Abstract
Oxidative and nitrosative damage are major contributors to cone cell death in retinitis pigmentosa (RP). In this study, we explored the effects of augmenting components of the endogenous antioxidant defense system in models of RP, rd1, and rd10 mice. Unexpectedly, overexpression of superoxide dismutase 1 (SOD1) in rd1 mice increased oxidative damage and accelerated cone cell death. With an elaborate mating scheme, genetically engineered rd10 mice with either inducible expression of SOD2, Catalase, or both in photoreceptor mitochondria were generated. Littermates with the same genetic background that did not have increased expression of SOD2 nor Catalase provided ideal controls. Coexpression of SOD2 and Catalase, but not either alone, significantly reduced oxidative damage in the retinas of postnatal day (P) 50 rd10 mice as measured by protein carbonyl content. Cone density was significantly greater in P50 rd10 mice with coexpression of SOD2 and Catalase together than rd10 mice that expressed SOD2 or Catalase alone, or expressed neither. Coexpression of SOD2 and Catalase in rd10 mice did not slow rod cell death. These data support the concept of bolstering the endogenous antioxidant defense system as a gene-based treatment strategy for RP, and also indicate that coexpression of multiple components may be needed.
Introduction
Retinitis pigmentosa (RP) is a group of diseases in which one of several different mutations results in death of rod photoreceptor cells. The loss of rods results in night blindness, but patients are still able to function well if illumination is adequate. However, once rods die, there is gradual loss of cones accompanied by constriction of visual fields and eventual blindness. If cone death could be prevented in patients with RP, blindness could be averted.
The outer portion of the retina consists solely of photoreceptors, and rods vastly outnumber cones. After rods die, oxygen utilization in the outer retina is reduced, but because choroidal vessels, unlike retinal vessels, are incapable of autoregulation to decrease blood flow when tissue oxygen levels are increased, the oxygen level in the outer retina becomes markedly elevated.1,2 After rods are eliminated, there is progressive oxidative and nitrosative damage to cones, which are major contributors to their death.3,4 In several models of RP in which rods die from different mutations, exogenous antioxidants slow cone cell death, indicating a potential therapeutic approach in all RP patients despite tremendous heterogeneity in pathogenic mutations.5
When free radicals are generated they interact with the first available acceptor they contact, and for antioxidants to prevent damage to critical molecules, they must be present in sufficiently high concentrations in correct cellular compartments to reduce chance meetings of radicals with those molecules. This is a difficult requirement for exogenous antioxidants that must penetrate into all cellular compartments and maintain high levels at all times. Bolstering the endogenous antioxidant defense system may provide a more efficient approach that could be used in a complimentary fashion. In this study, we sought to explore the effects of increasing levels of components of the antioxidant defense system in photoreceptors on cone survival in models of RP.
Results
Paradoxical effect of overexpression of SOD1 in rd1+/+ mice
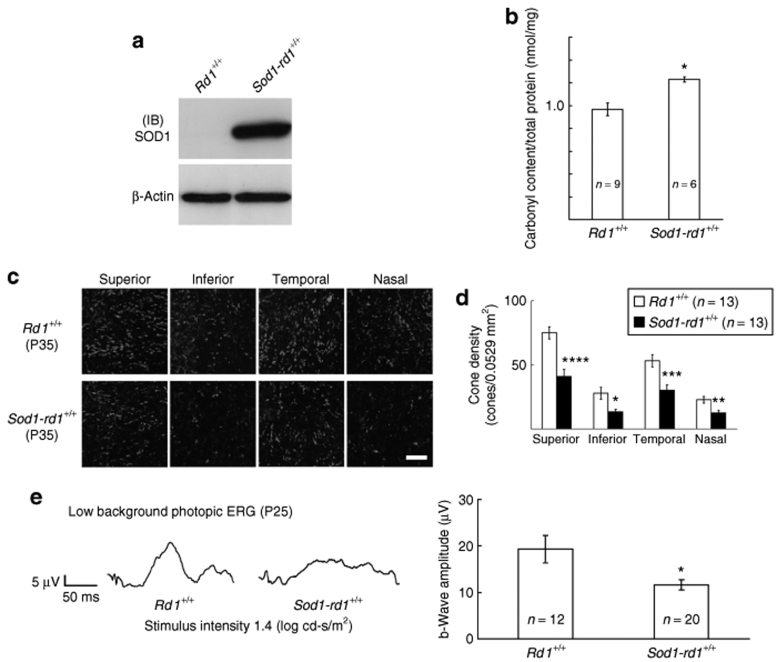
In order to determine if increased levels of superoxide dismutase 1 (SOD1) could slow or prevent cone cell death in a primary rod cell degeneration, transgenic mice in which the actin promoter drives expression of human SOD1 were crossed with rd1+/+ mice and offspring were crossed to obtain rd1+/+ mice that carry the Sod1 transgene (Sod1-rd1+/+ mice). At postnatal day (P) 25, there was strong expression of human SOD1 in Sod1-rd1+/+ mice and no detectable expression in rd1+/+ mice (Figure 1a), but surprisingly Sod1-rd1+/+ mice showed significantly greater carbonyl adducts on proteins in the retina than did rd1+/+ mice, indicating increased rather than decreased oxidative damage (Figure 1b). At P35, compared to rd1+/+ mice, Sod1-rd1+/+ mice showed reduced cone density in all four quadrants of the retina (Figure 1c,d). There was also a reduction in mean photopic b-wave amplitude in P35 Sod1-rd1+/+ mice compared to rd1+/+ mice, indicating that loss of cone cell function was accelerated by overexpression of SOD1 in rd1+/+ mice.
Figure 1.
Superoxide dismutase 1 (SOD1) overexpression significantly decreases cone function and cone cell number in rd1+/+ mice. Transgenic mice in which the actin promoter drives expression of human SOD1 were crossed with rd1+/+ mice and offspring were crossed to obtain rd1+/+ mice that carried the Sod1 transgene (Sod1-rd1+/+ mice). (a) At postnatal day (P) 25, rd1+/+, and Sod1-rd1+/+ mice were euthanized and retinal homogenates were run in western blots using an antibody directed against human SOD1. Immunoblots (IBs) showed strong expression of human SOD1 in Sod1-rd1+/+ and no detectable expression in rd1+/+ mice. Stripping and reprobing of IBs with an antibody directed against β-actin showed that loading was equivalent. (b) At P25, the mean (±SEM) number of carbonyl adducts determined by enzyme-linked immunosorbent assay of retinal homogenates showed a significant increase in oxidized proteins in Sod1-rd1+/+ mice (n = 6) compared to rd1 mice (n = 9;*P < 5.0 × 10−4 by unpaired Student's t-test). (c) At P35, compared to rd1 mice, Sod1-rd1+/+ mice appeared to show lower cone density in all four quadrants of the retina by confocal microscopy of peanut agglutinin–stained retinal flat mounts (scale bar = 50 µm) and this was confirmed by image analysis (d; *P < 2.0 × 10−4, **P < 0.02, ***P < 0.002, ****P < 0.01 by unpaired Student's t-test). (e) Representative wave forms from photopic electroretinograms (ERGs) done in low background illumination at P25 showed lower b-waves for Sod1-rd1+/+ mice than rd1+/+ mice and measurements confirmed a significant reduction in mean (±SEM) b-wave amplitude Sod1-rd1+/+ mice (*P < 0.05 by unpaired Welch's t-test).
Generation of transgenic mice with inducible expression of SOD2, Catalase, or both
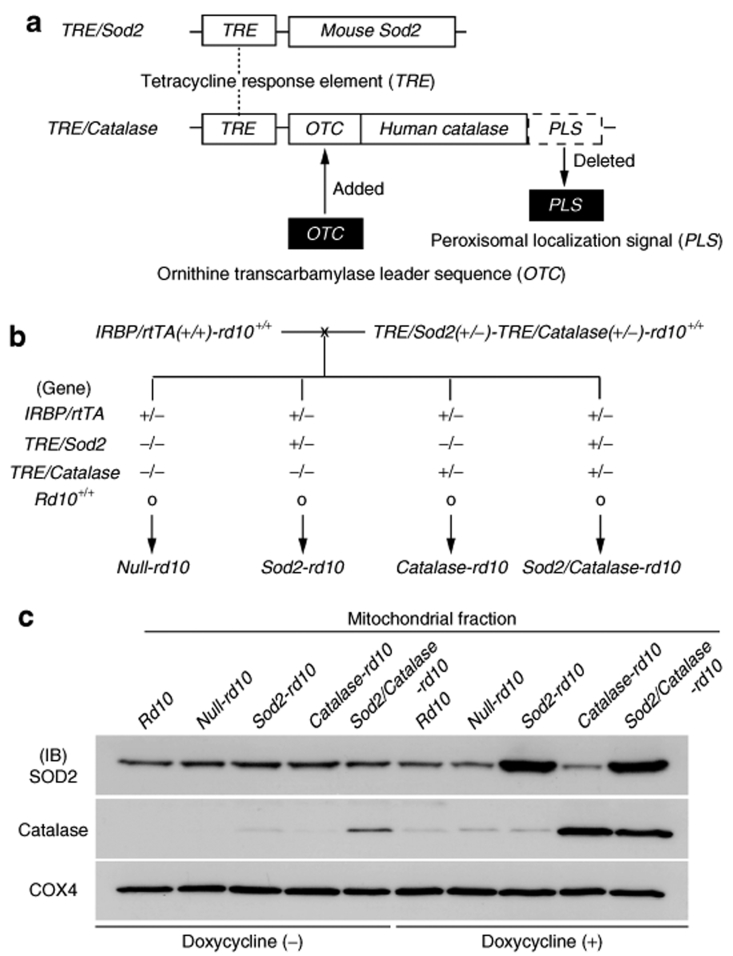
We have previously used the tet/on inducible system to test the effects of overexpressing many different proteins in photoreceptors.6,7,8,9,10 To explore the effects of overexpressing components of the antioxidant defense system, we generated tetracycline response element (TRE)/Sod2 mice and TRE/Catalase mice. The peroxisomal targeting signal was deleted from the Catalase transgene and an ornithine transcarbamylase signal sequence was added to direct the Catalase to mitochondria (Figure 2a). The reverse tetracycline transactivator/interphotoreceptor retinol–binding protein promoter (rtTA/IRBP) was used as the driver line, because it directs expression in both rods and cones. Rd10+/+ mice were used for these experiments, because retinal degeneration occurs more slowly in rd10+/+ mice than rd1+/+ mice. Mice homozygous at both the rtTA/IRBP and rd10 alleles were generated and crossed with mice homozygous at the rd10 allele, but heterozygous at the TRE/Sod2 and TRE/Catalase alleles and the possible offspring are shown in Figure 2b. The offsprings were genotyped and after weaning they were given normal drinking water or drinking water containing 2 mg/ml of doxycycline, and then mitochondrial fractions of retinal homogenates were run in immunoblots. A fairly consistent baseline level of murine SOD2 was seen in all samples except those from doxycycline-treated mice that carried the TRE/Sod2 transgene (Figure 2c). Likewise, strong bands for human Catalase were seen only in samples from doxycycline-treated mice that carried the TRE/Catalase transgene. All samples showed similar bands for COX4, which is expressed in mitochondria, indicating that roughly equivalent amounts of mitochondrial fractions had been loaded. These data demonstrate that mice with either inducible expression of SOD2, Catalase, or both in the mitochondria of photoreceptors had been generated.
Figure 2.
Rd10+/+ mice with inducible increased expression of superoxide dismutase 2 (SOD2) and Catalase in the mitochondria of photoreceptors. (a) Schematic diagram of the TRE/Sod2 and TRE/Catalase transgenes are shown. The tetracycline response element (TRE) was coupled to the full-length cDNA for mouse-Sod2. The ornithine transcarbamylase (OTC) leader sequence, which mediates mitochondrial localization, was ligated to the N terminus cDNA for human Catalase and the peroxisomal localization signal (PLS) was deleted from the C terminus prior to coupling to the TRE. Using these constructs, TRE/Sod2 and TRE/Catalase transgenic mice were generated. (b) Multiple crosses were done to generate TRE/Sod2(+/−)-TRE/Catalase(+/−)-rd10+/+ mice and homozygous interphotoreceptor retinol–binding protein promoter/reverse tetracycline transactivator-rd10+/+ mice (IRBP/rtTA(+/+)-rd10+/+ mice). These two types of mice were crossed to yield four groups of offspring, null-rd10+/+, Sod2-rd10+/+, Catalase-rd10+/+, and Sod2/Catalase-rd10+/+ mice for which the genotypes are shown. (c) Null-rd10+/+, Sod2-rd10+/+, Catalase-rd10+/+, and Sod2/Catalase-rd10+/+ mice were given normal drinking water or water supplemented with 2 mg/ml of doxycycline between postnatal day (P) 10 and P25. Mice were euthanized and the mitochondrial fractions of retinal homogenates were run in immunoblots using antibodies specific for murine SOD2, human Catalase, and murine cyclooxygenase 4 (COX4), which is known to localize to mitochondria. Background levels of murine SOD2 were seen in retinal mitochondria of all mice, but when treated with doxycycline, only Sod2-rd10+/+ and Sod2/Catalase-rd10+/+ mice showed a substantial increase in SOD2. Likewise, when treated with doxycycline Catalase-rd10+/+ and Sod2/Catalase-rd10+/+ showed strong bands for Catalase. Strong bands for COX4 were seen in the retinal mitochondria of all mice.
Rd10+/+ mice with induced expression of SOD2 and Catalase in photoreceptors show reduced superoxide radicals in the retina
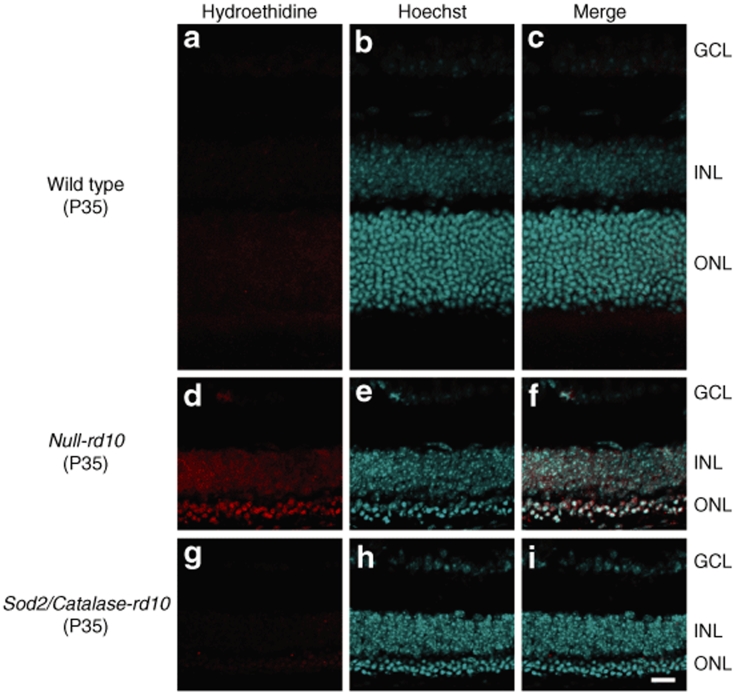
Hydroethidine is taken up into cells and in the presence of superoxide radicals is converted to ethidium, which binds DNA and emits red fluorescence providing a means to visualize production of superoxide radicals in situ.11 We previously utilized this technique to show that there is a striking increase in superoxide radicals in the outer retinas of P30 rd1+/+ mice in which rods have degenerated.12 At P35, wild-type mice showed minimal fluorescence in the retina when hydroethidine had been injected prior to death (Figure 3a) indicating low levels of superoxide radicals, but P35 rd10+/+ mice showed strong fluorescence in the outer retina indicating high levels of superoxide radicals (Figure 3b). In contrast, P35 rd10+/+ mice with coexpression of SOD2 and Catalase in the mitochondria of photoreceptors showed little fluorescence in the retina when hydroethidine had been injected prior to death (Figure 3c), indicating a large increase in the capacity to scavenge superoxide radicals.
Figure 3.
Cooverexpression of superoxide dismutase 2 (SOD2) and Catalase in mitochondria reduce superoxide radicals in the retinas of rd10+/+ mice. At postnatal day (P) 35, hydroethidine was injected intraperitoneally into wild-type mice (n = 4), null-rd10+/+ mice treated with doxycycline between P10 and P35 as described in Materials and Methods (n = 4), or Sod2/Catalase-rd10+/+ mice treated with doxycycline between P10 and P35 (n = 4) and after 18 hours the mice were euthanized and ocular sections were examined by confocal microscopy. Representative sections showed minimal fluorescence in the retinas of wild-type mice (a–c), strong fluorescence primarily in the remaining outer nuclear layer (ONL) and outer plexiform layer of the retinas of null-rd10+/+ mice (d–f), and minimal fluorescence in the retinas of Sod2/Catalase-rd10+/+ mice (g–i). This demonstrates a marked increase in superoxide radicals in the outer retina of mice after degeneration of rods that is reduced by coexpression of SOD2 and Catalase. Scale bar = 20 µm. GCL, ganglion cell layer; INL, inner nuclear layer.
Increased expression of Catalase and SOD2 significantly reduce carbonyl content in the retinas of rd10+/+ mice
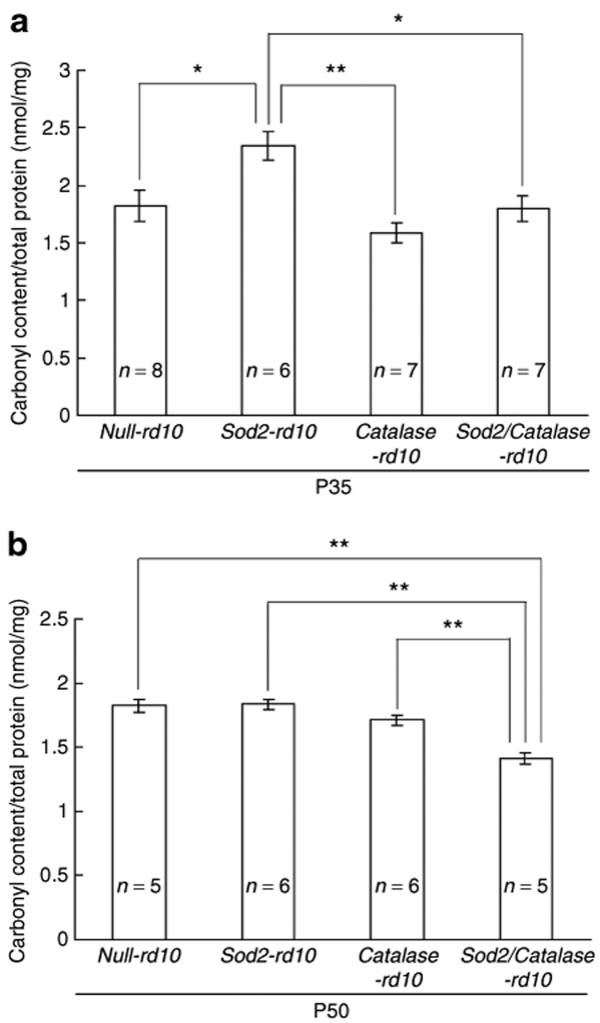
When proteins undergo oxidative damage, the most common modification is introduction of carbonyl groups into side chains,13 and enzyme-linked immunosorbent assay (ELISA) for carbonyl adducts provides a quantitative measure of oxidative damage.14,15 To determine if the increased capacity to neutralize superoxide radicals translated into protection from oxidative damage we measured carbonyl levels in the retina by ELISA. At P35, a time point when rod degeneration is just being completed in rd10+/+ mice, there was no difference in carbonyl levels in the retinas of mice with increased expression of Catalase or both Catalase and SOD2 in photoreceptor mitochondria compared to null-rd10+/+ mice that did not have increased expression of an antioxidant enzyme (Figure 4a). However, Sod2-rd10+/+ mice had significantly greater carbonyl content per mg retinal protein than null-rd10+/+, Catalase-rd10+/+, or Sod2/Catalase-rd10+/+ mice, indicating that increased production of SOD2 in photoreceptors increased oxidative damage in rd10+/+ mice. At P50, when cones have been present with no surrounding rods for ~2 weeks, carbonyl content per mg retinal protein was significantly less in Sod2/Catalase-rd10+/+ mice compared to null-rd10+/+, Sod2-rd10+/+, or Catalase-rd10+/+ mice (Figure 4b). This indicates that coexpression of SOD2 and Catalase, but not expression of either of them alone reduces oxidative damage in cones after rods have degenerated.
Figure 4.
Increased expression of Catalase and superoxide dismutase 2 (SOD2) significantly reduce carbonyl content in the retinas of postnatal day (P) 50 rd10+/+ mice. Starting at P10, the mothers of null-rd10+/+, Sod2-rd10+/+, Catalase-rd10+/+, and Sod2/Catalase-rd10+/+ mice and after weaning the mice themselves were treated with doxycycline. Mice were euthanized at P35 or P50 and protein carbonyl content was measured by enzyme-linked immunosorbent assay of retinal homogenates. At P35, the mean (±SEM) carbonyl content per mg retinal protein was significantly greater in Sod2-rd10+/+ mice than null-rd10+/+, Catalase-rd10+/+, or Sod2/Catalase-rd10+/+ mice (a; *P < 0.05; **P < 0.01 by Tukey–Kramer test). At P50, the mean (±SEM) carbonyl content per mg retinal protein was significantly less in Sod2/Catalase-rd10+/+ mice compared to null-rd10+/+, Sod2-rd10+/+, or Catalase-rd10+/+ mice (b; **P < 0.01 by Tukey–Kramer test).
Increased expression of SOD2 and Catalase in mitochondria of photoreceptors decreases cone cell death in rd10+/+ mice
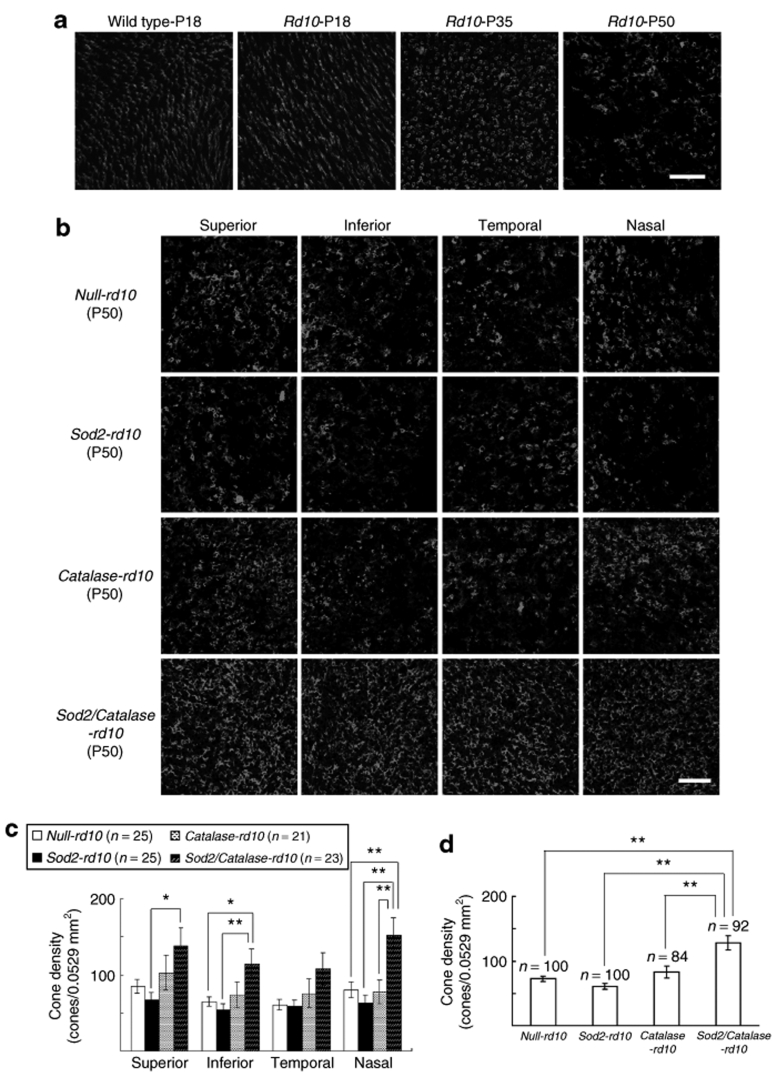
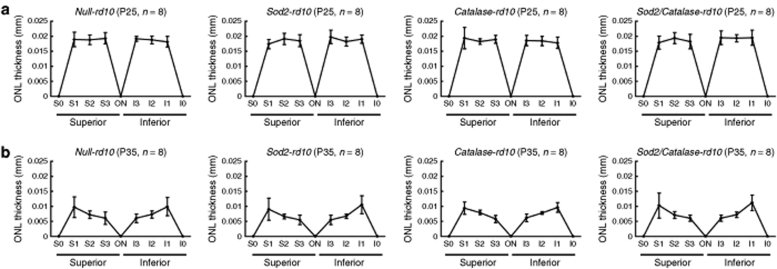
Fluorescence confocal microscopy of peanut agglutinin–stained retinal flat mounts provides a means of assessing cone cell density and, hence, cone survival, provided the same region of the retina is evaluated at different time points.4 In comparison to P18 wild-type mice, there is no difference in cone density in P18 or P35 rd10 mice (Figure 5a); however, between P35 and P50, there is substantial loss of cones. This is consistent with observations in multiple models of RP, indicating that cone density is relatively stable until rod degeneration is essentially complete, and then gradual loss of cones occurs.4,5 However, while the number of cones is similar in P18 and P35 rd10+/+ mice, cone morphology is abnormal at P35, because outer segments are missing and inner segments are flattened, indicating that cones are under considerable stress (Figure 5a). When mice were treated with doxycycline starting at P18, cone density at P50 was significantly greater in Sod2/Catalase-rd10+/+ mice compared to null-rd10+/+, Sod2-rd10+/+, or Catalase-rd10+/+ (Figure 5b–d). Cone density was not greater in Sod2-rd10+/+ or Catalase-rd10+/+ compared to null- rd10+/+ mice. This indicates that coexpression of SOD2 and Catalase in the mitochondria of cones, but not either alone, promotes cone survival after rods have degenerated in rd10+/+ mice. In contrast to this robust effect on cone survival, coexpression of SOD2 and Catalase, as well as expression of either alone, had no effect on rod survival in rd10+/+ mice as demonstrated by failure to prevent thinning of the outer nuclear layer (ONL) at P25 and P35 (Figure 6).
Figure 5.
Increased expression of superoxide dismutase 2 (SOD2) and Catalase in mitochondria of photoreceptors decreases cone cell death in rd10+/+ mice. (a) Fluorescence confocal microscopy of peanut agglutinin (PNA)-stained retinal flat mounts showed little difference in cone cell density in 0.0529 mm2 bins 0.5 mm superior to the center of the optic nerve in rd10+/+ mice at postnatal day (P) 18 or 35 compared to wild-type mice at P18, but by P50 there was an obvious reduction in cone density in rd10+/+ mice. At P18, outer segments were seen in wild-type and rd10+/+ mice, but at P35 and P50, rd10+/+ mice had flattened inner segments and no outer segments. Scale bar = 50 µm. (b) Starting at P10, the mothers of null-rd10+/+, Sod2-rd10+/+, Catalase-rd10+/+, and Sod2/Catalase-rd10+/+ mice were treated with doxycycline in their drinking water. After weaning, the mice themselves were treated with doxycycline. At P50, mice were euthanized and fluorescence microscopy of PNA-stained retinal flat mounts in 0.0529 mm2 bins 0.5 mm superior, inferior, temporal, and nasal to the center of the optic nerve are shown. Sod2/Catalase-rd10+/+ mice appeared to have greater cone density in all four regions of the retina compared to null-rd10+/+, Sod2-rd10+/+, and Catalase-rd10+/+ mice. Sod2-rd10+/+ mice appeared to have the lowest cone density. Scale bar = 50 µm. (c) Quantification of cone density by image analysis in each of the four 0.0529 mm2 bins showed that Sod2/Catalase-rd10+/+ mice had significantly greater mean (±SEM) cone density than Sod2-rd10+/+ mice in the superior, inferior, and nasal quadrants of the retina (*P < 0.05, **P < 0.01 by Tukey–Kramer test). Sod2/Catalase-rd10+/+ mice had significantly greater cone density than null-rd10+/+ mice in the inferior and nasal quadrants. Sod2/Catalase-rd10+/+ mice had significantly greater cone density than Catalase-rd10 mice in the nasal quadrant. Scale bar = 50 µm. (d) Cone density measurements from each of the four quadrants in each mouse were consolidated to provide a single cone density measurement per retina. The mean (±SEM) cone density per retina was significantly greater in P50 Sod2/Catalase-rd10+/+ mice compared to null-rd10+/+, Sod2-rd10+/+, or Catalase-rd10+/+ mice (**P < 0.01 by Tukey–Kramer test).
Figure 6.
Overexpression of superoxide dismutase 2 (SOD2) and/or Catalase does not prevent rod cell death in rd10+/+ mice. Rod cell death leads to progressive thinning of the outer nuclear layer (ONL) in rd10+/+ mice. Measurement of ONL thickness of doxycycline-treated mice showed no significant differences by Tukey–Kramer test between null-rd10+/+, Sod2-rd10+/+, Catalase-rd10+/+, and Sod2/Catalase-rd10+/+ mice at P25 (a) and P35 (b). The bars show the mean (±SD).
Increased expression of SOD2 and Catalase preserves cone cell function in P50 rd10+/+ mice
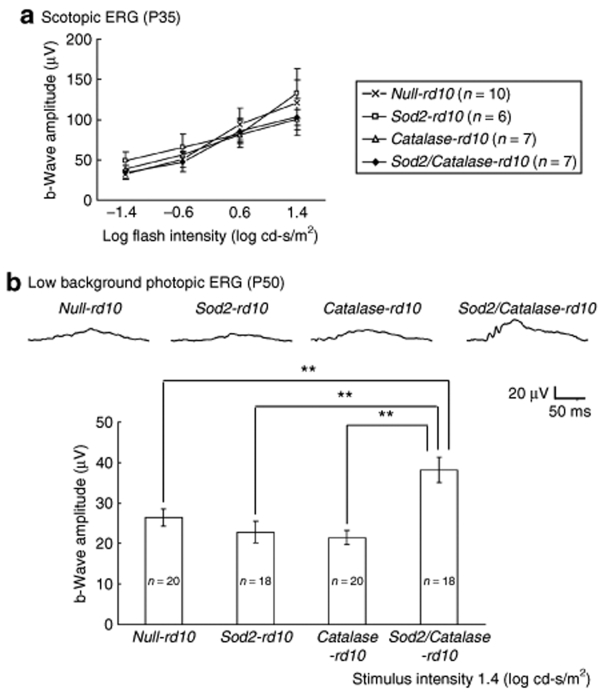
There was no difference in mean scotopic electroretinogram (ERG) b-wave amplitude at P35 in doxycycline-treated null-rd10+/+, Sod2-rd10+/+, Catalase-rd10+/+, and Sod2/Catalase-rd10+/+ mice, indicating that expression of SOD2 and/or Catalase had no effect on rod function in rd10+/+ mice (Figure 7a). At P50, low background photopic ERGs showed nearly flat waveforms in doxycycline-treated null-rd10+/+, Sod2-rd10+/+, and Catalase-rd10+/+ mice, but Sod2/Catalase-rd10+/+ mice showed a substantially better waveform and significantly greater mean photopic b-wave amplitude (Figure 7b). This indicates that coexpression of SOD2 and Catalase in mitochondria of photoreceptors, but not expression of either of them alone, preserves cone cell function after rods have degenerated in rd10+/+ mice.
Figure 7.
Increased expression of superoxide dismutase 2 (SOD2) and Catalase preserves some cone cell function at postnatal day (P) 50 in rd10+/+ mice. (a) Scotopic electroretinograms (ERGs) were done at P35 in null-rd10+/+, Sod2-rd10+/+, Catalase-rd10+/+, and Sod2/Catalase-rd10+/+ mice treated with doxycycline. The mean (±SEM) b-wave amplitude for four different stimulus intensities is plotted for each of four groups of mice and there were no significant differences. (b) Low background photopic ERGs were done as described in Materials and Methods at P50. Representative waveforms are shown for each of the four groups and illustrate a substantially better waveform in Sod2/Catalase-rd10+/+ mice compared to null-rd10+/+, Sod2-rd10+/+, or Catalase-rd10+/+ mice. The bars show mean (±SEM) photopic b-wave amplitude, which was significantly higher (**P < 0.01 by Tukey–Kramer test) for Sod2/Catalase-rd10+/+ mice compared to the other three types of mice.
Discussion
There is mounting evidence that after rods die in RP, cones are exposed to oxidative stress and gradually die from progressive oxidative and nitrosative damage.3,4,5,12 Systemically administered antioxidants or nitric oxide synthase inhibitors slow cone cell death and provide a new treatment strategy that may be applicable to all patients with RP, despite different pathogenic mutations. However, antioxidant dietary supplements are inefficient, because it is difficult to constantly maintain sufficient levels in all cellular compartments in target tissues. This problem is magnified in the retina, because access of many agents is reduced by the blood–retinal barrier. A complementary strategy is to circumvent delivery problems by local increased production of components of the endogenous antioxidant defense system. The SODs are key defenders against assault from oxidative stress in many tissues, including the retina, where deficiency of SOD1 markedly increases vulnerability to oxidative stress.16 Therefore, we first tested the concept of utilizing the endogenous antioxidant defense system in RP by exploring the effect of increased expression of SOD1 in rd1+/+ mice. Contrary to our initial expectations, rather than protecting cones in rd1+/+ mice, overexpression of SOD1 accelerated their loss of function and death (Figure 1). Similar toxic effects were seen when SOD1 or 2 were overexpressed in cultured retinal pigmented epithelial cells.10 It appears that excess production of H2O2 contributes to the toxic effects of overexpression of the SODs, because coexpression of the cytosolic form of glutathione peroxidase 4 (cGpx4) with SOD1 eliminated its toxicity. Coexpression of cGpx4 with SOD2 did not eliminate its toxicity, suggesting that it may be necessary to express a peroxide-metabolizing enzyme in the same cellular compartment as an overexpressed SOD to maximize benefit and minimize risk. Since oxidative stress is particularly severe in mitochondria in hyperoxic tissues and photoreceptors are packed with mitochondria, we decided to target this cellular compartment. In this study, we have demonstrated that increased expression of SOD2 and Catalase in the mitochondria of photoreceptors of rd10+/+ mice reduced superoxide radicals and oxidative damage in the retina, provided significant preservation of cone function, and reduced cone cell death. In contrast, overexpression of SOD2 or Catalase alone in the mitochondria of photoreceptors did not significantly reduce oxidative damage or cone cell death.
Various SODs have been overexpressed in other tissues in an attempt to reduce oxidative damage. Overexpression of SOD1 provides protection against oxidative stress in some situations,17,18,19,20 but increases the vulnerability of some tissues to other types of oxidative stress.21,22 Tissues with low levels of glutathione peroxidase might be expected to be intolerant to overexpression of SOD1, because an imbalance between SOD1 and glutathione peroxidase can increase levels of H2O2.23 This may be part of the explanation for the deleterious effects of overexpression SOD1 in models of RP, but it appears that the nature and severity of the oxidative stress is also important, because overexpression of SOD1 reduced oxidative damage from severe oxidative stress.16 In primary hippocampal neuron cultures, overexpression of SOD1 reduced cyanide toxicity, but increased toxicity from kainic acid or oxygen/glucose deprivation.24 Interestingly, the combination of increased expression of SOD1 and cyanide induced increased levels of glutathione peroxidase, whereas increased SOD1 and kainic acid did not. Therefore, it appears that the tissue, the type of oxidative stress, and its severity may all influence the impact of overexpression of SOD1.
In mice with experimental allergic encephalomyelitis and optic neuritis and also mice in which the NADH-ubiquinone oxidoreductase complex I of the respiratory chain has been knocked down in retinal ganglion cells, overexpression of SOD2 in ganglion cells reduced ganglion cell death and optic nerve degeneration.25,26 This differs from the situation in cones subjected to hyperoxia after death of rods in which we found that overexpression of SOD2 alone increased oxidative damage and failed to improve cone function or survival.
Mice deficient in SOD3 but not those deficient in SOD1, show increased susceptibility to lung damage from hyperoxia27 and brain damage from ischemia/reperfusion.28 Overexpression of SOD3 protects lungs from several types of injury, and it has been postulated that many insults lead to high levels of reactive oxygen species in the interstitial space of lungs, which could best be neutralized by SOD3, which is secreted.29,30,31 Similarly, high levels of reactive oxygen species have been demonstrated in the extracellular space in association with ischemia–reperfusion, and overexpression of SOD3 has provided benefit.32 However, deficiency of SOD3 does not increase susceptibility of the retina to paraquat or hyperoxia (A. Dong and P.A. Campochiaro, unpublished results), whereas deficiency of SOD1 markedly increases retinal susceptibility to those sources of oxidative stress,16 causing us to eliminate Sod3 as a possible transgene for our application. However, Sod3 gene transfer may have some potential usefulness for chronic inflammatory conditions affecting the inner retina; while overexpression of SOD3 alone had no significant effect on ganglion cell or axon loss in mice with chronic experimental allergic encephalomyelitis, when combined with overexpression of Catalase, the effects were greater than the effects of overexpression of Catalase alone.33
Thus, it appears that the effects of overexpressing SODs can vary considerably depending upon the situation. Our data indicate that overexpression of SOD1 or 2 alone in photoreceptors can exacerbate oxidative damage in cones after rods have degenerated and accelerate retinal degeneration. However, coexpression of SOD2 and Catalase in the mitochondria of photoreceptors strongly promotes cone survival and maintenance of cone function in a model of RP. This suggests that antioxidant gene therapy is a good therapeutic approach for RP, but must be designed and tested carefully before testing in clinical trials.
Materials and Methods
Generation of transgenic mice. Mice were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Research and the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice carrying a β-actin promoter/human Sod1 transgene [C57BL/6-TgN(SOD1)3Cje/J mice, Sod1(+/−) mice] were purchased from Jackson Laboratories (Bar Harbor, ME) and crossed with rd1+/+ mice in a C57BL/6 background to obtain Sod1(+/−)-rd1+/+ mice.
Full-length murine Sod2 cDNA was generated by reverse transcription–PCR of mouse retinal RNA, cloned into Topo TA cloning vector (Invitrogen, Carlsbad, CA), and sequenced. The BamHI and HindIII fragment was released from Topo TA vector and ligated into pTRE2 vector (Clontech, Mountain View, CA) containing the TRE. After sequencing, a fragment containing TRE, Sod2, and a 1.2 kb β-globin poly A signal was released from pTRE2 to provide the TRE/Sod2 construct that was used to generate transgenic mice in the Johns Hopkins University Transgenic Mouse Core Facility.
The MCAT plasmid, also known as poCAT, which contains human Catalase gene with the ornithine transcarbamylase leader sequence at its 5′ end and without the peroxisomal localization signal at its 3′ end to provide targeting to mitochondria; transgenic mice with ubiquitous expression Catalase in mitochondria have a long lifespan.34 The MCAT construct was ligated into pTRE2. After sequencing, a fragment containing TRE, MCAT, and a 1.2 kb β-globin poly A signal was released from pTRE2 to provide the TRE/Catalase construct that was used to generate transgenic mice in the Johns Hopkins University Transgenic Mouse Core Facility.
Founder mice were mated with C57BL/6 mice to generate founder lines. Mice from each line were crossed with mice from the IRBP/rtTA driver line to generate IRBP/rtTA-TRE/Sod2 and IRBP/rtTA-TRE/Catalase double transgenic mice. Mice from double transgenic lines were given 2 mg/ml in their drinking water and real-time PCR was done to identify IRBP/rtTA-TRE/Sod2 and IRBP/rtTA-TRE/Catalase lines with strong, inducible transgene expression.
Genotyping of mice. Genotyping was done by PCR of tail DNA using the following primers: human Sod1 (forward:5′-CATCAGCCC TAATCCATCTGA-3′, reverse:5′-CGCGACTAACAATCAAAGTGA-3′); TRE/Sod2 (forward:5′-CACGCTGTTTTGACCTCC-3′, reverse:5′-GCTT GATAGCCTCCAGCAAC-3′); TRE/Catalase (forward:5′-TCTGGAGAA GTGCGGAGATT-3′, reverse:5′-AGTCAGGGTGGACCTCAGTG-3′), and IRBP/rtTA (forward:5′-GTTTACCGATGCCCTTGGAATTGACGA GT-3′, reverse:5′-GATGTGGCGAGATGCTCTTGAAGTCTGGTA-3′). To distinguish homozygous rd1, heterozygous rd1, and wild-type mice, the PCR fragment generated with forward, 5′-CATCCCACCT GAGCTCACAGAAAG-3′ and reverse, 5′-GCCTACAACAGAGGAGCTT CTAGC-3′ was digested with DdeI or BsaAI. To distinguish homozygous rd10, heterozygous rd10, and wild-type mice, the PCR fragment generated with forward, 5′-CTTTCTATTCTCTGTCAGCAAAGC-3′ and reverse, 5′-CATGAGTAGGGTAAACATGGTCTG-3′ was digested with CfoI.
Mutant rd10 mice with inducible expression of SOD2, Catalase, or both. Rd10+/+ mice (Jackson Laboratories, Bar Harbor, ME) were used in an elaborate mating scheme to generate TRE/Sod2(+/−)-TRE/Catalase(+/−) rd10+/+ mice and IRBP/rtTA(+/−)-rd10+/+ mice. These mice were crossed to generate -rd+/+ mice that did not carry either the TRE/Sod2 or TRE/Catalase transgenes, but that which carried only the TRE/Sod2 transgene, or only the TRE/Catalase transgene, or that which carried both the TRE/Sod2 and TRE/Catalase transgenes. Starting at P10, mothers of these mice were given 2 mg/ml of doxycycline in their drinking water. At P21, the mice were separated from their mothers and given drinking water containing 2 mg/ml of doxycycline. Transgene product was measured by immunoblots of retinal homogenates at P25.
Immunoblots. For Sod1(+/−)-rd1+/+ mice, whole retinas were dissected and placed in 50 µl of lysis buffer (10 mmol/l Tris, pH 7.2, 0.5% Triton X-100, 50 mmol/l NaCl, and 1 mmol/l EDTA) containing a proteinase inhibitor mixture tablet (Roche, Indianapolis, IN). After three freeze/thaw cycles and homogenization, samples were microfuged at 14,000g for 5 minutes at 4 °C and the protein concentration of the supernatant was measured using a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). For all of the other mice, a Mitochondrial Isolation Kit for Tissue (Pierce, Rockford, IL) was used according to the manufacturer's instructions to isolate retinal mitochondria. For each sample, 20 µg of protein was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Hybond-ECL; Amersham Biosciences, Piscataway, NJ). Rabbit polyclonal antihuman SOD1 (1:1,000; Chemicon International, Temecula, CA), rabbit polyclonal anti-SOD2 (1:10,000; Abcam, Cambridge, MA), or rabbit polyclonal antihuman Catalase (1:2,000; Athens Research Technology, Athens, GA) were used as primary antibody. The secondary antibody was a horseradish peroxidase–coupled goat antirabbit IgG (1:1,000; Cell Signaling, Danvers, MA). Blots were incubated in SuperSignal Western Pico Lumino/Enhancer solution (Pierce, Rockford, IL) and exposed to X-ray film (Eastman-Kodak, Rochester, NY). To assess loading levels of protein, SOD1 blots were stripped and incubated with polyclonal rabbit anti-β-actin antibody (1:5,000; Cell Signaling, Danvers, MA) followed by horseradish peroxidase–coupled goat antirabbit IgG and other blots were stripped and incubated with mouse monoclonal anti-COX4 (1:5,000; Abcam, Cambridge, MA) followed by horseradish peroxidase–coupled antimouse IgG (1:2,000; Cell Signaling, Danvers, MA).
Assessment of superoxide radicals with hydroethidine. As previously described,12,35 in situ production of superoxide radicals was evaluated using hydroethidine, which in the presence of superoxide radicals is converted to ethidium, which binds DNA and emits red fluorescence at ~600 nm. Briefly, mice were given two 20-mg/kg intraperitoneal injections 30 minutes apart of freshly prepared hydroethidine (Invitrogen, Carlsbad, CA) and euthanized 18 hours after injection. Eyes were rapidly removed and 10-µm frozen sections were fixed in 4% paraformaldehyde for 20 minutes at room temperature, rinsed with phosphate-buffered saline (PBS), and counterstained for 5 minutes at room temperature with the nuclear dye Hoechst 33258 (1:10,000; Sigma, St Louis, MO). After rinsing in PBS, slides were mounted with Aquamount solution and evaluated for fluorescence (excitation: 543 nm, emission >590 nm) with a LSM 510 META confocal microscope. Images were captured using the same exposure time for each section.
ELISA for protein carbonyl content. Retinas were homogenized in lysis buffer and centrifuged at 16,000g for 5 minutes at 4 °C and the protein concentration of the supernatant was measured using a Bio-Rad Protein Assay Kit (Bio-Rad). Samples were adjusted to 4 mg/ml by dilution with Tris-buffered saline, and protein carbonyl content was determined by ELISA, as previously described.4,10
Measurement of cone cell density. Cone density was measured as previously described.4 Briefly, each mouse was euthanized, and eyes were carefully removed and were fixed in 4% paraformaldehyde for 3 hours or over night at 4 °C. After washing with PBS, the cornea, iris, and lens were removed. A small triangle cut was made at 12:00 in the retina for future orientation and after four cuts equidistant around the circumference, the entire retina was carefully dissected from the eye cup and any adherent retinal pigmented epithelium was removed. Retinas were placed in 10% normal goat serum in PBS for 30 minutes at room temperature, incubated for 1 hour at room temperature in 1:100 rhodamine-conjugated peanut agglutinin (Vector Laboratories, Burlingame, CA) in PBS containing 1% normal goat serum, and flat mounted. The retinas were examined with a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Oberkochen, Germany) with a Zeiss Plan-Apochromat 20×/0.75 NA objective using an excitation wavelength of 543 nm to detect rhodamine fluorescence. Images were acquired in the frame scan mode. The number of cones was determined by image analysis within four 230 mm × 230 mm squares located 1 mm (rd1 mice) or 0.5 mm (wild-type and rd10 mice) superior, inferior, temporal, and nasal to the center of the optic nerve. The investigator was masked with respect to experimental group.
Measurement of ONL thickness. ONL thickness was measured, as previously described.5 Mice were euthanized, a mark was placed at 12:00 at the corneal limbus, and eyes were removed and embedded in optimal cutting temperature compound. Ten-micrometer frozen sections were cut perpendicular to the 12:00 meridian through the optic nerve and fixed in 4% paraformaldehyde. The sections were stained with hematoxylin and eosin, examined with an Axioskop microscope (Zeiss, Thornwood, NY), and images were digitalized using a three-charge-coupled device color video camera (IK-TU40A; Toshiba, Tokyo, Japan) and a frame grabber. Image-Pro Plus software (Media Cybernetics, Silver Spring, MD) was used to outline the ONL. ONL thickness was measured at six locations, 25% (S1), 50% (S2), and 75% (S3) of the distance between the superior pole and the optic nerve and 25% (I1), 50% (I2), and 75% (I3) of the distance between the inferior pole and the optic nerve.
Recording of ERGs. An Espion ERG Diagnosys machine (DiagnoSYS LLL, Littleton, MA) was used to record ERGs as previously described.4,5,7,36 For scotopic recordings, mice were adapted to dark overnight, and for photopic recordings, mice were adapted to background white light at an intensity of 30 cd/m2 for 10 minutes. The mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (100 mg/kg body weight) and xylazine (5 mg/kg body weight). Pupils were dilated with Midrin P containing of 0.5% tropicamide and 0.5% phenylephrine, hydrochloride (Santen Pharmaceutical, Osaka, Japan). The mice were placed on a pad heated to 39 °C and platinum loop electrodes were placed on each cornea after application of Gonioscopic prism solution (Alcon Labs, Fort Worth, TX). A reference electrode was placed subcutaneously in the anterior scalp between the eyes and a ground electrode was inserted into the tail. The head of the mouse was held in a standardized position in a ganzfeld bowl illuminator that ensured equal illumination of the eyes. Recordings for both eyes were made simultaneously with electrical impedance balanced. Scotopic ERGs were recorded at six intensity levels of white light ranging from −3.00 to 1.40 log cd-s/m2. Six measurements were averaged at each flash intensity. Low background photopic ERGs were recorded at 1.48 log cd-s/m² under a background of 10 cd/m². Sixty photopic measurements were taken and the average value was recorded.
Statistical analysis. Statistical comparisons were done using Tukey–Kramer's test for multiple comparisons and unpaired Student's t-test or Welch's t-test for two comparisons. Differences test for multiple comparisons were judged statistically, significant at P < 0.05 or P < 0.01.
Acknowledgments
This work was supported by grants R01 EY05951 (P.A.C.) and P01 AG01751 (P.S.R.) from the National Institutes of Health and a gift from and William Lake. S.U. is a Bausch and Lomb Japan Vitreoretinal Research Fellow and was supported by The Osaka Medical Research Foundation for Incurable Diseases. P.A.C. is the George S. and Dolores Dore Eccles Professor of Ophthalmology and Neuroscience.
REFERENCES
- Yu DY, Cringle SJ, Su EN., and , Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Valter K, Walsh N, Lee D., and , Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest Ophthalmol Vis Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- Shen J, Yan X, Dong A, Petters RM, Peng YW, Wong F, et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L., and , Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natil Acad Sci USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K, Rogers BS., and , Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P, et al. Inducible expression of vascular endothelial growth factor in photoreceptors of adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160:711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye G, Zimmer J, Sung, Gehlbach P, Deering T, Nambu N, et al. Increased expression of BDNF preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neurosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, et al. Different effects of angiopoietin 2 in different vascular beds in the eye; new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Hackett SF., and , Campochiaro PA. Increased expression of glial cell line-derived neurotrophic factor protects against oxidative damage-induced retinal degeneration. J Neurochem. 2007;103:1041–1052. doi: 10.1111/j.1471-4159.2007.04839.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Oveson BC, Jo YJ, Lauer T, Usui S, Komeima K, et al. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage Antioxid Redox Signal 2008. epub ahead of print [DOI] [PMC free article] [PubMed]
- Pietch A, Dessy C, Havaux X, Feron O., and , Balligand JL. Differential regulation of nitric oxide synthases and their allosteric regulators in heart and vessels of hypertensive rats. Cardiovasc Res. 2003;57:456–467. doi: 10.1016/s0008-6363(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Komeima K, Usui S, Shen J, Rogers BS., and , Campochiaro PA. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic Biol Med. 2008;45:905–912. doi: 10.1016/j.freeradbiomed.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- Buss H, Chan TP, Sluis KB, Domigan NM., and , Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radical Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Hackett SF, Mincey A, Lai H., and , Campochiaro PA. Effects of different types of oxidative stress in RPE cells. J Cell Physiol. 2006;206:119–125. doi: 10.1002/jcp.20439. [DOI] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Akiyama H, Hackett SF, Lai H, et al. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006;208:516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Kostic V, Jackson LV, Naini AB, Simonetti S, Fahn S, et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neuosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Sheng P, Ali S, Rothman R, Carlson E., and , Epstein CJ. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;62:380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Reaume A, Scott R., and , Coyle JT. Effects of over- and under-expression of Cu,Zn-superoxide dismutase on the toxicity of glutamate analogs in transgenic mouse striatum. Brain Res. 1998;789:32–39. doi: 10.1016/s0006-8993(97)01469-8. [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Wu J, Catana AM, Eisenbud L, He SQ, Duan YY, et al. Lentivirus-mediated superoxide dismutase1 gene delivery protects against oxidative stress-induced liver injury in mice. Liver Int. 2007;27:1311–1322. doi: 10.1111/j.1478-3231.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., and , Groner Y. Impaired uptake in PC12 cells overexpressing human Cu/Zn-superoxide dismutase—implication for gene dosage effect in Down syndrome. Cell. 1988;52:259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- Rader RK., and , Lanthorn TH. Experimental ischemia induces a persistent depolarization blocked by decreased calcium and NMDA antagonists. Neurosci Lett. 1989;99:125–130. doi: 10.1016/0304-3940(89)90276-0. [DOI] [PubMed] [Google Scholar]
- de Haan JB, Cristiano F, Iannello R, Bladier C, Kelner MJ., and , Kola I. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet. 1996;5:283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Nimon V, Brooke S, Moore T, McLaughlin J., and , Sapolsky R. Gene therapy in the nervous system with superoxide dismutase. Brain Res. 2006;1088:12–18. doi: 10.1016/j.brainres.2006.02.109. [DOI] [PubMed] [Google Scholar]
- Qi X, Lewin AS, Sun L, Hauswirth WW., and , Guy J. SOD2 gene transfer protects against optic neuropathy induced by deficiency of complex I. Ann Neurol. 2004;56:182–191. doi: 10.1002/ana.20175. [DOI] [PubMed] [Google Scholar]
- Qi X, Lewin AS, Sun L, Hauswirth WW., and , Guy J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:681–691. doi: 10.1167/iovs.06-0553. [DOI] [PubMed] [Google Scholar]
- Carlsson LM, Jonsson J, Edlund T., and , Marklund SL. Mice lacking extracellular superoxide dismutase are more susceptible to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Brady TC, Pearlstein RD, Crapo JD., and , Warner DS. Extracellular superoxide dismutase deficiency worsens outcome from focal cerebral ischemia in the mouse. Neurosci Lett. 1999;267:13–16. doi: 10.1016/s0304-3940(99)00316-x. [DOI] [PubMed] [Google Scholar]
- Bowler RP, Nicks M, Warnick K., and , Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282:L719–L726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, et al. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E., and , Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L32–L40. doi: 10.1152/ajplung.00133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal RS, Muangman S, Layne MD, Melo L, Perrella MA, Lee RT, et al. Pre-emptive gene therapy using recombinant adeno-associated virus delivery of extracellular superoxide dismutase protects heart against ischemia reperfusion injury, improves ventricular function and prolongs survival. Gene Ther. 2004;11:962–969. doi: 10.1038/sj.gt.3302250. [DOI] [PubMed] [Google Scholar]
- Qi X, Sun L, Lewin AS, Hauswirth WW., and , Guy J. Long-term suppression of neurodegeneration in chronic experimental optic neuritis: antioxidant gene therapy. Invest Ophthalmol Vis Sci. 2007;48:5360–5370. doi: 10.1167/iovs.07-0254. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2008;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Ueno S, Pease ME, Wersinger DM, Masuda T, Vinores SA, Licht T, et al. Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J Cell Physiol. 2008;217:13–22. doi: 10.1002/jcp.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]