Abstract
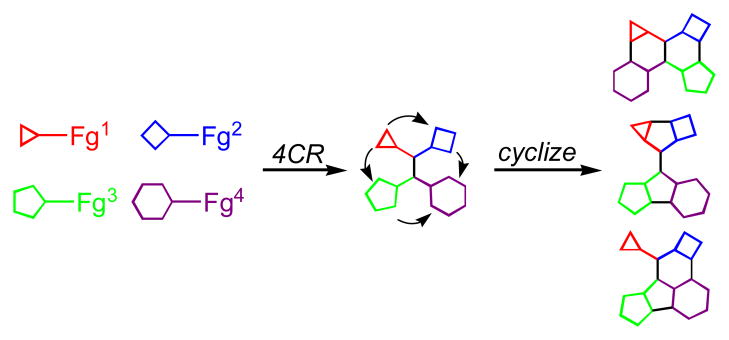
The sequencing of multicomponent reactions (MCRs) and subsequent cyclization reactions is a powerful stratagem for the rapid synthesis of diverse heterocyclic scaffolds. The optimal MCR is sufficiently flexible that it can be employed to generate adducts bearing a variety of functional groups that may then be selectively paired to enable different cyclization manifolds, thereby leading to a diverse collection of products. The growing interest in diversity-oriented synthesis has led to increased attention to this paradigm for library synthesis, which has inspired many advances in the design and implementation of MCRs for the construction of diverse heterocyclic scaffolds.
Keywords: multicomponent processes, cyclization, diversity-oriented synthesis, heterocyclic compounds, molecular diversity
Introduction
Diversity-oriented synthesis (DOS) continues to grow as an area of importance in the disciplines of organic synthesis and chemical biology.[1] A central goal of DOS is to create collections of structurally and stereochemically diverse molecules for evaluation in biological systems, hoping that a broader spectrum of diversity in the chemical library will lead to the generation of more information from biological screens. Of special interest are compounds which possess those molecular skeleta found in natural products and drug-like molecules. [2] Since the vast majority of natural products and drug-like compounds possess heterocyclic subunits, the ability to synthesize efficiently diverse heterocyclic compounds is critical.
Arguably, one of the most promising synthetic strategies for generating collections of small molecules by DOS involves the sequencing of multicomponent reactions (MCRs) with subsequent transformations, including cyclizations and refunctionalizations, that form new compounds possessing increased molecular complexity and diversity.[3] This process of sequencing MCRs with subsequent cyclizations is commonly referred to as the build/couple/pair strategy of DOS.[4] Ideally, the MCRs will be sufficiently versatile that each input for the MCR can incorporate a wide range of functionalities and substituents. Using the tactic of functional group pairing,[5] the MCR adduct is then selectively transformed in ring forming processes and refunctionalizations comprising the synthome[6] into the target heterocyclic structures. Moreover, the ring forming reactions should be chosen so that the products they generate still contain functional handles that can be further derivatized by other carbon-carbon or carbon-heteroatom bond forming reactions. The major advantage of combining MCRs with post-condensation modifications relative to other strategies for DOS is that it enables access to a number of functionalized heterocyclic scaffolds in a short number of steps.
The fields of DOS and MCRs have recently been extensively reviewed,[7] so this Concept Article will focus only on providing an overview of the applications of MCRs that have been sequenced with multiple ring-forming reactions that lead thereby to the synthesis of diverse heterocyclic scaffolds.
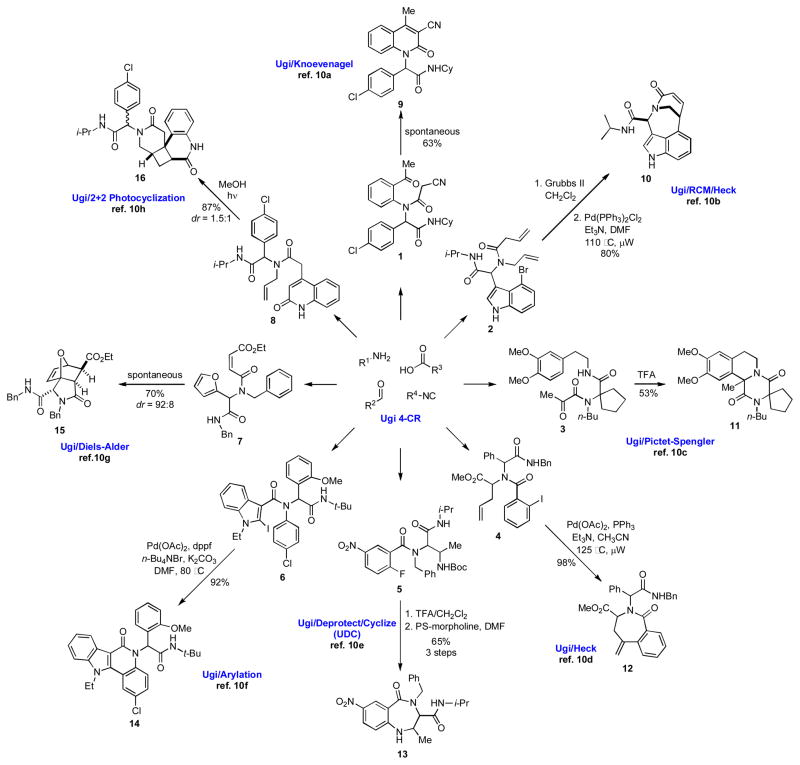
Applications of the Ugi Reaction
Since its original publication in 1959, the Ugi four component reaction (U-4CR) has emerged as the most well known and widely used MCR in organic synthesis.[3,8] The U-4CR is extraordinarily functional group tolerant, and each of the four components can contain functionality that can be used in subsequent transformations, thus allowing for access to a number of cyclization manifolds. It thus does not occasion surprise that a substantial body of work has been devoted to post condensation modifications of U-4CR adducts.[9] A brief overview showing some of the post-condensation cyclization manifolds accessible by the U-4CR is presented in Scheme 2.[10] Upon examining structures 9–16, it is evident that considerable structural diversity can be generated in only two or three steps by sequencing the U-4CR with different cyclization reactions.
Scheme 2.
An overview of the U-4CR post condensation cyclizations.
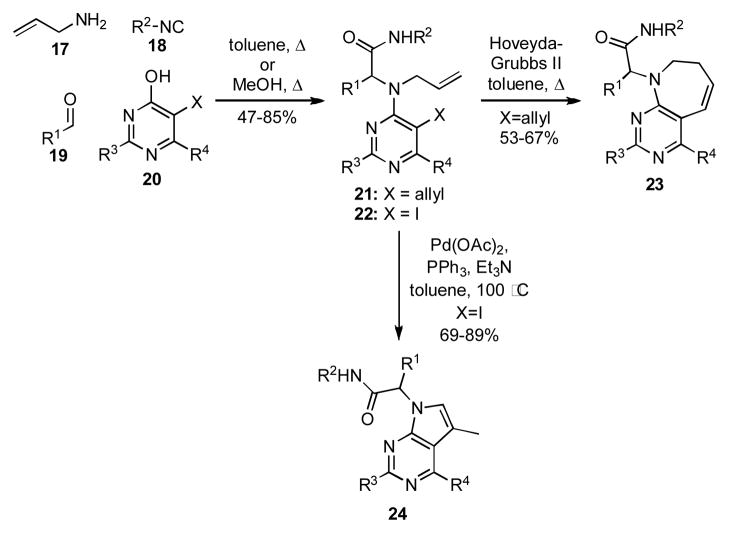
In 2005 El Kaïm and Grimaud reported a variation on the U-4CR referred to as the Ugi-Smiles coupling.[11] In the Ugi-Smiles process, an electron deficient phenol is substituted for the carboxylic acid normally used in the U-4CR. The Ugi-Smiles reaction works well with a variety of electron deficient phenols, including heterocyclic phenols like the pyrimidines 20 (Scheme 3). By incorporating different functional handles into 20, various cyclization modes could be accessed. When an allyl group was incorporated into 20 (X = allyl), the products that were formed underwent sequential ring closing metathesis (RCM) and double bond isomerization to give 23.[12] Similarly, an Ugi-Smiles/Heck cyclization pathway could be implemented when the iodopyrimidine 20 (X = I) was used, thus providing the pyrrolopyrimidine 24.[13] Only the RCM and Heck reaction have been used to date to manipulate the products of Ugi-Smiles reactions, but there is clearly the potential for sequencing the Ugi-Smiles condensation with other reactions analogous to what has been done with adducts formed by the U-4CR.
Scheme 3.
Sequential Ugi-Smiles/RCM or Ugi-Smiles/Heck
Applications of the Petasis 3CR
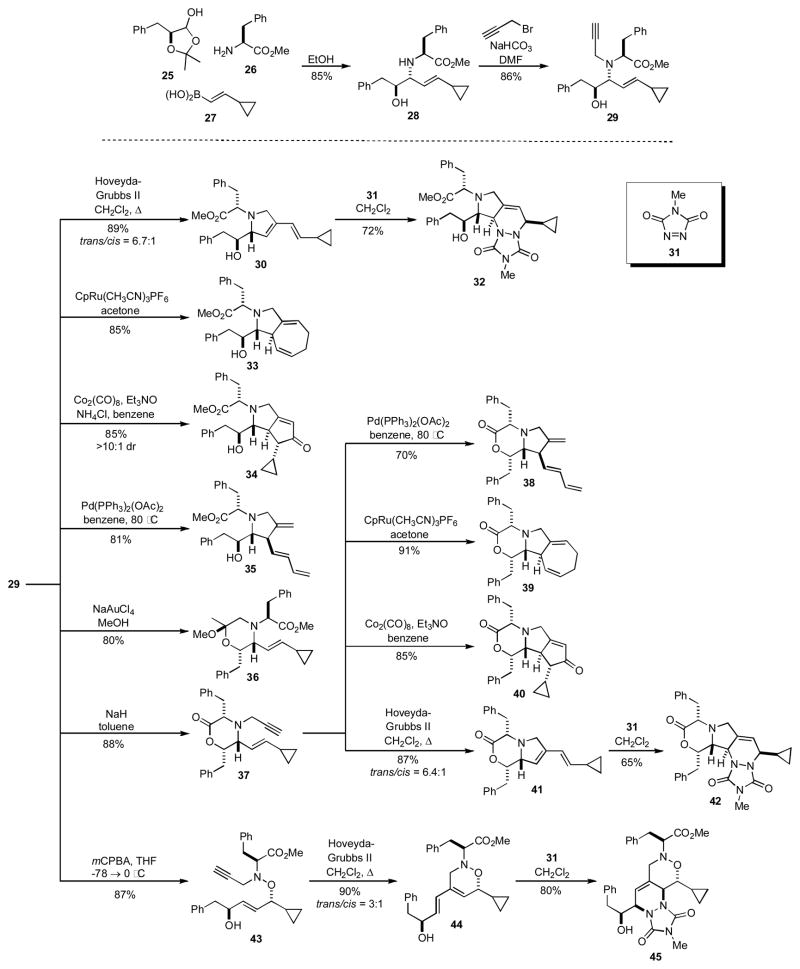
The Petasis three component reaction, which has also been termed a boronic acid Mannich reaction, consists of the coupling of an aldehyde, amine, and boronic acid.[14] Schreiber has shown that the Petasis reaction can be nicely exploited to synthesize a multifunctional template that is suitable for further elaboration by a number of pathways (Scheme 4).[15] For example, coupling of lactol 25, amine 26, and boronic acid 27 gave 28 in good yield. The adduct 28 is already highly functionalized, but in order to maximize its potential, a subsequent propargylation of 28 to give 29 was implemented to provide the desired multifunctional template. The need for a subsequent elaboration of the initial MCR product reveals one of the drawbacks of the Petasis 3CR and illustrates why employing MCRs that can incorporate all the requisite functionality into the product of the MCR are preferred. This modest shortcoming notwithstanding, the highly functionalized amine 29 was readily elaborated by seven different selective functional group pairing reactions to generate the structurally distinct scaffolds 30–45. Because several of these transformations exploit the enyne functionality found in 29, this example highlights the utility of enyne motifs for generating diverse structures.
Scheme 4.
Schreiber’s use of the Petasis 3CR for the synthesis of 15 distinct heterocyclic scaffolds.
This elegant application of the Petasis 3CR to DOS represents one of the few examples where the potential of a MCR product was fully realized. Every functional handle in 29 was utilized in a subsequent ring forming reaction to give the targeted heterocycles 30–45. This work nicely demonstrates the power of utilizing all of the functional handles in MCR adducts as a strategy for maximizing the number and types of scaffolds that can be accessed.
Applications of the van Leusen 3CR
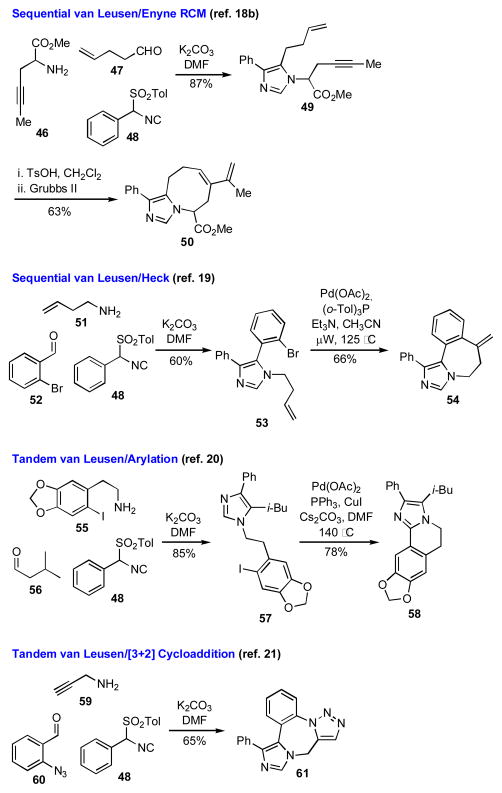
The van Leusen imidazole synthesis is a 3CR in which an aldehyde, an amine, and a substituted TosMIC reagent are combined to form a substituted imidazole in one pot. [16] When the substituents on the imidazole ring are suitably functionalized to allow subsequent cyclizations, polycyclic imidazole scaffolds, which are important owing to their presence in a number of natural products and biologically active compounds, may be readily prepared.[17]
The application of the van Leusen 3CR followed by subsequent ring closing reactions to the production of substituted imidazoles and fused imidazoles is nicely exemplified by the work of Djuric and co-workers (Scheme 5). By incorporating either two alkenes or an alkene and an alkyne into the starting materials, intermediates were prepared that could be cyclized by various RCM reactions.[18] For example, the imidazole 49 contains an enyne motif that was utilized in an enyne RCM to give 50. Compounds amenable to further transformation via palladium-catalyzed ring closures were also readily generated by using an aryl halide as one of the inputs. Tricycle 54 was prepared via a sequential van Leusen/Heck reaction sequence,[19] and compounds such as 58 could be accessed via a Pd catalyzed C-H activation.[20] When an appropriately disposed azide and alkyne functionalities were incorporated as inputs in the reaction, intermediates were generated that underwent a spontaneous azide-alkyne cycloaddition to give fused triazolo imidazole scaffolds such as 61.[21]
Scheme 5.
Diverse heterocyclic scaffolds accessible by the van Leusen 3CR.
The van Leusen 3CR has considerable promise for preparing compounds that may be converted into diverse imidazole containing structures. However, there is but a solitary example wherein functionality is incorporated into the isocyanide input that might be exploited in subsequent cyclizations.18a The expansion of this methodology to include functional handles on the TosMIC derivative would significantly expand the scope of targets accessible by the van Leusen 3CR.
Applications of the Amide/Aldeyhde/Dienophile Reaction
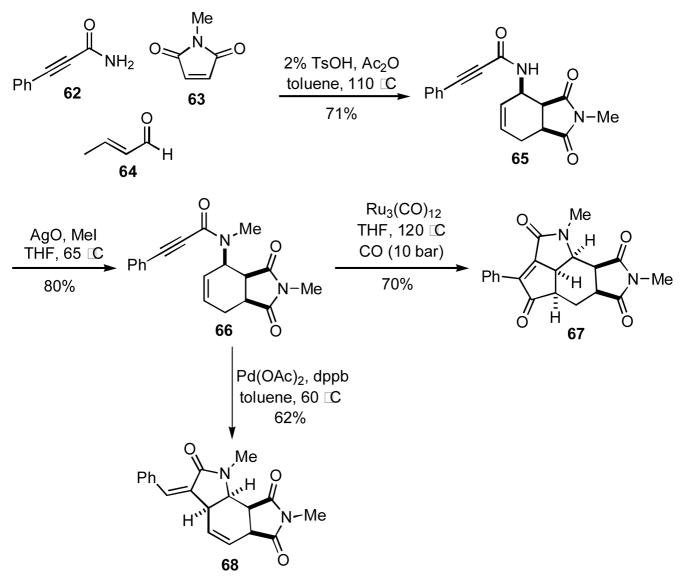
A novel MCR developed by Beller involving the coupling of an amide, aldehyde, and dienophile (AAD reaction) has been used to prepare compounds that may be elaborated by post-condensation modifications.[22] For example, the AAD reaction of amide 62 with crotonaldehyde (64) and maleimide 63 provided the enyne 65 (Scheme 6).[23] Subsequent methylation of 65 provided 66. As demonstrated by Schrieber (see Scheme 4), the enyne moiety is a versatile functional motif that may be transformed via different cyclizations manifolds by simply modifying reagents or catalysts to provide a variety of scaffolds. Accordingly, 66 could be converted into 67 via a ruthenium catalyzed Pauson-Khand reaction or into 68 by a palladium catalyzed Alder-ene reaction.
Scheme 6.
Sequential AAD/Pauson-Khand and AAD/Alder-ene reactions.
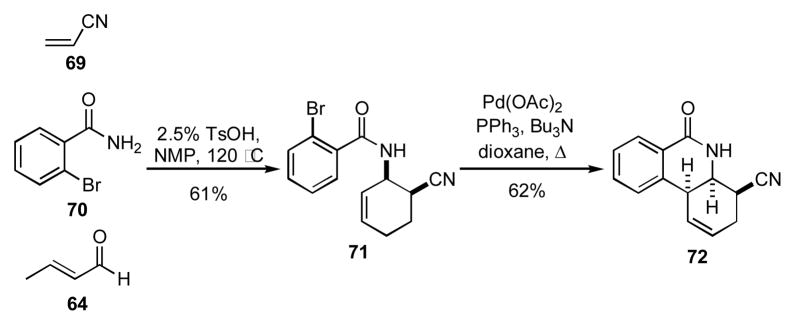
Alternatively, when the amide input also possessed an aryl halide group as found in 70, substrates similar to 71 could be synthesized (Scheme 7).[24] Subsequent cyclization of 71 via a Heck reaction afforded the phenanthridone 72. The phenanthridone core structure can be found in a number of alkaloids,25 thus highlighting the broader utility of this method to access natural product-like structures.
Scheme 7.
Sequential AAD/Heck reaction.
Applications of the α,γ-Difunctionalization Reaction of β-Ketoamides
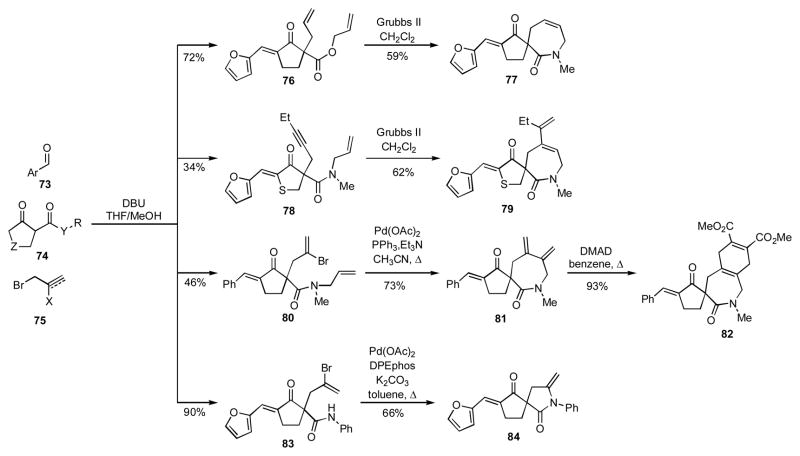
Spirocycles are present in a number of natural products and biologically active compounds, and they are consequently attractive targets for synthetic chemists.[26] Rodriguez has recently reported an interesting 3CR involving aldehydes 73, β-ketoesters or amides 74, and allylic or propargylic halides 75 that can be utilized for synthesizing spiroheterocyclic structures (Scheme 8).[ 27 ] By incorporating the appropriate functional groups in the intermediate adducts, a number of different cyclization modes could be accessed. For example, by a suitable combination of inputs, compounds 76 and 78 were prepared and cyclized via RCM into 77 and 79, respectively. A similar 3CR was employed to prepare the vinyl bromide 80 that was converted via an intermolecular Heck reaction into diene 81, which then underwent a Diels-Alder reaction with dimethyl acetylenedicarboxylate (DMAD) to give 82. Pairing of the vinyl bromide and amide functionalities in 83 under conditions developed by Buchwald[28] yielded 84.
Scheme 8.
α,γ-Difunctionalization reaction of β-ketoamides and its application to the synthesis of spiroheterocycles.
Applications of the 4-Component Triazinane Dione Synthesis
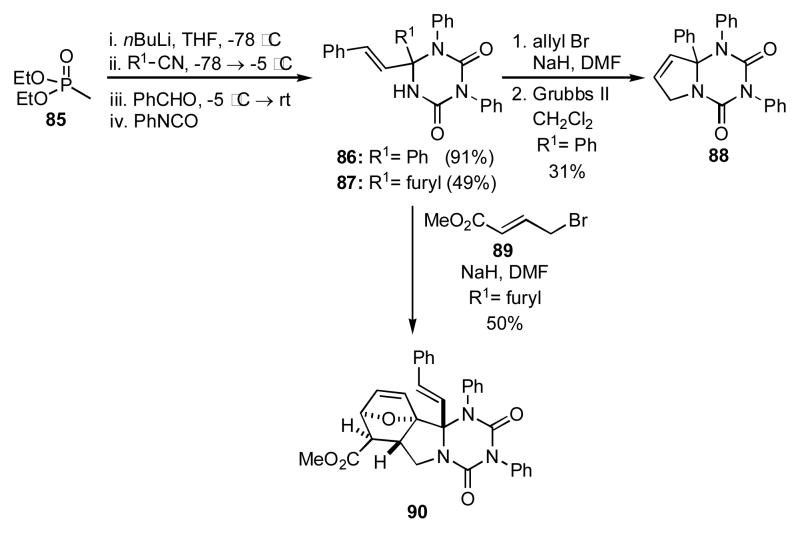
Orru has recently developed a 4CR for the synthesis of triazinane diones by a process that entails the sequential combination of a phosphonate, nitrile, aldehyde, and two equivalents of an isocyanate.[29] While it has not been shown that the triazinane dione products themselves can be directly manipulated into other more complex heterocycles, an additional alkylation step affords substrates suitable for further cyclizations (Scheme 9).[30] The utility of this 4CR for heterocyclic synthesis can be illustrated by the combination of phosphonate 85, benzonitrile, benzaldehyde, and phenyl isocyanate to yield 86 (R1 = Ph), which underwent N-allylation to give an intermediate that was transformed into 88 by RCM. Use of 2-furonitrile as the nitrile input gave 87 (R1 = furyl), which upon N-alkylation with 89 gave a compound that underwent a spontaneous intramolecular Diels-Alder to give 90. While the need for a second alkylation step detracts somewhat from the overall efficiency and convenience, this methodology shows some promise as a quick entry into a diverse library of structures having triazinane dione subunits.
Scheme 9.
A 4CR approach to the synthesis of diverse triazinane diones.
In all of these examples, only the alkyl halide and ester (or amide) incorporated functionality that was utilized in subsequent reactions. Further expansion of this methodology to access substrates suitable for additional modes of cyclization which utilize the aldehyde component would greatly enhance the usefulness of this methodology for the synthesis of diverse spiroheterocycles.
Applications of a Mannich-Type 4CR
In 2007 Martin published a 4CR that involved combining aldehydes, amines, acid chlorides, and nucleophiles to provide access to highly functionalized amide products that could be readily subjected to a variety of subsequent ring closures to give a diverse collection of heterocyclic scaffolds.[6] One of the important features of this approach to DOS is that there is considerable flexibility associated with the additional functionality that may be incorporated in each of the components. This allows for greater opportunity in the functional group pairing that is essential for transforming the initial adducts via different cyclization manifolds. A few examples will illustrate the potential of this novel strategy.
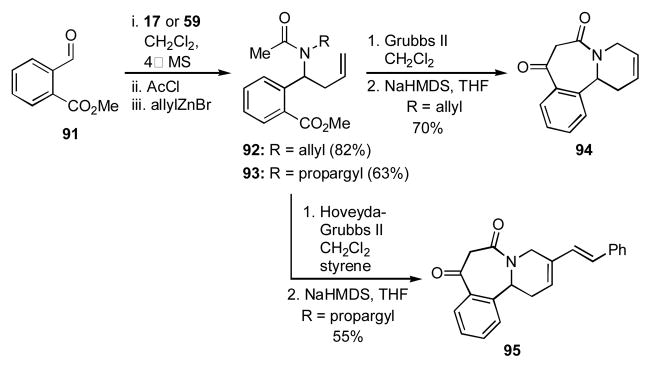
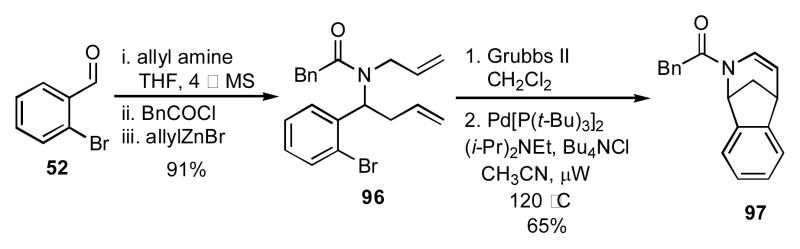
When aldehyde 91 was sequentially reacted with allylamine, acetyl chloride, and allylzinc bromide, the amide 92 was obtained (Scheme 10). The two olefins in 92 were then paired via RCM, and the amide and ester paired in a Dieckmann cyclization to give the benzazepine dione 94. Similarly, when propargyl amine was used as the amine input in the initial condensation reaction, the resulting amide 93 could be transformed by a tandem enyne RCM/cross-metathesis in the presence of styrene, a fifth input, followed by a Dieckmann cyclization to give the benzazepine 95. Changing the aldehyde component in the initial 4CR to o-bromobenzaldehyde (52) provided the intermediate amide 96, which was elaborated by a RCM and a subsequent Heck reaction to give the bridged azatricycle 97 (Scheme 11).
Scheme 10.
Sequential Mannich-type 4CR/RCM/Dieckmann Cyclization.
Scheme 11.
Sequential Mannich-type 4CR/RCM/Heck Cyclization.
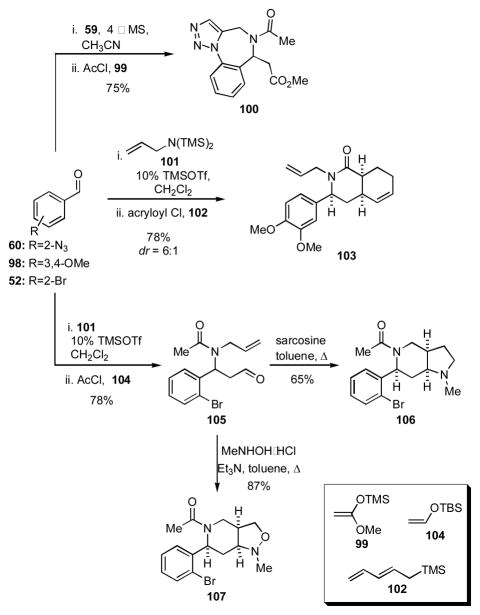
By judiciously varying the inputs in this Mannich-type 4CR, a number of different cycloaddition manifolds become accessible (Scheme 12). For example, when o-azidobenzaldehyde (60) and propargylamine are among the components in the 4CR, the intermediate amide thus formed may undergo spontaneous azide/alkyne [3+2] dipolar cycloaddition to give triazoles such as 100. Alternatively, use of acryloyl chloride as the acylating agent and 2,4-pentadienyltrimethylsilane (102) as the nucleophile can lead to intermediates that will undergo facile intramolecular Diels-Alder cycloaddition to give compounds related to 103. When the nucleophile is the silyl enol ether 104 and the amine or acylating agent contains a carbon-carbon double or triple bond, adducts such as 105 are produced. Such compounds may be used as precursors to azomethine ylides or nitrones that will undergo spontaneous [3+2] dipolar cycloadditions to give bicycles 106 and 107, respectively.
Scheme 12.
Sequential Mannich-type 4CR/Cycloaddition.
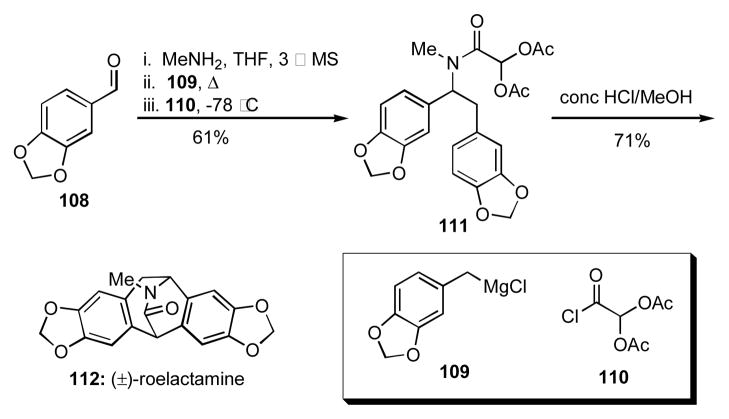
The utility of this Mannich-type 4CR for the facile synthesis of complex natural products has also been demonstrated (Scheme 13). Namely, the sequential reaction of piperonal (108) with methyl amine, the benzylic Grignard reagent 109, and the acid chloride 110 delivered 111, which underwent an acid catalyzed double cyclization to give the isopavine alkaloid (±)-roelactamine (112).
Scheme 13.
Application of the Mannich-type 4CR to the synthesis of (±)-roelactamine.
Conclusion
The sequencing of MCRs with subsequent ring forming reactions is truly one of the best methods for rapidly accessing heterocyclic scaffolds. When the MCRs that are employed are flexible enough to enable incorporation of a broad range of functional groups, and these functional groups are chosen so that a variety of post MCR transformations can be employed, then the structures of accessible scaffolds are greatly increased. The examples presented herein illustrate that with careful selection of the MCR inputs, a single MCR can be used to generate versatile intermediates that may be readily transformed into diverse collections of compounds. However, there remains the need to develop new MCRs that allow access to different scaffolds, as well as the need to find new functional group pairing processes to implement on the MCR products so compounds having natural product or drug like structures may be easily prepared.
Scheme 1.
Sequencing of MCRs with post condensation cyclizations to generate diverse scaffolds.
Acknowledgments
We thank the National Institutes of Health (GM 25439), Pfizer, Inc., Merck Research Laboratories, and the Robert A. Welch Foundation for their generous support of our research reported herein.
References
- 1.a) Burke MD, Schreiber SL. Angew Chem. 2004;116:48–60. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2004;43:46–58. [Google Scholar]; b) Spring DR. Org Biomol Chem. 2003;1:3867–3870. doi: 10.1039/b310752n. [DOI] [PubMed] [Google Scholar]; c) Tan DS. Nat Chem Biol. 2005;1:74–84. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]; d) Spandl RJ, Bender A, Spring DR. Org Biomol Chem. 2008;6:1149–1158. doi: 10.1039/b719372f. [DOI] [PubMed] [Google Scholar]
- 2.a) Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]; b) Constantino L, Barlocco D. Curr Med Chem. 2006;13:65–85. [PubMed] [Google Scholar]
- 3.a) Zhu J, Bienaymé H, editors. Multicomponent Reactions. Wiley-VCH; Weinheim: 2005. [Google Scholar]; b) Dömling A. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen TE, Schreiber SL. Angew Chem. 2008;120:52–61. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2008;47:48–56. [Google Scholar]
- 5.Comer E, Rohan E, Deng L, Porco JA., Jr Org Lett. 2007;9:2123–2126. doi: 10.1021/ol070606t. [DOI] [PubMed] [Google Scholar]
- 6.The synthome has been defined as the set of all reactions available to the chemist for synthesis. See: Sunderhaus JD, Dockendorff C, Martin SF. Org Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357.
- 7.See refs. 1,3, 8, and 9 and references therein.
- 8.a) Dömling A, Ugi I. Angew Chem. 2000;112:3300–3344. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.For reviews, see: Marcaccini S, Torroba T. In: Multicomponent Reactions. Zhu J, Bienaymé H, editors. Wiley-VCH; Weinheim: 2005. pp. 33–75.Tempest PA. Curr Oinp Drug Discovery Dev. 2005;8:776–788.Akritopoulou-Zanze I, Djuric SW. Heterocycles. 2007;73:125–146.
- 10.a) Marcaccini S, Pepino R, Pozo MC, Basurto S, García-Valverde M, Torroba T. Tetrahedron Lett. 2004;45:3999–4001. [Google Scholar]; b) Ribelin TP, Judd AS, Akritopoulou-Zanze I, Henry RF, Cross JL, Whittern DN, Djuric SW. Org Lett. 2007;9:5119–5122. doi: 10.1021/ol7023373. [DOI] [PubMed] [Google Scholar]; c) El Kaim L, Gageat M, Gaultier L, Grimaud L. Synlett. 2007:500–502. [Google Scholar]; d) Gracias V, Djuric SW. Tetrahedron Lett. 2004;45:417–420. [Google Scholar]; e) Tempest P, Pettus L, Gore V, Hulme C. Tetrahedron Lett. 2003;44:1947–1950. [Google Scholar]; f) Ma Z, Xiang Z, Luo T, Lu K, Xu Z, Chen J, Yang Z. J Comb Chem. 2006;8:696–704. doi: 10.1021/cc060066b. [DOI] [PubMed] [Google Scholar]; g) Paulvannan K. Tetrahedron Lett. 1999;40:1851–1854. [Google Scholar]; h) Akritopoulou-Zanze I, Whitehead A, Waters JE, Henry RF, Djuric SW. Org Lett. 2007;9:1299–1302. doi: 10.1021/ol070164l. [DOI] [PubMed] [Google Scholar]
- 11.El Kaïm L, Grimaud L, Oble J. Angew Chem. 2005;117:8175–8178. doi: 10.1002/anie.200502636. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2005;44:7961–7964. doi: 10.1002/anie.200502636. [DOI] [PubMed] [Google Scholar]
- 12.El Kaïm L, Grimaud L, Oble J. J Org Chem. 2007;72:5835–5838. doi: 10.1021/jo070706c. [DOI] [PubMed] [Google Scholar]
- 13.El Kaïm L, Gizzi M, Grimaud L. Org Lett. 2008;10:3417–3419. doi: 10.1021/ol801217a. [DOI] [PubMed] [Google Scholar]
- 14.Petasis NA. In: Multicomponent Reactions. Zhu J, Bienaymé H, editors. Wiley-VCH; Weinheim: 2005. pp. 199–223. [Google Scholar]
- 15.Kumagai N, Muncipinto G, Schreiber SL. Angew Chem. 2006;118:3717–3720. doi: 10.1002/anie.200600497. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2006;45:3635–3638. doi: 10.1002/anie.200600497. [DOI] [PubMed] [Google Scholar]
- 16.van Leusen AM, Wilderman J, Oldenzeil OH. J Org Chem. 1977;42:1153–1159. [Google Scholar]
- 17.a) Greenlee WJ, Siegel PKS. Annu Rep Med Chem. 1992;27:59–68. [Google Scholar]; b) Hodges JC, Hamby JM, Blankley CJ. Drugs Future. 1992;17:575–593. [Google Scholar]; c) Meanwell NA, Romine JL, Seiler SM. Drugs Future. 1994;19:361–385. [Google Scholar]
- 18.a) Gracias V, Gasiecki AF, Djuric SW. Org Lett. 2005;7:3183–3186. doi: 10.1021/ol050852+. [DOI] [PubMed] [Google Scholar]; b) Gracias V, Gasiecki AF, Djuric SW. Tetrahedron Lett. 2005;46:9049–9052. [Google Scholar]
- 19.Beebe X, Gracias V, Djuric SW. Tetrahedron Lett. 2006;47:3225–3228. [Google Scholar]
- 20.Gracias V, Gasiecki AF, Pagano TG, Djuric SW. Tetrahedron Lett. 2006;47:8873–8876. [Google Scholar]
- 21.Gracias V, Darczak D, Gasiecki AF, Djuric SW. Tetrahedron Lett. 2005;46:9053–9056. [Google Scholar]
- 22.Neumann H, von Wangelin AJ, Gördes D, Spannenberg A, Beller M. J Am Chem Soc. 2001;123:8398–8399. doi: 10.1021/ja010503k. [DOI] [PubMed] [Google Scholar]
- 23.a) Strübing D, Neumann H, Klaus S, Hübner S, Beller M. Tetrahedron. 2005;61:11333–11344. [Google Scholar]; b) Strübing D, Neumann H, Klaus S, Hübner S, Beller M. Tetrahedron. 2005;61:11345–11354. [Google Scholar]
- 24.von Wangelin AJ, Neumann H, Gördes D, Hübner S, Wendler C, Klaus S, Strübing D, Spannenberg A, Jiao H, El Firdoussi L, Thurow K, Stoll N, Beller M. Synthesis. 2005:2029–2038. [Google Scholar]
- 25.Kornienko A, Evidente A. Chem Rev. 2008;108:1982–2014. doi: 10.1021/cr078198u. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.a) Pradhan R, Patra M, Behera AK, Mishra BK, Behera RK. Tetrahedron. 2006;62:779–828. [Google Scholar]; b) El Bialy SAA, Braun H, Tietze LF. Synthesis. 2004:2249–2262. [Google Scholar]; c) Martin JD. In: Studies in Natural Products Chemistry. Atta-ur-Rahman, editor. Vol. 6. Elsevier; Amsterdam: 1990. p. 59. [Google Scholar]
- 27.Habib-Zahmani H, Viala J, Hacini S, Rodriguez J. Synlett. 2007:1037–1042. [Google Scholar]
- 28.Wolfe JP, Wagaw S, Buchwald SL. Org Lett. 1999;1:35–37. doi: 10.1021/ol9905351. [DOI] [PubMed] [Google Scholar]
- 29.Groenendaal B, Vugts DJ, Schmitz RF, de Kanter FJJ, Ruijter E, Groen MB, Orru RVA. J Org Chem. 2008;73:719–722. doi: 10.1021/jo701973d. [DOI] [PubMed] [Google Scholar]
- 30.Groenendaal B, Ruijter E, de Kanter FJJ, Lutz M, Spek AL, Orru RVA. Org Biomol Chem. 2008;6:3158–3165. doi: 10.1039/b807138a. [DOI] [PubMed] [Google Scholar]