Abstract
Fibrinogen (Fg) is a high molecular weight plasma adhesion protein and a biomarker of inflammation. Many cardiovascular and cerebrovascular disorders are accompanied by increased blood content of Fg. Increased levels of Fg result in changes in blood rheological properties such as increases in plasma viscosity, erythrocyte aggregation, platelet thrombogenesis, alterations in vascular reactivity and compromises in endothelial layer integrity. These alterations exacerbate the complications in peripheral blood circulation during cardiovascular diseases such as hypertension, diabetes and stroke. In addition to affecting blood viscosity by altering plasma viscosity and erythrocyte aggregation, growing experimental evidence suggests that Fg alters vascular reactivity and impairs endothelial cell layer integrity by binding to its endothelial cell membrane receptors and activating signalling mechanisms. The purpose of this review is to discuss experimental data, which demonstrate the effects of Fg causing vascular dysfunction and to offer possible mechanisms for these effects, which could exacerbate microcirculatory complications during cardiovascular diseases accompanied by increased Fg content.
Keywords: arteriolar constriction, endothelial cell layer permeability, endothelin-1, erythrocyte aggregation, tight junction proteins, Weibel-Palade body exocytosis
Fibrinogen (Fg) is a biomarker of inflammation (Ross 1999), which, when elevated, indicates the presence of inflammation and identifies individuals with a high risk for cardiovascular disorders. Fg is synthesized and assembled in hepatocytes and fibroblasts, and when secreted into the circulation, its plasma half-life ranges from 3 to 4 days (Collen et al. 1972, Martinez et al. 1974). Increased plasma Fg concentration typically accompanies hypertension development (Letcher et al. 1981, Lominadze et al. 1998) and stroke (D'Erasmo et al. 1993). Other inflammatory biomarkers such as interleukin (IL)-6 (Dalekos et al. 1996, Nakamura et al. 1996, Chae et al. 2001) and IL-1 (Tikkanen et al. 1995, Kannan et al. 1996, Nakamura et al. 1996, Yudkin et al. 1999), which are involved in the synthesis of Fg (Humphries 1995, Vasse et al. 1996), are also associated with elevation of blood pressure. These data suggest that Fg overproduction is involved in progression of hypertension and may even be involved in its development.
Many demographic and environmental factors (Krobot et al. 1992) as well as genetic factors (De Maat 2001) determine Fg levels. While the effect of most environmental factors is largely through the acute phase reaction, estimates based on twin studies suggest that 30–50% of the plasma Fg level is genetically determined (Hamsten et al. 1987, Humphries et al. 1987, Pankow et al. 1998). Two independent meta-analysis studies estimated that individuals in the upper tertile of Fg concentration had a 2.3-fold greater risk of subsequent cardiovascular disease than individuals in the lower tertile (Ernst & Resch 1993, Danesh et al. 2005). This creates a greater interest in Fg and an increased appreciation of its importance, role and implications in cardiovascular diseases.
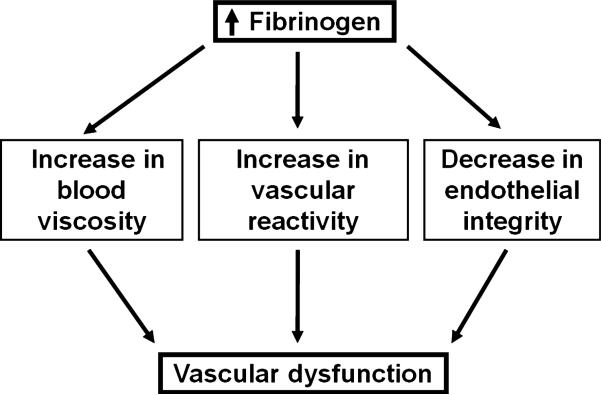
Recent studies indicate that increased Fg content affects microcirculation by increasing plasma viscosity, RBC aggregation and platelet thrombogenesis, altering vascular reactivity and compromising endothelial cell layer integrity. These changes lead to vascular dysfunction and exacerbate microcirculatory complications during cardiovascular diseases. The purpose of this review is to discuss the detrimental effects of elevated levels of Fg that cause vascular dysfunction (Fig. 1) and the possible mechanisms of these effects. Based on the results of our studies and studies of others, we present a new hypothesis to define a possible mechanism for Fg-induced vasoconstriction (Fig. 2). Some aspects of this mechanism need to be confirmed by further experimental data. Therefore, we hope that the discussion presented below may trigger ideas for additional experiments to clarify the mechanisms of microvascular dysfunction caused by an increased blood content of Fg.
Figure 1.
Schematic representation of fibrinogen-induced vascular dysfunction.
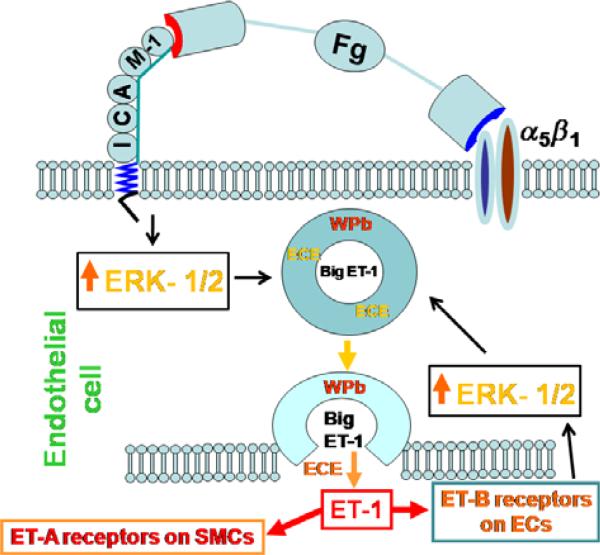
Figure 2.
Possible mechanism for fibrinogen (Fg)-induced vasoconstriction. Fg binds to endothelial intercellular adhesion molecule-1 (ICAM-1) and possibly α5β1 integrin, induces activation of extracellular signal-regulated kinase-1/2 (ERK-1/2) signalling causing exocytosis of Weibel-Palade bodies (WPbs), formation of endothelin-1 (ET-1), and thus subsequent vasoconstriction through involvement of endothelin type A and B receptors (ET-A and ET-B respectively). Notice a negative feedback loop that involves ET-B-induced activation of ERK-1/2 signalling. Printed with permission from Sen et al. (2009).
Effects of Fg in the microcirculation
Fg and blood viscosity
Increased total peripheral vascular resistance (TPVR) is typically associated with the development of cardiovascular and cerebrovascular diseases, many of which are accompanied by increased arterial pressure. Arterial pressure is dependent on cardiac output and TPVR, with the latter being determined by the calibre of resistance vessels and the intrinsic viscosity of blood. As cardiac output is generally normal in established hypertension (Lund-Johansen 1983), it is likely that elevated TPVR is commonly the main cause of increased arterial blood pressure. Major determinants of TPVR include vessel radius (Folkow 1978, Wiegman et al. 1979) and number of vessels (Bohlen 1989), which are decreased during hypertension.
Blood viscosity is one of the strongest predictors of adverse cardiovascular events such as hypertension, stroke and diabetes (Lowe et al. 1997). Increased blood viscosity is a result of changes in a number of variables, including increased haematocrit, erythrocyte aggregation and plasma viscosity (Dintenfass 1978, Letcher et al. 1981, Chien 1986, Chabanel et al. 1987, Chabanel & Chien 1990, London 1997, Lowe et al. 1997). Plasma viscosity is elevated in hypertensive patients, and is positively correlated with blood pressure (Koenig et al. 1989). A major determinant of plasma viscosity is the Fg content. Plasma Fg levels are higher (3.8 ± 0.1 mg mL−1) in patients with essential hypertension than in normotensive controls (2.8 ± 0.1 mg mL−1) (Letcher et al. 1981). Even when hypertension is mild, Fg levels are higher than in normotensive controls (Landin et al. 1990).
Increased plasma Fg concentration increases blood viscosity, and therefore increases blood flow shear stress (Lowe et al. 1997) that activates endothelial cells (Shyy & Chien 2002, Davies et al. 2003) and platelets (Ruggeri 1993). Activation of endothelial cells results in expression and/or activation of various adhesion molecules (Plow et al. 2000) and integrins (Languino et al. 1993). Some of these molecules, such as intercellular adhesion molecule-1 (ICAM-1) (Suehiro et al. 1997) and α5β1 integrin (Kern et al. 1986), are receptors for Fg.
Red blood cell (RBC) aggregation is a major determinant that affects blood flow in resistance vessels at low shear rates by increasing blood viscosity and inducing microcirculatory sludging and stagnation in small vessels (Chien et al. 1967). Although several convincing studies illustrate that an increase in RBC aggregation causes a decrease in local blood flow in microvessels (Zilliacus 1951, Bloch 1956, Knisely 1965), this concept was ignored for some time. More recently, several laboratories found that an increase in erythrocyte aggregation causes an elevation in total vascular resistance to blood flow (Gustafsson et al. 1981a,b, Maspers et al. 1990, Vicaut et al. 1994). Cabel et al. (1997) showed that RBC aggregation-induced blood flow viscosity changes venous resistance to flow. In addition, blood flow resistance was increased in arterioles and capillaries of the microcirculation by RBC hyperaggregability (Vicaut et al. 1994, Vicaut 1995) and a role of Fg was suggested (Vicaut 1995). In fact, many cardiovascular diseases and cerebrovascular disorders are characterized by both increased RBC aggregation (Zannad & Stoltz 1992, Mchedlishvili et al. 1993, Lominadze et al. 1998, Hacioglu et al. 2002) and plasma viscosity (Lip 1995, Qizilbash 1995, Lowe et al. 1997).
Two major theoretical models have been proposed for RBC aggregation by non-specific mechanisms (Rampling et al. 2004). The first, based on a macromolecular bridging model, is well accepted for describing the erythrocyte rouleaux formation and is based on the surface adsorption of macromolecules to form a bridging configuration between adjacent erythrocytes (Chien et al. 1967, Chien & Jan 1973). van der Waal's forces, hydrogen bonds and electrostatic attractions are believed to favour the adsorption of macromolecules (Chien et al. 1967). The other theory suggests that aggregation is induced by macromolecular depletion from the membrane surface (Evans & Needham 1988). According to the former non-specific adhesion theory, polymers and plasma proteins with a large molecular mass packed between adjacent erythrocytes, increasing the intercellular distance and inducing erythrocyte aggregation by decreasing the electrostatic repulsive forces between erythrocytes (Chien & Jan 1973). Therefore, the increased aggregation can also be explained by an increase in the adsorption area on the cell surface due to the larger size of the macromolecules (Chien & Jan 1973). In the latter nonspecific adhesion theory, aggregation is thought to be independent of both the molecular mass and surface adsorption. However, it is well documented that RBC aggregation is promoted mainly by proteins with higher molecular weight (Zannad & Stoltz 1992, Lip & Beevers 2007). In fact, Weng et al. (1996) showed that plasma proteins with a large molecular mass such as haptoglobin (86–400 kDa), C-reactive protein (105 kDa), ceruloplasmin (132 kDa) and Fg (340 kDa) exhibited strong effects on erythrocyte aggregation. However, proteins with a lower molecular weight such as α1-acid glycoprotein (40 kDa) and α1-antitrypsin (54 kDa) played no significant role in RBC aggregation (Putnam 1984). These discrepancies between the theory and facts point to the possible co-existence of a specific binding mechanism. Indeed, contrary to the accepted hypothesis that RBCs do not have receptors for adhesion proteins, specific receptors for ceruloplasmin were identified on the erythrocyte membrane (Barnes & Frieden 1984, Saenko & Yaropolov 1990). Presently, to our knowledge, no studies have been performed to determine whether plasma adhesion proteins involved in RBC aggregation have specific binding sites on the erythrocyte membrane.
Fibrinogen has a greater effect than other plasma proteins on RBC aggregation (Letcher et al. 1983, Chen & Schachter 1993, Game et al. 1996, Weng et al. 1996, Lominadze et al. 1998). We found that Fg can specifically bind erythrocyte membranes (Lominadze & Dean 2002). In hypertensive animals, the ability of erythrocytes to aggregate (RBC aggregability) was associated with hypertension (Lominadze et al. 1998, 2002). In addition, the composition of the RBC membrane changes with development of hypertension (Lominadze et al. 1998, 2002). These data suggest that with the development of hypertension, specific Fg receptors on the RBC membrane may be altered. For example, platelets specifically bind through their αIIbβ3 integrin (GPIIb/IIIa) to RBC membrane ICAM-4 (Hermand et al. 2003), which may compound the deleterious effects of RBC aggregation on blood rheological properties by promoting thrombosis.
Fg and vasoactivity
Despite the knowledge that Fg is a high risk factor for many cardiovascular and cerebrovascular disorders (Lowe et al. 1997), its effect on vascular reactivity is poorly understood. The ability to stimulate frog heart by the extracted clot liquors obtained after conversion of Fg to fibrin by thrombin was first mentioned in 1951 (Laki 1951). It was then found that fibrinopeptide-B, which is obtained as a result of Fg conversion to fibrin by thrombin, can increase bradykinin-induced contraction of isolated rat uterus in the oestrus cycle (Gladner et al. 1963). Further studies have shown short-lasting effects of fibrinopeptides A and B on arterial blood pressure in rats (Barczak-Osinska et al. 1983). Vasoactive effects of isolated peptides derived from plasmin digestion of fibrin and Fg were studied in various vascular beds including lung (Kern et al. 1986), heart (Mehta et al. 1985, Nichols et al. 1985), femoral artery (Saldeen et al. 1991) and mesenteric artery (Anderson et al. 1983).
The vasoactive effect of Fg as an intact protein was first shown by Hicks et al. (1996). They found that low concentrations of Fg (up to 2 μm) induced endothelium-dependent dilation of isolated saphenous vein rings. A maximum relaxation to Fg of about 60% was achieved at a concentration of 1.8 μm (Hicks et al. 1996). Diameters of the isolated rings were restored to a normal level in the presence of higher concentrations of Fg (5–6 μm). In this study, while the role of endothelial αvβ3 integrin was ruled out, Fg binding to endothelial ICAM-1 was suggested as a possible mechanism for initiation of signalling pathways leading to the synthesis of vasoactive mediators other than nitric oxide (NO) and prostacyclin (Hicks et al. 1996). Recent work by others showed that increased content of Fg causes dilation of small (0.8–1.4 mm in diameter) porcine coronary artery and human internal thoracic artery segments (Bas et al. 2008). In this study, 2 μm of Fg induced vasodilation of about only 20%, while approx. 60% relaxation was achieved at an Fg concentration of 12 μm (Bas et al. 2008). Fg-induced vasorelaxations were abolished by abciximab (platelet aggregation inhibitor that inhibits platelet GPIIb/IIIa) and diminished by endothelial denudation or NO synthase inhibitors (Bas et al. 2008). As abciximab binds αvβ3 integrin with the same affinity as GPIIb/IIIa, the authors suggested that Fg binding to vascular αvβ3 integrin leads to the synthesis of vasoactive mediators (Bas et al. 2008). Discrepancies between these studies may result from differences among the types of vessels, species and the avidities (avidity is the combined synergistic strength of bond affinities, thus it relates to the number of receptors) of Fg receptors on the endothelium.
Treatment of rat pulmonary artery rings by early digestion products of Fg did not cause vasoconstriction (Boutcher et al. 1996). However, in this work, responses of the vascular rings to Fg degradation products were tested in the presence of 4 × 10−8 m phenylephrine (PE) which induced 50% of the maximal contraction. Therefore, PE could have masked the effects of Fg digestion products on vasoconstriction. Arterial constriction induced by Fg binding to endothelial ICAM-1 was first demonstrated by Lominadze et al. (2005). It was also found that this Fg-induced vasoconstriction can be reversed by endothelin type A (ET-A) receptor inhibition (Lominadze et al. 2005). Further studies by the same group showed that elevated content of Fg increases production of endothelin-1 (ET-1) by means of enhanced exocytosis of Weibel-Palade bodies (WPbs) from endothelial cells (Sen et al. 2009). Activation of extracellular signal-regulated kinase-1/2 (ERK-1/2) by Fg binding to endothelial ICAM-1 was suggested to enhance production of ET-1 (Sen et al. 2009). Figure 2 presents a schematic representation of the hypothesis for the possible mechanism of Fg-induced vasoconstriction.
Other Fg receptors on endothelial cell membrane are αvβ3 and α5β1 integrins (Luscinskas & Lawler 1994). Mogford et al. (1996, 1997) showed that endothelial αvβ3 and α5β1 receptors induce opposite effects on vascular tone in response to Arg-Gly-Asp-Asn (RGDN)-containing peptide. The RGDN peptide induced dilation through a vascular smooth muscle cell (VSMC) αvβ3 integrin-mediated mechanism (Mogford et al. 1996), whereas α5β1 integrin mediated vascular constriction (Mogford et al. 1997). Arterioles denuded of a functional endothelium were unable to maintain the constriction induced by RGDN-containing peptide, indicating that the response resulted from the interaction of the RGD sequence with endothelial α5β1 integrin (Mogford et al. 1997). However, luminal application of fibronectin or RGDN peptide did not alter arteriolar diameter (Mogford et al. 1997). Therefore, the authors concluded that the constrictor response to the RGDN peptide was mediated by abluminal endothelial α5β1 integrin (Mogford et al. 1997). A role of ET-1 was suggested in the mechanism for RGD/α5β1 integrin interaction-induced vasoconstriction (Mogford et al. 1997). Reduction of VSMC intercellular Ca2+ (D'Angelo et al. 1997) and K+ channel activation (Platts et al. 1998) were implicated in RGD/αvβ3 integrin interaction-induced vasodilation (Mogford et al. 1996). The later studies by the same group showed that both α5β1 and αvβ3 integrins are required to produce myogenic vasoconstriction in skeletal muscle arterioles (Martinez-Lemus et al. 2005).
Fibrinogen contains two RGD sequences on its Aa chain (Suehiro et al. 1997). The Aα chain residues 572–574 are the binding sites for both αvβ3 (Smith et al. 1990) and α5β1 (Suehiro et al. 2000) integrins, suggesting that these integrins compete for binding to Fg. It was reported that plasma fibronectin does not bind luminal α5β1 integrin (Zanetti et al. 1994, Kano et al. 1996). However, binding of endothelial cells to immobilized Fg occurs through α5β1 integrin (Suehiro et al. 1997). In the absence of α5β1, αvβ3 integrin exists in an activated state with high affinity for Fg (Ly et al. 2003). De novo expression of α5β1 integrin suppresses αvβ3 integrin-mediated adhesive functions (Ly et al. 2003). These data suggest that there may be variations in the expression of α5β1 during pathologies, which lead to the modulation of αvβ3 integrin affinity for its ligands (Ly et al. 2003). In addition, while the 120 kDa fragment of fibronectin supports α5β1 binding, smaller fragments (<5 kDa) of the protein have greater affinity for αvβ3 integrin (Pytela et al. 1985). These results indicate that the functional role of αvβ3 integrin is greater if smaller protein fragments are present in the blood.
ICAM-1 was shown to have a role in Fg binding to endothelial cells in vitro (Porteri et al. 1998). As binding of Fg to ICAM-1 is independent of its RGD sequence (Suehiro et al. 1997, Pluskota & D'Souza 2000), these data indicate that ICAM-1 may also be involved in Fg binding to the vascular endothelium, and serve as a possible mechanism for Fg-induced vasoconstriction. This hypothesis is corroborated by studies showing that the binding affinity of Fg to ICAM-1 is higher than the binding to β1 integrin (D'Souza et al. 1996, Lominadze et al. 2005). In addition, α5β1 and other integrins have higher affinity for Fg only when they are in the activated state (Suehiro et al. 1997). These results suggest that Fg may bind ICAM-1 and α5β1 or αvβ3 integrins simultaneously, but with different binding affinity.
From these studies it appears that α5β1 and αvβ3 integrins compete for a binding site on Fg, while ICAM-1, when activated, has no competition from integrins. The discrepancies in the results of various studies demonstrating opposite effects of Fg-induced vasoactivity may be due to differences in experimental set-ups leading to differences in the expression of Fg receptors and/or differences in the vessels used in the studies resulting in different avidities of the Fg receptors. Further studies are required to resolve the discrepancies in Fg-induced vasodilatory and vasoconstrictory effects and to dissect the mechanisms of action of endothelial ICAM-1, α5β1 and αvβ3 integrin during inflammation-induced endothelial activation.
ICAM-1 and α5β1 integrin expression and/or activation
During hypertension, which is an inflammatory cardiovascular disease (Savoia & Schiffrin 2006, Wang et al. 2007), mRNA expression of ICAM-1 is upregulated (Buemi et al. 1997, DeSouza et al. 1997, Porteri et al. 1998, Becker et al. 2000). Although expression of α5β1 integrin was increased with ageing in the vasculature of hypertensive rats (Bezie et al. 1998, Intengan et al. 1999, Intengan & Schiffrin 2000), its expression was not different from that in age-matched WKY rats (Intengan et al. 1999). However, no direct evidence is available on microvascular endothelial surface expression or Fg binding activity of these Fg receptors during hypertension, diabetes or stroke. Interestingly, α5β1 integrin was strongly involved in the regulation of arterial stiffness due to its activation by higher shear stress during hypertension (Bezie et al. 1998). Changes in shear stress during hypertension may modulate the avidity and affinity of integrins including α5β1 (Urbich et al. 2000). Thus, it is possible that surface expression and activity of endothelial ICAM-1 and α5β1 integrin are increased during the development of cardiovascular and cerebrovascular diseases and contribute to the detrimental effects induced by increased content of Fg.
ICAM-1 and α5β1 integrin signalling involved in endothelial cell contractility
Activation of vascular endothelial cell intraluminal surface receptors triggers many signalling cascades, some of which result in alteration of vascular reactivity. ICAM-1 ligation mediated by antibody cross-linking in brain microvascular endothelial cells, induced tyrosine phosphorylation of focal adhesion kinase (FAK) and activated c-Jun N-terminal kinase (JNK), but not ERK (Etienne et al. 1998). In contrast, Fg binding to endothelial ICAM-1 resulted in activation (phosphorylation) of ERK1/2 (Pluskota & D'Souza 2000, Sen et al. 2009). Integrin signalling involves both ERK and JNK pathways (Schlaepfer & Hunter 1998, Giancotti & Ruoslahti 1999, Hynes 1999, Shyy & Chien 2002). Activation of integrins such as α5β1 leads to activation of multiple kinases (e.g. FAK, Src, Fyn, Shc) (Shyy & Chien 2002). Activation of FAK/Src that is associated with the actin cytoskeleton leads to JNK activation, and Fyn/Shc is linked to ERK activation (Giancotti & Ruoslahti 1999). In addition, activation of ICAM-1 induces activation of various cellular events leading to activation of members of the Rho small GTPase family, including RhoA, Cas, Crk, Cdc42 and Rac (Etienne et al. 1998), and results in activation of JNK (Giancotti & Ruoslahti 1999). RhoA, Cdc42 and Rac have distinct functions in regulating the actin-based cytoskeletal structure. RhoA enhances cell contractility and actin stress fiber formation, Cdc42 modulates filopodia formation, while Rac modulates membrane ruffling (Lim et al. 1996, Van Aelst & D'Souza-Schorey 1997). Activation of p38 mitogen-activated protein kinase (MAPK) (Buchsbaum et al. 2002) regulates F-actin formation (Garcia et al. 2002). Integrin activation is directly associated with members of the Rho family (Giancotti & Ruoslahti 1999). Fg binding ICAM-1 and possibly α5β1 integrin, activated ERK 1/2 signalling leading to the formation of F-actin and cellular contractility (Tyagi et al. 2008).
ET-1 and its receptors, Fg-induced release of ET-1 and role of NO
The vascular endothelium releases agents that affect vascular tone by inducing vasodilation [prostaglandin I (PGI2), NO and endothelium-derived hyperpolarizing factor] and vasoconstriction (thromboxane A2 and ET-1). In addition to modulating vascular tone (Burnstock & Ralevic 1994, Yanagisawa et al. 1998), these agents affect platelet adhesion and aggregation (Willoughby & Loscanzo 2002). During hypertension, although total production of NO was unchanged in hypertensive compared with normal rats, bioavailability of NO was decreased (Li & Joshua 1993). Greater tissue, but not plasma ET-1, content was found in deoxycorticosterone-acetate salt-treated hypertensive rats (Zhao et al. 2000). As NO is an antagonist of ET-1 (Yanagisawa 1994), reduced NO bioavailability may allow ET-1 to have a pronounced effect on vascular tone during hypertension. Therefore, increased vascular ET-1 production may be one of the early events resulting in decreased resting diameters of first- and second-order arterioles in rats with early stage of hypertension development compared with their appropriate normotensive control rats (Meininger et al. 1981, Harper & Bohlen 1984, Bohlen 1989). In addition, small arterioles of the rat skeletal muscle constrict more during hypertension than comparable sized vessels in normotensive rats (Bohlen 1979), suggesting that vascular reactivity is enhanced during hypertension.
Endothelins, first purified from cultured ECs by Yanagisawa et al. (1998), consist of three isoforms, ET-1, ET-2 and ET-3, each containing 21 amino acids (Inoue et al. 1989). The primary source of ET-1 is the endothelium, while ET-2 and ET-3 are present in nonvascular tissue (Russell et al. 1998a). ET-1, the most potent vasoconstrictor currently known, produces long-lasting arterial constriction in humans and rats (Deng et al. 1995). In the last two steps of its synthesis, a pre-propeptide is cleaved by a protease from big ET-1, which is cleaved by endothelin converting enzyme (ECE) to form ET-1 (Schmitz-Spanke & Schipke 2000). ET-1 is synthesized and segregated in the rough endoplasmic reticulum-Golgi system and is stored primarily in WPb (Fukushige et al. 2000). Activation of ECs by agonists such as thrombin, adrenaline or histamine causes exocytosis of WPbs and release of their contents into the blood circulation (Russell et al. 1998b). Recent data indicate that an elevated content of Fg enhances ET-1 production from cultured ECs (Sen et al. 2009). These results suggest that ET-1 is released from WPb through a regulatory pathway. However, there are indications that ET-1 is also discharged constitutively from the ECs (Nakamura et al. 1990).
The ET-A receptor (with greater affinity for ET-1) is located on VSMCs, while endothelin type B (ET-B) (a non-selective receptor) is primarily located on the ECs and VSMCs (Inoue et al. 1989, Deng et al. 1995, Russell et al. 1998b, Yanagisawa et al. 1998, Willoughby & Loscanzo 2002). ET-A and ET-B receptors regulate vascular tone in various blood vessels and vascular beds (Yanagisawa 1994) by mediating sodium and calcium ion channel function (Marsault et al. 1991) and by affecting ET-B, receptor-mediated release of NO and PGI2. Most of the ET-1 secretion (~75%) from cultured ECs is towards the VSMC (abluminal) side (Yoshimoto et al. 1991), where it binds to ET-A receptors on the VSMCs and causes vasoconstriction. Under normal physiological conditions, ET-1 and NO are constitutively released by the endothelium and provide a balance between vascular constrictor and dilator activity (Nakamura et al. 1990). However, during various pathologies such as hypertension, when bioavailability of NO is decreased (Li & Joshua 1993) and regulated production of ET-1 is increased due to increased Fg content, there is a tendency towards constriction. Interestingly, an in vivo study of dose-dependent, ET-1-induced microvascular constriction showed that microvessels did not constrict to low doses of ET-1 (Roberts et al. 1998). However, if the vessels were pre-treated with Nw-nitro-l-arginine methyl ester, the vascular constrictions in response to ET-1 were greatly enhanced and were significant even at low doses of ET-1 (Roberts et al. 1998). Because endothelin induces NO production (King et al. 1997, Srivastava & Magazine 1998, Ishizuka et al. 1999), NO may counterbalance and therefore prevent further increases in vascular constriction at higher doses of Fg.
Signalling in ET-1 production, and a role of F-actin
The role of MAPKs in ET-1-induced vasoconstriction during hypertension has been shown (Watts 2000). However, a complete understanding of the signalling mechanisms of ET-1 production remains unclear. Transcription of ET-1 depends on ERK activity (Morey et al. 1998). ET-1 gene expression in cultured ECs also involves the ERK pathway (Juan et al. 2004). Activation of ERK-1/2 is involved in Fg-induced regulated production of ET-1 (Sen et al. 2009). A role of the JNK-mediated pathway in production of prepro-ET-1 gene transcription has also been demonstrated (Yamakaw et al. 2002). However, the role of these MAPKs in ET-1 production during hypertension is not established. In several studies, activation of endothelial ICAM-1 caused activation of p38 MAP kinase and resulted in formation of F-actin through the small heat shock protein 27 (Hsp27) (Wang & Doerschuk 2001, Wang et al. 2005). As formation of F-actin is required for exocytosis of WPbs (Manneville et al. 2003), and increased content of Fg enhances formation of F-actin (Tyagi et al. 2008) and production of ET-1 (Sen et al. 2009), it is reasonable to hypothesize that Fg binding to endothelial ICAM-1 (and possibly α5β1 integrin) causes accumulation of WPbs in the vicinity of the EC membrane. Fg binding may also cause exocytosis of WPB through ERK (and possibly JNK and/or p38) signalling, which results in release of big ET-1 and ECE from these granules (Sen et al. 2009) (Fig. 2). Subsequently, formed ET-1 binds to ET-A receptors on VSMCs and causes vasoconstriction (Lominadze et al. 2005, Sen et al. 2009) (Fig. 2).
Fg and endothelial layer integrity; effect on tight junction proteins
Endothelial cells are connected to each other by a complex set of junctional proteins that comprise tight junctions, gap junctions and adherens junctions. Occludin, claudins and junctional adhesion molecules are the main tight junction proteins (TJPs) (Mehta & Malik 2006). TJPs create a paracellular barrier in endothelial cells forming a first layer of protection that separates them from blood (Förster 2008). Vascular hyperperme-ability triggered by inflammation in the heart, brain or lung promotes oedema, exacerbates disease progression and impairs recovery (Weis 2008).
Although digestion of Fg by plasmin in vivo is quite rare (Boutcher et al. 1996, Gaffney 2001), increased vascular permeability has been found to result from the interaction of thrombin and Fg (Johnson et al. 1983, 1985). It has been shown that the Fg degradation product, fragment D, increases endothelial layer permeability in vitro (Ge et al. 1991, 1992). Infusion of fragment D into conscious rabbits increased vascular permeability to albumin (Manwaring & Curreri 1981). However, another study showed that infusion of fragment D alone in sheep did not alter pulmonary transvascular fluid and protein exchange (Johnson et al. 1985). Vascular leakage was increased only if thrombin-induced pulmonary microembolization preceded fragment D infusion, suggesting a role of increased capillary hydrostatic pressure (Johnson et al. 1985).
A recent study by Guo et al. (2009) suggested that binding of the Fg-γ C terminus to endothelial αvβ3 integrin caused leakage of mesenteric microvessels through a RhoA-dependent mechanism. Another study indicated that elevated content of un-degraded Fg enhanced the formation of filamentous actin (F-actin), leading to increased endothelial layer leakage to albumin (Tyagi et al. 2008). In addition, leakage of Fg itself through the EC layer was also increased if its content was elevated (Tyagi et al. 2008), confirming the possibility that Fg leakage through the vascular wall was induced by different agonists (Pedersen et al. 1991) or even diseases (Areekul 1986). A role for Fg binding to endothelial ICAM-1 and α5β1 integrin was suggested in the Fg-induced increased EC layer permeability (Tyagi et al. 2008). In this study, an effect of Fg-to-α5β1 integrin binding on EC permeability, confirmed the results of Qiao et al. (1995), indicating that the RGD peptide increases endothelial hydraulic conductivity. These data provide an explanation for Ancrod being ineffective in treating myocardial ischaemia-reperfusion, as it reduces blood content of Fg, but at the same time increases the content of its degradation products, which may exacerbate vascular injury (Zacharowski et al. 2006).
Recently published data indicate that the integrity of the EC monolayer was impaired by elevated un-degraded Fg (Patibandla et al. 2009). A role for activation of ERK-1/2 following binding of Fg to endothelial ICAM-1 was suggested as a possible mechanism for disruption of EC layer integrity and increased permeability to albumin (Tyagi et al. 2008, Patibandla et al. 2009). In addition, increased content of Fg caused a decrease in expression of the actin-associated proteins, occludin, zonula ocluden-1 and zonula ocluden-2 (Patibandla et al. 2009). Fg binding to endothelial ICAM-1 was implicated in the alteration of TJPs (Patibandla et al. 2009) and activation of matrix metalloproteinases (MMPs) (Patibandla PK, Tyagi N, Tyagi SC, Dean WL, Roberts AM, Lominadze D, unpublished data), confirming the results indicating that ICAM-1 is a key player in activation of MMP-9 during T lymphoma/EC interaction (Aoudjit et al. 1998). Taken together, these data suggest that an elevated content of Fg may increase permeability of the EC layer through formation of F-actin, downregulation of TJPs, and activation of MMPs. The activation of MMPs may cause digestion of adherens junction proteins (e.g. VE-cadherin) and this sequence of events may result in increased leakage of Fg to the sub-endothelium and its accumulation in this subcellular compartment.
Of the endothelial α5β1 and αvβ3 integrins (Luscinskas & Lawler 1994) that act as Fg receptors (Plow et al. 2000), only α5β1 integrin has been reported to reside at the endothelial cell-to-cell contact border (Lampugnani et al. 1991). αvβ3 integrin was not found in intercellular contact regions (Lampugnani et al. 1991). Fg binds to endothelial α5β1 and αvβ3 integrins through its two RGD sequences on the Aα chain (Suehiro et al. 1997), and to ICAM-1 through a discrete region of the γ chain (Altieri et al. 1995). Therefore, depending on the Fg binding sites (ICAM-1, α5β1 integrin, αvβ3 integrin) or their relative expression levels on the EC surface, it is possible that different physiological responses may be produced. Further studies are necessary to delineate the effects of intact Fg on vascular endothelium and SMCs, and the resultant vascular responses.
Fibrinogen-induced albumin leakage may also occur through transendothelial extravasation. Binding of Fg to ECs and activation of ERK signalling triggers albumin extravasation by caveolae via an absorptive (receptor-mediated) or fluid-phase pathway (John et al. 2003). Albumin can be easily taken up by caveolae (Predescu & Palade 1993). ET-1 may also have an effect on both paracellular and transendothelial transport mechanisms. However, Fg and ET-1 may utilize different mechanisms and have different potencies in inducing macromolecular leakage through the EC layer. Further experiments are needed to determine the roles and mechanism of Fg and ET-1 in macromolecular leakage through the EC monolayer.
Increases in microvascular permeability may lead to deposition of Fg in the subendothelial matrix. A strong association between elevated levels of Fg and formation of atherosclerotic plaques has been found (Bennett 2001, Lowe & Rumley 2001). Increased binding of Fg to the vascular endothelium and its leakage into the subendothelial matrix may provide favourable conditions for its conversion to fibrin by thrombin, and its generation, which is increased during hypertension (Iida et al. 2008) and diabetes (Cohen et al. 2002). Increased levels and/or activity of the plasminogen system have not been observed during a majority of the cardiovascular and cerebrovascular diseases. For example, activity of tissue plasminogen activator is diminished in brains of patients with Alzheimer's disease and mouse models of the disease (Ledesma et al. 2000). Thus, an increase in blood content of Fg, in the context of decreased or unaltered activity of the plasminogen system, leads to an enhanced deposition of Fg on the vascular endothelium and/or the subendothelial matrix. Immobilized Fg is then converted to fibrin by thrombin, as its degradation system can no longer counterbalance excess formation of fibrin. Enhanced deposition of fibrin exacerbates circulatory complications of cardiovascular diseases, such as the development of atherosclerotic plaques (Smith 1994), neurovascular damage and neuroinflammation (Paul et al. 2007). In addition, even a subtle effect of plasmin on Fg or fibrin, leading to the formation of their degradation products such as fragment D, will amplify the destructive effects initiated by an increased content of Fg.
Summary and conclusions
The data reviewed here strongly indicate that an increased Fg content may lead to significant deleterious effects in the microcirculation. As cardiovascular and cerebrovascular diseases such as hypertension, diabetes and stroke are accompanied by increased blood levels of Fg, its detrimental effects may be more pronounced during these inflammatory diseases. Binding of Fg to its endothelial receptors may activate signalling cascades that alter vasoactivity through disruption of ET-1/NO bioavailability, increased EC layer permeability, increased formation of F-actin and disruption of the TJPs, and enhanced activity of MMPs that may digest adherens junction proteins. In addition, increased levels of Fg increase plasma viscosity and RBC aggregation, causing an increase in blood viscosity. This process may lead to an increase in blood shear stress, activation of vascular adhesion molecules and integrins, and platelets. These events may lead to even greater binding of Fg to vascular endothelium and an enhanced platelet thrombogenesis.
Lastly, the authors emphasize the potential clinical importance of increased blood Fg content. Understanding the mechanisms of destructive effects of Fg exacerbating complications of vascular dysfunction during cardiovascular diseases should allow for the development of interventions that diminish these complications.
Acknowledgments
Supported in part by NIH grants to D.L. (HL-80394) and to S.C.T. (HL-71010 and NS-051568).
Footnotes
Conflict of interest
There is no conflict of interest in this study.
References
- Altieri D, Duperray A, Plescia J, Thornton G, Languino L. Structural recognition of a novel fibrinogen gamma chain sequence (117-133) by intercellular adhesion molecule-1 mediates leukocyte-endothelium interaction. J Biol Chem. 1995;270:696–699. doi: 10.1074/jbc.270.2.696. [DOI] [PubMed] [Google Scholar]
- Anderson R, Saldeen K, Saldeen T. A fibrin(ogen) derived pentapeptide induces vasodilation, prostacyclin release and an increase in cyclic AMP. Thromb Res. 1983;30:213–218. doi: 10.1016/0049-3848(83)90074-9. [DOI] [PubMed] [Google Scholar]
- Aoudjit F, Potworowski EF, St-Pierre Y. Bi-directional induction of matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 during T lymphoma/endothelial cell contact: implication of ICAM-1. J Immunol. 1998;160:2967–2973. [PubMed] [Google Scholar]
- Areekul S. Plasmodium coatneyi: alterations of transcapillary escape rate and capillary permeability to fibrinogen in rhesus monkeys. Exp Parasitol. 1986;61:304–310. doi: 10.1016/0014-4894(86)90185-2. [DOI] [PubMed] [Google Scholar]
- Barczak-Osinska J, Buczko W, Wisniewski K. The effect of products of degradation of fibrinogen A and B on the arterial blood pressure in the rat. Pol J Pharmacol Pharm. 1983;35:279–283. [PubMed] [Google Scholar]
- Barnes G, Frieden E. Ceruloplasmin receptors of erythrocytes. Biochem Biophys Res Commun. 1984;125:157–162. doi: 10.1016/s0006-291x(84)80348-4. [DOI] [PubMed] [Google Scholar]
- Bas M, Kirchhartz N, Hochfeld J, Tüllmann C, Kumpf S, Suvorava T, Oppermann M, Hafner D, Bier H, Hoffmann TK, Balz V, Kojda G. Potential role of vasomotor effects of fibrinogen in bradykinin-induced angioedema. J Allergy Clin Immunol. 2008;121:969–975. doi: 10.1016/j.jaci.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Becker B, Heindl B, Kupatt C, Zahler S. Endothelial function and hemostasis. Z Kardiol. 2000;89:160–167. doi: 10.1007/pl00007320. [DOI] [PubMed] [Google Scholar]
- Bennett J. Platelet-fibrinogen interactions. Ann N Y Acad Sci. 2001;936:340–354. doi: 10.1111/j.1749-6632.2001.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Bezie Y, Lacolley P, Laurent S, Gabella G. Connection of smooth muscle cells to elastic lamellae in aorta of spontaneously hypertensive rats. Hypertension. 1998;32:166–169. doi: 10.1161/01.hyp.32.1.166. [DOI] [PubMed] [Google Scholar]
- Bloch E. Microscopic observations of the circulating blood in the bulbar conjunctiva in man in health and disease. Ergeb Anat Entwicklungsgesch. 1956;35:1–98. [PubMed] [Google Scholar]
- Bohlen HG. Arteriolar closure mediated by hyperresponsiveness to norepinephrine in hypertensive rats. Am J Physiol. 1979;236:H157–H164. doi: 10.1152/ajpheart.1979.236.1.H157. [DOI] [PubMed] [Google Scholar]
- Bohlen H. The microcirculation in hypertension. J Hypertens. 1989;7:S117–S124. [PubMed] [Google Scholar]
- Boutcher P, Gaffney P, Raut S, O'Regan R, McLoughlin P. Effects of early plasmin digests of fibrinogen on isometric tension development in isolated rings of rat pulmonary artery. Thromb Res. 1996;81:231–239. doi: 10.1016/0049-3848(95)00240-5. [DOI] [PubMed] [Google Scholar]
- Buchsbaum R, Connolly B, Feig L. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol. 2002;22:4073–4085. doi: 10.1128/MCB.22.12.4073-4085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemi M, Allegra A, Aloisi C, Corica F, Alonci A, Ruello A, Gaetano M, Nicola F. Cold pressor test raises serum concentrations of ICAM-1, VCAM-1, and E-selectin in normotensive and hypertensive patients. Hypertension. 1997;30:845–847. doi: 10.1161/01.hyp.30.4.845. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg. 1994;47:527–543. doi: 10.1016/0007-1226(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Cabel M, Meiselman H, Popel A, Johnson P. Contribution of red blood cell aggregation to venous vascular resistance in skeletal muscle. Am J Physiol Heart Circ Physiol. 1997;272:H1020–H1032. doi: 10.1152/ajpheart.1997.272.2.H1020. [DOI] [PubMed] [Google Scholar]
- Chabanel A, Chien S. Blood viscosity as a factor in human hypertension. In: Laragh H, Brenner BM, editors. Hypertension Pathophysiology, Diagnosis and Management. Raven Press; New York: 1990. pp. 329–337. [Google Scholar]
- Chabanel A, Schachter D, Chien S. Increased rigidity of red cell membrane in young spontaneously hypertensive rats. Hypertension. 1987;10:603–607. doi: 10.1161/01.hyp.10.6.603. [DOI] [PubMed] [Google Scholar]
- Chae C, Lee R, Rifai N, Ridker P. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- Chen C, Schachter D. Elevation of plasma immunoglobulin A in the spontaneously hypertensive rat. Hypertension. 1993;21:731–738. doi: 10.1161/01.hyp.21.5.731. [DOI] [PubMed] [Google Scholar]
- Chien S. Blood rheology in myocardial infarction and hypertension. Biorheology. 1986;23:633–653. doi: 10.3233/bir-1986-23614. [DOI] [PubMed] [Google Scholar]
- Chien S, Jan K-M. Ultrastructural basis of the mechanism of rouleaux formation. Microvasc Res. 1973;5:155–166. doi: 10.1016/0026-2862(73)90068-x. [DOI] [PubMed] [Google Scholar]
- Chien S, Usami S, Dellenback RJ, Gregersen MI, Nanninga LB, Guest MM. Blood viscosity: influence of erythrocyte aggregation. Science. 1967;157:829–831. doi: 10.1126/science.157.3790.829. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Gonzales R, Davis-Gorman G, Copeland J, McDonagh P. Thrombin activity and platelet microparticle formation are increased in type 2 diabetic platelets: a potential correlation with caspase activation. Thromb Res. 2002;107:217–221. doi: 10.1016/s0049-3848(02)00334-1. [DOI] [PubMed] [Google Scholar]
- Collen D, Tytgat G, Claeys H, Piessens R. Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. Br J Haematol. 1972;22:681–700. doi: 10.1111/j.1365-2141.1972.tb05715.x. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Mogford JE, Davis GE, Davis MJ, Meininger GA. Integrin-mediated reduction in vascular smooth muscle [Ca2 + ]i induced by RGD-containing peptide. Am J Physiol Heart Circ Physiol. 1997;272:H2065–H2070. doi: 10.1152/ajpheart.1997.272.4.H2065. [DOI] [PubMed] [Google Scholar]
- D'Erasmo E, Acca M, Celi F, Medici F, Palmerini T, Pisani D. Plasma fibrinogen and platelet count in stroke. J Med. 1993;24:185–191. [PubMed] [Google Scholar]
- D'Souza S, Byers-Ward V, Gardiner E, Wang H, Sung S-S. Identification of an active sequence within the first immunoglobulin domain of intercellular cell adhesion molecule-1 (ICAM-1) that interacts with fibrinogen. J Biol Chem. 1996;271:24270–24277. doi: 10.1074/jbc.271.39.24270. [DOI] [PubMed] [Google Scholar]
- Dalekos G, Elisaf M, Papagalanis N, Tzallas C, Siam-opoulos K. Elevated interleukin-1 beta in the circulation of patients with essential hypertension before any drug therapy: a pilot study. Eur J Clin Invest. 1996;26:936–939. doi: 10.1111/j.1365-2362.1996.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- Davies P, Zilberberg J, Helmke B. Spatial microstimuli in endothelial mechanosignaling. Circ Res. 2003;92:359–370. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- De Maat M. Effects of diet, drugs, and genes on plasma fibrinogen levels. Ann N Y Acad Sci. 2001;2001:936. doi: 10.1111/j.1749-6632.2001.tb03537.x. [DOI] [PubMed] [Google Scholar]
- Deng L-Y, Li JS, Schiffrin EL. Endothelin receptor subtypes in resistance arteries from humans and rats. Cardiovasc Res. 1995;29:532–535. [PubMed] [Google Scholar]
- DeSouza C, Dengel D, Maeko R, Cox K, Seals D. Elevated levels of circulating cell adhesion molecules in uncomplicated essential hypertension. Am J Hypertens. 1997;10:1335–1341. doi: 10.1016/s0895-7061(97)00268-9. [DOI] [PubMed] [Google Scholar]
- Dintenfass L. Blood-pressure and blood viscosity in coronary heart-disease. Lancet. 1978;2:993–994. doi: 10.1016/s0140-6736(78)92558-8. [DOI] [PubMed] [Google Scholar]
- Ernst E, Resch K. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- Etienne S, Adamson P, Greenwood J, Strosberg A, Cazaubon S, Couraud P. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161:5755–5761. [PubMed] [Google Scholar]
- Evans E, Needham D. Attraction between lipid bilayer membranes in concentrated solutions of nonadsorbing polymers: comparison of mean-field theory with measurements of adhesion energy. Macromolecules. 1988;21:1822–1831. [Google Scholar]
- Folkow B. The fourth Volhard lecture: cardiovascular structural adaptation; its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med. 1978;55:3s–22s. doi: 10.1042/cs055003s. [DOI] [PubMed] [Google Scholar]
- Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige H, Doi Y, Kudo H, Kayashima K, Kiynaga H, Nagata T, Itoh H, Fujimoto S. Synthesis and receptor sites of endothelin-1 in the rat liver vasculature. Anat Rec. 2000;259:437–445. doi: 10.1002/1097-0185(20000801)259:4<437::AID-AR70>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Gaffney P. Fibrin degradation products: a review of structures found in vitro and in vivo. Ann N Y Acad Sci. 2001;936:594–610. [PubMed] [Google Scholar]
- Game L, Voegel J-C, Schaaf P, Stoltz J. Do physiological concentrations of IgG induce a direct aggregation of red blood cells: comparison with fibrinogen. Biochim Biophys Acta. 1996;1291:138–142. doi: 10.1016/0304-4165(96)00056-6. [DOI] [PubMed] [Google Scholar]
- Garcia J, Wang P, Schaphorst K, Becker P, Borbiev T, Liu F, Birukova A, Jacobs K, Bogatcheva N, Verin A. Critical involvement of p38 MAP kinase in pertussis toxin-induced cytoskeletal reorganization and lung permeability. FASEB J. 2002;16:1064–1076. doi: 10.1096/fj.01-0895com. [DOI] [PubMed] [Google Scholar]
- Ge M, Ryan TJ, Lum H, Malik AB. Fibrinogen degradation product fragment D increases endothelial monolayer permeability. Am J Physiol Lung Cell Mol Physiol. 1991;261:L283–L289. doi: 10.1152/ajplung.1991.261.4.L283. [DOI] [PubMed] [Google Scholar]
- Ge M, Tang G, Ryan T, Malik A. Fibrinogen degradation product fragment D induces endothelial cell detachment by activation of cell-mediated fibrinolysis. J Clin Invest. 1992;90:2508–2516. doi: 10.1172/JCI116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gladner J, Murtaugh P, Folk J, Laki K. Nature of peptides released by thrombin. Ann N Y Acad Sci. 1963;104:47–52. doi: 10.1111/j.1749-6632.1963.tb17651.x. [DOI] [PubMed] [Google Scholar]
- Guo M, Daines D, Tang J, Shen Q, Perrin RM, Takada Y, Yuan SY, Wu MH. Fibrinogen-{gamma} C-terminal fragments induce endothelial barrier dysfunction and microvascular leak via integrin-mediated and RhoA-dependent mechanism. Arterioscler Thromb Vasc Biol. 2009;29:394–400. doi: 10.1161/ATVBAHA.108.180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson L, Appelgren L, Myrvold HE. Blood flow and in vivo apparent viscosity in working and non-working skeletal muscle of the dog after high and low molecular weight dextran. Circ Res. 1981a;48:465–469. doi: 10.1161/01.res.48.4.465. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Appelgren L, Myrvold HE. Effects of increased plasma viscosity and red blood cell aggregation on blood viscosity in vivo. Am J Physiol Heart Circ Physiol. 1981b;241:H513–H518. doi: 10.1152/ajpheart.1981.241.4.H513. [DOI] [PubMed] [Google Scholar]
- Hacioglu G, Yalcin O, Bor-Kucukatay M, Ozkaya G, Baskurt O. Red blood cell rheological properties in various rat hypertension models. Clin Hemorheol Microcirc. 2002;26:27–32. [PubMed] [Google Scholar]
- Hamsten A, Iselius L, de Faire U, Blomback M. Genetic and cultural inheritance of plasma fibrinogen concentration. Lancet. 1987;2:988–991. doi: 10.1016/s0140-6736(87)92557-8. [DOI] [PubMed] [Google Scholar]
- Harper S, Bohlen H. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension. 1984;6:408–419. doi: 10.1161/01.hyp.6.3.408. [DOI] [PubMed] [Google Scholar]
- Hermand P, Gane P, Huet M, Jallu V, Kaplan C, Sonneborn HH, Cartron JP, Bailly P. Red cell ICAM-4 is a novel ligand for platelet-activated alpha IIbbeta 3 integrin. J Biol Chem. 2003;278:4892–4898. doi: 10.1074/jbc.M211282200. [DOI] [PubMed] [Google Scholar]
- Hicks R, Golledge J, Mir-Hasseine R, Powell J. Vasoactive effects of fibrinogen on saphenous vein. Nature. 1996;379:818–820. doi: 10.1038/379818a0. [DOI] [PubMed] [Google Scholar]
- Humphries S. Genetic regulation of fibrinogen. Eur Heart J. 1995;16:16–20. doi: 10.1093/eurheartj/16.suppl_a.16. [DOI] [PubMed] [Google Scholar]
- Humphries S, Cook M, Dubowitz M, Stirling Y, Meade TW. Role of genetic variation at the fibrinogen locus in determination of plasma fibrinogen concentrations. Lancet. 1987;1:1452–1455. doi: 10.1016/s0140-6736(87)92205-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9:M33–M37. [PubMed] [Google Scholar]
- Iida M, Yamamoto M, Yamazaki M, Sawaguchi M, Honjo H, Kodama I, Kamiya K. Association of aortic valve sclerosis with thrombin generation in hypertensive patients. J Hum Hypertens. 2008;22:781–787. doi: 10.1038/jhh.2008.68. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. Human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intengan H, Schiffrin E. Structure and mechanical properties of resistance arteries in hypertension; role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- Intengan H, Thibault G, Li J-S, Schiffrin E. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats: effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation. 1999;100:2267–2275. doi: 10.1161/01.cir.100.22.2267. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Takamizawa-Matsumoto M, Suzuki K, Kurita A. Endothelin-1 enhances vascular cell adhesion molecule-1 expression in tumor necrosis factor a-stimulated vascular endothelial cells. Eur J Pharmacol. 1999;369:237–245. doi: 10.1016/s0014-2999(99)00042-4. [DOI] [PubMed] [Google Scholar]
- John T, Vogel S, Tiruppathi C, Malik A, Minshall R. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284:L187–L196. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- Johnson A, Tahamont MV, Malik AB. Thrombin-induced lung vascular injury. Roles of fibrinogen and fibrinolysis. Am Rev Respir Dis. 1983;128:38–44. doi: 10.1164/arrd.1983.128.1.38. [DOI] [PubMed] [Google Scholar]
- Johnson A, Garcia-Szabo R, Kaplan J, Malik A. Fibrin degradation products increase lung transvascular fluid filtration after thrombin-induced pulmonary microembolism. Thromb Res. 1985;37:543–554. doi: 10.1016/0049-3848(85)90100-8. [DOI] [PubMed] [Google Scholar]
- Juan S, Chen J, Chen C, Lin H, Cheng C, Liu J, Hsieh M, Chen Y, Chao H, Chen T, Chan P, Cheng T. 17{beta}-Estradiol inhibits cyclic strain-induced endothelin-1 gene expression within vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1254–H1261. doi: 10.1152/ajpheart.00723.2003. [DOI] [PubMed] [Google Scholar]
- Kannan H, Tanaka Y, Kunitake T, Ueta Y, Hayashida Y, Yamashita H. Activation of sympathetic outflow by recombinant human interleukin-1 beta in conscious rats. Am J Physiol. 1996;270:R479–R485. doi: 10.1152/ajpregu.1996.270.2.R479. [DOI] [PubMed] [Google Scholar]
- Kano Y, Katoh K, Masuda M, Fujiwara K. Macromolecular composition of stress fiber-plasma membrane attachment sites in endothelial cells in situ. Circ Res. 1996;79:1000–1006. doi: 10.1161/01.res.79.5.1000. [DOI] [PubMed] [Google Scholar]
- Kern D, Saldeen K, Saldeen T, Malik A. Pulmonary vascular effects of a fibrin(ogen)-derived vasoactive peptide. Thromb Res. 1986;42:783–788. doi: 10.1016/0049-3848(86)90114-3. [DOI] [PubMed] [Google Scholar]
- King J, Srivastava K, Stefano G, Bilfinger T, Bahou W, Magazine H. Human monocyte adhesion is modulated by endothelin B receptor-coupled nitric oxide release. J Immunol. 1997;158:880–886. [PubMed] [Google Scholar]
- Knisely M. Intravascular erythrocyte aggregation (blood sludge). In: Renkin E, Michel C, editors. Handbook of Physiology; A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts. American Physiological Society; Bethesda, MD: 1965. pp. 2249–2292. [Google Scholar]
- Koenig W, Sund M, Ernst E, Matrai A, Keil U, Rosenthal J. Is increased plasma viscosity a risk factor for high blood pressure? Angiology. 1989;40:153–163. doi: 10.1177/000331978904000301. [DOI] [PubMed] [Google Scholar]
- Krobot K, Hense H, Cremer P, Eberle E, Keil U. Determinants of plasma fibrinogen: relation to body weight, waist-to-hip ratio, smoking, alcohol, age, and sex. Results from the second MONICA Augsburg survey 1989–1990. Arterioscler Thromb. 1992;12:780–788. doi: 10.1161/01.atv.12.7.780. [DOI] [PubMed] [Google Scholar]
- Laki K. The action of thrombin on fibrinogen. Science. 1951;114:435–436. doi: 10.1126/science.114.2965.435. [DOI] [PubMed] [Google Scholar]
- Lampugnani M, Resnati M, Dejana E, Marchisio P. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin K, Tengborn L, Smith U. Elevated fibrinogen and plasminogen activator inhibitor (PAI-1) in hypertension are related to metabolic risk factors for cardiovascular disease. J Intern Med. 1990;227:273–278. doi: 10.1111/j.1365-2796.1990.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Languino L, Plescia J, Duperrray A, Brian A, Plow E, Geltosky J, Alteri D. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- Ledesma M, Da Silva J, Crassaerts K, Delacourte A, De Strooper B, Dotti C. Brain plasmin enhances APP alpha-cleavage and Abeta degradation and is reduced in Alzheimer's disease brains. EMBO Rep. 2000;1:530–535. doi: 10.1093/embo-reports/kvd107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher R, Chien S, Pickering T, Sealey J, Laragh J. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. Am J Med. 1981;70:1195–1202. doi: 10.1016/0002-9343(81)90827-5. [DOI] [PubMed] [Google Scholar]
- Letcher R, Chien S, Pickering T, Laragh J. Elevated blood viscosity in patients with borderline essential hypertension. Hypertension. 1983;5:757–762. doi: 10.1161/01.hyp.5.5.757. [DOI] [PubMed] [Google Scholar]
- Li F, Joshua I. Decreased arteriolar endothelium-derived relaxing factor production during the development of genetic hypertension. Clin Exp Hypertens. 1993;15:511–526. doi: 10.3109/10641969309041626. [DOI] [PubMed] [Google Scholar]
- Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signaling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- Lip G. Fibrinogen and cardiovascular disorders. Q J Med. 1995;88:155–165. [PubMed] [Google Scholar]
- Lip G, Beevers D. Abnormalities of rheology and coagulation in hypertension. J Hum Hypertens. 2007;8:693–702. [PubMed] [Google Scholar]
- Lominadze D, Dean W. Involvement of fibrinogen specific binding in erythrocyte aggregation. Clin Exp Hypertens. 2002;517:41–44. doi: 10.1016/s0014-5793(02)02575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominadze D, Joshua I, Schuschke D. Increased erythrocyte aggregation in spontaneously hypertensive rats. Am J Hypertens. 1998;11:784–789. doi: 10.1016/s0895-7061(98)00056-9. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Schuschke D, Joshua I, Dean W. Increased ability of erythrocytes to aggregate in spontaneously hypertensive rats. Clin Exp Hypertens. 2002;24:397–406. doi: 10.1081/ceh-120005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominadze D, Tsakadze N, Sen U, Falcone J, D'Souza S. Fibrinogen- and fragment D-induced vascular constriction. Am J Physiol. 2005;288:H1257–H1264. doi: 10.1152/ajpheart.00856.2004. [DOI] [PubMed] [Google Scholar]
- London M. The role of blood rheology in regulating blood pressure. Clin Hemorheol Microcirc. 1997;17:93–106. [PubMed] [Google Scholar]
- Lowe G, Rumley A. Fibrinogen and its degradation products as thrombotic risk factors. Ann N Y Acad Sci. 2001;936:560–565. doi: 10.1111/j.1749-6632.2001.tb03544.x. [DOI] [PubMed] [Google Scholar]
- Lowe G, Lee A, Rumley A, Price J, Fowkes F. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol. 1997;96:168–173. doi: 10.1046/j.1365-2141.1997.8532481.x. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen P. The hemodynamics of hypertension. In: Robertson J, editor. Handbook of Hypertension. Elsevier; Amsterdam: 1983. pp. 151–173. [Google Scholar]
- Luscinskas F, Lawler J. Integrins as dynamic regulators of vascular function. FASEB J. 1994;8:929–938. doi: 10.1096/fasebj.8.12.7522194. [DOI] [PubMed] [Google Scholar]
- Ly D, Zazzali K, Corbett S. De novo expression of the integrin alpha5beta1 regulates alphavbeta3-mediated adhesion and migration on fibrinogen. J Biol Chem. 2003;278:21878–21885. doi: 10.1074/jbc.M212538200. [DOI] [PubMed] [Google Scholar]
- Manneville J, Etienne-Manneville S, Skehel P, Carter T, Ogden D, Ferenczi M. Interaction of the actin cytoskeleton with microtubules regulates secretory organelle movement near the plasma membrane in human endothelial cells. J Cell Sci. 2003;116:3927–3938. doi: 10.1242/jcs.00672. [DOI] [PubMed] [Google Scholar]
- Manwaring D, Curreri P. Cellular mediation of respiratory distress syndrome induced by fragment D. Ann Chir Gynaecol. 1981;70:304–307. [PubMed] [Google Scholar]
- Marsault R, Vigne P, Breittmayer J, Frelin C. Kinetics of vasoconstrictor action of endothelins. Am J Physiol. 1991;261:C987–C993. doi: 10.1152/ajpcell.1991.261.6.C986. [DOI] [PubMed] [Google Scholar]
- Martinez J, Holburn R, Shapiro S, Erslev A. Fibrinogen Philadelphia: a hereditary hypodysfibrinogenemia characterized by fibrinogen hypercatabolism. J Clin Invest. 1974;53:600–611. doi: 10.1172/JCI107595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. {alpha}v{beta}3- and {alpha}5{beta}1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- Maspers M, Björnberg J, Mellander S. Relation between capillary pressure and vascular tone over the range from maximum dilatation to maximum constriction in cat skeletal muscle. Acta Physiol Scand. 1990;140:73–83. doi: 10.1111/j.1748-1716.1990.tb08977.x. [DOI] [PubMed] [Google Scholar]
- Mchedlishvili G, Beritashvili N, Lominadze D, Tsinamdzvrishvili B. Technique for direct and quantitative evaluation of erythrocyte aggregability in blood samples. Biorheology. 1993;30:153–161. doi: 10.3233/bir-1993-30206. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik A. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mehta J, Wargovich T, Nichols W, Saldeen K, Wallin R, Saldeen T. Peptide 6A, a fibrin(ogen) degradation product, increases coronary blood flow. Am J Physiol. 1985;249:H457–H462. doi: 10.1152/ajpheart.1985.249.3.H457. [DOI] [PubMed] [Google Scholar]
- Meininger G, Harris P, Joshua I, Miller F, Wiegman D. Microvascular pressures in skeletal muscle of one-kidney one-clip renovascular hypertensive rats. Microcirculation. 1981;1:237–254. [Google Scholar]
- Mogford J, Davis G, Platts S, Meininger G. Vascular smooth muscle alpha v beta 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res. 1996;79:821–826. doi: 10.1161/01.res.79.4.821. [DOI] [PubMed] [Google Scholar]
- Mogford J, Davis G, Meininger G. RGDN peptide interaction with endothelial alpha5beta1 integrin causes sustained endothelial-dependent vasoconstrictionto rat skeletal muscle arterioles. J Clin Invest. 1997;100:1647–1653. doi: 10.1172/JCI119689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey A, Razandi M, Pedram A, Hu R, Prins B, Levin E. Oestrogen and progesterone inhibit the stimulated production of endothelin-1. Biochem J. 1998;15:1097–1105. doi: 10.1042/bj3301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Naruse M, Naruse K, Demura H, Uemura H. Immunocytochemical localization of endothelin in culturedbovineendothelialcells. Histochemistry. 1990;94:475–477. doi: 10.1007/BF00272609. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Kohsaka T, Johns E. Neuro-regulation of interleukin-6 gene expression in the spontaneously hypertensive rat kidney. J Hypertens. 1996;14:839–845. doi: 10.1097/00004872-199607000-00006. [DOI] [PubMed] [Google Scholar]
- Nichols W, Mehta J, Wargovich T, Saldeen K, Wallin R, Saldeen T. Fibrin(ogen)-derived peptide B beta 30-43 increases coronary blood flow in the anesthetized dog. Thromb Res. 1985;39:223–229. doi: 10.1016/0049-3848(85)90110-0. [DOI] [PubMed] [Google Scholar]
- Pankow J, Folsom A, Province M, Rao D, Williams R, Eckfeldt J, Sellers T. Segregation analysis of plasminogen activator inhibitor-1 and fibrinogen levels in the NHLBI family heart study. Arterioscler Thromb Vasc Biol. 1998;18:1559–1567. doi: 10.1161/01.atv.18.10.1559. [DOI] [PubMed] [Google Scholar]
- Patibandla PK, Tyagi N, Dean WL, Tyagi SC, Roberts AM, Lominadze D. Fibrinogen induces alterations of endothelial cell tight junction proteins. J Cell Physiol. 2009;221:195–203. doi: 10.1002/jcp.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K, Rigby P, Goldie R. Quantitative assessment of increased airway microvascular permeability to 125I-labelled plasma fibrinogen induced by platelet activating factor and bradykinin. Br J Pharmacol. 1991;104:128–132. doi: 10.1111/j.1476-5381.1991.tb12396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts SH, Mogford JE, Davis MJ, Meininger GA. Role of K+ channels in arteriolar vasodilation mediated by integrin interaction with RGD-containing peptide. Am J Physiol Heart Circ Physiol. 1998;275:H1449–H1454. doi: 10.1152/ajpheart.1998.275.4.H1449. [DOI] [PubMed] [Google Scholar]
- Plow E, Haas T, Zhang L, Loftus J, Smith J. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- Pluskota E, D'Souza S. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial cell survival. Eur J Biochem. 2000;267:4693–4704. doi: 10.1046/j.1432-1327.2000.01520.x. [DOI] [PubMed] [Google Scholar]
- Porteri E, Rizzoni D, Piccoli A, Castellano M, Bettoni G, Muiesan M, Pasini G, Guelfi D, Zulli R, Rosei E. Effects of hypotensive and non-hypotensive doses of manidipine on structure, responses to endothelin-1 and ICAM-1 production in mesenteric small resistance arteries of spontaneously hypertensive rats. Blood Press. 1998;7:324–330. doi: 10.1080/080370598437204. [DOI] [PubMed] [Google Scholar]
- Predescu D, Palade G. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am J Physiol. 1993;265:H725–H733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- Putnam FW. The Plasma Proteins: Structure, Function, and Genetic Control. Academic Press; New York: 1984. [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci USA. 1985;82:5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao R, Yan W, Lum H, Malik A. Arg-Gly-Asp peptide increases endothelial hydraulic conductivity: comparison with thrombin response. Am J Physiol Cell Physiol. 1995;269:C110–C117. doi: 10.1152/ajpcell.1995.269.1.C110. [DOI] [PubMed] [Google Scholar]
- Qizilbash N. Fibrinogen and cerebrovascular disease. Eur Heart J. 1995;16:42–46. doi: 10.1093/eurheartj/16.suppl_a.42. [DOI] [PubMed] [Google Scholar]
- Rampling M, Meiselman H, Neu B, Baskurt O. Influence of cell-specific factors on red blood cell aggregation. Biorheology. 2004;41:91–112. [PubMed] [Google Scholar]
- Roberts A, Slaaf D, Joshua I. Potentiation of pulmonary arteriolar vasoconstriction to endothelin-1 by inhibition of nitric oxide synthesis in the intact lung. Microcirculation. 1998;5:289–298. [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. Mechanisms of shear-induced platelet-adhesion and aggregation. Thromb Haemost. 1993;70:119–123. [PubMed] [Google Scholar]
- Russell F, Skepper J, Davenport A. Endothelin peptide and converting enzymes in human endothelium. J Cardiovasc Pharmacol. 1998a;31:S19–S21. doi: 10.1097/00005344-199800001-00008. [DOI] [PubMed] [Google Scholar]
- Russell F, Skepper J, Davenport A. Human endothelial cell storage granules: a novel intracellular site for isoforms of the endothelin-converting enzyme. Circ Res. 1998b;83:314–321. doi: 10.1161/01.res.83.3.314. [DOI] [PubMed] [Google Scholar]
- Saenko E, Yaropolov A. Studies on receptor interaction of ceruloplasmin with human red blood cells. Biochem Int. 1990;20:215–225. [PubMed] [Google Scholar]
- Saldeen K, Nichols W, Lawson D, Andersson R, Saldeen T, Mehta J. Fibrin(ogen) degradation product peptide 6A increases femoral artery blood flow in dogs. Acta Physiol Scand. 1991;142:339–344. doi: 10.1111/j.1748-1716.1991.tb09166.x. [DOI] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Schmitz-Spanke S, Schipke J. Potential role of endothelin-1 and endothelin antagonists in cardiovascular diseases. Basic Res Cardiol. 2000;95:290–298. doi: 10.1007/s003950070048. [DOI] [PubMed] [Google Scholar]
- Sen U, Tyagi N, Patibandla PK, Dean WL, Tyagi SC, Roberts AM, Lominadze D. Fibrinogen-induced endothelin-1 production from endothelial cells. Am J Physiol Cell Physiol. 2009;296:C840–C847. doi: 10.1152/ajpcell.00515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyy J-J, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- Smith E. Fibrin deposition and fibrin degradation products in atherosclerotic plaques. Thromb Res. 1994;75:329–335. doi: 10.1016/0049-3848(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA. Interaction of integrins alpha v beta 3 and glycoprotein IIb-IIIa with fibrinogen. Differential peptide recognition accounts for distinct binding sites. J Biol Chem. 1990;265:12267–12271. [PubMed] [Google Scholar]
- Srivastava K, Magazine H. Thrombin receptor activation inhibits monocyte spreading by induction of ET(B) receptor-coupled nitric oxide release. J Immunol. 1998;161:5039–5044. [PubMed] [Google Scholar]
- Suehiro K, Gailit J, Plow E. Fibrinogen is a ligand for integrin alpha beta5beta1 on endothelial cells. J Biol Chem. 1997;272:5360–5366. doi: 10.1074/jbc.272.8.5360. [DOI] [PubMed] [Google Scholar]
- Suehiro K, Mizuguchi J, Nishiyama K, Iwanaga S, Farrell DH, Ohtaki S. Fibrinogen binds to integrin alpha(5)beta(1) via the carboxyl-terminal RGD site of the Aalpha-chain. J Biochem. 2000;128:705–710. doi: 10.1093/oxfordjournals.jbchem.a022804. [DOI] [PubMed] [Google Scholar]
- Tikkanen I, Uhlenius N, Tikkanen T, Miettinen A, Tornroth T, Fyhrquist F, Holthofer H. Increased renal expression of cytokines and growth factors induced by DOCA-NaCl treatment in Heymann nephritis. Nephrol Dial Transplant. 1995;10:2192–2198. doi: 10.1093/ndt/10.12.2192. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Roberts A, Dean W, Tyagi S, Lominadze D. Fibrinogen induces endothelial cell permeability. Mol Cell Biochem. 2008;307:13–22. doi: 10.1007/s11010-007-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Walter D, Zeiher A, Dimmler S. Laminar shear stress upregulates integrin expression: role in endothelial cell adhesion and apoptosis. Circ Res. 2000;87:683–689. doi: 10.1161/01.res.87.8.683. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vasse M, Paysant J, Soria J, Collet J, Vannier J, Soria C. Regulation of fibrinogen biosynthesis by cytokines, consequences on the vascular risk. Haemostasis. 1996;26:331–339. doi: 10.1159/000217313. [DOI] [PubMed] [Google Scholar]
- Vicaut E. Opposite effects of red blood cell aggregation on resistance to blood flow. J Cardiovasc Surg. 1995;36:361–368. [PubMed] [Google Scholar]
- Vicaut E, Hou X, Decuypère L, Taccoen A, Duvelleroy M. Red blood cell aggregation and microcirculation in rat cremaster muscle. Int J Microcirc. 1994;14:14–21. doi: 10.1159/000178201. [DOI] [PubMed] [Google Scholar]
- Wang Q, Doerschuk C. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol. 2001;166:6877–6884. doi: 10.4049/jimmunol.166.11.6877. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yerukhimovich M, Gaarde W, Popoff I, Doerschuk C. MKK3 and -6-dependent activation of p38 MAP kinase is required for cytoskeletal changes in pulmonary microvascular endothelial cells induced by ICAM-1 ligation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L359–L369. doi: 10.1152/ajplung.00292.2004. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, Jacques PF, Meigs JB, Rifai N, Selhub J, Robins SJ, Newton-Cheh C, Vasan RS. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–438. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- Watts SW. 5-Hydroxytryptamine-induced potentiation of of endothelin-1- and norepinephrine-induced contraction is mitogen-activated protein kinase pathway dependent. Hypertension. 2000;35:244–248. doi: 10.1161/01.hyp.35.1.244. [DOI] [PubMed] [Google Scholar]
- Weis S. Vascular permeability in cardiovascular disease and cancer. Curr Opin Hematol. 2008;15:243–249. doi: 10.1097/MOH.0b013e3282f97d86. [DOI] [PubMed] [Google Scholar]
- Weng X, Cloutier G, Beaulieu R, Roederer G. Influence of acute-phase proteins on erythrocyte aggregation. Am J Physiol Heart Circ Physiol. 1996;271:H2346–H2352. doi: 10.1152/ajpheart.1996.271.6.H2346. [DOI] [PubMed] [Google Scholar]
- Wiegman DL, Joshua IG, Morff RJ, Harris PD, Miller FN. Microvascular responses to norepinephrine in renovascular and spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 1979;236:H545–H548. doi: 10.1152/ajpheart.1979.236.4.H545. [DOI] [PubMed] [Google Scholar]
- Willoughby S, Loscanzo J. Vascular control of platelet function. In: Gresele P, Page CP, Fuster V, Vermylen J, editors. Platelets; In Thrombotic and Non-Thrombotic Disorders; Pathophysiology, Pharmacology and Therapeutics. Cambridge University Press; New York: 2002. pp. 432–455. [Google Scholar]
- Yamakaw K, Kitamura K, Nonoguchi H, Takasu N, Miller R, Tomita K. G alpha13 induces preproET-1 gene expression via JNK. Hypertens Res. 2002;25:427–432. doi: 10.1291/hypres.25.427. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M. The endothelium system. A new target for the therapeutic intervention. Circulation. 1994;89:1320–1322. doi: 10.1161/01.cir.89.3.1320. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1998;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Ishizaki T, Saski T, Murota S-I. Effect of carbon dioxide and oxygen on endothelin production by cultured porcine cerebral endothelial cells. Stroke. 1991;22:378–383. doi: 10.1161/01.str.22.3.378. [DOI] [PubMed] [Google Scholar]
- Yudkin J, Kumari M, Humphries S, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 1999;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zacharowski K, Zacharowski P, Reingruber S, Petzelbauer P. Fibrin(ogen) and its fragments in the pathophysiology and treatment of myocardial infarction. J Mol Med. 2006;84:1434–1440. doi: 10.1007/s00109-006-0051-7. [DOI] [PubMed] [Google Scholar]
- Zanetti A, Conforti G, Hess S, Martin-Padura I, Ghibaudi E, Preissner KT, Dejana E. Clustering of vitronectin and RGD peptides on microspheres leads to engagement of integrins on the luminal aspect of endothelial cell membrane. Blood. 1994;84:1116–1123. [PubMed] [Google Scholar]
- Zannad F, Stoltz J-F. Blood rheology in arterial hypertension. J Hypertens. 1992;10:S69–S78. [PubMed] [Google Scholar]
- Zhao H, Joshua I, Porter J. Microvascular responses to endothelin in deoxycorticosterone acetate-salt hypertensive rats. Am J Hypertens. 2000;13:819–826. doi: 10.1016/s0895-7061(00)00260-0. [DOI] [PubMed] [Google Scholar]
- Zilliacus H. Intravascular erythrocyte aggregation and the sedimentation reaction in local inflammation in the tissues. Acta Med Scand. 1951;140:149–151. doi: 10.1111/j.0954-6820.1951.tb10165.x. [DOI] [PubMed] [Google Scholar]