensive care | |
| Cytostatics | |
| Radiation | |
| Indwelling catheters |
Patients with irritable bowel syndrome most frequently suffer from intermittent persistent watery diarrhea, meteorism, flatulence, and abdominal pain—just the same as the dominant symptoms for patients with intestinal candidosis (e41). It is, however, unclear whether pathogenic yeasts are responsible for these symptoms in a proportion of patients with irritable bowel syndrome. A controlled study published in 1992 found no correlation between Candida colonization of the intestine and the symptoms of irritable bowel syndrome (e42). However, there are doubts about the quality of the methods used and there have been no more recent studies on this topic.
It has been known since the 1990s that Candida albicans may be involved in the occurrence of allergies. Thus, some cell wall components (mannans and mannoproteins) and enzymes (SAP, enolase) of C. albicans are potentially immunogenic and allergenic (19, e43– e50). Animal experiments have confirmed this association. Canadida albicans colonization of the mouse gut was promoted by antibiotic treatment. This enhanced pulmonary hypersensitivity towards nasally administered foreign protein (ovalbumin) or to spores of Aspergillus fumigatus. On the other hand, animals not colonized with C. albicans exhibited no hypersensitivity (16, e24).Moreover, Candida albicans provoked mast cell-mediated hyperpermeability of the intestinal mucosa in another mouse model (e51). The well known association between reduced barrier function of the intestinal mucosa (“leaky gut”), disturbances in the gut-associated immune system, changes in the intestinal microflora, and atopic diseases such as neurodermatitis, may bring special problems for patients with corresponding genetic susceptibility and intestinal Candida albicans colonization (19). However, this concept requires additional clinical studies.
According to current knowledge, all that remains of Truss’s Candida hypersensitivity syndrome is the assumption of a relationship with the irritable bowel syndrome and the knowledge that Candida in the intestine may function as an “allergy trigger factor.”
Therapeutic possibilities
Antimycotics of various substance classes are available for therapy as described in the guidelines. These may be administered orally or parenterally, depending on the localization and severity of the Candida infection (20, 21, e52, e53) (table 3).
Table 3. Antimycotics and mechanisms of action.
| Substance classes | Active substance | Administration | Action on yeast cells |
| Antimycotics for mucocutaneous candidoses | |||
| Polyenes | Nystatin | oral | Changes in permeability of cytoplasmic membrane from complex binding to ergosterol |
| Natamycin | oral | Changes in permeability of cytoplasmic membrane by blocking ergosterol | |
| Amphotericin B | oral | Changes in permeability of cytoplasmic membrane from oxidative damage | |
| Azole derivatives | |||
| – Imidazoles | Miconazole | oral | Damage to cytoplasmic membrane from inhibition of ergosterol biosynthesis |
| Ketoconazole | oral | ||
| – Triazoles | Fluconazole | oral | Inhibition of 14-α -demethylase |
| Itraconazole | oral | ||
| Voriconazole | oral | ||
| Antimycotics for invasive candidoses | |||
| Polyenes | Amphotericin B | IV | Changes in permeability of cytoplasmic membrane from oxidative damage |
| Triazoles | Fluconazole | IV | Damage to cytoplasmic membrane from inhibition of ergosterol biosynthesis |
| Itraconazole | IV | ||
| Posaconazole | IV | Inhibition of 14-α -demethylase | |
| Voriconazole | IV | ||
| Nucleoside analogues | 5-fluorocytosine | IV | Inhibition of biosynthesis of RNA and DNA |
| (5-FC, flucytosine) | |||
| Echinocandins | Anidulafungin | IV | Incomplete cell wall synthesis due to inhibition of β -(1,3)-D-glucan synthase |
| Caspofungin | IV | ||
| Micafungin | IV | ||
(compiled from 20, 21, e52, e53) IV: intravenous
Nystatin is the most frequently used non-absorbable antimycotic for candidoses of the orogastrointestinal tract. Orally administered topically active nystatin and systemically active fluconazole are also established components of the prophylaxis of Candida infections in the critically ill (22, e54, e55) and in premature babies (e56– e59). Nystatin—a polyene—is cheaper and has the advantage of causing fewer side effects than fluconazole—an absorbable azole derivative (e53, e60). There have been occasional reports of allergic reactions to nystatin (e61). Therapeutic failures may be due to decreased sensitivity or acquired resistance to azole antimycotics (20, e53). Care should be taken to avoid re-infections (“ping pong effect”) by partner contact, or through dental prostheses, tooth brushes, mouthpieces, or dummies. Yeasts can however circumvent elimination by antimycotics by triggering the escape mechanisms we have described or by embedding themselves in biofilms. New therapeutic approaches are being discussed (5).
It is known that physiological intestinal microflora can provide protection against Candida infections in the orogastrointestinal tract (1, 3, e62). This focuses attention on probiotics. Twelve-month administration of a combination preparation with 8 bacterial strains to 10 pouchitis patients—in comparison to placebo therapy—gave the desired maintenance of remission and also significantly reduced the diversity of intestinal fungi (p<0.002) (e63). Controlled animal experiments have found that gastric ulceration promoted by Candida was attenuated after oral administration of L. acidophilus and was accompanied by more than 60% reduction in Candida colonization (23). A double blind placebo-controlled study with 80 premature babies found a significant reduction (p = 0.01) in intestinal Candida colonization after oral administration of L. rhamnosus for 12 months (24). An analogous study with 276 elderly patients and treatment for 16 weeks also found a significant decrease of Candida counts (p = 0.004) (25). Further controlled studies with probiotics are expected.
There are no reliable findings on the necessity for the adjuvant use of special nutritional forms (“anti-fungus diets”). It is much more sensible to use a—generally recommended—mixed diet with high fibre content and reduced sugar to stabilize the microecological system in the gut.
Key Messages.
Candida albicans strains can in principle be classified as facultatively pathogenic yeasts.
102 to 104 CFU Candida are found per g stool in more than half the adult population, so that this cannot be equated with intestinal mycosis.
-
Depending on the stability of the innate host barriers (mucosa, immune system, intestinal microflora), intestinal Candida colonization may lead to
superficial candidosis (restricted to the epidermal and mucosal surfaces),
locally restricted invasive candidosis, or
invasive systemic candidosis.
The indications most closely linked to Candida colonization are irritable bowel syndrome and certain allergic reactions, although an association has been proven. There is no epidemiological or interventional evidence for the existence of a general and clinically demonstrable Candida hypersensitivity syndrome.
Topically active antimycotics (such as nystatin preparations) are available for the treatment of superficial infections of the orogastrointestinal tract. Modulation of intestinal microflora with probiotics can suppress Candida colonization.
Acknowledgments
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Footnotes
Conflict of interest statement
Until 2005, Dr. Schulze was an employee of the company Ardeypharm GmbH.
Dr. Sonnenborn is head of the section for biological research at Ardeypharm GmbH.
References
- 1.Kommission „Methoden und Qualitätssicherung in der Umweltmedizin“. Pathogenetische Bedeutung der intestinalen Candidabesiedelung. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2004;47:587–600. doi: 10.1007/s00103-004-0860-1. [DOI] [PubMed] [Google Scholar]
- 2.Schulze J, Sonnenborn U, Ölschläger T, Kruis W. Probiotika Mikroökologie, Mikrobiologie, Qualität, Sicherheit und gesundheitliche Effekte. Stuttgart: Hippokrates; 2008. pp. 4–16. [Google Scholar]
- 3.Bernhardt H, Knoke M. Mycological aspects of gastrointestinal microflora. Scand J Gastroenterol. 1997;32(Suppl 222):102–106. doi: 10.1080/00365521.1997.11720731. [DOI] [PubMed] [Google Scholar]
- 4.Hube B. From commensal to pathogen: stage- and tissue-specific gene expression of Candida albicans. Curr Opin Microbiol. 2004;7:336–341. doi: 10.1016/j.mib.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, Brown GD, Kullberg BJ, Gow NAR. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Immunol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 6.Raska M, Belakova J, Krupka M, Weigl E. Candidiasis - Do we need to fight or to tolerate the Candida fungus? Folia Microbiol. 2007;52:297–312. doi: 10.1007/BF02931313. [DOI] [PubMed] [Google Scholar]
- 7.Mathews HL, Witek-Janusek L. Host defense against oral, esophageal, and gastrointestinal candidiasis. In: Calderone RA, editor. Candida and candidiasis. Washington: ASM Press; 2002. pp. 179–192. [Google Scholar]
- 8.Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piispanen AE, Hogan DA. PEPped up: induction of Candida albicans virulence by bacterial cell wall fragments. Cell Host Microbe. 2008;4:1–2. doi: 10.1016/j.chom.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Thewes S, Kretschmar M, Park H, Schaller M, Filler SG, Hube B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol Microbiol. 2007;63:1606–1628. doi: 10.1111/j.1365-2958.2007.05614.x. [DOI] [PubMed] [Google Scholar]
- 11.Morschhäuser J, Köhler G, Ziebuhr W, Blum-Oehler G, Dobrindt U, Hacker J. Evolution of microbial pathogens. Phil Trans R Soc Lond. 2000;355:695–704. doi: 10.1098/rstb.2000.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 13.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 14.Soll DR. Phenotypic switching. In: Calderone RA, editor. Candida and Candidiasis. Washington: ASM Press; 2002. pp. 123–142. [Google Scholar]
- 15.Bastidas RJ, Heitman J. Trimorphic stepping stones pave the way to fungal virulence. PNAS. 2009;106:351–352. doi: 10.1073/pnas.0811994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–38. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XL, Lee RTH, Fang HM, Wang YM, Li R, Zou H, et al. Bacterial pepti-doglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase cyr1p. Cell Host Microbe. 2008;4:28–39. doi: 10.1016/j.chom.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Polakova S, Blume C, Zarate JA, Mentel M, Jorck-Ramberg D, Stenderup J, et al. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. PNAS. 2009;106:2688–2693. doi: 10.1073/pnas.0809793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita E, Hide M, Yoneya Y, Kannbe M, Tanaka A, Yamamoto S. An assessment of the role of Candida albicans antigen in atopic dermatitis. J Dermatol. 1999;26:282–287. doi: 10.1111/j.1346-8138.1999.tb03473.x. [DOI] [PubMed] [Google Scholar]
- 20.Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of Candidiasis. CID. 2004;38:161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 21.Reinel D, Plettenberg A, Seebacher C, et al. Orale Candidose. Leitlinie der Deutschen Dermatologischen Gesellschaft und der Deutschsprachigen Mykologischen Gesellschaft. JDDG. 2008;7:593–597. doi: 10.1111/j.1610-0387.2008.06801.x. [DOI] [PubMed] [Google Scholar]
- 22.Cruciani M, de Lalla F, Mengoli C. Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysis. Intens Med Care. 2005;31:1479–1487. doi: 10.1007/s00134-005-2794-y. [DOI] [PubMed] [Google Scholar]
- 23.Zwolinska-Wcislo M, Brozozowski T, Mach T, et al. Are probiotics effective in the treatment of fungal colonization of the gastrointestinal tract? Experimental and clinical studies. J Physiol Pharmacol. 2006 57;(Suppl 9):35–49. [PubMed] [Google Scholar]
- 24.Manzoni P, Mostert M, Leonessa ML, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric coloniza-tion by Candida species in preterm neonates: A randomized study. Clin Infect Dis. 2006;42:1735–1742. doi: 10.1086/504324. [DOI] [PubMed] [Google Scholar]
- 25.Hatakka K, Ahola AJ, Yli-Knuuttila, et al. Probiotics reduce the pre-v-alence of oral Candida in the elderly—a randomized controlled trial. J Dent Res. 2007;86:125–130. doi: 10.1177/154405910708600204. [DOI] [PubMed] [Google Scholar]
- e1.Truss CO. Tissue injury induced by Candida albicans: mental and neurological manifestations. J Orthomol Psychiatry. 1978;7:17–37. [Google Scholar]
- e2.Truss CO. Restoration of immunologic competence to Candida albicans. J Orthomol Psychiatry. 1980;9:287–301. [Google Scholar]
- e3.Truss CO. The role of Candida albicans in human illness. J Orthomol Psychiatry. 1981;10:228–238. [Google Scholar]
- e4.Eckardt VF, Rösch W. Pilze im Darm. Krankheitserreger oder Kommensale? Dtsch Arztebl. 1995;92:A2324–A-2326. [Google Scholar]
- e5.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol. 2005;59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- e6.Farah CS, Elahi S, Drysdale K, Pang G, et al. Primary role for CD4+ T lymphocytes in recovery from oropharyngeal candidiasis. Infect Immun. 2002;70:724–731. doi: 10.1128/iai.70.2.724-731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Walker LA, MacCallum DM, Bertram G, Gow NA, Odds FC, Brown AJ. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet Biol. 2009;46:210–219. doi: 10.1016/j.fgb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Kruppa M. Quorum sensing and Candida albicans. Mycoses. 2008;52:1–10. doi: 10.1111/j.1439-0507.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- e9.Romani L, Bistoni F, Puccetti P. Adaptation of Candida albicans to the host environment: the role of morphogenesis in virulence and survival in mammalian hosts. Curr Opin Microbiol. 2003;6:338–343. doi: 10.1016/s1369-5274(03)00081-x. [DOI] [PubMed] [Google Scholar]
- e10.Calderone RA. Taxonomy and biology of Candida. In: Calderone RA, editor. Candida and Candidiasis. Washington: ASM Press; 2002. pp. 15–27. [Google Scholar]
- e11.Ott SJ, Kühbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, Drews O, Weichert W, Timmis KN, Schreiber S. Fungi and inflammatory bowel disease: alterations of composition and diversity. Scand J Gastroenterol. 2008;43:831–841. doi: 10.1080/00365520801935434. [DOI] [PubMed] [Google Scholar]
- e12.Calderone RA. Virulence factors of Candida albicans. In: Calderone RA, editor. Fungal pathogenesis: principles and clinical applications. New York: Marcel Dekker Inc; 2001. pp. 3–13. [Google Scholar]
- e13.Magee PT, Chibana H. The genomes of Candida albicans and other Candida species. In: Calderone RA, editor. Candida and candidiasis. Washington: ASM Press; 2002. pp. 293–304. [Google Scholar]
- e14.Nobile CL, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- e15.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- e16.Grubb SEW, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect Immun. 2008;76:4370–4377. doi: 10.1128/IAI.00332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e17.Shea JM, Del Poeta M. Lipid signaling in pathogenic fungi. Curr Opin Microbiol. 2006;9:352–358. doi: 10.1016/j.mib.2006.06.003. [DOI] [PubMed] [Google Scholar]
- e18.Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun. 2007;75:2126–2135. doi: 10.1128/IAI.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. PNAS. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e20.Ramirez-Zavala B, Reuß O, Park YN, Ohlsen K, Morschhäuser J. Environmental induction of white-opaque switching in Candida albicans. PLOS Pathog. 2008;4:e1000089. doi: 10.1371/journal.ppat.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e21.Zordan RE, Galgoczy DJ, Johnson AD. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. PNAS. 2006;103:12807–12812. doi: 10.1073/pnas.0605138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e22.Berman J. Morphogenesis and cell cycle progression in Candida albicans. Curr Opin Microbiol. 2006;9:595–601. doi: 10.1016/j.mib.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e24.Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e25.Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, Lopez-Ribot JL, Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. PNAS. 2009;106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Shen J, Cowen LE, Griffin AM, Chan L, Kohler JR. The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. PNAS. 2008;105:20918–20923. doi: 10.1073/pnas.0809147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e27.Filler SG, Sheppard DC. Fungal invasion of normally non- phagocytic host cells. PLOS Pathog. 2006;2:e129. doi: 10.1371/journal.ppat.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e28.Dalkilic E, Aksebzeci T, Kocatürk I, Aydin N, Koculu B. The investigation of pathogenity and virulence of Candida. In: Tümbay E, Seeliger HPR, Ang Ö, editors. Candida and Candidamycosis. Vol. 50. New York: Plenum Press; 1991. pp. 167–174. [Google Scholar]
- e29.Maschmeyer G. The changing epidemiology of invasive fungal infections: new threats. Int J Antimicrob Agents. 2006 27;(Suppl 1):3–6. doi: 10.1016/j.ijantimicag.2006.03.006. [DOI] [PubMed] [Google Scholar]
- e30.Tan TY, Tan AL, Tee NWS, Ng LSY. A retrospective analysis of anti-fungal susceptibilities of Candida bloodstream isolates from Singapore hospitals. Ann Acad Med Singapore. 2008;37:835–840. [PubMed] [Google Scholar]
- e31.Chauhan N, Latge JP, Calderone R. Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat Rev Microbiol. 2006;4:435–444. doi: 10.1038/nrmicro1426. [DOI] [PubMed] [Google Scholar]
- e32.Nett J, Andes D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol. 2006;9:340–345. doi: 10.1016/j.mib.2006.06.007. [DOI] [PubMed] [Google Scholar]
- e33.Alam SA, Tahir M, De MN. Candida as a cause of diarrhoea in children. Bangladesh Med Res Council Bull. 1977;3:32–36. [PubMed] [Google Scholar]
- e34.Berkefeld I, Wahn V, Koletzko S. Candida-assoziierte chronische Durchfälle bei selektiv verminderter zellulärer Immunität gegen Candida. Monatsschr Kinderheilk. 1991;139:528. [Google Scholar]
- e35.Cimbaluk D, Scudiere J, Butsch J, Jakate S. Invasive candidal enterocolitis followed shortly by fatal cerebral hemorrhage in immuno-compromised patients. J Clin Gastroenterol. 2005;39:795–797. doi: 10.1097/01.mcg.0000177237.82382.b8. [DOI] [PubMed] [Google Scholar]
- e36.Danna PL, Urban C, Bellin E, Rahal JJ. Role of Candida in pathogenesis of antibiotic-associated diarrhoea in elderly in-patients. Lancet. 1991;337:511–514. doi: 10.1016/0140-6736(91)91296-7. [DOI] [PubMed] [Google Scholar]
- e37.Gupta TP, Ehrinpreis MN. Candida-associated diarrhea in hospitalized patients. Gastroenterology. 1990;98:780–785. doi: 10.1016/0016-5085(90)90303-i. [DOI] [PubMed] [Google Scholar]
- e38.Klingspor L, Stitzing G, Johansen K, Murtaza A, Holmberg K. Infantile diarrhoea and malnutrition associated with Candida in a developing community. Mycoses. 1993;36:19–24. doi: 10.1111/j.1439-0507.1993.tb00682.x. [DOI] [PubMed] [Google Scholar]
- e39.Mathaba LT, Paxman AE, Ward PB, Warmington JR. Genetically distinct strains of Candida albicans with elevated secretory protein-ase production are associated with diarrhoea in hospitalized patients. J Gastroenterol Hepatol. 2000;15:53–60. doi: 10.1046/j.1440-1746.2000.02053.x. [DOI] [PubMed] [Google Scholar]
- e40.Micames CG, Bentley R, Onken J. Image 1—Invasive candidal enterocolitis. Gastroenterology. 2007;133:391–731. doi: 10.1053/j.gastro.2007.06.051. [DOI] [PubMed] [Google Scholar]
- e41.Bodey GP, Sobel JD. Lower gastrointestinal candidiasis. In: Bodey GP, editor. Candidiasis: Pathogenesis, diagnosis and treatment. New York: Raven Press; 1993. pp. 205–223. [Google Scholar]
- e42.Middleton SJ, Coley A, Hunter JO. The role of faecal Candida albicans in the pathogenesis of food-intolerant irritable bowel syndrome. Postgrad Med J. 1992;68:453–454. doi: 10.1136/pgmj.68.800.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e43.Akiyama K, Shida T, Yasueda H, Mita H, Yamamoto T, Yamaguchi H. Atopic asthma caused by Candida albicans acid protease: case reports. Allergy. 1994;49:778–781. doi: 10.1111/j.1398-9995.1994.tb02102.x. [DOI] [PubMed] [Google Scholar]
- e44.Akiyama K, Shida T, Yasueda H, Mita H, Yanagihara Y, Hasegawa M, et al. Allergenicity of acid protease secreted by Candida albicans. Eur J Allergy Clin Immunol. 1996;51:887–892. doi: 10.1111/j.1398-9995.1996.tb04489.x. [DOI] [PubMed] [Google Scholar]
- e45.Ito K, Ishiguro A, Kanbe T, Tanaka K, Torii S. Detection of IgE antibody against Candida albicans enolase and its crossreactivity to Saccharomyces cerevisiae enolase. Clin Exp Allergy. 1995;25:522–528. doi: 10.1111/j.1365-2222.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- e46.Ito K, Ishiguro A, Kanbe T, Tanaka K, Torii S. Characterization of IgE-binding epitopes on Candida albicans enolase. Clin Exp Allergy. 1995;25:529–535. doi: 10.1111/j.1365-2222.1995.tb01090.x. [DOI] [PubMed] [Google Scholar]
- e47.Kanbe T, Utsunomiya K, Ishiguro A. A crossreactivity at the immunoglobulin E level of the cell wall mannoproteins of Candida albicans with other pathogenic Candida and airborne yeast species. Clin Exp Allergy. 1997;27:1449–1457. [PubMed] [Google Scholar]
- e48.Savolainen J, Rantala A, Nermes M, Lehtonen L, Viander M. Enhanced IgE response to Candida albicans in postoperative invasive candidiasis. Clin Exp Allergy. 1996;26:452–460. [PubMed] [Google Scholar]
- e49.Savolainen J, Kosonen J, Lintu P, Viander M, Pene J, Kalimo K, et al. Candida albicans mannan- and protein-induced humoral, cellular and cytokine responses in atopic dermatitis patients. Clin Exp Allergy. 1999;29:824–831. doi: 10.1046/j.1365-2222.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- e50.Savolainen J, Lintu P, Kosonen J, Kortekangas-Savolainen O, Viander M, Pene J, et al. Pityrosporum and Candida specific and nonspecific humoral, cellular and cytokine responses in atopic dermatitis patients. Clin Exp Allergy. 2001;31:125–134. [PubMed] [Google Scholar]
- e51.Yamaguchi N, Sugita R, Miki A, Takemura N, Kawabata J, Watanabe J, Sonoyama K. Gastrointestinal Candida colonisation promotes sensitisation against food antigens by affecting the mucosal barrier in mice. Gut. 2006;55:954–960. doi: 10.1136/gut.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e52.Morschhäuser J, Blum-Oehler G, Hacker J. Virulenz- und Resistenzmechanismen pathogener Candida-Spezies. Med Welt. 1997;48:352–357. [Google Scholar]
- e53.Segal E. Candida, still number one—what do we know and where are we going from there? Mycoses. 2005 48;(Suppl 1):3–11. doi: 10.1111/j.1439-0507.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- e54.Normand S, Francois B, Dardé ML, et al. Oral nystatin prophylaxis of Candida spp. colonization in ventilated critically ill patients. Intens Care Med. 2005;31:1508–1513. doi: 10.1007/s00134-005-2807-x. [DOI] [PubMed] [Google Scholar]
- e55.Wong PN, Lo KY, Tong GMW, Chan SF, Lo MW, Mak SK, et al. Prevention of fungal peritonitis with nystatin prophylaxis in patients receiving CAPD. Perit Dial Int. 2007;27:531–536. [PubMed] [Google Scholar]
- e56.Isaacs D. Fungal prophylaxis in very low birth weight neonates: nystatin, fluconazole or nothing? Curr Opin Infect Dis. 2008;21:246–250. doi: 10.1097/QCO.0b013e3282f8adab. [DOI] [PubMed] [Google Scholar]
- e57.Kaufman DA. Prevention of invasive Candida infections in preterm infants: the time is now. Expert Rev Anti Infect Ther. 2008;6:393–399. doi: 10.1586/14787210.6.4.393. [DOI] [PubMed] [Google Scholar]
- e58.Manzoni P, Leonessa ML, Monetti C, Farina D, Gomirato G. Prevention strategies in patients at high-risk for Candida infections: data from a neonatal intensive care setting. Intens Care Med. 2006;32 doi: 10.1007/s00134-006-0165-y. [DOI] [PubMed] [Google Scholar]
- e59.Ozturk MA, Gunes T, Koklu E, Cetin N, Koc N. Oral nystatin prophylaxis to prevent candidiasis in neonatal intensive care unit. Mycoses. 2006;49:484–492. doi: 10.1111/j.1439-0507.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- e60.Eggimann P, Wolff M, Garbino J. Oral nystatin as antifungal prophylaxis in critically ill patients: an old SDD tool to be renewed? Intens Care Med. 2005;31:1466–1468. doi: 10.1007/s00134-005-2806-y. [DOI] [PubMed] [Google Scholar]
- e61.Martinez FV, Pamplona MPM, Garcia EC, Urzaiz AG. Delayed hypersensitivity to oral nystatin. Contact Derm. 2007;57:200–201. doi: 10.1111/j.1600-0536.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- e62.Bengmark S. Ecological control of gastrointestinal tract. The role of probiotic flora. Gut. 1998;42:2–7. doi: 10.1136/gut.42.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e63.Kühbacher T, Ott SJ, Helwig U, et al. Bacterial and fungal micro-biota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]